Abstract
Background
Increased access to and indications for genetic testing will lead to more women undergoing risk-reducing salpingo-oophorectomy (RRSO), with a potential impact on sexual function.
Aim
Our objective was to prospectively investigate (1) sexual function in women with pathogenic variant (PV) in BRCA1/2 genes, before and 1 year after RRSO, and to compare with a healthy age-matched control group and (2) to study if testosterone levels correlate with sexual functioning after RRSO.
Methods
A prospective observational follow-up study of 43 BRCA1/2-PV carriers planned for RRSO and 73 healthy-age matched controls. Data including personal medical history, the Female Sexual Function Index (FSFI) and blood samples for analysis of testosterone by tandem mass spectrometry and free androgen index (FAI) were collected before and 1 year after surgery or at inclusion (controls).
Outcomes
Sexual function and testosterone levels following RRSO.
Results
Median age in the RRSO group was 42 years at baseline, 55.8% were premenopausal and 53.5% had a history of breast cancer. The RRSO group had significantly lower median FSFI total score (P < .001), lower scores of all 6 FSFI domains (P < .001), as well as a higher proportion of female sexual dysfunction (FSD) (P < .001) compared to the control group at 1 year after surgery. In the RRSO group, users of menopausal hormone therapy (MHT) had a significantly higher median FSFI total score compared with the nonusers both at baseline (P = .023) and follow-up (P = .010). The proportion of FSD was significantly higher in the non-MHT group at both baseline (P = .041) and follow-up (P = .009). FAI was significantly lower in the RRSO group when compared to the controls at 1-year follow-up (P = .041); however, no significant correlations between testosterone levels and FSFI scores were found.
Clinical implications
The results highlight the need to counsel BRCA1/2-PV carriers before RRSO and offer a structured follow-up and support addressing sexual function and impact of MHT use.
Strengths and Limitations
The main strength of this study is its prospective design with age-matched controls. Limitation is a small sample size.
Conclusion
Our findings show that sexual function deteriorated 1 year after RRSO independent of testosterone levels, and the proportion with impaired sexual function was higher compared to healthy age-matched controls.
Keywords: risk-reducing salpingo-oophorectomy, BRCA, menopausal hormone therapy, sexual function, testosterone, free androgen index, female sexual function index
Introduction
Women with pathogenic variant (PV) of breast cancer susceptibility genes BRCA1 and BRCA2 are predisposed to breast and ovarian cancer.1 There is no effective screening for ovarian cancer, and therefore, current guidelines recommend risk-reducing salpingo-oophorectomy (RRSO) at 35-40 years of age for BRCA1 and 40-45 years for BRCA2.1,2 Removal of the ovaries and the fallopian tubes reduces the risk of epithelial ovarian cancer and all-cause mortality in this high-risk group.3,4
RRSO in premenopausal women will induce surgical menopause, which is associated with early onset of menopausal symptoms and impaired sexual functioning, as well as increased risk of cardiovascular disease, bone loss, and cognitive dysfunction.5,6 To reduce negative health effects, current guidelines recommend menopausal hormone therapy (MHT) after RRSO until the age of natural menopause if there are no contraindications.7-9 Several studies have reported that MHT can alleviate adverse effects on sexual function but not to presurgical levels.5,10-13
Besides estrogen loss, RRSO also results in 25-50% reduction of serum testosterone in both pre- and postmenopausal women.14 Low testosterone may impair sexual life.15 Moreover, testosterone treatment has been shown to improve sexual desire in postmenopausal women with decreased libido.16 The association between sexual dysfunction and endogenous androgen levels in women have been explored with diverging results.15,17-22
The objective of this study was to prospectively investigate sexual function in BRCA1/2-PV carriers. Does sexual function in BRCA1/2 carriers deteriorates 1 year after RRSO in comparison to a healthy age-matched control group? Do testosterone levels correlate with sexual functioning after RRSO?
Materials and Methods
Study design and participants
This is a prospective observational follow-up study. Women scheduled for RRSO at the Department of Obstetrics and Gynecology, Karolinska University Hospital, were invited to participate in the study. The indication for preventive surgery was a PV in the BRCA1 or BRCA2-gene. Exclusion criteria were not being able to read and understand the study information. Recruitment and 1-year follow up lasted between October 2011 and March 2020.
Controls were recruited at screening for cervical dysplasia, contraceptive counseling, or via social media. Inclusion criteria for the controls were BMI 18-29 kg m−2, comparable age as the RRSO women (±1 year) at 1-year follow-up, sexually active, ability to understand the study information and at least have one ovary in situ. Exclusion criteria were severe illness, pregnancy, current breastfeeding, hormonal contraception, systemic MHT, prior cancer or known hereditary cancer in the family.
The Regional Ethical Review Board in Stockholm approved the study (2010/661-31/1; amendment 2016/799-32). Written informed consent was obtained from all participants.
Study procedures
All participants completed questions on their medical and reproductive history including ongoing medication and lifestyle factors. Sexual function was assessed using a validated questionnaire as described below. Women in the RRSO group completed the questionnaire at baseline before surgery and approximately 1 year after surgery. Serum was stored at −20o until analysis. Due to practical reasons, the blood sampling could not be performed standardized according to menstrual cycle day or time of the day. The controls completed the questionnaire and gave a blood sample at one time point.
Sexual function
Sexual function was assessed by the Female Sexual Function Index (FSFI), which is a validated questionnaire for healthy women, as well as for cancer survivors.23,24 It contains 19-items assessing 6 domains of sexual functioning reported for the last month: desire, arousal, lubrication, orgasm, satisfaction, and pain. The total score (2–36) is obtained by summing the 6 domains. A higher score means better sexual functioning. A score below 26.55 is considered as female sexual dysfunction (FSD).24,25 A sensitivity analysis, including only participants considered to be sexually active, was performed (Supplemental Data 1).26
Hormone analyses
Total testosterone was measured by liquid chromatography tandem mass spectrometry (LC–MS/MS) and serum levels of sex hormone-binding globulin (SHBG) were determined by electrochemiluminescence immunoassay (ELISA). Free androgen index (FAI) was calculated with the equation total testosterone/SHBG × 100.
Power calculation
A power calculation was conducted prior to the study which had a primary outcome of a change in sexual function following RRSO, as measured by the FSFI subscales. Based on the available data a sample size of 30 would provide 80% power at a 2-sided 5% level of significance to detect a clinical difference in sexual function between groups.23,25
Statistics
Descriptive characteristics are presented as median and interquartile range for numerical variables and as frequencies and percentages for categorical variables. In the cross-sectional analyses of the demographic characteristics and FSFI, differences between the RRSO and the control group were investigated using Wilcoxon rank-sum test for numerical variables and Fisher’s exact test for categorical variables. In the longitudinal analyses, Wilcoxon signed-rank test was used for numerical variables and McNemar’s test was used for the categorized total FSFI score.
The association between the FSFI score at follow-up and FSFI total score at baseline, history of breast cancer, menopausal status at baseline, MHT use postoperatively and age was studied using median regression with the default settings of the rq function of the quantreg R package.27 To investigate the correlation between testosterone levels and sexual functioning, Spearman’s correlation coefficients were calculated and median regression analysis was performed.
Subgroup analyses were performed according to menopausal status at baseline, history of breast cancer and MHT use at follow-up. For individuals with isolated missing data in the FSFI questionnaire, imputation of the mean value for the domain was applied.28,29 If more than half of the data within the domains was missing, the participant was excluded from the analysis. The full FSFI score was calculated for women with no missing domains.
A P <.05 was considered statistically significant. All data from the questionnaires and the clinical trial have been analyzed in R version 4.2.2.30
In the Supplemental Data, we provide a complementary methods section with details about sexual function and hormonal measurements, MHT, bias and statistical analysis.
Results
Out of the 65 invited BRCA1/2-PV carriers planned for RRSO, 54 were eligible and included (Figure 1). Drop-outs are described in Figure 1. Forty-three participants in the RRSO group completed the study. In the loss to follow-up analysis, demographic data was similar between groups (data not shown). Seventy-five age-matched controls met the inclusion criteria and consented to participate. Of them 73 women, with complete questionnaires, were included in the analyses (Figure 1).
Figure 1.
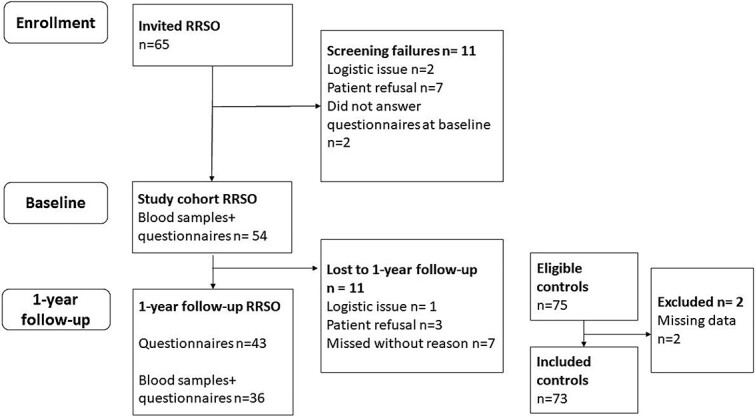
Flowchart.
The characteristics of the study participants are presented in Table 1. At baseline, median age in the RRSO group was 42.0 years, 55.8% were premenopausal, and 53.5 % had a personal history of breast cancer. At 1-year follow-up, there was no change in partnership status and no new cases of breast cancer developed during the study period (Supplementary Table 1); however, there was a significant increase in MHT use. Of the women with no history of breast cancer (n = 20), 11 premenopausal and 2 postmenopausal women were MHT users. There were no significant differences between the RRSO group at 1-year follow-up and the controls regarding age, BMI, parity, or education. However, more controls were in partnership and employed.
Table 1.
Characteristics of study participants.
| RRSO baseline |
RRSO 1 year |
Controls | P value | |
|---|---|---|---|---|
| n = 43 | n = 43 | n = 73 | ||
| Age years (median(IQR)) | 42.0 (40-57.5) | 43.4 (41.2-58.5) | 44 (40-58) | .601 |
| BMI kg m−2 (median(IQR)) | 24.3 (22.0-26.5) | 23.6 (22.1-26.4) | 23.1 (21.5-25.3) | .254 |
| Children n (%) | .783 | |||
| Yes | 37 (86.0) | 37 (86.0) | 64 (87.7) | |
| No | 6 (14.0) | 6 (14.0) | 9 (12.3) | |
| Partner n (%) | .005 | |||
| Yes | 36 (83.7) | 31 (72.1) | 68 (93.2) | |
| No | 6 (14.0) | 10 (23.3) | 5 (6.8) | |
| Missing | 1 (2.3) | 2 (4.7) | ||
| Employment n (%) | .017 | |||
| Employed | 40 (93.0) | 39 (90.7) | 73 (100.0) | |
| Unemployed | 2 (4.7) | 3 (7.0) | 0 (0.0) | |
| Unknown | 1 (2.3) | 1 | ||
| Menopausal status n (%) | <.001 | |||
| Premenopausal | 24 (55.8) | 0 (0) | 49 (67.1) | |
| Postmenopausal | 18 (41.9) | 43 (100) | 24 (32.9) | |
| Missing | 1 (2.3) | |||
| Hysterectomy at time of RRSO n | NA | |||
| Yes | 8 | |||
| No | 35 | |||
| History of breast cancer n (%) | <.001 | |||
| Yes | 23 (53.5) | 23 (53.5) | 0 | |
| No | 20 (46.5) | 20 (46.5) | 73 | |
| MHT use n (%) | <.001 | |||
| Systemica | 4 (9.3) | 14 (32.5) | 0 | |
| Local | 1 (2.3) | 1 (2.3) | 5 (6.8) | |
| None | 38 (88.4) | 28 (65.1) | 68 (93.2) | |
| Time since RRSO, years (median IQR) | 1.11 (1.04, 1.21) | NA |
BMI, body mass index; IQR, inter quartal range; MHT, menopausal hormone therapy; RRSO, risk-reducing salpingo-oophorectomy.
P-value: Wilcoxon rank-sum test for age and BMI otherwise Fisher’s exact test for group difference (RRSO group 1 year vs. controls).
aSystemic MHT use = includes oral contraception, oral estrogen therapy (ET) with or without systemic progesterone, transdermal ET with or without systemic progesterone or LNG-IUD.
Sexual function in the total cohorts
In the RRSO group, the domain score orgasm (P = .023) and pain (P = .032) decreased significantly, and there was a tendency of decline (P = .062) in median FSFI total score from 26.6 points at baseline to 23.0 points one year after RRSO (Table 2). When comparing the RRSO group with the control group at 1 year, the RRSO group had significantly lower median FSFI total score (P < .001), as well as lower scores of all 6 domains (P < .001). The proportion of FSD in the RRSO group tended to increase after RRSO (P = .070). The proportion of FSD was significantly higher in the RRSO group at 1-year follow-up compared to the control group (P < .001).
Table 2.
FSFI and hormone levels.
| Total cohort | |||||
|---|---|---|---|---|---|
| Variables | RRSO baseline | RRSO 1 year | P 1 | Controls | P 2 |
| n = 39 | n = 39 | n = 67 | |||
| FSFI total score (2-36) | 26.6 (15.6-29.1) | 23.0 (4.2-28.1) | .062 | 30.2 (26.6-32.5) | <.001 |
| Desire (1.2-6) | 2.4 (1.2-3.6) | 2.4 (1.2-3.0) | .123 | 3.0 (3.0-4.2) | <.001 |
| Arousal (0-6) | 3.9 (1.7-5.0) | 3.6 (0.6-4.7) | .074 | 5.1 (3.9-5.7) | <.001 |
| Lubrication (0-6) | 4.5 (2.0-5.6) | 3.6 (0.0-5.7) | .196 | 5.7 (4.8-6.0) | <.001 |
| Orgasm (0-6) | 4.8 (2.0-5.6) | 3.6 (0.0-5.2) | .023 | 5.6 (4.4-6.0) | <.001 |
| Satisfaction (0.8-6) | 3.6 (2.4-5.2) | 3.6 (2.4-5.2) | .813 | 5.2 (4.6-5.6) | <.001 |
| Pain (0-6) | 5.4 (0.0-6.0) | 3.2 (0.0-6.0) | .032 | 6.0 (5.6-6.0) | <.001 |
| Proportion with sexual dysfunction FSFI total score < 26.55 (%) | 17 (43.6) | 26 (66.7) | .070a | 17 (25.4) | <.001b |
| n = 35 | n = 35 | n = 58 | |||
| Total testosterone (nmol L−1) | 0.5 (0.4-0.8) | 0.5 (0.4-0.7) | .522 | 0.6 (0.4-0.8) | .36 |
| SHBG (nmol L−1) | 74 (51-94) | 77 (51-113) | .212 | 66 (46-89) | .176 |
| FAI (nmol L−1) | 0.7 (0.4-1.1) | 0.6 (0.5-1) | .588 | 0.9 (0.6-1.3) | .041 |
All values are presented as median (IQR) except FSD (female sexual dysfunction) presented in n (%).
FSFI, Female Sexual Function Index; FAI, free androgen index; RRSO, risk-reducing salpingo-oophorectomy; SHBG, sexual hormone-binding globulin.
P 1: Wilcoxon’s signed-rank test for comparison between baseline and 1-year follow-up.
P 2: Wilcoxon rank-sum test for group difference (RRSO group 1 year vs. controls).
aMcNemar's Chi-squared test with continuity correction.
bFisher’s exact test for group difference (RRSO group 1 year vs. controls).
A sensitivity analysis for women in the cohort who were sexually active (RRSO, n = 34 and controls, n = 59) showed similar results (Supplementary Table 2).
Sexual function in subgroups
At baseline, premenopausal women had a significantly higher median FSFI total score than postmenopausal women (P = .022) (Figure 2a, Supplementary Table 3). At follow-up, the premenopausal women declined significantly in median FSFI total score (P = .044), whereas the women who were postmenopausal did not change, and there was no difference between the groups.
Figure 2.
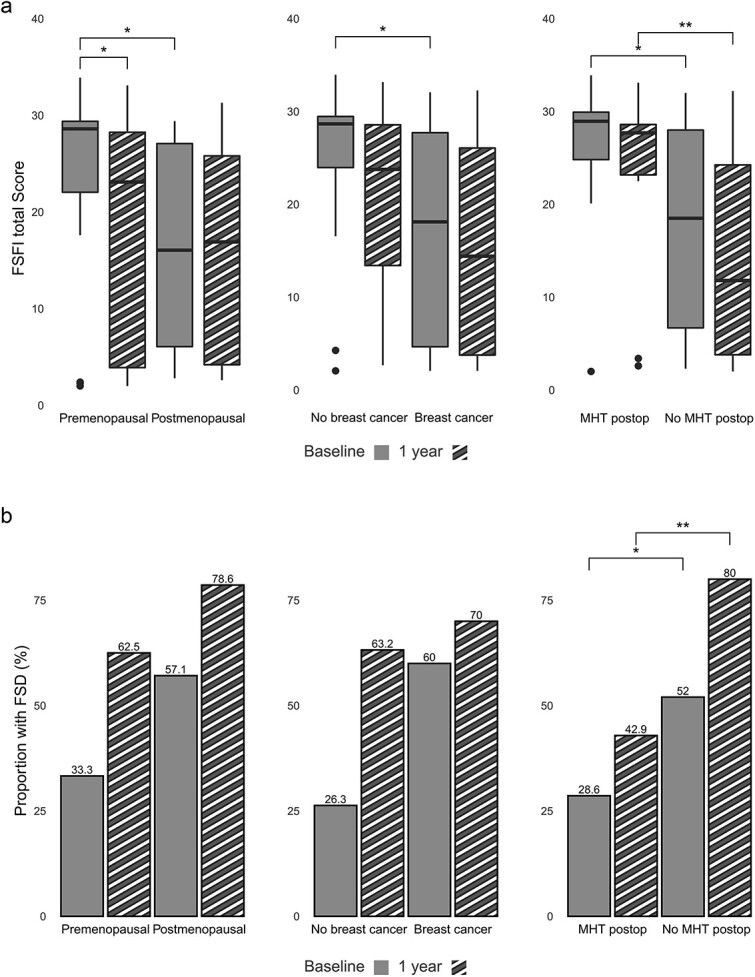
(a) FSFI total score divided by menopausal status, history of breast cancer and use of MHT postoperatively. FSFI, female sexual function index; MHT, = menopausal hormone therapy, *P < .05-.01, **P < .01-.001. (b) Proportion of women with female sexual dysfunction at baseline and 1-year divided by menopausal status, history of breast cancer and use of MHT postoperatively. FSD, female sexual dysfunction; MHT, menopausal hormone therapy, *P < .05-.01, **P < .01-.001.
Women with no history of breast cancer had a significantly higher median FSFI score at baseline compared to the women with a history of breast cancer (P = .015) (Figure 2a, Supplementary Table 3). At follow-up, there was no significant difference between the groups.
Women reporting MHT use at follow-up had a significantly higher median FSFI total score at baseline compared with the nonusers (P = .023), as well as at follow-up (P = .010) (Figure 2a, Supplementary Table 3). The proportion of FSD was significantly higher in the non-MHT group at both baseline (P = .041) and follow-up (P = .009) (Figure 2b).
Regression analysis showed a significant association between a higher total FSFI score at baseline and a lower total FSFI score at follow-up (0.89 (CI 0.68-1.02)). Furthermore, MHT postoperatively was associated with a significantly higher total FSFI score at follow-up (3.93 (CI 0.16-16.55)).
Hormone levels
In the RRSO group, the median levels of total testosterone, SHBG and FAI were unchanged from baseline to 1 year after RRSO (Table 2). However, FAI was significantly lower in the RRSO group when compared to the controls at 1-year follow-up (P = 0.041). There were no significant correlations between total testosterone and FAI and FSFI scores in any group (data not shown).
Discussion
The main findings in this study were a significantly impaired sexual function and a higher proportion of FSD in BRCA1/2-PV carriers compared to healthy controls of the same age at 1-year after RRSO. Furthermore, we found that MHT after RRSO significantly counteracted a decline in sexual function. Although FAI was significantly lower in the RRSO group when compared to the controls at 1 year follow-up, neither circulating testosterone nor FAI were associated with sexual function in the RRSO group or the controls.
To our knowledge, this is the only prospective study including both pre- and postmenopausal women undergoing RRSO compared to age-matched controls. Our findings, with a high frequency of sexual dysfunction after RRSO (67%), suggest that BRCA1/2-PV carriers need a structured follow-up addressing sexual problems. In premenopausal women, RRSO induces an estrogen deficiency causing vaginal atrophy with dryness and pain at sexual activity. This could partly explain the impaired sexual function. Furthermore, in the RRSO group, 53.5% of the women had a prior history of breast cancer with risk of iatrogenic menopause from cytotoxic treatment. This may explain the higher proportion of postmenopausal women in the RRSO group, compared to controls, and the lower levels of sexual function at baseline.31 In addition, this is one of few studies that prospectively examines the impact of MHT use in different domains of female sexual function following RRSO.12,13 At follow-up, women in the RRSO group with MHT reported a significantly better sexual function as well as significantly higher levels in all sexual domains, except satisfaction, compared to the nonusers. This indicates that MHT improves sexual function after RRSO. However, type of MHT used in the RRSO group was heterogenous and with different routes of administration. The findings must therefore be interpreted with caution. Other studies also report worse sexual functioning after RRSO. Some studies only included premenopausal women and controls from a high-risk population rather than healthy controls.13,32,33 Premenopausal women and women with no personal history of breast cancer had higher presurgical levels of sexual function and experienced a greater decline in sexual function compared to postmenopausal women and those with a history of breast cancer. These results are all in line with previous findings.10,12,34 In the RRSO cohort the median total FSFI score at follow-up was significantly associated with FSFI at baseline and use of MHT postoperatively. Presurgical levels of FSFI seem to be important in predicting sexual function after RRSO.
There is a risk that the concern for a decline in sexual function may deter BRCA1/2-PV carriers from a potentially life-saving preventive surgery. To minimize negative effects, MHT is recommended to women after RRSO up to natural age of menopause if no contraindications.7,8 However, women may be hesitant in using MHT due to safety concerns and their inherited high risk of breast cancer.35 Development of guidelines and long-term safety data on MHT following RRSO are needed.
Women experiencing FSD following RRSO commonly ask about testosterone treatment. While MHT effectively counteracts effects on vaginal tissue and reduces vasomotor symptoms following estrogen loss, testosterone is thought to play a more important role in sexual desire.15,21 We did not detect a decrease in testosterone levels in the RRSO group at follow-up. Neither did we find any association between endogenous testosterone and female sexual function in the RRSO group nor among controls. Explanations could be potential confounders influencing the testosterone levels such as; blood samples not standardized to morning hours nor menstrual cycle day (preferably in the follicular phase in premenopausal women), previous chemotherapy, ongoing antiestrogen therapies, and the use of MHT affecting SHBG. Our results confirm previous studies where endogenous levels of testosterone have not been directly related to female sexual function suggesting that other factors may also be important.18-20 Only a few studies have explored testosterone levels in women following RRSO. One prospective study by van Winden et al.36 found a significant association between sexual function and reduced testosterone levels in postmenopausal women. However, two other studies could not find any association between testosterone levels and sexual function following RRSO.17,18
The strengths of this study are its prospective design and controls of similar age, BMI, parity, smoking, and education, as well as determining testosterone levels by tandem mass spectrometry. A cancer diagnoses as well as breast cancer treatment may affect relationship status and employment. This reflects the complexity of the patient group where some women already have a personal history of breast cancer before the RRSO. Moreover, at baseline women in the RRSO group with no personal history of breast cancer reported similar levels of FSD as the controls. Controls showed similar levels of FSD as in the general population.13,37
The small sample size collected over an extended time period suggests that our findings should be interpreted with caution. However, even if the loss to follow-up analysis showed no demographic differences between groups we cannot exclude that the women lost to follow-up or not completing FSFI were less sexually interested. Another limitation is the lack of evaluation of sexual-related distress, central in FSD. Women with a history of breast cancer generally have a contraindication to MHT use, and in the analysis concerning MHT this can be considered as a confounder. Due to the small sample size, we were in the regression analyses unable to address several factors that may be of importance for sexual function in women such as BMI, partnership, depression, anxiety, body image, personal distress related to sexual problems, and use of antidepressants.38
Conclusion
Our study suggests that sexual function is impaired after RRSO and the proportion of FSD 1 year after RRSO is larger compared to healthy controls. MHT mitigates the sexual problems but does not restore them to baseline levels. The study highlights the need for counseling of women before and after RRSO, including evidence-based information on MHT use. The importance of endogenous testosterone for female sexual function after RRSO needs to be further explored in larger studies.
Supplementary Material
Acknowledgments
The authors thank colleagues and research staff C. Klynning, B. Legerstam, L. Blomberg, H. Fagreaus, and A. Wickström, at Karolinska University Hospital Research Unit and Danderyd Hospital Research Unit for help with inclusion of patients and data collection as well as Henrike Häbel at the Department of Learning, Informatics, Management and Ethics (LIME), Karolinska Institutet for help and support with the statistical analysis.
Contributor Information
Åsa Ehlin von Kartaschew, LIVIO, 115 42 Stockholm, Sweden; Department of Women’s and Children’s Health, Karolinska Institutet, 117 77 Stockholm, Sweden.
Angelica Lindén Hirschberg, Department of Women’s and Children’s Health, Karolinska Institutet, 117 77 Stockholm, Sweden; Clinical Department of Gynecology and Reproductive Medicine, Karolinska University Hospital, 141 86 Huddinge, Sweden.
K Gemzell-Danielsson, Department of Women’s and Children’s Health, Karolinska Institutet, 117 77 Stockholm, Sweden; Clinical Department of Gynecology and Reproductive Medicine, Karolinska University Hospital, 141 86 Huddinge, Sweden.
Angelique Flöter Rådestad, Department of Women’s and Children’s Health, Karolinska Institutet, 117 77 Stockholm, Sweden; Department of Hereditary Cancer, Karolinska University Hospital, 117 76 Stockholm, Sweden.
Author contributions
Å.E.: Methodology, Investigation, Data Analysis, Data Curation, Writing—Original Draft, Writing—Review & Editing, Project Administration;
A.H.L.: Methodology, Data analysis, Writing—Review & Editing;
K.G.D.: Methodology, Data analysis, Writing—Review & Editing, Supervision;
A.F.R.: Design, Methodology, Investigation, Resources, Data analysis Data Curation, Writing—Original Draft, Writing—Review & Editing, Supervision, Project Administration, Funding Acquisition.
Funding
Financial support was provided through the Regional Agreement on Medical Training and Clinical Research (ALF) between the Stockholm County Council and the Karolinska Institutet.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1. Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2021;19(1):77-102. 10.6004/jnccn.2021.0001 [DOI] [PubMed] [Google Scholar]
- 2. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240-1253. 10.1016/S0140-6736(18)32552-2 [DOI] [PubMed] [Google Scholar]
- 3. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14(1):150. 10.1186/s12905-014-0150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Finch AP, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32(15):1547-1553. 10.1200/JCO.2013.53.2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tucker PE, Cohen PA. Review article: sexuality and risk-reducing Salpingo-oophorectomy. Int J Gynecol Cancer. 2017;27(4):847-852. 10.1097/IGC.0000000000000943 [DOI] [PubMed] [Google Scholar]
- 6. Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483-491. 10.3109/13697137.2015.1020484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manchanda R, Gaba F, Talaulikar V, et al. Risk-reducing Salpingo-oophorectomy and the use of hormone replacement therapy below the age of natural menopause: scientific impact paper No. 66 October 2021: scientific impact paper No. 66. BJOG. 2022;129(1):e16-e34. 10.1111/1471-0528.16896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pillay OC, Manyonda I. The surgical menopause. Best Pract Res Clin Obstet Gynaecol. 2022;81:111-118. 10.1016/j.bpobgyn.2022.03.001 [DOI] [PubMed] [Google Scholar]
- 9. Sinno AK, Pinkerton J, Febbraro T, et al. Hormone therapy (HT) in women with gynecologic cancers and in women at high risk for developing a gynecologic cancer: a Society of Gynecologic Oncology (SGO) clinical practice statement: this practice statement has been endorsed by the North American Menopause Society. Gynecol Oncol. 2020;157(2):303-306. 10.1016/j.ygyno.2020.01.035 [DOI] [PubMed] [Google Scholar]
- 10. Finch A, Metcalfe KA, Chiang JK, et al. The impact of prophylactic salpingo-oophorectomy on menopausal symptoms and sexual function in women who carry a BRCA mutation. Gynecol Oncol. 2011;121(1):163-168. 10.1016/j.ygyno.2010.12.326 [DOI] [PubMed] [Google Scholar]
- 11. Johansen N, Liavaag AH, Tanbo TG, Dahl AA, Pripp AH, Michelsen TM. Sexual activity and functioning after risk-reducing salpingo-oophorectomy: impact of hormone replacement therapy. Gynecol Oncol. 2016;140(1):101-106. 10.1016/j.ygyno.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 12. Hall E, Finch A, Jacobson M, et al. Effects of bilateral salpingo-oophorectomy on menopausal symptoms and sexual functioning among women with a BRCA1 or BRCA2 mutation. Gynecol Oncol. 2019;152(1):145-150. 10.1016/j.ygyno.2018.10.040 [DOI] [PubMed] [Google Scholar]
- 13. Islam RM, Davis SR, Bell RJ, et al. A prospective controlled study of sexual function and sexually related personal distress up to 12 months after premenopausal risk-reducing bilateral salpingo-oophorectomy. Menopause. 2021;28(7):748-755. 10.1097/GME.0000000000001766 [DOI] [PubMed] [Google Scholar]
- 14. Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90(7):3847-3853. 10.1210/jc.2005-0212 [DOI] [PubMed] [Google Scholar]
- 15. Wahlin-Jacobsen S, Pedersen AT, Kristensen E, et al. Is there a correlation between androgens and sexual desire in women? J Sex Med. 2015;12(2):358-373. 10.1111/jsm.12774 [DOI] [PubMed] [Google Scholar]
- 16. Davis SR, Worsley R, Miller KK, Parish SJ, Santoro N. Androgens and female sexual function and dysfunction--findings from the fourth international consultation of sexual medicine. J Sex Med. 2016;13(2):168-178. 10.1016/j.jsxm.2015.12.033 [DOI] [PubMed] [Google Scholar]
- 17. Tucker PE, Bulsara MK, Salfinger SG, Tan JJ, Green H, Cohen PA. Prevalence of sexual dysfunction after risk-reducing salpingo-oophorectomy. Gynecol Oncol. 2016;140(1):95-100. 10.1016/j.ygyno.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 18. Johansen N, Liavaag AH, Morkrid L, Michelsen TM. Hormone levels and sexual functioning after risk-reducing Salpingo-oophorectomy. Sex Med. 2018;6(2):143-153. 10.1016/j.esxm.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tucker PE, Bulsara MK, Salfinger SG, Tan JJ, Green H, Cohen PA. The effects of pre-operative menopausal status and hormone replacement therapy (HRT) on sexuality and quality of life after risk-reducing salpingo-oophorectomy. Maturitas. 2016;85:42-48. 10.1016/j.maturitas.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 20. Zheng J, Islam RM, Skiba MA, Bell RJ, Davis SR. Associations between androgens and sexual function in premenopausal women: a cross-sectional study. Lancet Diabetes Endocrinol. 2020;8(8):693-702. 10.1016/S2213-8587(20)30239-4 [DOI] [PubMed] [Google Scholar]
- 21. Randolph JF Jr, Zheng H, Avis NE, Greendale GA, Harlow SD. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab. 2015;100(1):258-266. 10.1210/jc.2014-1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davis SR, Davison SL, Donath S, Bell RJ. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294(1):91-96. 10.1001/jama.294.1.91 [DOI] [PubMed] [Google Scholar]
- 23. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191-208. 10.1080/009262300278597 [DOI] [PubMed] [Google Scholar]
- 24. Baser RE, Li Y, Carter J. Psychometric validation of the female sexual function index (FSFI) in cancer survivors. Cancer. 2012;118(18):4606-4618. 10.1002/cncr.26739 [DOI] [PubMed] [Google Scholar]
- 25. Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31(1):1-20. 10.1080/00926230590475206 [DOI] [PubMed] [Google Scholar]
- 26. Meston CM, Freihart BK, Handy AB, Kilimnik CD, Rosen RC. Scoring and interpretation of the FSFI: what can be learned from 20 years of use? J Sex Med. 2020;17(1):17-25. 10.1016/j.jsxm.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 27. Koenker R. quantreg: Quantile Regression. R package version 5.94. 2022. https://CRAN.R-project.org/package=quantreg
- 28. Bell ML, Fairclough DL, Fiero MH, Butow PN. Handling missing items in the hospital anxiety and depression scale (HADS): a simulation study. BMC Res Notes. 2016;9(1):479. 10.1186/s13104-016-2284-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Everhov ÅH, Flöter Rådestad A, Nyberg T, Smedby KE, Bergmark K, Lindén HA. Serum androgen levels and sexual function before and one year after treatment of uterine cervical cancer: a pilot study. J Sex Med. 2016;13(3):413-424. 10.1016/j.jsxm.2015.12.022 [DOI] [PubMed] [Google Scholar]
- 30. R Core Team A Language and Environment for Statistical Computing: Version 4.0.1. R Foundation for Statistical Computing; 2022. [Google Scholar]
- 31. Nappi RE, Lachowsky M. Menopause and sexuality: prevalence of symptoms and impact on quality of life. Maturitas. 2009;63(2):138-141. 10.1016/j.maturitas.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 32. Vermeulen RFM, Beurden MV, Kieffer JM, et al. Hormone replacement therapy after risk-reducing salpingo-oophorectomy minimises endocrine and sexual problems: a prospective study. Eur J Cancer. 2017;84:159-167. 10.1016/j.ejca.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 33. Fang CY, Cherry C, Devarajan K, Li T, Malick J, Daly MB. A prospective study of quality of life among women undergoing risk-reducing salpingo-oophorectomy versus gynecologic screening for ovarian cancer. Gynecol Oncol. 2009;112(3):594-600. 10.1016/j.ygyno.2008.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Panjari M, Bell RJ, Davis SR. Sexual function after breast cancer. J Sex Med. 2011;8(1):294-302. 10.1111/j.1743-6109.2010.02034.x [DOI] [PubMed] [Google Scholar]
- 35. Gordhandas S, Norquist BM, Pennington KP, Yung RL, Laya MB, Swisher EM. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol. 2019;153(1):192-200. 10.1016/j.ygyno.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 36. van Winden LJ, Vermeulen RFM, van den Noort V, et al. Changes in sex steroids and relation with menopausal complaints in women undergoing risk-reducing Salpingo-oophorectomy. J Endocr Soc. 2022;6(6):bvac069. 10.1210/jendso/bvac069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lammerink EAG, de Bock GH, Pascal A, et al. A survey of female sexual functioning in the general Dutch population. J Sex Med. 2017;14(7):937-949. 10.1016/j.jsxm.2017.04.676 [DOI] [PubMed] [Google Scholar]
- 38. Zheng J, Skiba MA, Bell RJ, Islam RM, Davis SR. The prevalence of sexual dysfunctions and sexually related distress in young women: a cross-sectional survey. Fertil Steril. 2020;113(2):426-434. 10.1016/j.fertnstert.2019.09.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


