Abstract
Background:
Degenerative cervical myelopathy (DCM) occurs when spondylotic changes compress the spinal cord and cause neurologic dysfunction. Because of a lack of comparative data on nonoperative care versus surgery for DCM, it has been difficult to support patients through the shared decision-making process regarding treatment options. Our objective was to synthesize the best available data in a manner that helps clinicians and patients to weigh the differences between nonoperative care and surgery at different ages and disease severity. The 2 patient-centered questions we sought to answer were (1) “am I more likely to experience worsening myelopathy with nonoperative care, or need more surgery if I have my myelopathy treated operatively?” and (2) “how much better will my quality of life be with nonoperative care versus surgery?”
Methods:
We used a health economic technique, microsimulation, to model head-to-head comparisons of nonoperative care versus surgery for DCM. We incorporated the best available data, modeled patients over a lifetime horizon, used direct comparators, and incorporated uncertainty in both natural history and treatment effect.
Results:
Patients with mild DCM at baseline who were ≥75 years of age were less likely to neurologically decline under nonoperative care than to undergo a second surgery if the index surgery was an anterior cervical discectomy and fusion (ACDF), cervical disc arthroplasty (ADR), or posterior cervical decompression and instrumented fusion (PDIF). Using quality-adjusted life-years (QALYs), our results suggest that surgery for DCM may be superior to nonoperative care. However, for all patients except those with severe DCM who are of middle age or younger (depending on the procedure, ≤50 to ≤60 years of age), the lower bound of the 95% confidence interval for the estimated difference in QALYs was <0.
Conclusions:
In most patient groups, neurologic progression with nonoperative management is more likely than the need for additional cervical surgery following operative management, with the exception of patients 75 to 80 years of age and older with mild DCM. Furthermore, on average, surgery for DCM tends to improve quality of life. However, patients with DCM who are older than middle age should be aware that the estimates of the quality-of-life benefit are highly uncertain, with a lower bound of <0.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
Cervical spondylosis is a common condition, with a prevalence that varies according to patient age (e.g., 70% of 70-year-old patients)1. Progressive neurologic dysfunction may develop in the form of radiculopathy and/or myelopathy. A previous study found that approximately 1 in 4 nonmyelopathic patients with spondylotic cord compression developed clinical myelopathy at a median of 44 months of follow-up2. Degenerative cervical myelopathy (DCM) occurs when spondylotic changes compress the spinal cord3,4 and is characterized by fine-motor dysfunction of the hands, upper-extremity sensory changes, gait dysfunction, and/or bladder/bowel incontinence.
To protect against progressive neurologic dysfunction as well as catastrophic spinal cord injury, current clinical practice guidelines recommend surgical decompression for patients with moderate-to-severe symptomatic DCM, the consideration of surgery in addition to structured rehabilitation for mildly symptomatic patients, and close observation for patients with asymptomatic spinal cord compression5,6. Unadjusted cervical surgery rates, for all diagnoses, are accelerating in the United States, and increased 206% between 1992 and 20057.
Cervical spine surgery is primarily offered to halt the progression of DCM and has been shown to improve neurologic deficits as measured by the modified Japanese Orthopaedic Association (mJOA) scale8-11. Although surgery is often effective, 1 in 4 patients will not achieve the minimally important difference (MID) on the mJOA, and approximately 15% of surgical patients will experience a major complication10-12. Moreover, some studies have found that the risk of worsening myelopathy following surgery is nearly equivalent to the annual probability of acute spinal cord injury in nonoperatively treated patients with DCM (see Appendix 1 Table A1). Given that surgery is primarily offered to arrest, and not ameliorate, myelopathy, operative management should be reserved for patients in whom the risk of DCM progression is greater than the risks of surgery.
There is limited evidence to inform the effectiveness of surgery versus nonoperative care for DCM. A widely cited guideline from the AO Spine North America and the Cervical Spine Research Society (AO/CSRS) found only 1 comparative study on surgery versus nonoperative care for DCM6. Given the low-quality evidence on treatment for DCM, shared decision-making should be used to ensure that patients’ decisions reflect their values and preferences13.
Shared decision-making is a process by which clinicians and patients jointly deliberate treatment options and related risks14,15. Although considered a key element of high-quality care, shared decision-making has been shown to be underutilized in surgical practice16. A potential barrier to the implementation of shared decision-making in DCM is the paucity of comparative studies and relevant data on the (un)certainty around relative benefits and risks of treatment options.
In this study, we used a microsimulation strategy to simulate head-to-head comparisons of nonoperative care versus surgery for DCM. Microsimulation is an established health economic technique that is particularly useful when controlled experiments are rare or unethical, as it allows investigators to leverage existing data to draw unique conclusions17. This technique is now widely used to study chronic diseases, infectious diseases, and cancer, and to guide public-policy decisions18-22. Results are presented in a manner that helps clinicians and patients weigh the differences between nonoperative care and surgery, including the associated uncertainties, as part of the shared decision-making process. This approach empowers patients to make decisions in line with their values and preferences.
Materials and Methods
Overview of Decision Model
We used microsimulation to build realistic models of both operative and nonoperative care for DCM that account for risk factors and changing event rates over time18,23. Reporting was conducted in accordance with Consolidated Health Economic Evaluation Reporting Standards 202224.
Health Economic Analysis Plan and Rationale and Description of Model
Analyses were planned a priori to support shared-decision-making conversations. It has been shown that patients have difficulty understanding probabilities and uncertainty around outcomes25. Therefore, our analyses were designed to answer 2 clear questions: (1) Am I more likely to experience worsening myelopathy with nonoperative care, or need more surgery if I have my myelopathy treated operatively? (2) How much better will my quality of life be with nonoperative care versus surgery?
The first question was structured to address the U.S. Food and Drug Administration (FDA) definition of “success” for the evaluation of total artificial disc Investigational Device Exemption (IDE) applications26. Many of these IDE applications included patients with DCM, and therefore, we felt these criteria to be relevant. The FDA instructs that “success” be based on improvement in pain, improvement in function, the absence of a new neurologic deficit, the absence of a secondary surgical intervention, and the absence of a serious adverse event. We sought to simulate this composite end point using the best available data. Comprehensive data were only available for neurologic deficits and secondary surgical interventions.
We implemented a probabilistic microsimulation model, through Monte Carlo sampling of the Bayesian posterior distributions for model parameters estimated by the meta-analyses, on the risks of neurologic progression and second surgery using R (R Foundation for Statistical Computing)27. Since Bayesian analysis yields probability distributions jointly for all parameters28, it was not necessary to directly specify covariance.
In Analysis 1, we computed the time to neurologic progression (TTNP) for patients undergoing nonoperative care, and the time to second surgery (TTSS) for patients undergoing initial surgery. No event occurred if that time (TTNP or TTSS) was greater than the predicted survival.
In Analysis 2, we computed the difference in predicted quality-adjusted life-years (QALYs).
Study Population
The simulation was run with different baseline ages (40, 45, 50, 55, 60, 65, 70, 75, 80, and 85 years) and baseline DCM severity (mild, with an mJOA of 15 to 17; moderate, with an mJOA of 12 to 14; and severe, with an mJOA of ≤11). In total, we simulated 3,000,000 patients undergoing nonoperative care, and 12,000,000 patients undergoing surgical treatment.
Comparators
In each simulation, we followed 100,000 patients with DCM undergoing each of the following methods of care: (1) nonoperative care, (2) anterior cervical discectomy and fusion (ACDF), (3) cervical disc arthroplasty (ADR), (4) cervical laminoplasty (LAMP), and (5) posterior cervical decompression and instrumented fusion (PDIF).
Time Horizon
Life expectancy was predicted using 2017 to 2019 Canadian life tables29. The simulation was run until patient death.
Selection and Measurement of Outcomes
For patients undergoing nonoperative care, we simulated the time to neurologic progression, TTNP. For patients undergoing surgical treatment, we simulated the time to second surgery, TTSS.
Valuation of Outcomes
Utilities for the QALY calculation were obtained from a general-population direct utility valuation study30.
Analytics and Assumptions
In Analysis 1, we computed the TTNP with nonoperative care, and the TTSS for patients undergoing initial surgery. No event occurred if that time (TTNP or TTSS) was greater than the predicted survival.
In Analysis 2, we computed the difference in predicted QALYs.
mJOA scores were stochastically generated for each simulated patient. Nonoperatively treated patients remained in their baseline mJOA health state until the TTNP, when they experienced a stochastic neurologic decline. Neurologic decline was implemented as an mJOA change corresponding to the MID for disease severity: (1) 1-point decline for baseline mild DCM, (2) 2-point decline for baseline moderate DCM, and (3) 3-point decline for baseline severe DCM12. Change scores for the mJOA were implemented using a multinomial distribution as shown in Appendix 2 Table A2b.
Characterizing Heterogeneity and Distributional Effects
A meta-analysis of the natural history of DCM was used to populate our decision model31. A Bayesian Gompertz survival regression model was used to simulate the TTNP for each simulated patient, one-by-one.
A meta-analysis of the TTSS for patients undergoing ACDF, ADR, LAMP, and PDIF was also used to populate our model32. The log-normal survival regression model was used to simulate the TTSS for each simulated patient, one-by-one.
Characterizing Uncertainty
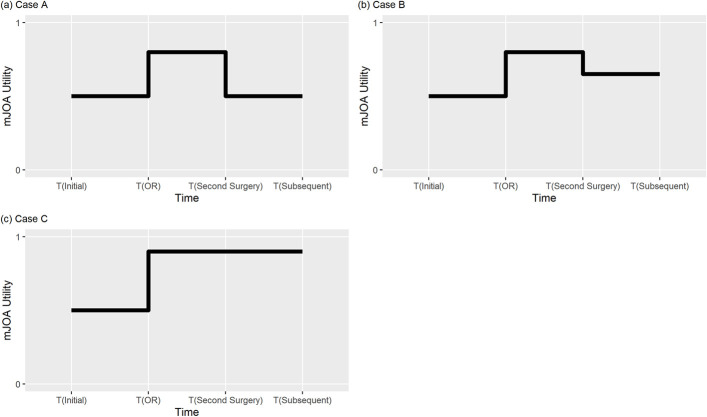
Modeling for surgically treated patients was run using 3 separate patterns of neurologic change affected by surgery (Fig. 1). In all cases, surgical patients immediately experienced a neurologic improvement in mJOA score after the first surgery. The magnitude of change was drawn from a probability distribution for change scores obtained from the pooled published data (see Appendix 2 Tables A2a and A2b). Our model permitted an immediate change in the mJOA to a perfect score of 18 (for example, the maximum allowable change was 9 points for a severely myelopathic patient with a baseline mJOA score of 9). In Case A, at TTSS, patients reverted to their baseline mJOA health state; for example, this corresponds to patients with adjacent-segment disease developing worsening myelopathy without neurologic improvement following the second surgery. In Case B, at TTSS, patients were assigned a utility between the baseline mJOA health state and a neurologically improved mJOA health state; for example, this corresponds to patients with adjacent-segment disease developing worsening myelopathy, with neurologic improvement following the second surgery. In Case C, at TTSS, patients did not experience a decline in mJOA health state; for example, this corresponds to patients undergoing a second surgery for pseudarthrosis.
Fig. 1.
Modeling of quality of life for surgically treated patients. In all cases, surgical patients immediately experienced a neurologic improvement in the mJOA score after the first surgery (OR). The magnitude of change was drawn from a probability distribution for change scores obtained from the pooled published data (Appendix 2). In Case A, at the time to second surgery (TTSS), patients reverted to their baseline mJOA health state; for example, this corresponds to patients with adjacent-segment disease developing worsening myelopathy without neurologic improvement following the second surgery. In Case B, at TTSS, patients were assigned a utility between the baseline mJOA health state and neurologically improved mJOA health state; for example, this corresponds to patients with adjacent-segment disease developing worsening myelopathy, with neurologic improvement following the second surgery. In Case C, at TTSS, patients did not experience a decline in mJOA health state; for example, this corresponds to patients undergoing a second surgery for pseudarthrosis.
Results
Study Parameters
The 10-year neurologic progression-free survival for patients with mild and moderate-to-severe DCM was 68% and 46%, respectively. The 10-year second-surgery-free survival for ACDF, ADR, LAMP, and PDIF was 88%, 84%, 99%, and 86%, respectively. Life expectancy for the simulated cohort is shown in Table I. The distribution of mJOA scores for simulated patients with mild, moderate, and severe DCM is shown in Table II.
TABLE I.
Life Expectancy of the Simulated Cohort
| Baseline Age (yr) | Life Expectancy (yr) |
|---|---|
| 40 | 83.9 |
| 45 | 84.3 |
| 50 | 84.6 |
| 55 | 85.1 |
| 60 | 85.6 |
| 65 | 86.4 |
| 70 | 87.4 |
| 75 | 88.8 |
| 80 | 90.5 |
| 85 | 92.8 |
TABLE II.
Distribution of mJOA Scores
| mJOA Score | Mild DCM Cohort | Moderate DCM Cohort | Severe DCM Cohort |
|---|---|---|---|
| 17 | 11.8% | ||
| 16 | 29.4% | ||
| 15 | 58.8% | ||
| 14 | 22.9% | ||
| 13 | 33.3% | ||
| 12 | 43.8% | ||
| 11 | 30.5% | ||
| 10 | 34.1% | ||
| 9 | 35.4% |
Neurological Progression and Second Surgeries
The percentage of nonoperatively treated patients expected to neurologically decline before those treated operatively required a second surgery (TTNP < TTSS or death) is shown in Table III. Patients with mild DCM at baseline who were ≥75 years of age were less likely to neurologically decline under nonoperative care than to undergo a second surgery if the index surgery was an ACDF, ADR, or PDIF. Once ≥80 years of age, patients with mild DCM at baseline were also more likely to undergo a second surgery after LAMP. For patients with moderate-to-severe DCM at baseline, neurologic progression after nonoperative care was more likely than second surgery for all age groups.
TABLE III.
Percentage of Patients with TTNP Less Than TTSS or Death for Surgery Versus Nonoperative Care*
| Cohort Age (yr) | Mild DCM (%) | Moderate-to-Severe DCM (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| ACDF | ADR | LAMP | PDIF | ACDF | ADR | LAMP | PDIF | |
| 40 | 54.51 | 53.35 | 57.00 | 53.85 | 93.98 | 91.75 | 98.90 | 93.11 |
| 45 | 54.37 | 53.23 | 56.88 | 53.75 | 93.76 | 91.53 | 98.64 | 92.87 |
| 50 | 54.19 | 53.05 | 56.67 | 53.60 | 93.36 | 91.18 | 98.25 | 92.48 |
| 55 | 53.89 | 52.78 | 56.35 | 53.35 | 92.82 | 90.65 | 97.61 | 91.97 |
| 60 | 53.41 | 52.36 | 55.82 | 52.80 | 91.85 | 89.74 | 96.55 | 91.09 |
| 65 | 52.76 | 51.66 | 55.06 | 52.23 | 90.39 | 88.38 | 95.03 | 89.74 |
| 70 | 51.62 | 50.59 | 53.83 | 51.11 | 88.18 | 86.27 | 92.61 | 87.59 |
| 75 | 49.79 | 48.96 | 51.98 | 49.55 | 84.82 | 83.11 | 88.94 | 84.28 |
| 80 | 47.31 | 46.51 | 49.26 | 47.04 | 79.97 | 78.29 | 83.56 | 79.59 |
| 85 | 43.94 | 43.20 | 45.58 | 43.67 | 73.53 | 72.03 | 76.40 | 73.15 |
A proportion of <50% indicates that nonoperative care is superior; a proportion of >50% indicates that surgery is superior. TTNP = time to neurologic progression, TTSS = time to second surgery, DCM = degenerative cervical myelopathy, ACDF = anterior cervical discectomy and fusion, ADR = cervical disc arthroplasty, LAMP = laminoplasty, and PDIF = posterior cervical decompression and instrumented fusion.
QALYs
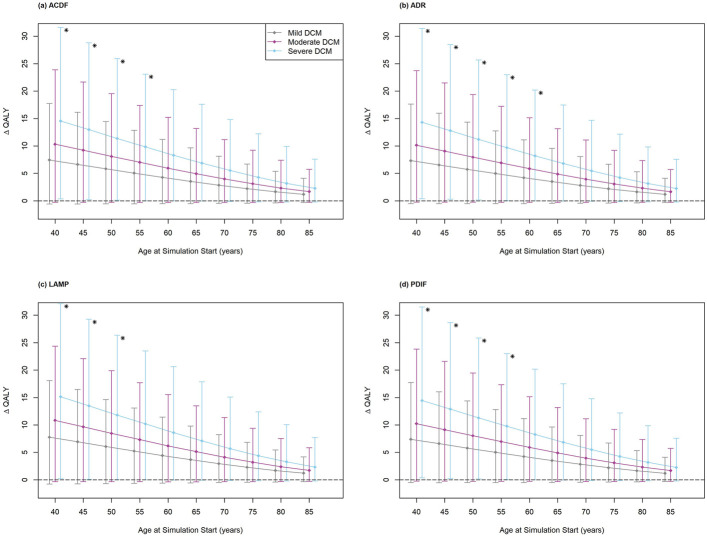
The differences in QALYs between nonoperative care and surgery for patients with baseline mild, moderate, and severe myelopathy are shown in Figure 2 and Appendix Tables A3, A4, and A5. Under all simulation conditions, for all baseline DCM severities, the mean point estimates of the QALY difference (ΔQALY) were positive, indicating that surgery was favored over nonoperative care. ΔQALYs increased with baseline DCM severity. ΔQALYs were also inversely related to baseline cohort age.
Fig. 2.
Difference in quality-adjusted life-years (ΔQALYs) for surgery versus nonoperative care among simulated patients undergoing anterior cervical discectomy and fusion (ACDF) (Fig. 2-A), cervical disc arthroplasty (ADR) (Fig. 2-B), cervical laminoplasty (LAMP) (Fig. 2-C), or posterior cervical decompression and instrumented fusion (PDIF) (Fig. 2-D). Asterisks indicate that the lower bound of the 95% confidence interval for the estimated ΔQALY was >0 (indicating surgery was superior). The lower bound was >0 only for patients with severe DCM undergoing ACDF at an age of ≤55 years, ADR at an age of ≤60 years, LAMP at an age of ≤50 years, and PDIF at an age of ≤55 years. Whiskers indicate the 95% confidence interval.
However, there was high uncertainty in the QALY estimates. For the majority of simulations, the lower bound of the 95% confidence interval for the estimated QALY was <0 (indicating nonoperative care was superior). The lower bound was >0 only for patients with severe DCM undergoing ACDF at an age of ≤55 years, ADR at an age of ≤60 years, LAMP at an age of ≤50 years, and PDIF at an age of ≤55 years (Fig. 2).
Discussion
Because of a lack of comparative data on nonoperative care versus surgery for DCM, it has been difficult to support patients through the shared decision-making process regarding treatment options. When the quality of evidence is low, it is also important to articulate the (un)certainty around the relative benefits and harms of treatment options so that patients can make decisions that align with their values and preferences. In this study, we synthesized the best available evidence to simulate head-to-head comparisons of nonoperative care versus surgery for DCM.
The first question we sought to answer was “am I more likely to experience worsening myelopathy with nonoperative care, or need more surgery if I have my myelopathy treated operatively?” Our findings suggest that worsening myelopathy is less likely than second surgery following ACDF, ADR, or PDIF for patients ≥75 years of age with mild DCM, and following LAMP for patients ≥80 years of age with mild DCM. Patients with moderate-to-severe myelopathy, and younger patients with mild myelopathy, are more likely to experience worsening myelopathy than to need a second surgery.
The second question we sought to answer was “how much better will my quality of life be with nonoperative care versus surgery?” Using QALYs, our results suggest that, for patients overall, surgery for DCM may be superior to nonoperative care. However, for all patients except those with severe DCM who are of middle age or younger (depending on the procedure, ≤50 to ≤60 years of age), the lower bound of the 95% confidence interval for the estimated ΔQALY was negative, which indicates that nonoperative care would be superior.
Reconciling Our Findings with Current Guidelines
Using the GRADE approach37, the AO/CSRS DCM guideline makes a “strong” recommendation in favor of surgery for patients with moderate-to-severe DCM6. When assessing the effectiveness of interventional procedures with the GRADE approach, randomized trials are initially classified as high-certainty evidence and observational studies, as low-certainty evidence; however, both may be further downgraded because of the risk of bias, imprecision, indirectness, inconsistency, and small study effects33. The AO/CSRS guideline identified only 1 randomized trial exploring surgery versus nonoperative care for moderate DCM and only observational data for severe DCM34. The GRADE approach typically does not allow for strong recommendations when the supporting evidence is only low or very low in certainty, and no exceptions were met for the DCM guidelines35. Thus, by the GRADE approach, the AO/CSRS guidelines should, at most, have suggested a weak recommendation for surgery over nonoperative care. This conclusion is consistent with our findings of high uncertainty regarding the benefit of surgery among all DCM severities.
Strengths
Our QALY estimates align with those reported in a previous decision analysis on DCM36. However, in addition to stratifying results on the basis of age and procedure, our decision analysis incorporated 2 methodologic improvements that increase the fidelity and granularity of our model. We derived transition probabilities for neurologic deterioration and reoperation from meta-analyses. These studies incorporated data from 529 patients regarding natural history and 73,811 patients regarding second surgeries. Such extensive data input offers a more comprehensive and widely applicable perspective than prior studies. Additionally, the use of parametric survival curves allowed us to incorporate time-dependency in our decision model. Furthermore, in our decision analysis, the utility of neurologic deterioration was obtained from a direct general population utility valuation study for the mJOA30. In contrast, previous work used a fixed value of 0.045, on the utility scale, for neurologic deterioration. Our approach allowed modeling of the differential impact of components of the mJOA on quality of life, and the differential marginal utility of change as related to DCM severity.
Limitations
Our analysis was constrained by the available data. In the natural history meta-analysis used to populate our decision model31, only 63 (12%) of the patients had baseline severe DCM. In the second-surgery meta-analysis used to populate our model, we were unable to control for surgical indications (DCM versus radiculopathy versus neck pain versus deformity). As a consequence of using a lifetime horizon, the survival curves for TTNP and TTSS were extrapolated beyond the observed data. However, these model inputs were of high quality; it is important to note that of the 13 primary studies incorporated in the meta-analyses used to populate this model, only 1 study had elements at high risk for bias. Limited available data also did not allow us to incorporate demographic variables such as level of education, activity, or work status.
Conclusions
Our health economic simulation comparing the effectiveness of nonoperative care versus surgery for DCM provides data that can be used in conversations of shared decision-making between patients and physicians. In most patient groups, neurologic progression is more likely than the need for additional cervical surgery, with the exception of patients 75 to 80 years of age and older with mild DCM. Furthermore, on average, surgery for DCM tends to improve quality of life. However, patients with DCM who are older than middle age should be aware of high uncertainty in the estimates of the quality-of-life benefit.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJSOA/A696).
Footnotes
Investigation performed at the Division of Orthopedic Surgery, Hamilton General Hospital, McMaster University, Hamilton, Ontario, Canada
Disclosure: No external funding was received for this work. The Article Processing Charge for open access publication was funded internally by the university. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A697).
Contributor Information
Mohamed Sarraj, Email: mohamed.sarraj@medportal.ca.
Jason Busse, Email: bussejw@mcmaster.ca.
Daipayan Guha, Email: guhad@mcmaster.ca.
Mohit Bhandari, Email: bhandam@mcmaster.ca.
References
- 1.Brinjikji W, Luetmer PH, Comstock B, Bresnahan BW, Chen LE, Deyo RA, Halabi S, Turner JA, Avins AL, James K, Wald JT, Kallmes DF, Jarvik JG. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015. Apr;36(4):811-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson JR, Barry S, Fischer DJ, Skelly AC, Arnold PM, Riew KD, Shaffrey CI, Traynelis VC, Fehlings MG. Frequency, timing, and predictors of neurological dysfunction in the nonmyelopathic patient with cervical spinal cord compression, canal stenosis, and/or ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 2013. Oct 15;38(22)(Suppl 1):S37-54. [DOI] [PubMed] [Google Scholar]
- 3.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine (Phila Pa 1976). 2015. Jun 15;40(12):E675-93. [DOI] [PubMed] [Google Scholar]
- 4.Badhiwala JH, Ahuja CS, Akbar MA, Witiw CD, Nassiri F, Furlan JC, Curt A, Wilson JR, Fehlings MG. Degenerative cervical myelopathy - update and future directions. Nat Rev Neurol. 2020. Feb;16(2):108-24. [DOI] [PubMed] [Google Scholar]
- 5.Parthiban J, Alves OL, Chandrachari KP, Ramani P, Zileli M. Value of Surgery and Nonsurgical Approaches for Cervical Spondylotic Myelopathy: WFNS Spine Committee Recommendations. Neurospine. 2019. Sep;16(3):403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehlings MG, Tetreault LA, Riew KD, Middleton JW, Aarabi B, Arnold PM, Brodke DS, Burns AS, Carette S, Chen R, Chiba K, Dettori JR, Furlan JC, Harrop JS, Holly LT, Kalsi-Ryan S, Kotter M, Kwon BK, Martin AR, Milligan J, Nakashima H, Nagoshi N, Rhee J, Singh A, Skelly AC, Sodhi S, Wilson JR, Yee A, Wang JC. A Clinical Practice Guideline for the Management of Patients With Degenerative Cervical Myelopathy: Recommendations for Patients With Mild, Moderate, and Severe Disease and Nonmyelopathic Patients With Evidence of Cord Compression. Global Spine J. 2017. Sep;7(3)(Suppl):70S-83S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang MC, Kreuter W, Wolfla CE, Maiman DJ, Deyo RA. Trends and variations in cervical spine surgery in the United States: Medicare beneficiaries, 1992 to 2005. Spine (Phila Pa 1976). 2009. Apr 20;34(9):955-61; discussion 962-3. [DOI] [PubMed] [Google Scholar]
- 8.Fehlings MG, Wilson JR, Kopjar B, Yoon ST, Arnold PM, Massicotte EM, Vaccaro AR, Brodke DS, Shaffrey CI, Smith JS, Woodard EJ, Banco RJ, Chapman JR, Janssen ME, Bono CM, Sasso RC, Dekutoski MB, Gokaslan ZL. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 2013. Sep 18;95(18):1651-8. [DOI] [PubMed] [Google Scholar]
- 9.Fehlings MG, Ibrahim A, Tetreault L, Albanese V, Alvarado M, Arnold P, Barbagallo G, Bartels R, Bolger C, Defino H, Kale S, Massicotte E, Moraes O, Scerrati M, Tan G, Tanaka M, Toyone T, Yukawa Y, Zhou Q, Zileli M, Kopjar B. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine International study on 479 patients. Spine (Phila Pa 1976). 2015. Sep 1;40(17):1322-8. [DOI] [PubMed] [Google Scholar]
- 10.Fehlings MG, Badhiwala JH, Ahn H, Farhadi HF, Shaffrey CI, Nassr A, Mummaneni P, Arnold PM, Jacobs WB, Riew KD, Kelly M, Brodke DS, Vaccaro AR, Hilibrand AS, Wilson J, Harrop JS, Yoon ST, Kim KD, Fourney DR, Santaguida C, Massicotte EM, Kopjar B. Safety and efficacy of riluzole in patients undergoing decompressive surgery for degenerative cervical myelopathy (CSM-Protect): a multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. Lancet Neurol. 2021. Feb;20(2):98-106. [DOI] [PubMed] [Google Scholar]
- 11.Ghogawala Z, Terrin N, Dunbar MR, Breeze JL, Freund KM, Kanter AS, Mummaneni PV, Bisson EF, Barker FG 2nd, Schwartz JS, Harrop JS, Magge SN, Heary RF, Fehlings MG, Albert TJ, Arnold PM, Riew KD, Steinmetz MP, Wang MC, Whitmore RG, Heller JG, Benzel EC. Effect of Ventral vs Dorsal Spinal Surgery on Patient-Reported Physical Functioning in Patients With Cervical Spondylotic Myelopathy: A Randomized Clinical Trial. JAMA. 2021. Mar 9;325(10):942-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tetreault L, Nouri A, Kopjar B, Côté P, Fehlings MG. The Minimum Clinically Important Difference of the Modified Japanese Orthopaedic Association Scale in Patients with Degenerative Cervical Myelopathy. Spine (Phila Pa 1976). 2015. Nov;40(21):1653-9. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt G. GRADE weak or conditional recommendations mandate shared decision-making. Author Response. J Clin Epidemiol. 2018. Oct;102:147-8. [DOI] [PubMed] [Google Scholar]
- 14.Sommovilla J, Kopecky KE, Campbell T. Discussing Prognosis and Shared Decision-Making. Surg Clin North Am. 2019. Oct;99(5):849-58. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm. Washington (DC): National Academies Press; 2001. [Google Scholar]
- 16.de Mik SML, Stubenrouch FE, Balm R, Ubbink DT. Systematic review of shared decision-making in surgery. Br J Surg. 2018. Dec;105(13):1721-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klevmarken A. Microsimulation. A Tool for Economic Analysis. International Journal of Microsimulation. 2022;15(1):6-14. [Google Scholar]
- 18.Krijkamp EM, Alarid-Escudero F, Enns EA, Jalal HJ, Hunink MGM, Pechlivanoglou P. Microsimulation Modeling for Health Decision Sciences Using R: A Tutorial. Med Decis Making. 2018. Apr;38(3):400-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005. Aug 16;143(4):251-64. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson NM, Cummings DAT, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006. Jul 27;442(7101):448-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennessy DA, Flanagan WM, Tanuseputro P, Bennett C, Tuna M, Kopec J, Wolfson MC, Manuel DG. The Population Health Model (POHEM): an overview of rationale, methods and applications. Popul Health Metr. 2015. Sep 3;13(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mühlberger N, Kurzthaler C, Iskandar R, Krahn MD, Bremner KE, Oberaigner W, Klocker H, Horninger W, Conrads-Frank A, Sroczynski G, Siebert U. The ONCOTYROL Prostate Cancer Outcome and Policy Model: Effect of Prevalence Assumptions on the Benefit-Harm Balance of Screening. Med Decis Making. 2015. Aug;35(6):758-72. [DOI] [PubMed] [Google Scholar]
- 23.Wu O. Microsimulation Model for Health Economic Evaluation of Public Health Policies: An Imperfect but Useful Tool. Circulation. 2021. Oct 26;144(17):1377-9. [DOI] [PubMed] [Google Scholar]
- 24.Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, Caulley L, Chaiyakunapruk N, Greenberg D, Loder E, Mauskopf J, Mullins CD, Petrou S, Pwu RF, Staniszewska S. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. Pharmacoeconomics. 2022. Jun;40(6):601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonner C, Trevena LJ, Gaissmaier W, Han PKJ, Okan Y, Ozanne E, Peters E, Timmermans D, Zikmund-Fisher BJ. Current Best Practice for Presenting Probabilities in Patient Decision Aids: Fundamental Principles. Med Decis Making. 2021. Oct;41(7):821-33. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health. Preparation and Review of Investigational Device Exemption Applications (IDEs) for Total Artificial Discs. 2008. Accessed 2024 Jul 11. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/preparation-and-review-investigational-device-exemption-applications-ides-total-artificial-discs
- 27.R Core Team. R: A Language and Environment for Statistical Computing. Accessed 2024 Jul 11. https://www.R-project.org/
- 28.Pahuta MA, Werier J, Wai EK, van Walraven C, Coyle D. Back to Bayesian: A strategy to enhance prognostication of metastatic spine disease. Int J Clin Pract. 2019. Apr;73(4):e13322. [DOI] [PubMed] [Google Scholar]
- 29.Statistics Canada. Table 13-10-0114-01 Life Expectancy and Other Elements of the Life Table, Canada, All Provinces except Prince Edward Island. 2020. Accessed 2024 Jul 11. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310011401
- 30.Jiang EX, Fisk FE, Taliaferro K, Pahuta MA. Calculating Ex-ante Utilities From the Modified Japanese Orthopedic Association Score: A Prerequisite for Quantifying the Value of Care for Cervical Myelopathy. Spine (Phila Pa 1976). 2022. Apr 1;47(7):523-30. [DOI] [PubMed] [Google Scholar]
- 31.Sarraj M, Hache P, Foroutan F, Oitment C, Marion TE, Guha D, Pahuta M. Natural history of degenerative cervical myelopathy: a meta-analysis and neurologic deterioration survival curve synthesis. Spine J. 2024. Jan;24(1):46-56. [DOI] [PubMed] [Google Scholar]
- 32.Sarraj M, Hache P, Foroutan F, Oitment C, Marion TE, Guha D, Pahuta M. Long-Term Survivorship of Cervical Spine Procedures; A Survivorship Meta-Analysis and Meta-Regression. Global Spine J. 2023. Apr;13(3):840-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schumemann H, Brozek J, Guyatt G, Oxman A. GRADE Handbook. 2013. Accessed 2024 Jul 11. https://gdt.gradepro.org/app/handbook/handbook.html#h.svwngs6pm0f2
- 34.Kadaňka Z, Bednarík J, Vohánka S, Vlach O, Stejskal L, Chaloupka R, Filipovicová D, Surelová D, Adamová B, Novotný O, Nemec M, Smrcka V, Urbánek I. Conservative treatment versus surgery in spondylotic cervical myelopathy: a prospective randomised study. Eur Spine J. 2000. Dec;9(6):538-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, Rind D, Montori VM, Brito JP, Norris S, Elbarbary M, Post P, Nasser M, Shukla V, Jaeschke R, Brozek J, Djulbegovic B, Guyatt G. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013. Jul;66(7):726-35. [DOI] [PubMed] [Google Scholar]
- 36.Witiw CD, Tetreault LA, Smieliauskas F, Kopjar B, Massicotte EM, Fehlings MG. Surgery for degenerative cervical myelopathy: a patient-centered quality of life and health economic evaluation. Spine J. 2017. Jan;17(1):15-25. [DOI] [PubMed] [Google Scholar]
- 37.Cochrane Training. GRADE approach. Accessed 2024 Oct 3. https://training.cochrane.org/grade-approach