Abstract
Objective
Prehension of the position of the microcatheter tip under fluoroscopy during cerebral aneurysm embolization is critical to prevent intraoperative rupture of the aneurysm, even if the first marker at the tip is obscured by coils in the aneurysm. This study presents our initial experience with a sub-marker catheter, which includes an additional marker positioned 5 mm from the tip, designed to facilitate accurate positioning of the microcatheter tip.
Methods
We analyzed cases of cerebral aneurysms treated with sub-marker catheters at our hospital from July 2022 to September 2023. Single catheter embolization served as the primary treatment option, with balloon-assisted or stent-assisted techniques utilized only when necessary.
Results
During the study period, 18 patients with cerebral aneurysms were treated using sub-marker catheters. The median age of these patients was 65 years, comprising 8 men and 10 women. The aneurysms had a median maximum diameter of 6.2 mm, ranging from 5.0 to 16.8 mm. Among the 18 treated patients, 14 had unruptured aneurysms and 4 had ruptured aneurysms. Treatment methods included single catheter embolization in 10 patients, double catheter embolization in 3, stent-assisted embolization in 3, balloon-assisted embolization in 1, and flow diverter placement combined with coil embolization in 1. The sub-marker was consistently visible under fluoroscopy, aiding the precise positioning of the microcatheter tip without interference from the coils. No complications occurred, and successful embolization was achieved in all cases.
Conclusion
The sub-marker catheter appears valuable for safely performing aneurysm embolization.
Keywords: coil embolization, intracranial aneurysm, sub-marker microcatheter
Introduction
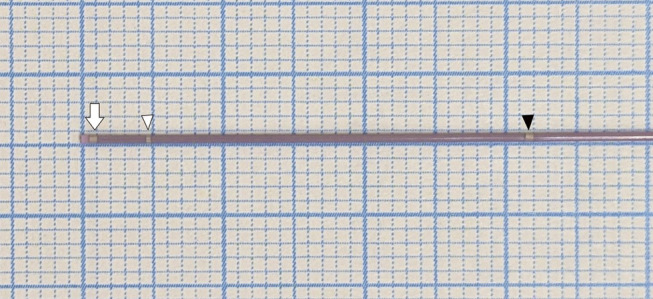
During cerebral aneurysm embolization, the accurate identification of the position of the first marker at the tip of the microcatheter using fluoroscopy is crucial. Understanding the degree of pressure exerted on the aneurysm wall by the microcatheter and coils is essential to prevent intraoperative rupture of the aneurysm. When the aneurysm is packed with coils, the first marker at the microcatheter tip may overlap with the coils, complicating its fluoroscopic visibility. Although conventional microcatheters feature a second marker located 3 cm from the tip for coil detachment, this marker is often too far from the tip to accurately predict its position within a coil-filled aneurysm. The sub-marker catheter (Komichi; HI-LEX Corporation, Hyogo, Japan) introduces a sub-marker 5 mm from the tip alongside the conventional second marker (Fig. 1). Even when an aneurysm is filled with coils, the sub-marker allows prediction of the microcatheter tip position. This microcatheter equipped with a sub-marker is unprecedented. This report presents our initial experience using a sub-marker catheter for the endovascular treatment of cerebral aneurysms.
Fig. 1. Magnified view of the tip of the Komichi catheter (HI-LEX Corporation). Komichi is a sub-marker catheter that, in addition to the second marker, features a new marker 5 mm from the tip. The white arrow indicates the first marker, the white arrowhead indicates the sub-marker and the black arrowhead indicates the second marker.
Materials and Methods
From July 2022 to September 2023, we enrolled patients with cerebral aneurysms who underwent endovascular treatment at our hospital. We investigated clinical factors such as age, sex, and aneurysm size by reviewing patient medical records. Endovascular treatment was performed under general anesthesia. Single catheter embolization was the primary treatment option, supplemented by techniques such as balloon-assisted or stent-assisted embolization when necessary. The sub-marker catheter, used as the microcatheter, featured a distal outer diameter of 1.7 Fr, an inner lumen of 0.017 inches, compatibility with a 0.014-inch guidewire, and an effective length of 157 cm. Its shaft, constructed with tungsten braiding, enhanced visibility under fluoroscopy. Patients with unruptured cerebral aneurysms were pre-treated with 100 mg of aspirin and 75 mg of clopidogrel starting 2 weeks before the procedure. During the procedure, systemic heparinization was maintained (activated coagulation time 250–300 seconds). For patients with ruptured cerebral aneurysms, heparinization was initiated only after achieving hemostasis with coils. Postoperative antiplatelet therapy with 100 mg of cilostazol, administered twice daily, was initiated to prevent both vasospasm and thrombotic complications. The postoperative embolization status was evaluated using the Raymond classification.1) This study was approved by the Clinical Research Review Committee of the Tokai University School of Medicine.
Results
During the study period, 18 patients with cerebral aneurysms were treated using a sub-marker catheter. The median age of these patients was 65 years; there were 8 men and 10 women. The median maximum diameter of the aneurysms was 6.2 mm, with a minimum size of 5.0 mm and a maximum size of 16.8 mm. There were 14 unruptured aneurysms and 4 ruptured aneurysms. The locations of the aneurysms included 6 in the internal carotid artery, 3 in the anterior communicating artery, 4 in the middle cerebral artery, and 5 in the basilar and vertebral arteries. The treatment approaches varied: single catheter embolization was used in 10 patients, double catheter embolization—involving both a 2-marker catheter and a sub-marker catheter—in 3, stent-assisted embolization in 3, balloon-assisted embolization in 1, and flow diverter placement with coil embolization in 1. In all cases, the sub-marker catheter was reshaped using steam. In all cases, the sub-marker was visible under fluoroscopy during the procedure, allowing for embolization while facilitating the prediction of the microcatheter tip position. In all cases, the sub-marker did not enter the coil mass within the aneurysm during the procedure. Specifically, in a large aneurysm with a maximum diameter of 20 mm, which was treated with both flow diverter placement and coil embolization, the sub-marker was initially located within the aneurysm before the insertion of coils. During the first coil insertion, the catheter turned back toward the orifice of the aneurysm, positioning the sub-marker in the parent artery just outside the aneurysm. Throughout the remaining embolization process, the sub-marker stayed in the parent artery, clearly visible under fluoroscopy. According to the operator, the operability of the catheter was comparable to that of conventional microcatheters. There were no complications, and embolization was successfully completed in all patients. Immediate postoperative embolization results included complete occlusion in 4 cases, neck remnants in 13 cases, and dome filling in 1 case. No cases of aneurysm retreatment occurred during the study period (Table 1).
Table 1. Clinical data of 18 patients using a sub-maker catheter.
| No | Age | Sex | Type | Aneurysm location |
Aneurysm maximum size (mm) |
Treatment | Results | Complication |
|---|---|---|---|---|---|---|---|---|
| 1 | 71 | F | Unruptured | ICA paraclinoid | 20.0 | FD | BF | None |
| 2 | 55 | F | Unruptured | ICA-Pcom | 5.1 | Single catheter | NR | None |
| 3 | 61 | F | Unruptured | ICA top | 5.0 | Single catheter | CO | None |
| 4 | 78 | F | Unruptured | MCA | 6.2 | Single catheter | NR | None |
| 5 | 53 | F | Unruptured | ICA-Ach | 5.2 | Single catheter | CO | None |
| 6 | 63 | M | Unruptured | MCA | 5.0 | Single catheter | NR | None |
| 7 | 78 | M | Unruptured | A-com | 5.9 | Single catheter | NR | None |
| 8 | 66 | M | Ruptured | A-com | 6.9 | Single catheter | NR | None |
| 9 | 81 | M | Ruptured | MCA | 9.0 | Single catheter | NR | None |
| 10 | 38 | M | Ruptured | A-com | 4.8 | Single catheter | CO | None |
| 11 | 66 | F | Unruptured | MCA | 5.8 | Stent assist | NR | None |
| 12 | 64 | F | Unruptured | VA | 8.4 | Stent assist | CO | None |
| 13 | 57 | F | Unruptured | VA | 9.6 | Stent assist | NR | None |
| 14 | 56 | M | Unruptured | ICA-Pcom | 6.2 | Double catheter | NR | None |
| 15 | 73 | M | Unruptured | VA-SCA | 10.2 | Double catheter | NR | None |
| 16 | 51 | M | Ruptured | BA top | 9.5 | Double catheter | NR | None |
| 17 | 73 | F | Unruptured | ICA-Pcom | 12.3 | Balloon Assist | NR | None |
| 18 | 68 | F | Unruptured | BA top | 7.1 | Simple | NR | None |
Ach, anterior choroidal artery; A-com, anterior communicating artery; BA, basilar artery; BF, body filling; CO, complete occlusion; F, female; FD, flow diverter; ICA, internal carotid artery; M, male; MCA, middle cerebral artery; NR, neck remnant; Pcom, posterior communicating artery; SCA, superior cerebellar artery; VA, vertebral artery
Illustrative case 1
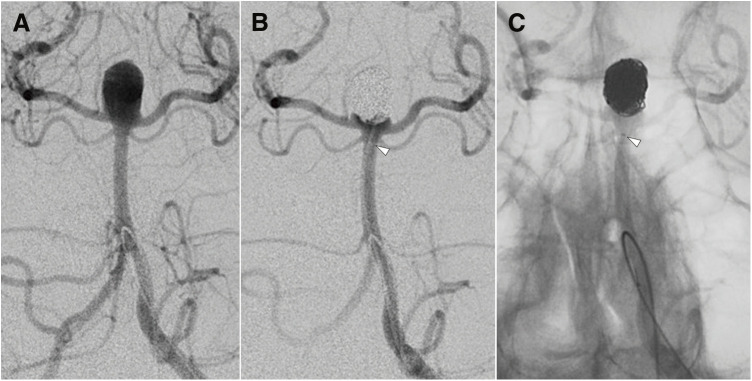
Case 16: A 51-year-old man presented with a subarachnoid hemorrhage, as revealed by a head CT scan. Cerebral angiography identified a ruptured basilar artery tip aneurysm, measuring 9.5 mm in maximum diameter and 6.3 mm in neck width. Figure 2A and 2B show angiograms (anterior–posterior working projection view) before and after embolization, respectively. Figure 2C shows the mask image in the working angle anterior–posterior view following coil insertion. Although the position of the first marker was obscured by the coil mass, the visibility of the sub-marker facilitated easy prediction of the microcatheter tip position.
Fig. 2. Cerebral angiogram of Case 16. (A) A vertebral angiogram (anterior–posterior working projection view) showing a ruptured basilar artery tip aneurysm measuring 9.5 mm in maximum diameter and 6.3 mm at the neck. (B) A vertebral angiogram after coil embolization. (C) A mask image of a vertebral angiogram after coil embolization. The white arrowheads (B and C) indicates the sub-marker. Even though the position of the first marker was obscured by the coil mass, the location of the sub-marker allowed for easy prediction of the microcatheter tip position.
Illustrative case 2
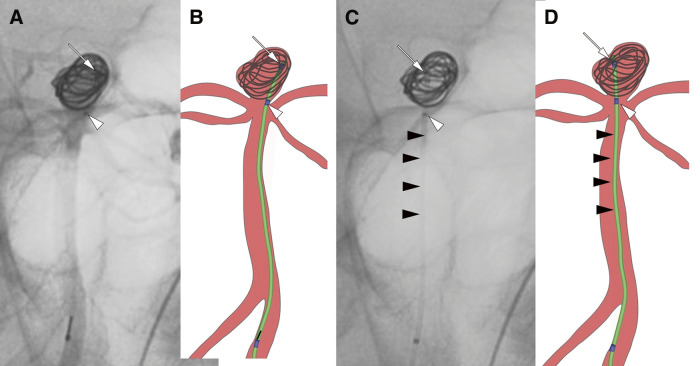
Case 18: A 68-year-old woman underwent cerebral angiography, which revealed an unruptured basilar artery tip aneurysm with a maximum diameter of 7.1 mm. Figure 3A shows a mask image of vascular imaging after insertion of the first coil, and Fig. 3B is an illustration of Fig. 3A. Figure 3C shows a mask image following detachment of the first coil. Although the first marker on the microcatheter was difficult to discern because of the coil mass, the sub-marker and microcatheter shaft indicated that the microcatheter tip, initially positioned on the left side of the aneurysm before coil detachment, shifted to the right side afterward, nearing the wall of the aneurysm. Figure 3D is an illustration of Fig. 3C. The presence of the sub-marker allowed for easy prediction of the microcatheter tip position.
Fig. 3. Cerebral angiogram of Case 18. (A) A mask image of a vertebral angiogram (anterior–posterior working projection view) after the insertion of the first coil. (B) An illustration of the mask image (A) after the first coil insertion. (C) A mask image of a vertebral angiogram following the detachment of the first coil. (D) An illustration of the mask image (C) after the first coil detachment. The white arrows (A–D) indicates the first marker, the white arrowheads (A–D) points to the sub-marker, and the black arrowheads (C and D) shows the microcatheter shaft. Although the first marker on the microcatheter was obscured by the coil mass, the sub-marker and microcatheter shaft allowed verification that the microcatheter tip, initially on the left side of the aneurysm before coil detachment, had moved to the right side afterward.
Discussion
The sub-marker catheter proved useful in predicting the position of the microcatheter tip in all 18 cases of aneurysm embolization. Intraoperative rupture during cerebral aneurysm embolization has been reported to occur in 1.4%–2.6% of cases.1–3) Given the serious consequences that can arise from such events, it is considered one of the most critical complications to prevent. One cause of intraoperative rupture is the difficulty in visualizing the catheter tip under fluoroscopy once the aneurysm is filled with coils, which may lead to perforation of the aneurysm wall by the catheter or coils. When the first marker is visible, it allows for confirmation of the catheter tip position and the prediction of the force and direction with which the coils deployed from the catheter compress the aneurysm wall. The advantage of the sub-marker is that it enables the prediction of the catheter tip position and the relationship between the coils and the aneurysm wall, even when the first marker is obscured by the filled coils. Visual confirmation enables assistants and supervisors to predict the position of the catheter tip and the forces acting on the coils, thereby allowing them to provide feedback to the operator. This enhances the educational aspect and ensures safer aneurysm embolization procedures. Furthermore, the tungsten braid of the microcatheter shaft facilitates visualization of the microcatheter’s trajectory. On the other hand, a disadvantage of the sub-marker is that it adds another marker that must be monitored. It requires careful attention to avoid confusion with other catheter markers during procedures like balloon-assisted or double catheter techniques. Particularly when employing the double catheter technique, it is preferable to use a 2-marker catheter in combination with a sub-marker catheter rather than 2 sub-marker catheters to avoid marker misidentification.
Despite concerns regarding operability issues associated with the hardness of the sub-markers and tungsten braids, this series showed no operational difficulties. In cases of large aneurysms, when the microcatheter tip is deeply positioned within the aneurysm, the sub-marker may become obscured within the coil mass. In such cases, the second marker located 3 cm from the catheter tip can be used as a reference instead of the sub-marker.
Conclusion
The sub-marker catheter is valuable for safely performing aneurysm embolization procedures.
Acknowledgment
The authors would like to express their sincere gratitude to Dr. Kittipong Srivatanakul for developing the sub-marker catheter.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1).Oishi H, Yamamoto M, Shimizu T, et al. Endovascular therapy of 500 small asymptomatic unruptured intracranial aneurysms. AJNR Am J Neuroradiol 2012; 33: 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Pierot L, Spelle L, Vitry F. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke 2008; 39: 2497–2504. [DOI] [PubMed] [Google Scholar]
- 3).Shigematsu T, Fujinaka T, Yoshimine T, et al. Endovascular therapy for asymptomatic unruptured intracranial aneurysms: JR-NET and JR-NET2 findings. Stroke 2013; 44: 2735–2742. [DOI] [PubMed] [Google Scholar]