Uterus transplantation (UTx) is a treatment option for women with absolute uterine factor infertility. More than 100 UTx procedures have been performed, and the procedure is being accepted into clinical practice.1-3 However, rejection, which is typically asymptomatic, occurs in approximately 60% of patients4; specific biomarkers for uterine function and rejection are lacking.5
Current practice for assessing UTx graft rejection involves assessing cervical biopsies for T-lymphocyte infiltration between the stroma and epithelium and appraising perivascular inflammation, focal capillary disruption, and interstitial hemorrhage.4 Here, we describe a prospective kinetic multiparameter evaluation of a UTx patient to document insight into the immune mechanisms involved and the biomarkers associated with rejection.
CASE DESCRIPTION
Our 34-y-old patient had Mayer-Rokitansky-Küster-Hauser syndrome. She was transplanted with her mother’s uterus under immunosuppressive treatments (basilixumab and intravenous corticosteroids for induction, followed by tacrolimus, mycophenolate mofetil, and oral corticosteroids6). Immunological profiles in her cervical biopsies and serum and vaginal microbiome analyses were compared with samples from 6 childbearing, nonpregnant, and not transplanted women with no comorbidities who underwent surgery for benign uterine polyps removed during the follicular phase of the menstrual cycle. Sample collections were consistent with the MARNI study protocol and the principles of the Declaration of Helsinki, as well as French law; the study was approved by the Institutional Review Board of Sud-Ouest et Outre-Mer in August 2020 (institutional review board number ID-RCB No: 2020-A00737-32) and registered as a clinical trial (No. NCT04615221). Informed consent was obtained from all study participants.
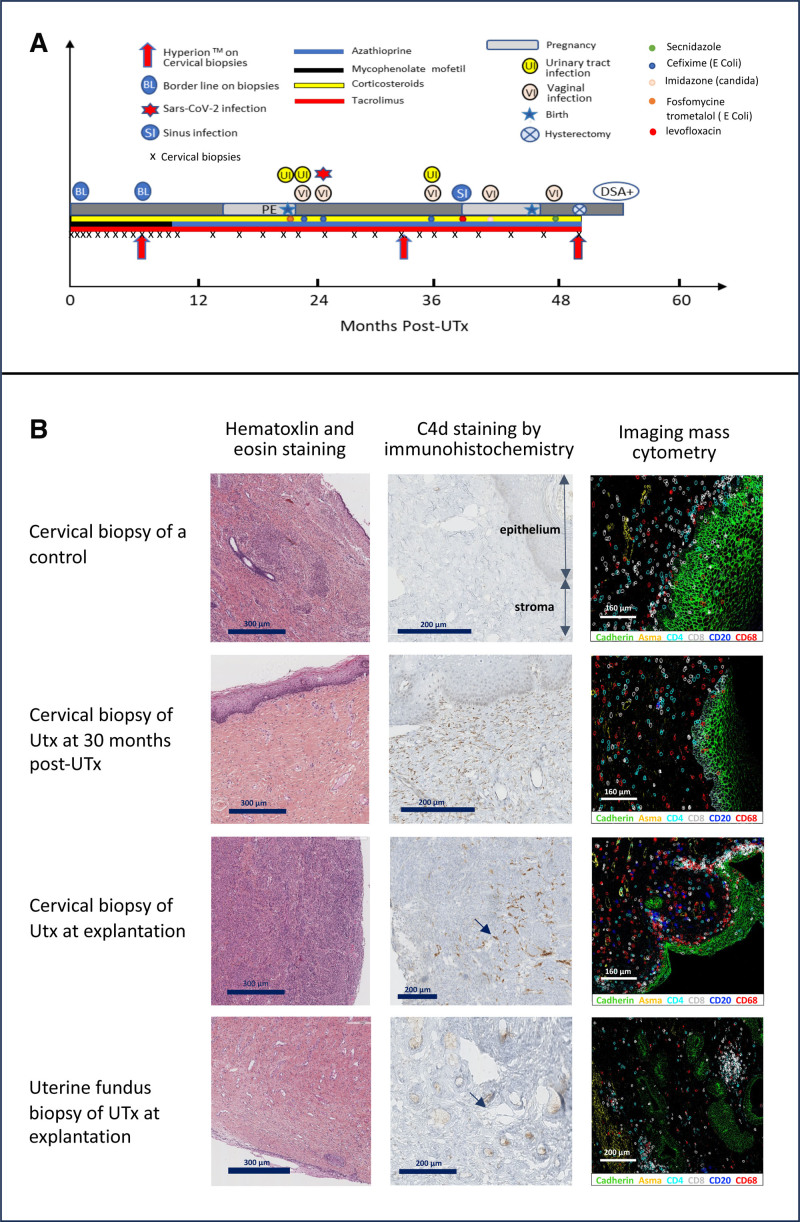
Figure 1A summarizes our patient’s clinical course. Her first menstrual cycle occurred 14 d after transplantation, and her first embryo transfer at 15 mo posttransplant resulted in a healthy pregnancy until 32 wk of gestation when preeclampsia with kidney failure necessitated a cesarean section; a healthy girl weighing 1850 g was delivered. Seventeen months after this delivery, she achieved a second pregnancy after a second embryo transfer, which progressed until 34 wk of gestation when persistent contractions occurred, necessitating a cesarean section; a healthy girl (2550 g) was delivered. The patient underwent uterus explantation 4 mo later, at 50 mo after UTx.
FIGURE 1.
A, Clinical course of the UTx patient (PE and kidney failure; DSA+). B, Comparison of cervical biopsies from randomly selected healthy, nonpregnant controls and the UTx patient at 30 mo post-UTx and at UTx explantation, and a uterine fundus biopsy of the graft at explantation. Arrows shows C4d staining in vessels. Asma, alpha smooth muscle actin; CD4, T4 lymphocyte; CD8, T8 lymphocyte; CD20, B lymphocyte; CD68, macrophage; DSA, donor-specific antibody; PE, preeclampsia; UTx, uterus transplantation.
Cervical biopsies (n = 29) performed weekly, monthly, and then quarterly post-UTx showed no signs of rejection according to Mölne’s classification.4 They were comparable with those of controls (Figure 1B). Two borderline lesions were observed at day 15 and month 8 (untreated). Nevertheless, at uterus explantation (month 50), c4d was positive on endothelium vessels of both cervical and uterine samples. All our recipient cervical biopsies were negative for c4d staining (Figure S1, SDC, http://links.lww.com/TXD/A715). Serum donor-specific antibodies (DSAs) were positive for the first time at month 52 (DR1: 1200 median fluorescence intensity [MFI]; DQ5: 1800 MFI), and again at months 55 and 58 (A11b, DQ5, and DR1; >20 000 MFI).
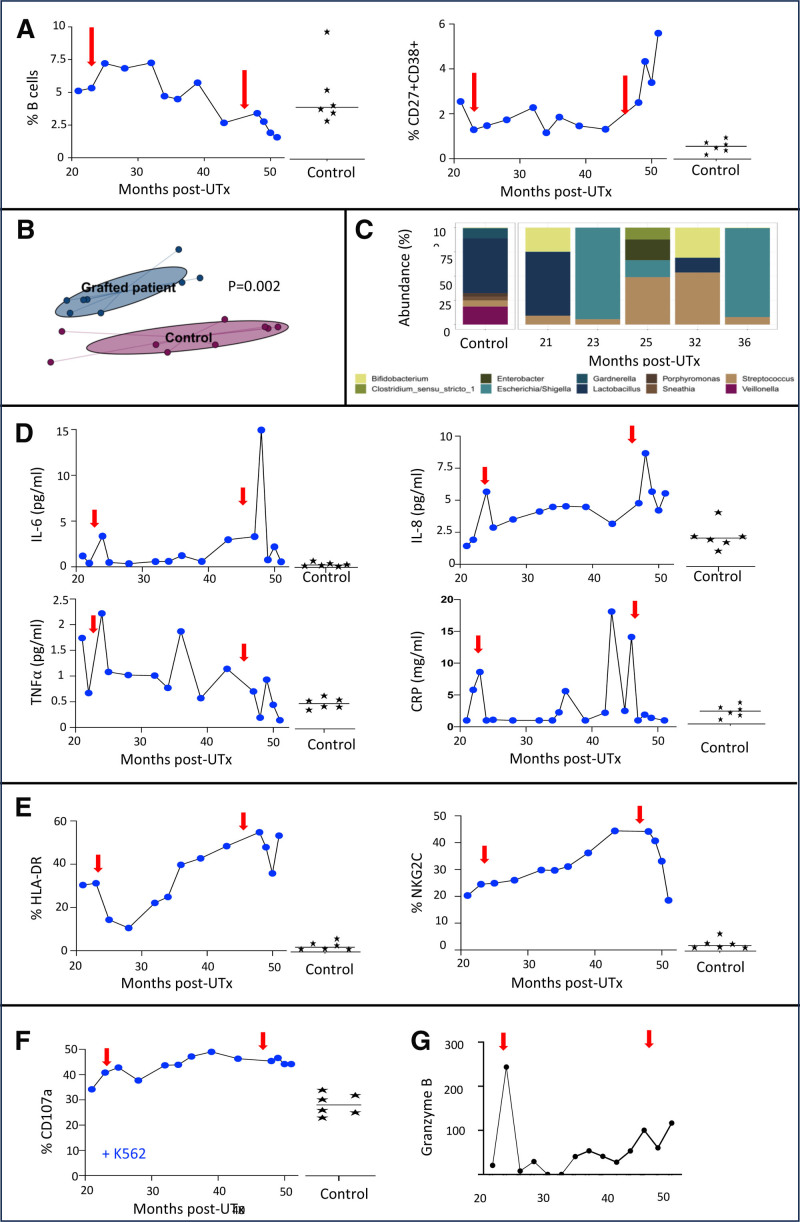
Imaging mass cytometry of cervical biopsies (Tables S1 and S2, SDC, http://links.lww.com/TXD/A715) revealed immune condensation foci, including CD8, CD4, CD20, and macrophages of the explanted uterus at month 50, which were not present before hysterectomy or in controls (Figure 1B). A significant increase in CD27+CD38+ plasmablasts was observed starting from 49 mo post-UTx. However, the incidence of B lymphocytes was within the range of control values although it decreased from around 35 mo post-UTx (Figure 2A).
FIGURE 2.
Kinetics of blood biological markers and the vaginal microbiome in the UTx patient vs healthy controls (n = 6). A, Frequency of CD27+ and CD38+ and B cells. B, Non–multidimensional scaling. C, Abundance of the top genes in the vaginal microbiome. Incidence of inflammatory cytokines and CRP (D), HLA-DR and NKG2C (E), CD107a among CD3−CD56+ NK cells with K567 (F), and CRP (G). Red arrow indicates delivery. CRP, C-reactive protein; IL, interleukin; NK, natural killer; TNF-α, tumor necrosis factor-α; UTx, uterus transplantation.
Longitudinal analyses with a beta-diversity approach using Bray-Curtis distance couplet with an 5mining Massive Data Sets algorithm and the PERMANOVA test revealed a statistically significant separation between the vaginal microbiome of our patient and the controls (P = 0.002; Figure 2B). The microbiomes of our patient at month 21 post-UTx and the controls were dominated by Lactobacillus, whereas microbiome of our patient was also composed of Bifidobacterium (Figure 2C), which became largely dysbiotic after delivery at month 23. The predominance of Escherichia/Shigella at months 23 and 36 post-UTx was correlated with clinical signs of vaginal infections (5 successfully treated during the graft life; Figure 1A).
Due to the complex interplay among microbiota, vaginal infection, and inflammation,7 we investigated sera concentrations of interleukin (IL)-6, IL-8, tumor necrosis factor-α, C-reactive protein (CRP), IL-4, and IL-10 cytokines. Compared with controls, a peak of IL-6 and IL-8 occurred in our patient at month 23 after her delivery, which was more marked at month 48 (Figure 2D), corresponding to the last vaginal infection just before hysterectomy. In contrast to the level of CRP, which peaked at month 23 and again at months 35, 43, and 46, tumor necrosis factor-α exhibited a more fluctuating, albeit declining, trend throughout her treatment course (Figure 2D). Levels of IL-4 and IL-10 remained close to baseline (data not shown). Her cytomegalovirus status was positive (IgG+ and IgM–) and no reinfection was observed during the graft life.
Analysis of the immune microenvironment in blood by flow cytometry (Table S3, SDC, http://links.lww.com/TXD/A715) revealed profound alterations in the CD3−CD56+ natural killer (NK) cell compartment compared with controls (Figure 2E). Progressive and significant increases in the cell-activation HLA-DR and activating NKG2C occurred until 48 mo post-UTx (Figure 2E). These trends indicate the presence of adaptive “NK-like” cells that are physiologically endowed with potent anti-infectious functions.8 Thus, CD107a expression revealed a higher degranulation capacity of these cells than controls and increased similarly to NKG2C expression, whether with or without stimulation by coculturing with K562 target cells (Figure 2F). Consistent with these observations, increased expression at month 49 of granzyme B, a key marker of degranulation and a noninvasive biomarker of rejection in solid transplantation,9 was detected by quantitative Polymerase Chain Reaction in cervical biopsies, vaginal smears, and peripheral blood until hysterectomy (Figure 2G).
DISCUSSION
The positivity of c4d on the graft vessels during explantation may indicate a potential humoral rejection. The persistent presence of functional activating adaptive “NK-like” cells in our patient, with pathologic levels of several inflammatory markers and cytokines, such as IL-6, IL-8, and CRP associated with repeated infections, suggest silent or chronic viral and bacterial infections. These findings need confirmation in a cohort of UTx patients.
Humoral rejection is a feared complication in organ transplantation. Antibodies bind to graft vascular endothelial cells, activating the complement system leading to tissue necrosis.10 Acute antibody-mediated rejection (AMR) is well defined in kidney transplantation with established histologic criteria, which include c4d and non-c4d criteria, associating with DSAs.5 No consensus about the diagnosis of AMR is available in UTx. In the current report, c4d positivity was observed during explantation and a weak DSA positivity 2 mo after explantation. Together, these observations raise the possibility of a beginning of humoral rejection at the time of uterus explantation. Nevertheless, the possibility also exists that the DSA positivity after explantation was due to cessation of immunosuppression, and c4d staining alone is not specific enough and may reflect other processes like infection. Only a few cases of AMR have been reported in UTx.11,12 Inflammatory responses in 2 cases in the first UTx series may reflect chronic rejection as in the composite vascularized tissue graft model.13 No signs of clear chronic rejection were observed in our case, although the appearance of immune condensation foci showed some inflammation14
The 2 pregnancies in our UTx recipient may have favored rejection due to sensitization associated with the semiallogenic fetus.15 Recent studies showed that innate immunity and particularly NK cells play a role in chronic and humoral rejection with the concept of “missing self.”16 Priming of NK-cell rejection could be favored by infections.17 This complex interplay between infection and immune response described in our case should encourage active vaginal infection treatment in UTx to avoid rejection. Our functional activating adaptive “NK-like” cells show an appropriate response to infection. Their progressive increase could indicate subclinical persistent infections due to immunosuppression. More markers (like CD457 and KIR) are needed to confirm that the identified NK cells were really adaptive.
The microbiome appears to be altered in organ transplantation and involved in rejection.18 In our case, a vaginal microbiome alteration may have been responsible for repetitive infections in direct relation to the graft. Therefore, the influence of the vaginal microbiome should also be explored in future UTx studies.19
We identified granzyme B as a potential biomarker of rejection as it progressively increased, particularly at the end of the graft life, and is well known in solid organ transplantation.20 The limitation of this marker could be its specificity: the peak observed during the first pregnancy was attributed to preeclampsia.21
Although this is the first study investigating immunological and infection trends post-UTx, the findings must be interpreted in the context of potential limitations. The data were derived from only 1 UTx patient who experienced several infections as well as the potential beginning of humoral rejection, preeclampsia, and kidney failure (albeit conditions that affect up to 16% of UTx patients). Some bias may have been introduced by biopsies taken in both the luteal and follicular phases of the menstrual cycle in our recipient, while they were taken exclusively in the follicular phase of the controls. Moreover, bias may have been introduced by the controls being neither transplanted nor pregnant.
Complete analyses of the materno-fetal interface and immunological profile in UTx recipients are required to help develop noninvasive approaches to identify UTx rejection, prevent other complications associated with these pregnancies, and evaluate the impact on the offspring.
ACKNOWLEDGMENTS
The authors thank the patients for the collection of their samples, the Department of Clinic Research, Foch Hospital, especially F. Couppey, for administrative supervision, and the Foch pathology team for analysis of the biopsies.
Supplementary Material
Footnotes
The authors declare no conflicts of interest.
M.C., J.M.-A., M.L., M.B., and V.V. designed the study. M.C., E.R., R.S., and M.L.M. contributed to clinical and biological data acquisition. M.C., R.V., N.T., V.M., M.P., A.V., M.T., Z.E.B., C.R., and V.V. performed the experimental work, contributed to data acquisition, analysis, and interpretation. M.C., C.R., and V.V. wrote the article. All authors contributed to reviewing the article. All authors contributed to the article and approved the submitted version.
Institutional Review Board of Sud-Ouest et Outre-Mer in August 2020 (IRB number ID-RCB No. 2020-A00737-32).
Research reported in this publication was partially supported by the University of Versailles Saint Quentin en Yvelines transplantation chair and the Foch Foundation.Informed consent was obtained from the patient and the controls for this publication.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
Clinical trial: NCT04615221.
Contributor Information
Remy Villette, Email: rem.villette@gmail.com.
Maxime Petit, Email: maxime.petit@epfl.ch.
Nadine Tarantino, Email: nadine.tarantino@sorbonne-universite.fr.
Veronique Morin, Email: veronique.morin@sorbonne-universite.fr.
Angélique Vinit, Email: angelique.vinit@sorbonne-universite.fr.
Emmanuel Roux, Email: emmanuel.roux94@gmail.com.
Morgan Tourne, Email: m.tourne@hopital-foch.com.
Camélia Radulescu, Email: cm.radulescu@hopital-foch.com.
Zakhia El Beaino, Email: z.el-beaino@hopital-foch.com.
Mathilde Le Marchand, Email: m.le-marchand@hopital-foch.com.
Renaud Snanoudj, Email: rsnanoudj@gmail.com.
Catherine Racowsky, Email: catherine.racowsky@gmail.com.
Martin Larsen, Email: martin.larsen@sorbonne-universite.fr.
Mats Brännström, Email: mats.brannstrom@obgyn.gu.se.
Jean-Marc Ayoubi, Email: jm.ayoubi@hopital-foch.com.
Vincent Vieillard, Email: vincent.vieillard@sorbonne-universite.fr.
REFERENCES
- 1.Brannstrom M, Racowsky C, Carbonnel M, et al. Uterus transplantation: from research, through human trials and into the future. Hum Reprod Update. 2023;29:521–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brannstrom M, Tullius SG, Brucker S, et al. Registry of the international society of uterus transplantation: first report. Transplantation. 2023;107:10–17. [DOI] [PubMed] [Google Scholar]
- 3.Johannesson L, Richards E, Reddy V, et al. The first 5 years of uterus transplant in the US: a report from the United States uterus transplant consortium. JAMA Surg. 2022;157:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molne J, Broecker V, Ekberg J, et al. Monitoring of human uterus transplantation with cervical biopsies: a provisional scoring system for rejection. Am J Transplant. 2017;17:1628–1636. [DOI] [PubMed] [Google Scholar]
- 5.Loupy A, Haas M, Solez K, et al. The Banff 2015 kidney meeting report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017;17:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayoubi JM, Carbonnel M, Kvarnstrom N, et al. Case report: post-partum SARS-CoV-2 infection after the first French uterus transplantation. Front Surg. 2022;9:854225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabee S, Passmore JS, Heffron R, et al. The complex link between the female genital microbiota, genital infections, and inflammation. Infect Immun. 2021;89:e00487–e00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjorkstrom NK, Strunz B, Ljunggren HG. Natural killer cells in antiviral immunity. Nat Rev Immunol. 2022;22:112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heng B, Li Y, Shi L, et al. A Meta-analysis of the significance of granzyme B and perforin in noninvasive diagnosis of acute rejection after kidney transplantation. Transplantation. 2015;99:1477–1486. [DOI] [PubMed] [Google Scholar]
- 10.Colvin RB. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046–1056. [DOI] [PubMed] [Google Scholar]
- 11.Flyckt R, Falcone T, Quintini C, et al. First birth from a deceased donor uterus in the United States: from severe graft rejection to successful cesarean delivery. Am J Obstet Gynecol. 2020;223:143–151. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Johannesson L, Findeis SK, et al. Clinicopathological analysis of uterine allografts including proposed scoring of ischemia reperfusion injury and T-cell-mediated rejection-Dallas UtErus transplant study: a pilot study. Transplantation. 2022;106:167–177. [DOI] [PubMed] [Google Scholar]
- 13.Cendales LC, Kanitakis J, Schneeberger S, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant. 2008;8:1396–1400. [DOI] [PubMed] [Google Scholar]
- 14.Carbonnel M, Petit M, Tarantino N, et al. Analysis of immunological biomarkers associated with rejection after uterus transplantation in human. Transplantation. 2024. [DOI] [PubMed] [Google Scholar]
- 15.Suah AN, Tran DV, Khiew SH, et al. Pregnancy-induced humoral sensitization overrides T cell tolerance to fetus-matched allografts in mice. J Clin Invest. 2021;131:e140715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hidalgo LG, Sis B, Sellares J, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant. 2010;10:1812–1822. [DOI] [PubMed] [Google Scholar]
- 17.Hanna J, Mandelboim O. When killers become helpers. Trends Immunol. 2007;28:201–206. [DOI] [PubMed] [Google Scholar]
- 18.Fricke WF, Maddox C, Song Y, et al. Human microbiota characterization in the course of renal transplantation. Am J Transplant. 2014;14:416–427. [DOI] [PubMed] [Google Scholar]
- 19.Jones BP, Saso S, L’Heveder A, et al. The vaginal microbiome in uterine transplantation. BJOG. 2020;127:230–238. [DOI] [PubMed] [Google Scholar]
- 20.Yadav B, Prasad N, Agarwal V, et al. Hidden granzyme B-mediated injury in chronic active antibody-mediated rejection. Exp Clin Transplant. 2020;18:778–784. [DOI] [PubMed] [Google Scholar]
- 21.Du M, Wang W, Huang L, et al. Natural killer cells in the pathogenesis of preeclampsia: a double-edged sword. J Matern Fetal Neonatal Med. 2022;35:1028–1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.