Abstract
Background:
To systematically review the efficacy and safety of second-line medications for status epilepticus (SE).
Methods:
Electronic searches were conducted in PubMed, Embase, and The Cochrane Library for randomized controlled trials of second-line medications for SE from inception to January 2024. Two reviewers independently screened literature, extracted data, and assessed the risk of bias of included studies. Network meta-analysis was performed using R 4.2.2 software.
Results:
A total of 23 randomized controlled trials were analyzed, examining the efficacy of 5 different treatment regimens: levetiracetam (LEV), phenytoin (PHT), fosphenytoin (FPHT), valproate (VPA), and phenobarbital (PHB). The results of the network meta-analysis indicated that the seizure control rate ranking was as follows: PHB (98.1%) > LEV (60.7%) > FPHT (40.3%) > PHT (33.0%) > VPA (17.8%). The surface under the cumulative ranking (SUCRA) values revealed that PHB had the highest ranking (SUCRA, 91.8%), followed by VPA (SUCRA, 69.3%), PHT (SUCRA, 56.1%), and FPHT (SUCRA, 5.9%) for the recurrence of seizures within 24 hours. Subgroup analysis revealed that PHB was most effective for seizure control in both pediatric and adult populations, VPA demonstrated superior efficacy in children across various indicators, LEV was deemed the safest option for children and elderly individuals, and VPA was identified as the safest choice for adult patients.
Conclusions:
PHB continues to be a prominent option for managing SE, although its safety profile warrants careful consideration. Meanwhile, both VPA and LEV offer distinctive advantages in the treatment of SE, with each demonstrating commendable safety profiles.
Keywords: network meta-analysis, randomized controlled trial, second-line medications, status epilepticus
1. Introduction
Status epilepticus (SE) is a common emergency in neurology, characterized by prolonged seizures or multiple seizures without returning to a normal level.[1,2] SE can lead to neuronal damage, respiratory and circulatory system complications, and other adverse outcomes, with high rates of morbidity and mortality.[3] Statistics show that the mortality rate of SE is 9% to 22%, and it increases with age.[4,5] Early control of SE can reduce neuronal damage and lower the risk of related complications and mortality, making the timely termination of the convulsive state the primary goal of SE treatment.[6]
Currently, the first-line treatment for SE is benzodiazepines, which can control about 70% of seizures.[7] However, despite the effectiveness of benzodiazepines as initial therapy, a significant proportion of patients may continue to experience seizures, necessitating the use of second-line treatments. Since phenytoin (PHT) was used for SE as a second-line treatment in 1970, several intravenous preparations, including fosphenytoin (FPHT), phenobarbital (PHB), valproate (VPA), and levetiracetam (LEV), have been recommended by guidelines as second-line treatments for SE.[8] Despite the array of available options, determining the safest and most effective medication regimen for SE remains a challenge. Each of these medications possesses unique pharmacokinetic and pharmacodynamic properties, as well as varying profiles of efficacy and safety. Factors such as patient comorbidities, concurrent medications, and the underlying etiology of SE further complicate treatment selection.
For example, while PHB has a long history of use in the management of SE and is associated with rapid seizure control, its sedative properties and potential for respiratory depression limit its utility, particularly in critically ill patients or those with compromised respiratory function.[9,10] On the other hand, newer agents like LEV offer the advantage of rapid onset of action and favorable side effect profiles but may lack the robust evidence base of older antiepileptic drugs (AED).[11]
Furthermore, the lack of head-to-head randomized controlled trials (RCTs) directly comparing these agents in the context of SE poses a significant challenge in determining their relative efficacy and safety. Much of the existing evidence stems from observational studies, retrospective analyses, or extrapolations from trials conducted in other seizure populations, limiting the strength, and generalizability of conclusions drawn.
In light of these challenges, there is an urgent need for well-designed prospective studies, ideally employing network meta-analysis techniques, to compare the efficacy, safety, and tolerability of various second-line treatment regimens for SE. By synthesizing data from multiple sources and accounting for indirect treatment comparisons, network meta-analysis offers a powerful tool for informing clinical decision-making and guideline development in this critical area of epilepsy management.
2. Materials and methods
Given that this study employs a network meta-analysis methodology utilizing data that has already been published, there is no need to seek ethical clearance.
2.1. Inclusion and exclusion criteria
Studies were included if the following inclusion criteria (PICOS) were met: population (P): the study population consists of patients with SE who have not achieved control following the administration of initial AEDs. SE is characterized by seizures lasting longer than 5 minutes, with no specific restrictions based on patient demographics such as gender, age, race, onset time, or duration of illness; interventions (I): the drug regimens under examination include two-by-two comparisons or multiple comparisons of LEV, PHT/FPHT, VPA, and PHB; outcomes (O): the primary outcome indicators include the SE control rate, defined as the proportion of SE attacks ceasing within 60 minutes, and the recurrences of seizures within 24 hours. Secondary outcome indicators encompass the rate of additional AED treatment required and the incidence of adverse events (AEs); study (S): all relevant RCTs were included.
Exclusion criteria include the following: (1) repetitive studies, (2) incomplete or inaccurate study data that is not extractable, and (3) literature not written in Chinese or English.
2.2. Literature search strategy
A systematic search was conducted in multiple electronic databases, including PubMed, Embase, and The Cochrane Library to identify RCTs on second-line medications for SE from the inception of the databases to January 2024. Additionally, references of included studies were screened to gather supplementary information. Search terms included #1 levetiracetam, #2 valproate, #3 phenytoin OR fosphenytoin, #4 phenobarbital OR luminal, #5 #1 OR #2 OR #3 OR #4, #6 status epilepticus, #7 random, #8 #5 AND #6 AND #7.
2.3. Literature screening and data extraction
Two researchers conducted a thorough review of the literature, extracting data, and cross-checking their findings independently. Disagreements were resolved through discussion or consultation with a third party. The screening process involved initially reviewing titles to exclude obviously irrelevant literature, followed by a more detailed examination of abstracts and full texts to determine inclusion. In cases where additional information was required, the researchers contacted the original study authors via email or phone to obtain crucial but uncertain data. The data extraction process encompassed the retrieval of various components, such as the basic information of included studies (e.g., study title, first author, published journal), baseline characteristics and intervention measures of study subjects, key elements for assessing the risk of bias, and outcome indicators along with result measurement data of interest.
2.4. Risk of bias assessment of included studies
Two researchers conducted an independent evaluation of the risk of bias in the studies included in the analysis and subsequently verified the findings. The assessment of bias utilized the RCT bias risk assessment tool as outlined in the Cochrane Handbook 5.1.0. Assessed by 2 investigators according to the Cochrane Handbook of Systematic Reviews criteria for evaluating risk of bias in RCTs, with decisions made through discussion between third parties or corresponding authors in case of disagreement. The evaluation included: (1) random sequences generation; (2) allocation concealment; (3) performance bias (blinding of participants and personnel); (4) detection bias (blinded of outcome assessment); (5) incomplete outcome data; (6) reporting bias; (7) other bias. The risk of bias was assessed according to the above 7 risk of bias, with low risk of bias, high risk of bias, lack of information or uncertainty about the bias.
2.5. Statistical analysis
Evidence network diagrams were constructed utilizing R software to visually represent the direct and indirect comparison relationships among various treatment regimens. Bayesian network meta-analysis was conducted utilizing the gemtc package in R 4.1.0 software, employing 4 Markov chains with 50,000 iterations and 20,000 burn-in iterations to mitigate the impact of initial values. Consistency tests were carried out utilizing the node-splitting method. If the statistical analysis showed that the discrepancy between direct and indirect comparison outcomes was not significant (P ≥ .05), it suggested a high level of consistency, prompting the utilization of a consistency model analysis. In cases where certain nodes exhibited a P < .05, signifying local inconsistency, direct comparisons for those nodes were conducted using Stata 15.1 software. The iterative convergence was assessed through the potential scale reduction factor, with a value falling between 1 and 1.05 denoting acceptable convergence. In the analysis of binary variables, the risk ratio was employed as the effect measure statistic, accompanied by its corresponding 95% confidence interval. The calculation of the surface under the cumulative ranking curve (SUCRA) and the visualization of the SUCRA curve were facilitated by the utilization of the ranking probability matrix table generated from R software. A higher SUCRA value signifies a superior ranking of the treatment regimen. Subgroup analysis was performed based on age, with statistical significance denoted by a P-value of <.05. Funnel plots were drawn for each outcome indicator and the risk of bias was assessed by Egger test, if P > .05, the risk of publication bias is small, and vice versa, the risk of publication bias is large.
3. Results
3.1. Literature screening process and results
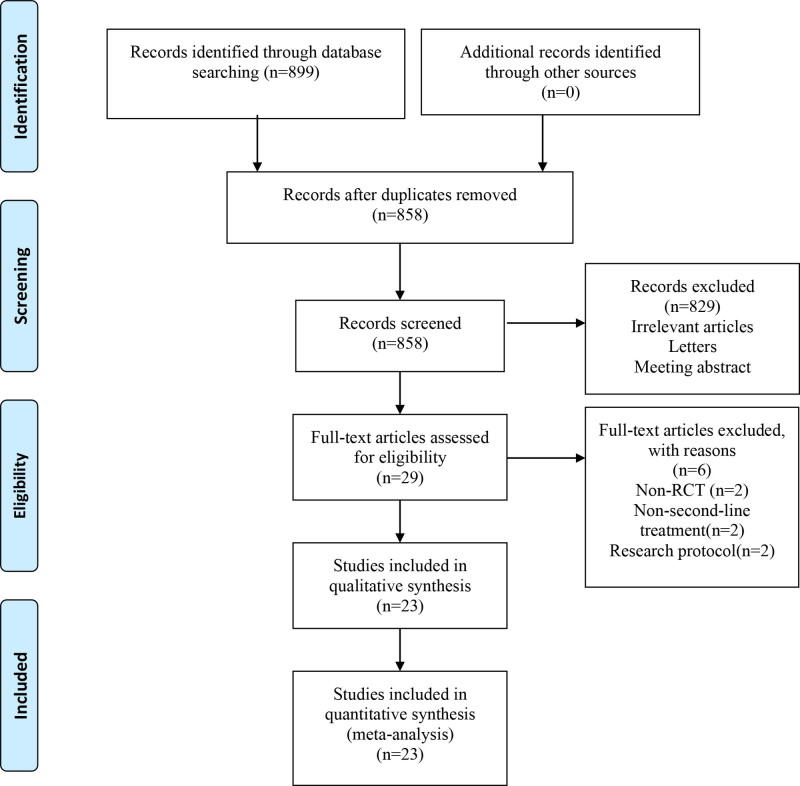
A total of 859 relevant articles were initially identified. After a step-by-step screening process, 23 RCTs[12–34] were finally included, involving 3554 patients. The literature screening process and results are shown in Figure. 1.
Figure 1.
Flow diagram of the literature selection process.
3.2. General characteristics and risk of bias evaluation of included studies
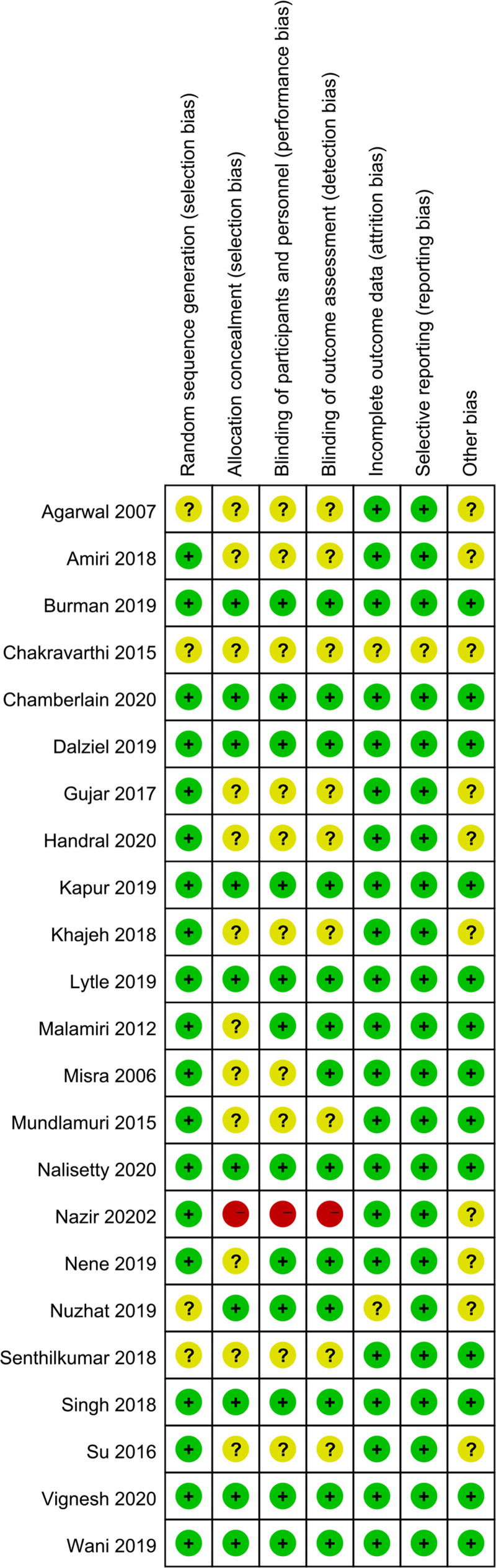
The general characteristics of the included studies are shown in Table 1. Publication year ranged from 2006 to 2020. Diagnosis included SE, convulsive status epilepsy and generalized convulsive status epilepsy. Age of the patients ranged 2.6 to 81.5. Number of the patients ranged from 15 to 152. And the results of the risk of bias evaluation are shown in Figures 2 and 3.
Table 1.
General characteristic of the included studies.
| Study | Country | Diagnosis | Mean age (years) | Number of patients | Sex (male/female) | Treatment 1 | Treatment 2 | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 1 | Treatment 2 | |||||||
| Chamberlain (2020) | America | SE | 6.1 ± 4.3 | 85 | 71 | 124/101 | LEV | FPHT | 1, 4 | |
| 69 | 71 | VPA | FPHT | |||||||
| 85 | 69 | LEV | VPA | |||||||
| 42.6 ± 14.1 | 71 | 54 | 108/78 | LEV | FPHT | 1, 4 | ||||
| 61 | 54 | VPA | FPHT | |||||||
| 71 | 61 | LEV | VPA | |||||||
| 73.8 ± 7.2 | 19 | 17 | 30/21 | LEV | FPHT | 1, 4 | ||||
| 15 | 17 | VPA | FPHT | |||||||
| 19 | 15 | LEV | VPA | |||||||
| Nalisetty (2020) | India | SE | 2.6 ± 2.8 | 32 | 29 | 16/16 | 16/13 | LEV | FPHT | 1, 2, 3, 4 |
| Handral (2020) | India | CSE | 3.4 ± 3.4 | 58 | 58 | 32/26 | 36/22 | LEV | FPHT | 1, 2, 4 |
| Nazir (2020) | India | SE | 5.1 ± 3.9 | 50 | 50 | 36/14 | 35/15 | LEV | PHT | 2 |
| 50 | 50 | 33/17 | 35/15 | VPA | PHT | |||||
| 50 | 50 | 36/14 | 33/17 | LEV | VPA | |||||
| Vignesh (2020) | India | CSE | 4.5 ± 3.8 | 32 | 35 | 18/14 | 19/16 | LEV | PHT | 1, 3, 4 |
| 35 | 35 | 21/14 | 19/16 | VPA | PHT | |||||
| 32 | 35 | 18/14 | 21/14 | LEV | VPA | |||||
| Kapur (2019) | America | SE | 32.8 ± 25.6 | 145 | 118 | 77/68 | 71/47 | LEV | FPHT | 1, 2, 4 |
| 121 | 118 | 65/56 | 71/47 | VPA | FPHT | |||||
| 145 | 121 | 77/68 | 65/56 | LEV | VPA | |||||
| Lytle (2019) | America | CSE | 27 (1.3–59) | 152 | 134 | 75/77 | 72/62 | LEV | PHT | 1, 3, 4 |
| Dalziel (2019) | Australia | CSE | 3.9 ± 3.8 | 119 | 114 | 59/60 | 53/61 | LEV | PHT | 1, 3, 4 |
| New Zealand | ||||||||||
| Wani (2019) | India | SE | 4.1 ± 3.7 | 52 | 52 | 32/20 | 34/18 | LEV | PHT | 1, 2, 3, 4 |
| Burman (2019) | South Africa | CSE | 2.3 (1.3–5.5) | 36 | 33 | 18/18 | 18/15 | PHB | PHT | 1 |
| Nuzhat (2019) | Pakistan | GCSE | 3.5 ± 0.2 | 300 | 300 | 216/84 | 190/110 | LEV | PHT | 1, 4 |
| Nene (2019) | India | GCSE | 67.5 ± 7.4 | 50 | 50 | – | LEV | VPA | 1, 3, 4 | |
| Senthilkumar (2018) | India | CSE | 2.8 ± 2.9 | 25 | 25 | 18/7 | 16/9 | LEV | FPHT | 1, 2, 4 |
| Khajeh (2018) | Iran | SE | 4.4 ± 4.7 | 40 | 40 | 15/25 | 13/27 | PHB | VPA | 1 |
| Singh (2018) | India | SE | 7.2 ± 2.5 | 50 | 50 | 32/18 | 26/24 | LEV | PHT | 2 |
| Amiri (2018) | Iran | SE | 42.9 ± 16.7 | 55 | 55 | 27/28 | 24/31 | PHT | VPA | 1, 4 |
| Gujar (2017) | Oman | SE | 37.8 ± 18.0 | 22 | 30 | 13/9 | 21/9 | LEV | PHT | 1, 4 |
| Su (2016) | China | GCSE | 41.7 ± 17.1 | 37 | 36 | 19/18 | 20/16 | PHB | VPA | 1, 2, 4 |
| Mundlamuri (2015) | India | GCSE | 33.7 ± 17.0 | 50 | 50 | 32/18 | 28/22 | LEV | PHT | 1, 3, 4 |
| 50 | 50 | 28/22 | 28/22 | VPA | PHT | |||||
| 50 | 50 | 32/18 | 28/22 | LEV | VPA | |||||
| Chakravarthi (2015) | India | SE | 35.4 ± 16.0 | 22 | 22 | 12/10 | 15/7 | LEV | PHT | 1, 2, 4 |
| Malamiri (2012) | Iran | CSE | 5.5 (3.0–16.0) | 30 | 30 | 21/9 | 16/14 | PHB | VPA | 1, 2, 4 |
| Agarwal (2007) | India | SE | 27.2 ± 15.9 | 50 | 50 | 32/18 | 35/15 | PHT | VPA | 1, 4 |
| Misra (2006) | U.K. | SE | 1.0–85.0 | 33 | 35 | 17/16 | 24/11 | PHT | VPA | 1, 4 |
AED = antiepileptic drugs, CSE = convulsive status epilepsy, FPHT = fosphenytoin, GCSE = generalized convulsive status epilepsy, LEV = levetiracetam, PHB = phenobarbital, PHT = phenytoin, VPA = valproic acid, SE = status epilepticus. 1 = SE control rate, 2 = the recurrences of seizures within 24 hours. 3 = rate of additional AED treatment required; 4 = the incidence of adverse events.
Figure 2.
Risk of bias graph of the included studies.
Figure 3.
Risk of bias summary of the included studies.
3.3. Results of network meta-analysis
3.3.1. Evaluation of iterative convergence
Iterative convergence was evaluated for the 4 outcome indicators. The results showed that the potential scale reduction factors were all between 1 and 1.05, indicating good data convergence after 50,000 iterations (Figure S1, Supplemental Digital Content, http://links.lww.com/MD/N836).
3.3.2. SE control rate
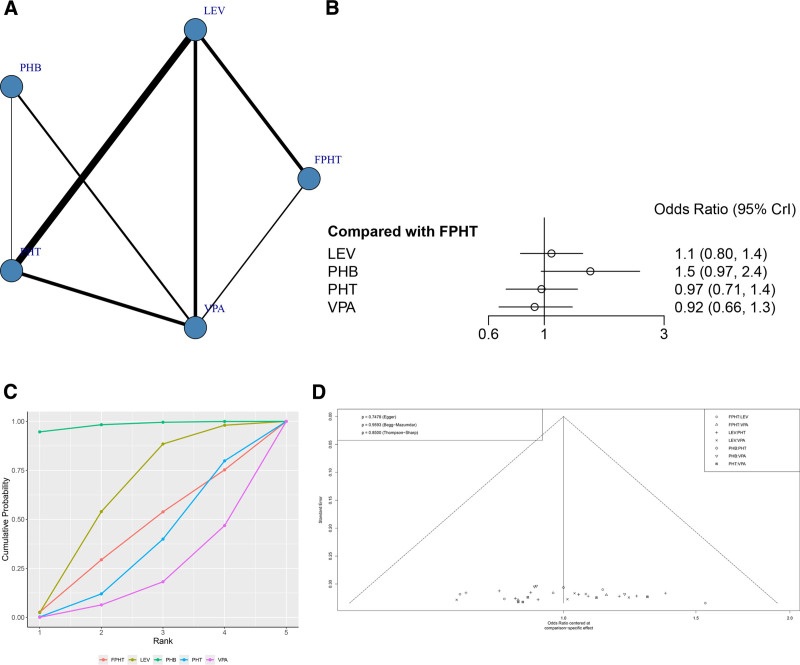
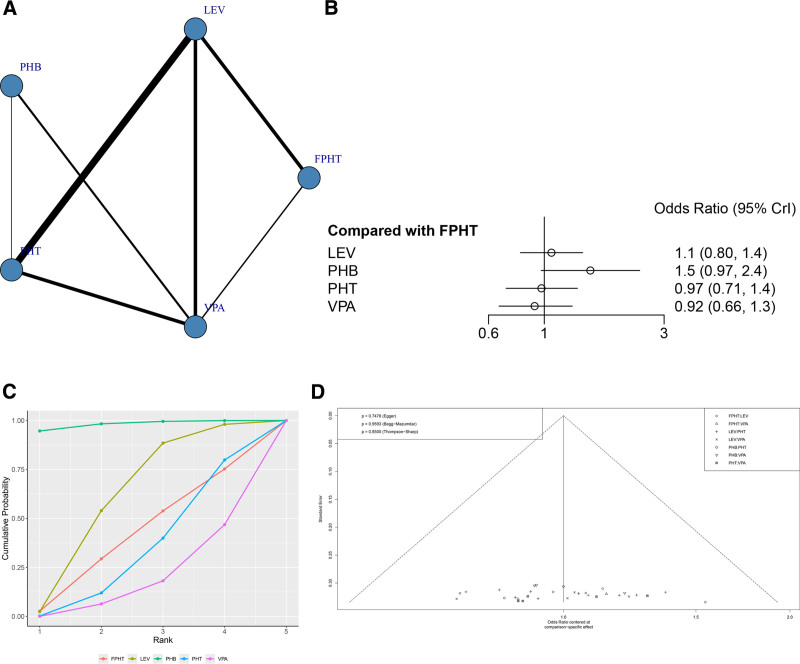
A total of 21 RCTs reported the SE control rate (Fig. 4A). The node-splitting method showed that the P values between direct and indirect comparisons were all > .05, indicating no statistically significant difference (Figure S2, Supplemental Digital Content, http://links.lww.com/MD/N836). The network meta-analysis results showed that no statistically significant difference was found when the 2 interventions were compared against each other (Fig. 4B and Table 2).
Figure 4.
(A) Network structure diagrams of SE control rate. (B) Forest plot of the SE control rate as compared with FPHT. (C) SUCRA probabilities of different treatments for SE control rate. (D) Funnel plot of the different treatments for SE control rate. FPHT = fosphenytoin, SE = status epilepticus, SURCA = surface under the cumulative ranking curve.
Table 2.
Efficacy of different comparisons for SE control rate by ORs and corresponding 95% CIs.
| FPHT | ||||
| 0.94 (0.71, 1.25) | LEV | |||
| 0.66 (0.42, 1.02) | 0.7 (0.48, 1.01) | PHB | ||
| 1.03 (0.74, 1.43) | 1.1 (0.91, 1.33) | 1.56 (1.09, 2.25) | PHT | |
| 1.1 (0.78, 1.52) | 1.17 (0.93, 1.47) | 1.67 (1.21, 2.31) | 1.07 (0.84, 1.34) | VPA |
CI = confidence interval, FPHT = fosphenytoin, LEV = levetiracetam, PHB = phenobarbital, PHT = phenytoin, VPA = valproic acid.
The SUCRA results showed that PHB might be the treatment regimen with the highest SE control rate: PHB (98.1%) > LEV (60.7%) > FPHT (40.3%)>PHT (33.0%) > VPA (17.8%), as shown in Figure 4C. It can be seen that there is no publication bias only on SE control rate (P = .7478, Fig. 4D).
3.3.3. Recurrences of seizures within 24 hours
A total of 11 RCTs reported recurrences of seizures within 24 hours (Fig. 5A). The node-splitting method showed that the P values between direct and indirect comparisons were >.05, indicating no statistically significant difference (Figure S3, Supplemental Digital Content, http://links.lww.com/MD/N836). The network meta-analysis results showed no statistically significant difference in the recurrences of seizures within 24 hours between the 2 groups (Fig. 5B and Table 3). The SUCRA shows that PHB ranked first (SUCRA, 91.8%), VPA ranked second (SUCRA, 69.3%), PHT ranked third (SURCA, 56.1%) and FPHT ranked the last (SUCRA, 5.9%, Fig. 5C). It can be seen that the funnel plot is basically symmetrical, and the P-value of Egger test is .4541, indicating that there is no obvious publication bias in this study (Fig. 5D).
Figure 5.
(A) Network structure diagrams of SE control rate. (B) Forest plot of the SE control rate as compared with FPHT. (C) SUCRA probabilities of different treatments for the recurrences of seizures within 24 hours. (D) Funnel plot of the different treatments for the recurrences of seizures within 24 hours. FPHT = fosphenytoin, SE = status epilepticus, SURCA = surface under the cumulative ranking curve.
Table 3.
Efficacy of different comparisons for the recurrences of seizures within 24 hours by ORs and corresponding 95% CIs.
| FPHT | ||||
| 1.18 (0.87, 1.58) | LEV | |||
| 1.91 (1.02, 3.62) | 1.63 (0.91, 2.96) | PHB | ||
| 1.34 (0.94, 1.92) | 1.14 (0.92, 1.43) | 0.7 (0.38, 1.25) | PHT | |
| 1.45 (1.02, 2.06) | 1.23 (0.95, 1.6) | 0.76 (0.44, 1.27) | 1.08 (0.83, 1.4) | VPA |
CI = confidence interval, FPHT = fosphenytoin, LEV = levetiracetam, PHB = phenobarbital, PHT = phenytoin, VPA = valproic acid.
3.3.4. Rate of further AED treatment needed
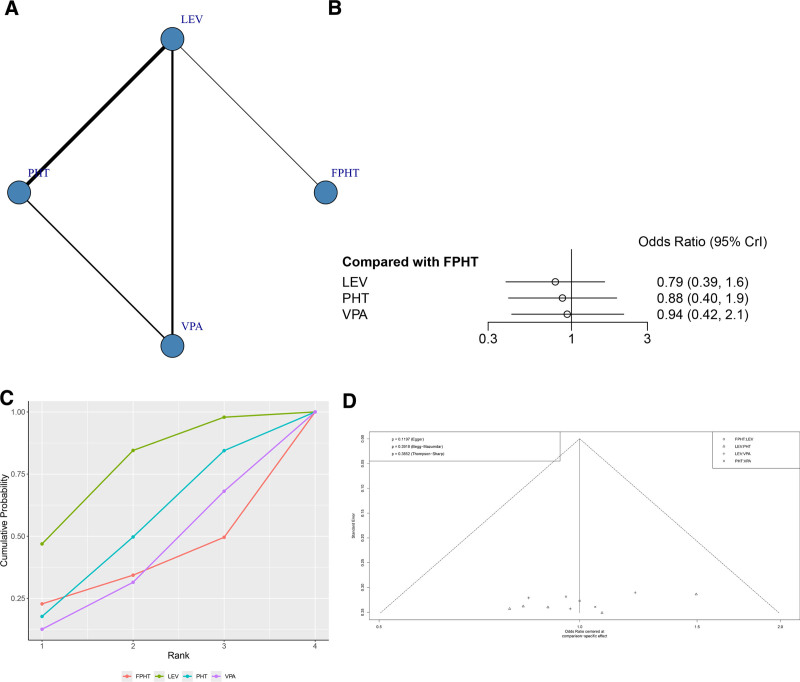
A total of 11 RCTs reported rate of further AED treatment needed (Fig. 6A). The node-splitting method showed that the P values between direct and indirect comparisons were >.05, indicating no statistically significant difference (Figure S4, Supplemental Digital Content, http://links.lww.com/MD/N836). The network meta-analysis results showed no statistically significant difference in the rate of further AED treatment needed between the 2 groups (Fig. 6B and Table 4). The SUCRA results showed that the rate of further AED treatment needed might be lowest in the LEV group: LEV (76.4%) > PHT (50.6%) > VPA (37.4%)>FPHT (35.6%) (Fig. 6C). It can be seen that the funnel plot is basically symmetrical, and the P-value of Egger test is .4541, indicating that there is no obvious publication bias in this study (Fig. 6D).
Figure 6.
(A) Network structure diagrams of the rate of additional AED treatment required. (B) Forest plot of the rate of additional AED treatment required as compared with FPHT. (C) SUCRA probabilities of different treatments for rate of additional AED treatment required. (D) Funnel plot of the different treatments for rate of additional AED treatment required. AED = antiepileptic drugs, FPHT = fosphenytoin, SURCA = surface under the cumulative ranking curve.
Table 4.
Efficacy of different comparisons for the rate of additional AED treatment required by ORs and corresponding 95% CIs.
| FPHT | |||
| 1.27 (0.62, 2.6) | LEV | ||
| 1.15 (0.52, 2.5) | 0.9 (0.65, 1.24) | PHT | |
| 1.07 (0.47, 2.41) | 0.84 (0.57, 1.24) | 0.93 (0.61, 1.42) | VPA |
AED = antiepileptic drugs, CI = confidence interval, FPHT = fosphenytoin, LEV = levetiracetam, PHT = phenytoin, VPA = valproic acid.
3.3.5. Incidence of AEs
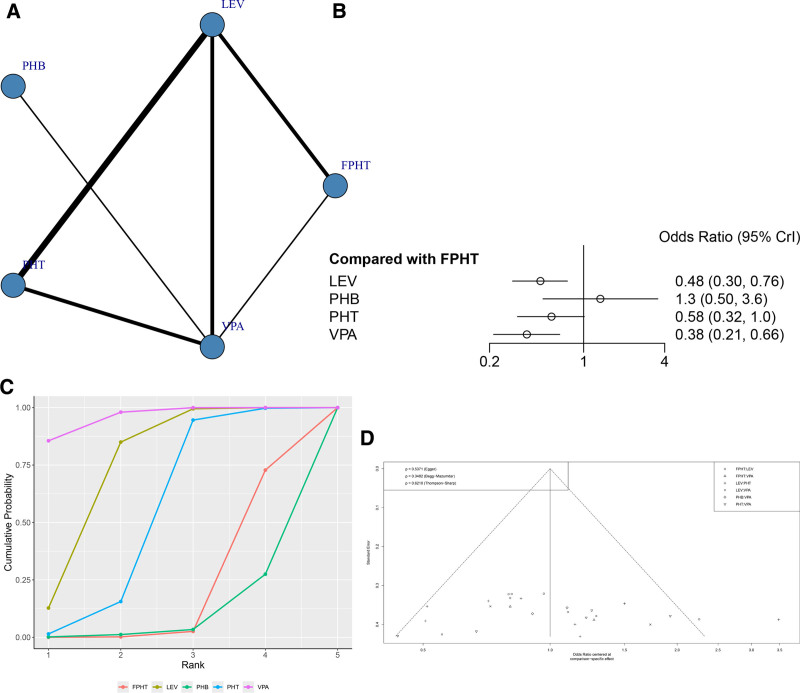
A total of 19 RCTs reported the incidence of AEs (Fig. 7A). The node-splitting method showed that the P values between direct comparisons of VPA and PHT and indirect comparisons were >.05, indicating no statistically significant difference (Figure S5, Supplemental Digital Content, http://links.lww.com/MD/N836). The network meta-analysis results showed that the incidence of adverse drug event (ADE) was lower for LEV than FPHT, VPA had a lower incidence of AEs than FPHT with statistically significant differences (Fig. 7B and Table 5). The SUCRA results showed that the incidence of AEs might be lowest in the VPA group: VPA (95.9%) > LEV (74.3%) > PHT (52.8%)>FPHT (18.9%)>PHB (8.1%) (Fig. 7C). It can be seen that the funnel plot is basically symmetrical, and the P-value of Egger test is .5371, indicating that there is no obvious publication bias in this study (Fig. 7D).
Figure 7.
(A) Network structure diagrams of the incidence of AEs. (B) Forest plot of the incidence of AEs as compared with FPHT. (C) SUCRA probabilities of different treatments for the incidence of AEs. (D) Funnel plot of the different treatments for the incidence of AEs. AEs = adverse events, FPHT = fosphenytoin, SURCA = surface under the cumulative ranking curve.
Table 5.
Efficacy of different comparisons for the incidence of adverse events by ORs and corresponding 95% CIs.
| FPHT | ||||
| 2.1 (1.32, 3.37) | LEV | |||
| 0.75 (0.28, 1.99) | 0.36 (0.14, 0.88) | PHB | ||
| 1.74 (0.99, 3.1) | 0.83 (0.58, 1.2) | 2.32 (0.94, 5.77) | PHT | |
| 2.64 (1.51, 4.68) | 1.26 (0.83, 1.92) | 3.53 (1.6, 7.86) | 1.52 (0.99, 2.33) | VPA |
CI = confidence interval, FPHT = fosphenytoin, LEV = levetiracetam, PHT = phenytoin, VPA = valproic acid.
3.4. Subgroup analysis results
Subgroup analysis was conducted for each outcome indicator based on age demographics. The findings indicated that PHB may be the optimal treatment regimen for children and adult patients in terms of SE control rate, while LEV may be more effective for elderly patients. In a mixed patient population, VPA demonstrated the most favorable effect. Additionally, for adult patients, the SE control rates of LEV, PHT, and VPA were all inferior to PHB. Moreover, VPA was identified as the treatment regimen associated with the lowest 24-hour epilepsy recurrence rate for children and mixed patient cohorts. VPA may represent the treatment regimen associated with the lowest rate of subsequent AED therapy required for pediatric patients. In terms of ADE occurrence, LEV may be considered the most secure treatment option for children and elderly individuals, whereas VPA may be deemed the safest choice for adults and patients with a combination of age groups (Table 6).
Table 6.
Subgroup analysis of the results.
| Indication | Number of studies | SURCA |
|---|---|---|
| SE control rate | ||
| Children | 12 | PHB(95.5%) > VPA(42.1) > LEV(41.7) > PHT(20.2) > FPHT(20.2) |
| Adult | 7 | PHB(99.3%) > LEV(55.6) > VPA(31.8) > PHT(14.9) > FPHT(10.8) |
| Elderly | 2 | LEV(70.9%) > VPA(46.9) > PHT(30.9) |
| Mixed | 2 | VPA(77.9%) > LEV(55.9) > PHT(25.5) |
| Epilepsy recurrence rate | ||
| Children | 7 | VPA(80.5%) > LEV(45.9) > PHT(40.9) > PHB(33.9) > FPHT(16.9) |
| Adult | 2 | |
| Mixed | 2 | VPA(75.5%) > LEV(55.6) > PHT(22.9) |
| Rate of further AED treatment needed | ||
| Children | 5 | VPA(78.9%) > LEV(55.9) > PHT(23.2) |
| Incidence of ADEs | ||
| Children | 10 | LEV(93.6) > PHT(52.3) > VPA(42.6%) > PHB(10.6) > FPHT(8.5) |
| Adult | 7 | VPA(92.2%) > PHB(72.6) > LEV(40.3) > PHT(25.6) > FPHT(18.9) |
| Elderly | 2 | LEV(75.6%) > VPA(69.2) > PHT(8.3) |
| Mixed | 2 | VPA(82.6%) > LEV(66.9) > PHT(10.3) |
ADE = adverse drug events, AED = antiepileptic drugs, FPHT = fosphenytoin, LEV = levetiracetam, PHB = phenobarbital, PHT = phenytoin, VPA = valproic acid, SE = status epilepticus, SURCA = surface under the cumulative ranking curve.
4. Discussion
PHT is a well-established medication endorsed in second-line treatment protocols for SE, demonstrating evident efficacy and extensive clinical application. Nevertheless, its administration rate is constrained by the occurrence of adverse reactions. FPHT, a prodrug of PHT, allows for administration at a threefold increased rate compared to PHT. However, as FPHT necessitates hydrolysis into PHT to manifest therapeutic effects, its efficacy and safety profile do not surpass that of PHT.[35] Research indicates that high doses of PHB effectively manage SE and reduce the likelihood of seizure recurrence, while VPA exhibits favorable tolerability.[36] The emergence of lacosamide (LEV) as a novel AED has garnered significant clinical interest attributed to its reduced adverse reaction profile and expedited administration. Two substantial, methodologically rigorous RCTs have investigated the comparative efficacy and safety of LEV, PHT, and VPA as second-line treatments for SE, revealing comparable outcomes in terms of efficacy and AED-related AEs.[16,20] However, certain RCTs have indicated that LEV may offer superior safety and efficacy compared to PHT or VPA.[26,33] Consequently, there exists substantial debate regarding the optimal second-line therapeutic approach for SE.
The findings of this study suggest that the efficacy rankings for seizure control rate are as follows: PHB > LEV > VPA > PHT; the rankings for 24-hour epilepsy recurrence rate are VPA > PHB > LEV > PHT; the rankings for the rate of further AED treatment needed are LEV > PHT > VPA; and in terms of safety, the ADE incidence of LEV and VPA was lower than that of PHT, with VPA > LEV > PHT > PHB.
The subgroup analysis indicates that PHB is the most effective treatment for seizure control in both children and adults, while VPA is preferred for other efficacy indicators in children. The analysis further highlights the significant adverse reactions associated with PHB, resulting in its limited use among elderly patients and a lack of reports on its use in this population within the study. VPA emerges as the optimal treatment option for children in terms of efficacy indicators. In terms of safety profiles, LEV is deemed the safest option for children and elderly patients, while VPA is considered the safest choice for adults and mixed patient populations.
The growing utilization of LEV in pediatric and geriatric populations can be attributed to their reduced tolerance to VPA, which is associated with a higher prevalence of ADEs in these age groups.[31] Additionally, as a novel AED, LEV offers the benefits of ease of administration and a decreased likelihood of adverse reactions. Recent research has indicated that PHB stimulates gamma-aminobutyric acid (GABA) receptors, resulting in heightened frequency of chloride channel opening and prolonged duration of channel opening. Furthermore, it has been demonstrated that PHB can decrease glutamate release. While PHB exhibits superior efficacy in managing SE compared to other AED due to its multifaceted antiepileptic mechanism, its increased likelihood of adverse reactions restricts its clinical utility. In contrast, VPA and LEV present fewer adverse reactions and benefits in the treatment of SE. However, VPA is constrained by a narrow therapeutic window, and variations in blood drug concentrations among different individuals may compromise safety, particularly in special populations. LEV exhibits minimal impact on hepatic and renal function within the standard therapeutic range, and there is no clear correlation between dosage and adverse reactions. This attribute makes it a favorable choice for pediatric and geriatric patient populations.
The study is constrained by various limitations, such as the absence of detailed reporting on specific randomization methods, allocation concealment, and blinding in certain included RCTs, which may introduce biases in selection and measurement. Furthermore, the predominant focus on studies conducted in Asian regions implies potential regional constraints. Moreover, the 2015 redefinition of SE by the International League Against Epilepsy, which previously characterized it as seizures lasting longer than 30 minutes, has introduced clinical heterogeneity as a result of inconsistent definitions utilized in different studies. The limited sample sizes in several studies may have compromised the reliability of trial outcomes. Furthermore, variations in the age distribution of patients across the literature have posed challenges in the comparison of findings between studies. Moreover, significant variations were observed in the drug dosages administered to various cohorts of patients.
5. Conclusions
In summary, current research indicates that PHB continues to be a prominent option for managing SE, although its safety profile warrants careful consideration. Meanwhile, both VPA and LEV offer distinctive advantages in the treatment of SE, with each demonstrating commendable safety profiles. VPA is particularly recommended for adult patients, while LEV is often preferred for pediatric and elderly populations.
Author contributions
Funding acquisition: Ziyi Wei.
Investigation: Shaokang Peng.
Methodology: Shaokang Peng, Ziyi Wei.
Project administration: Ziyi Wei.
Software: Xiangshu Cheng.
Validation: Qishun Zhang.
Visualization: Qishun Zhang, Xiangshu Cheng.
Writing – original draft: Qishun Zhang.
Writing – review & editing: Xiangshu Cheng.
Supplementary Material
Abbreviations:
- AEs
- adverse events
- AED
- antiepileptic drugs
- FPHT
- fosphenytoin
- LEV
- levetiracetam
- PHB
- phenobarbital
- PHT
- phenytoin
- RCTs
- randomized controlled trials
- SE
- status epilepticus
- SURCA
- surface under the cumulative ranking curve
- VPA
- valproate
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Zhang Q, Peng S, Wei Z, Cheng X. Comparative efficacy and safety of second-line medications for status epilepticus: A network meta-analysis. Medicine 2024;103:46(e40333).
Contributor Information
Qishun Zhang, Email: zhangqishun090@qq.com.
Shaokang Peng, Email: pengshaokang90@qq.com.
Ziyi Wei, Email: weiziyi787@qq.com.
References
- [1].Benghanem S, Robieux EP, Neligan A, et al. Status epilepticus: what’s new for the intensivist. Curr Opin Crit Care. 2024. [DOI] [PubMed] [Google Scholar]
- [2].Dedeoglu O, Akça H, Emeksiz S, et al. Management of status epilepticus by different pediatric departments: neurology, intensive care, and emergency medicine. Eur Neurol. 2023;86:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang S, Wu X, Xue T, et al. Efficacy and safety of levetiracetam versus valproate in patients with established status epilepticus: a systematic review and meta-analysis. Heliyon. 2023;9:e13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rollo E, Romozzi M, Dono F, et al. Treatment of benzodiazepine-refractory status epilepticus: a retrospective, cohort study. Epilepsy Behav. 2023;140:109093. [DOI] [PubMed] [Google Scholar]
- [5].Wachiropathum P, Nabangchang C, Likasitthananon N, Suwanpakdee P. Efficacy of oral perampanel in status epilepticus and acute repetitive seizures in children at a tertiary care hospital in Thailand. Epilepsy Behav. 2021;118:107964. [DOI] [PubMed] [Google Scholar]
- [6].Müller A, Schmiedeknecht A, Mende M, et al. Treatment of established status epilepticus in the elderly - a study protocol for a prospective multicenter double-blind comparative effectiveness trial (ToSEE). BMC Neurol. 2020;20:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kienitz R, Kay L, Beuchat I, et al. Benzodiazepines in the management of seizures and status epilepticus: a review of routes of delivery, pharmacokinetics, efficacy, and tolerability. CNS Drugs. 2022;36:951–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brigo F, Bragazzi NL, Lattanzi S, Nardone R, Trinka E. A critical appraisal of randomized controlled trials on intravenous phenytoin in convulsive status epilepticus. Eur J Neurol. 2018;25:451–63. [DOI] [PubMed] [Google Scholar]
- [9].Trinka E. Phenobarbital in Status epilepticus - Rediscovery of an effective drug. Epilepsy Behav. 2023;141:109104. [DOI] [PubMed] [Google Scholar]
- [10].Jain P, Aneja S, Cunningham J, Arya R, Sharma S. Treatment of benzodiazepine-resistant status epilepticus: systematic review and network meta-analyses. Seizure. 2022;102:74–82. [DOI] [PubMed] [Google Scholar]
- [11].Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans affairs status epilepticus cooperative study group. N Engl J Med. 1998;339:792–8. [DOI] [PubMed] [Google Scholar]
- [12].Agarwal P, Kumar N, Chandra R, Gupta G, Antony AR, Garg N. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure. 2007;16:527–32. [DOI] [PubMed] [Google Scholar]
- [13].Amiri-Nikpour MR, Nazarbaghi S, Eftekhari P, et al. Sodium valproate compared to phenytoin in treatment of status epilepticus. Brain Behav. 2018;8:e00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burman RJ, Ackermann S, Shapson-Coe A, Ndondo A, Buys H, Wilmshurst JM. A Comparison of parenteral phenobarbital vs. parenteral phenytoin as second-line management for pediatric convulsive status epilepticus in a resource-limited setting. Front Neurol. 2019;10:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chakravarthi S, Goyal MK, Modi M, Bhalla A, Singh P. Levetiracetam versus phenytoin in management of status epilepticus. J Clin Neurosci. 2015;22:959–63. [DOI] [PubMed] [Google Scholar]
- [16].Chamberlain JM, Kapur J, Shinnar S, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. 2020;395:1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dalziel SR, Borland ML, Furyk J, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet. 2019;393:2135–45. [DOI] [PubMed] [Google Scholar]
- [18].Gujjar AR, Nandhagopal R, Jacob PC, et al. Intravenous levetiracetam vs phenytoin for status epilepticus and cluster seizures: a prospective, randomized study. Seizure. 2017;49:8–12. [DOI] [PubMed] [Google Scholar]
- [19].Handral A, Veerappa BG, Gowda VK, Shivappa SK, Benakappa N, Benakappa A. Levetiracetam versus fosphenytoin in pediatric convulsive status epilepticus: a randomized controlled trial. J Pediatr Neurosci. 2020;15:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kapur J, Elm J, Chamberlain JM, et al. Randomized trial of three anticonvulsant medications for status epilepticus. N Engl J Med. 2019;381:2103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khajeh A, Yaghoubinia F, Yaghoubi S, Fayyazi A, Miri Aliabad G. Comparison of the effect of phenobarbital versus sodium valproate in management of children with status epilepticus. Iran J Child Neurol. 2018;12:85–93. [PMC free article] [PubMed] [Google Scholar]
- [22].Lyttle MD, Rainford NEA, Gamble C, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet. 2019;393:2125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Malamiri RA, Ghaempanah M, Khosroshahi N, Nikkhah A, Bavarian B, Ashrafi MR. Efficacy and safety of intravenous sodium valproate versus phenobarbital in controlling convulsive status epilepticus and acute prolonged convulsive seizures in children: a randomised trial. Eur J Paediatr Neurol. 2012;16:536–41. [DOI] [PubMed] [Google Scholar]
- [24].Misra UK, Kalita J, Patel R. Sodium valproate vs phenytoin in status epilepticus: a pilot study. Neurology. 2006;67:340–2. [DOI] [PubMed] [Google Scholar]
- [25].Mundlamuri RC, Sinha S, Subbakrishna DK, et al. Management of generalised convulsive status epilepticus (SE): A prospective randomised controlled study of combined treatment with intravenous lorazepam with either phenytoin, sodium valproate or levetiracetam--Pilot study. Epilepsy Res. 2015;114:52–8. [DOI] [PubMed] [Google Scholar]
- [26].Nalisetty S, Kandasamy S, Sridharan B, Vijayakumar V, Sangaralingam T, Krishnamoorthi N. Clinical effectiveness of levetiracetam compared to fosphenytoin in the treatment of benzodiazepine refractory convulsive status epilepticus. Indian J Pediatr. 2020;87:512–9. [DOI] [PubMed] [Google Scholar]
- [27].Nazir M, Tarray RA, Asimi R, Syed WA. Comparative Efficacy of IV Phenytoin, IV Valproate, and IV Levetiracetam in Childhood Status Epilepticus. J Epilepsy Res. 2020;10:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nene D, Mundlamuri RC, Satishchandra P, et al. Comparing the efficacy of sodium valproate and levetiracetam following initial lorazepam in elderly patients with generalized convulsive status epilepticus (GCSE): a prospective randomized controlled pilot study. Seizure. 2019;65:111–7. [DOI] [PubMed] [Google Scholar]
- [29].Noureen N, Khan S, Khursheed A, et al. Clinical efficacy and safety of injectable levetiracetam versus phenytoin as second-line therapy in the management of generalized convulsive status epilepticus in children: an open-label randomized controlled trial. J Clin Neurol. 2019;15:468–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Singh K, Aggarwal A, Faridi MMA, Sharma S. IV Levetiracetam versus IV phenytoin in childhood seizures: a randomized controlled trial. J Pediatr Neurosci. 2018;13:158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Su Y, Liu G, Tian F, et al. Phenobarbital versus valproate for generalized convulsive status epilepticus in adults: a prospective randomized controlled trial in China. CNS Drugs. 2016;30:1201–7. [DOI] [PubMed] [Google Scholar]
- [32].Vignesh V, Rameshkumar R, Mahadevan S. Comparison of phenytoin, valproate and levetiracetam in pediatric convulsive status epilepticus: a randomized double-blind controlled clinical trial. Indian Pediatr. 2020;57:222–7. [PubMed] [Google Scholar]
- [33].Wani G, Imran A, Dhawan N, Gupta A, Giri JI. Levetiracetam versus phenytoin in children with status epilepticus. J Family Med Prim Care. 2019;8:3367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Senthilkumar C, Selvakumar P, Kowsik M. Randomized controlled trial of levetiracetam versus fosphenytoin for convulsive status epilepticus in children. Int J Pediatr Res. 2018;5:237–42. [Google Scholar]
- [35].Nakamura K, Ohbe H, Matsui H, et al. Phenytoin versus fosphenytoin for second-line treatment of status epilepticus: propensity score matching analysis using a nationwide inpatient database. Seizure. 2020;80:124–30. [DOI] [PubMed] [Google Scholar]
- [36].Brigo F, Del Giovane C, Nardone R, et al. Intravenous antiepileptic drugs in adults with benzodiazepine-resistant convulsive status epilepticus: a systematic review and network meta-analysis. Epilepsy Behav. 2019;101:106466. [DOI] [PubMed] [Google Scholar]