Abstract
Background
The impact of pathogen reduction technology (PRT) on metabolic and hemostatic profile of treated platelets remains a subject of debate. Platelets Additive Solutions (PASs) are suggested as more appropriate storage medium compared to plasma. To investigate this in terms of zero heterogeneity PRT-treated and control apheresis platelet concentrates (PCs), collected from the same donors and stored in PAS and plasma respectively, were analyzed.
Materials and methods
In the first arm of the study six double dose-apheresis PCs were produced, split and stored in plasma, while in the second arm six split double dose-apheresis PCs from the same donors, were produced and stored in PAS. Control and PRT-treated PCs resulted in both arms. Metabolic and hemostatic markers were evaluated in all the examined groups on days 1, 3 and 5.
Results
A time dependent increased metabolism both in PAS and plasma-stored PCs was evident in PRT-treated PCs. However, the metabolic profile was better preserved in PCs stored in PAS, as higher pH (6.8 vs 6.5, p=0.007) and lower lactate levels (12.6 vs 17.8 mmol/L, p=0.009) were documented in PRT-treated PAS-PCs compared to plasma-PCs, on day 5. A time dependent decreased hemostatic capacity regardless the storage medium was evident in PRT-treated PCs, (PAS-PCs MCF, p=0.004 and plasma-PCs MCF, p=0.007). Similar results were obtained in control PCs.
Discussion
The use of PAS preserves the metabolic profile of PCs more adequately compared to plasma but has no effect on the hemostatic profile. The clinical relevance of these findings needs further investigation.
Keywords: Mirasol-treated apheresis platelets, ROTEM, pathogen reduction technology, storage medium
INTRODUCTION
The development of pathogen reduction technologies (PRTs) is a well-recognized practice in transfusion medicine. The main benefit of their use is minimizing the risk of pathogen transmission via transfusion. This is achieved by using UV-light along with a photosensitive agent a combination that prevents the replication of pathogens in platelet concentrates (PCs). Treatment causes chemical modifications in DNA and changes in molecular structure, affecting both pathogens and white blood cells in the product1–6. Nowadays, the two most prevalent PRT systems use either psoralen along with UVA light [Intercept, (Cerus Corporation, Concord, CA, USA)] or riboflavin along with broad spectrum UVB light [Mirasol, (Terumo BCT, Lakewood, CO, USA)]7,8. A reasonable concern, based on current literature, is that treated platelets are associated with significantly reduced post transfusion recovery and survival. Nevertheless, they seem to retain sufficient in vivo treatment efficacy9.
It is well established that platelet efficiency decreases during storage due to platelet storage lesions (PSL), which include activation, morphology changes, surface receptor activation, membrane destruction and proteolysis10. This natural process seems to be exacerbated with the utilization of PRT-treatment, either by accelerating or inducing further storage lesions. PRT-treated platelets also display increased metabolism patterns, such as elevated lactate production and glucose consumption11. During storage, platelet metabolism takes place mostly through aerobic respiration, for which oxygen is essential. As storage time proceeds, aerobic respiration is down regulated and anaerobic respiration predominates, resulting to elevated concentrations of lactic acid and consequently to reduced pH values. It is well documented that pH levels below 6.2 can result in irreversible platelet damage12.
The occurrence of PSL is multifactorial and involves the collection method, the storage medium and the processing techniques. Platelet storage remains a challenge in transfusion services with main objective being the maintenance of metabolic properties along with minimal platelet activation, for a longer time period13,14. Platelet additive solutions (PASs) have been developed in an effort to store platelets in a medium which would mitigate the loss of quality during storage and have lower allergen potential than plasma15. PASs possibly retain metabolic properties better than plasma, due to the higher buffering capacity offered by the platelet storage medium16,17. The use of PASs provides acetate, which display dual role acting as an extra metabolic fuel and also as a buffer. Furthermore, magnesium and potassium, present in PASs, are considered to have protective capacities regarding platelet aggregation and activation. As a result, PAS is proposed as a mean that preserves more adequately platelet properties for an extended storage period, when PCs in plasma cannot expand their storage period13,14. Contradictive reports also exist, where the inferiority of PASs is disputed, as no significant differences in PCs stored in PAS and in plasma are noted12,18.
Whereas, the metabolic profile of treated PCs has been extensively investigated, in several storage mediums, using different PRTs, different collecting techniques and for different time periods, the hemostatic ability of PRT-treated platelets is a subject in need of further research. Furthermore, the effects of PAS and plasma on PSL have not been fully elucidated. In order to attain these goals, a pool of shared platelet-donors, previously recruited in two different studies19,20 was created. In particular, the same donors have donated, at two different timepoints, apheresis platelets stored once in PAS and once in plasma. A series of measurements was conducted, in an attempt to assess the effects of the different storage mediums on the functional and hemostatic properties of PRT-treated and control platelets. In this context, untreated platelets stored in T-PAS+ were compared with untreated platelets stored in plasma, and treated platelets stored in T-PAS+ were compared with treated platelets in plasma.
MATERIALS AND METHODS
Platelet collection and in vitro testing
Initially, six double dose-apheresis PCs were produced, split and stored in plasma19. Afterwards, the same donors repeated the donation of six splitted double dose-apheresis PCs stored in T-PAS+20. Platelet apheresis was performed with Trima collection device (Trima, Accel Terumo BCT, Lakewood, CO, USA) while a protocol of platelet yield of 6.5×1011 and 40 mL plasma was selected for the first group. For the second group, the protocol included the collection of 6.5×1011 platelets per bag, suspended in 65% mL PAS and 35% mL plasma, along with 35 mL platelet poor plasma (PPP) in a separate bag to be used in further tests as needed. Apheresis-PCs were collected in the Blood Bank Unit of “Attikon” University Hospital, while the same operator was responsible for the apheresis procedure in all cases. The process duration was within the limits suggested by the manufacturers’ instructions.
In both studies, platelet units were kept undisturbed for two hours at a temperature of 22–24°C to allow dissociation of any platelet aggregates, after which they were agitated for one hour before being divided into two platelet aliquots of roughly the same size. At the time of apheresis-platelet collection, the total platelet count was determined. Afterwards, one bag was kept as a control unit (C) and the other one was merged with a riboflavin kit in low light conditions, in an illumination bag. Then, this aliquot was treated with UV light in a Mirasol device (M), according to the manufacturers’ instructions. The bag was UV-treated while linearly agitated at 120 cpm, at a product temperature below 37°C. The target energy to be delivered was 6.24 J/mL, after which the bag was removed.
Both control and PRT-treated aliquots suspended in plasma and respectively in PAS were stored in a linear agitator at 20–24°C for five days. On storage days 1 (for immediate effects assessment), 3 and 5, platelet samples were collected from each bag using an aseptic technique. Analysis was completed within 4 hours after sampling. Testing included platelet count determination, pH, pO2, pCO2 (blood gases) and metabolism markers such as lactate, glucose, and lactate dehydrogenase (LDH). Platelet aggregation (LTA aggregometry) and platelet viscoelastic properties (ROTEM) were also measured.
Both studies were approved by the “Attikon” General University Hospital’s institutional review board (28/01/2020 and 30/07/2021). Before platelet apheresis procedure, an informed written consent was obtained.
Metabolism assays
Platelet counts were performed on a Sysmex XE-2100 analyzer (Roche, Lincolnshire, IL, USA). Regarding PCs samples stored in PAS, blood gas analysis was performed by a GEM-Premier 5000 blood gas analyzer (Instrumentation Laboratory, Bedford, MA, USA). Blood gas analysis of PCs samples stored in plasma was undertaken on a Cobas®b123 POC blood gas analyzer (Roche Diagnostic Ltd, Rotkreuz, Switzerland). Metabolism markers were also assessed by GEM Premier 5000 and by Cobas®b123 POC analyzer for samples in PAS and plasma respectively. LDH was measured by Cobas8000 (Roche, Lincolnshire, IL, USA) analyzer in PCs stored both in plasma and PAS.
Platelet aggregation
Blood samples were centrifuged at 200 × g for 10 min to obtain platelet rich plasma (PRP). Subsequently, the specimen was re-centrifuged at 2,000 × g for 15 min to obtain platelet-poor plasma (PPP). Platelet count of C and M samples was adjusted between 200×109/L and 300×109/L with PPP. PPP was used to set a 100% upper limit, and platelet rich plasma to set a 0% baseline before the addition of the agonist. Aggregation was performed by a Biodata-PAP-4 aggregometer (Bio-Data Corporation, Horsham, PA, USA). ADP 2.0×10-5 M (Bio-Data Corporation) was used as an agonist. The tests were conducted as previously reported21. A sample of 450 μL PRP was transferred into a transparent cuvette to be incubated at 37°C for 3 min. Consequently, 50 μL of agonist were added and the aggregation pattern was allowed to proceed for 10 min.
Rotational thromboelastometry (ROTEM)
ROTEM (Tem Innovations GmbH, Munich, Germany) was used in order to evaluate platelet viscoelastic properties. Platelet samples C and M were diluted at 1:5 using fresh donor plasma, and the remaining plasma was ultracentrifuged and frozen at −40°C in aliquots in order to be used on days 3 and 5. Samples were analyzed, according to the manufacturers’ instructions, within 2h after sample collection and EXTEM test was performed22. In EXTEM assay, recombinant tissue factor was used to activate the extrinsic coagulation pathway. The following parameters were recorded: 1) clotting time (CT-sec), 2) clot formation time (CFT-sec) along with clot amplitude recorded at 10 mm width (A10), and 3) maximum clot firmness (MCF-mm). Maximum clot elasticity was calculated using the following formula: MCE=(MCF×100)/(100-MCF).
Statistical analysis
The variables were described using median values with interquartile ranges (IQR). The two-sample Wilcoxon rank-sum (Mann-Whitney) test was used to compare parameters between platelets suspended in plasma and T-PAS+. Variable values on days 1, 3 and 5 were assessed using the Kruskal-Wallis equality-of-populations rank test. p<0.05 was considered statistically significant for all tests. Stata 16 was used for statistical analyses (Stata Corp., College Station, TX, USA).
RESULTS
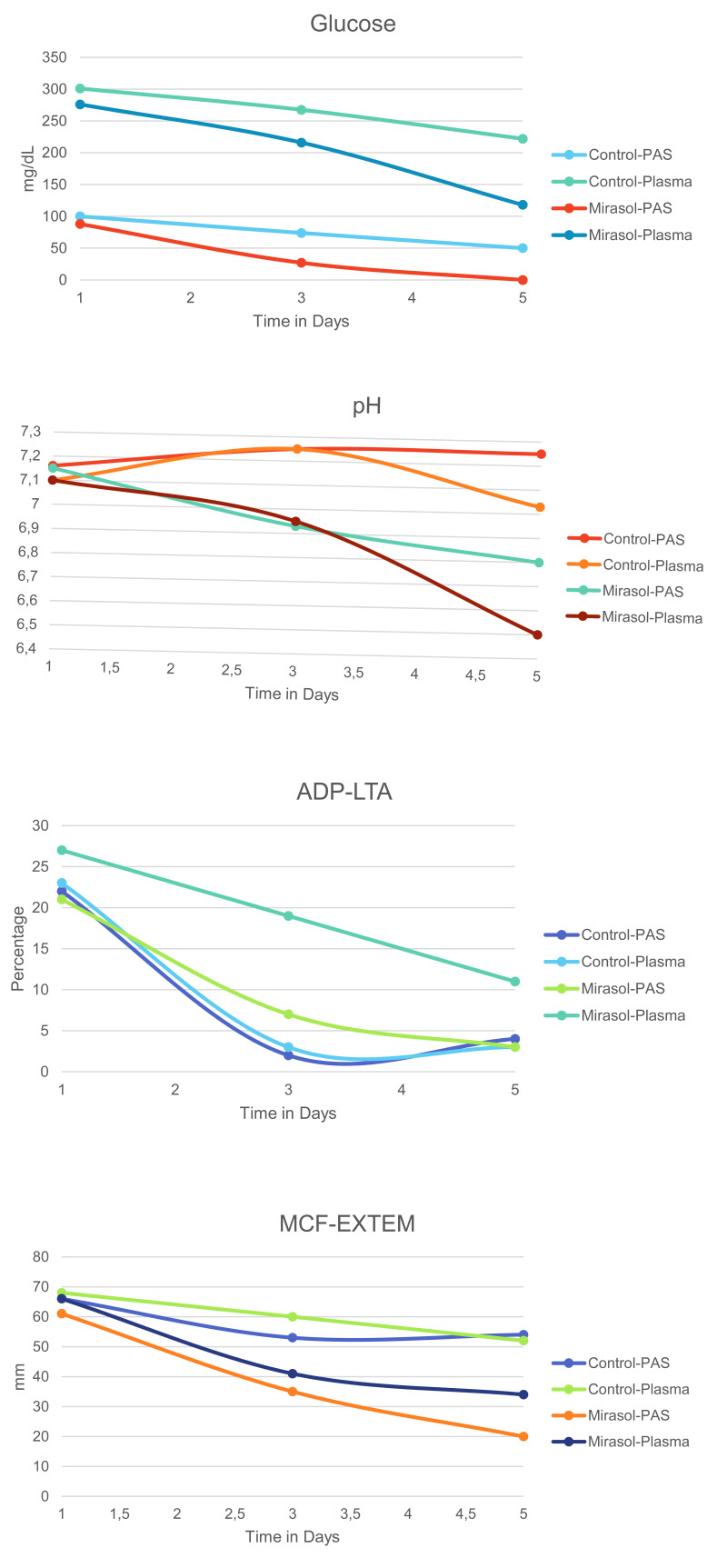
The comparison of metabolic variables and hemostatic parameters between untreated PCs suspended in plasma and T-PAS+ is shown in Table I and II, respectively. Regarding the untreated PCs, platelet concentration remained stable over time in both PAS-PCs and plasma-PCs groups (p=0.85 and p=0.67 respectively). Similarly, blood gas levels remained unchanged over time in both groups, with the exception of PCO2 levels in which a time dependent decrease was evident in PAS-PCs (p=0.007). On the other hand, lactate levels increased over time in both PAS-PCs and plasma-PCs (p=0.001 and p=0.004 respectively), while glucose levels declined during storage period in both groups (p=0.002 and p=0.007 respectively). No significant difference in LDH values was noted over time in the two groups (p=0.18 for PAS-PCs and p=0.67 for plasma-PCs). PAS seems to have a key role in preservation of pH levels, as PH was higher in PAS-PCs compared to plasma-PCs on day 5 (medians: 7.25 vs 7.03, p=0.008). Lactate levels were also better preserved in PAS-PCs, as lower lactate levels were documented in PAS-PCs compared to plasma-PCs on day 3 (medians: 5.1 vs 7.2 mmol/L, p=0.013) and day 5 (medians: 7.1 vs 12.1 mmol/L, p=0.008). Regarding the hemostatic profile of untreated-PCs suspended in PAS and plasma (Table II), no considerable differences were found between the two groups. A time dependent reduction in aggregation capacity as evaluated by LTA aggregometry with the use of ADP as an agonist was revealed in both PAS-PCs and plasma-PCs (p=0.011 and p=0.009 respectively). Similarly, a time dependent reduction in clot firmness as reflected by MCF in ROTEM analysis was evident in both PAS-PCs and plasma-PCs (p=0.041 and p=0.004 respectively).
Table I.
Metabolic parameters on days 1, 3 and 5 in control platelets stored in PAS and plasma
| Parameter/Day | Control-median (IQR) | p-value | |
|---|---|---|---|
| PAS | Plasma | ||
| Concentration (10 3 /uL), 1 | 116.0 (108–126) | 127.9 (116.1–132.9) | 0.11 |
| Concentration (10 3 /uL), 3 | 115.0 (108.5–122.5) | 123.3 (120.3–132.9) | 0.21 |
| Concentration (10 3 /uL), 5 | 120.5 (118–120.5) | 128.6 (124.0–131.7) | 0.046 |
| p-value | 0.85 | 0.67 | |
| pH, 1 | 7.16 (7.12–7.19) | 7.10 (7.10–7.20) | 0.52 |
| pH, 3 | 7.25 (7.24–7.26) | 7.25 (7.15–7.30) | 0.62 |
| pH, 5 | 7.25 (7.24–7.27) | 7.03 (7.03–7.20) | 0.008 |
| p-value | 0.009 | 0.17 | |
| pO 2 (mmHg), 1 | 55 (52–72) | 34.6 (29.9–74.8) | 0.46 |
| pO 2 (mmHg), 3 | 75 (68–108) | 39.1 (30.6–47.3) | 0.027 |
| pO 2 (mmHg), 5 | 74 (56–86) | 51.0 (34.2–86.9) | 0.46 |
| p-value | 0.40 | 0.93 | |
| pCO 2 (mmHg), 1 | 19 (17–20) | 52.8 (38.1–55.5) | 0.016 |
| pCO 2 (mmHg), 3 | 12 (11–13) | 23.5 (20.1–25.6) | 0.014 |
| pCO 2 (mmHg), 5 | 11 (10–12) | 21.8 (20.9–22) | 0.008 |
| p-value | 0.007 | 0.11 | |
| Glucose(mg/dL), 1 | 100 (95–106) | 301 (298–320) | 0.009 |
| Glucose(mg/dL), 3 | 74 (66–83) | 267.5 (234–287.5) | 0.014 |
| Glucose(mg/dL), 5 | 50 (47–51) | 222 (184–246) | 0.009 |
| p-value | 0.002 | 0.007 | |
| Lactate(mmol/L), 1 | 1.5 (1.4–1.6) | 2.8 (1.9–3.0) | 0.09 |
| Lactate(mmol/L), 3 | 5.1 (4.4–5.1) | 7.2 (6.8–9.7) | 0.013 |
| Lactate(mmol/L), 5 | 7.1 (6.7–7.1) | 12.1 (11.2–12.5) | 0.008 |
| p-value | 0.001 | 0.004 | |
| LDH(IU/L), 1 | 53 (40–72) | 106 (63–141) | 0.17 |
| LDH(IU/L), 3 | 81 (69–143) | 102 (67–118) | 0.88 |
| LDH(IU/L), 5 | 76 (75–88) | 120 (93–121) | 0.17 |
| p-value | 0.18 | 0.67 | |
p-values in bold stand for statistically significant results (p<0.05). IQR: interquartile range; LDH: lactate dehydrogenase.
Table II.
Hemostatic parameters on days 1, 3 and 5 in control platelets stored in PAS and plasma
| Parameter/Day | Control-median (IQR) | p-value | |
|---|---|---|---|
| PAS | Plasma | ||
| LTA ADP (%), 1 | 22 (9–38) | 23 (16–57) | 0.60 |
| LTA ADP (%), 3 | 2 (0–3) | 3 (2.5–4.5) | 0.25 |
| LTA ADP (%), 5 | 4 (3–8) | 3 (2–3) | 0.28 |
| p-value | 0.011 | 0.009 | |
| CT EXTEM (sec), 1 | 78 (71–94) | 59 (49–59) | 0.11 |
| CT EXTEM (sec), 3 | 61 (59–78) | 63.5 (46.5–72.5) | 0.53 |
| CT EXTEM (sec), 5 | 69 (55–74) | 59 (45–61) | 0.46 |
| p-value | 0.42 | 0.90 | |
| CFT EXTEM (sec), 1 | 53 (43–60) | 45 (45–47) | 0.46 |
| CFT EXTEM (sec), 3 | 55 (53–58) | 42 (38–54) | 0.14 |
| CFT EXTEM (sec), 5 | 61 (61–64) | 46 (45–58) | 0.027 |
| p-value | 0.34 | 0.55 | |
| A10 EXTEM (mm), 1 | 62 (55–63) | 66 (66–67) | 0.09 |
| A10 EXTEM (mm), 3 | 53 (51–58) | 59.5 (57–62) | 0.08 |
| A10 EXTEM (mm), 5 | 54 (51–55) | 52 (52–53) | 0.83 |
| p-value | 0.10 | 0.004 | |
| MCF EXTEM (mm), 1 | 66 (58–67) | 68 (68–69) | 0.07 |
| MCF EXTEM (mm), 3 | 53 (52–59) | 60 (57–63) | 0.14 |
| MCF EXTEM (mm), 5 | 54 (51–56) | 52 (52–53) | 0.91 |
| p-value | 0.041 | 0.004 | |
| MCE EXTEM, 1 | 198 (138–202) | 215 (212–227) | 0.07 |
| MCE EXTEM, 3 | 114 (110–143) | 151.5 (133.5–172) | 0.14 |
| MCE EXTEM, 5 | 119 (104–125) | 110 (110–115) | 0.91 |
| p-value | 0.059 | 0.004 | |
p-values in bold stand for statistically significant results (p<0.05). IQR: interquartile range; LTA: light transmission aggregometry; EPI: epinephrine; ADP: adenosine diphosphate; CT: clotting time; CFT: clot formation time; A10: amplitude 10 min after CT; A20: amplitude 20 min after CT; A30: amplitude 30 min after CT; MCF: maximum clot firmness; LI60:lysis index at 60 minutes; MCE: maximum clot elasticity.
The comparison of metabolic variables and hemostatic parameters between treated-PCs suspended in plasma and T-PAS+ is shown in Table III and IV, respectively. Concentration did not significantly change over time in both PAS and plasma PRT-treated platelets (p=0.61 and p=0.41 respectively). Regarding blood gas levels, PCO2 levels exhibited a time dependent reduction in both groups (p=0.021 and p=0.010 respectively). pH levels decreased over time in both PAS and plasma PRT-treated platelets (p=0.003 and p=0.002 respectively), however the metabolic profile was better preserved in PAS-PCs, as pH levels on the 5th day of storage was higher in PAS-PCs compared to plasma-PCs (medians: 6.80 vs 6.50, p=0.007). Glucose showed a significant reduction over time for both Mirasol-PCs irrespective of the suspension medium (p=0.002 for PAS-PCs and p=0.003 for plasma-PCs). In Mirasol-PCs stored in PAS, glucose was depleted on day 5, whereas in plasma-stored PCs glucose levels were better preserved (medians: 0 vs 118 mg/dL, p=0.005). Lactate production rose steadily during storage for both groups (p=0.003 for both PAS-PCs and plasma-PCs). LDH values did not have a considerable change during the 5-day storage period for any group (p=0.09 for PAS-PCs and p=0.17 for plasma-PCs). Regarding the results of the hemostatic parameters (Table IV), aggregation capacity (LTA) with ADP as an agonist, significantly decreased over time in both groups (p=0.008 for PAS-PCs and p=0.015 for plasma-PCs), with higher aggregation capacity on day 5 for plasma-PCs (medians: 11 vs 3, p=0.045). Regarding the viscoelastic methods, a time-dependent decrease in both treated groups was documented for EXTEM-A10 (p=0.004 for PAS-PCs and p=0.005 for plasma-PCs), EXTEM-MCF (p=0.004 for PAS-PCs and p=0.007 for plasma-PCs), and EXTEM-MCE (p=0.004 for PAS-PCs and p=0.007 for plasma-PCs). It is noteworthy that increased clot strength was observed in plasma PRT-treated platelets as compared to PAS platelets on the first storage day (medians: 66 vs 61 mm, p=0.011). The levels of the most critical platelet parameters of all PCs over time are shown Figure 1.
Table III.
Metabolic parameters on days 1, 3 and 5 in Mirasol-treated platelets stored in PAS and plasma
| Parameter/Day | Mirasol-median (IQR) | p-value | |
|---|---|---|---|
| PAS | Plasma | ||
| Concentration (10 3 /uL), 1 | 119 (86.5–119) | 112.4 (101–113) | 0.91 |
| Concentration (10 3 /uL), 3 | 115 (109.5–115) | 115.5 (107.3–126.5) | 0.45 |
| Concentration (10 3 /uL), 5 | 85.5 (85.5–103) | 115 (111.3–121.8) | 0.046 |
| p-value | 0.61 | 0.41 | |
| pH, 1 | 7.15 (7.11–7.17) | 7.10 (7.10–7.30) | 0.82 |
| pH, 3 | 6.93 (6.87–6.99) | 6.95 (6.90–7.01) | 0.62 |
| pH, 5 | 6.80 (6.80–6.87) | 6.50 (6.50–6.70) | 0.007 |
| p-value | 0.003 | 0.002 | |
| pO 2 (mmHg), 1 | 72 (48–72) | 49.7 (21.3–63.5) | 0.46 |
| pO 2 (mmHg), 3 | 94 (90–104) | 41.9 (38.4–55.2) | 0.027 |
| pO 2 (mmHg), 5 | 109 (107–123) | 56.2 (44.7–110.6) | 0.17 |
| p-value | 0.18 | 0.67 | |
| pCO 2 (mmHg), 1 | 15 (14–16) | 42.3 (27.9–45.9) | 0.009 |
| pCO 2 (mmHg), 3 | 12 (10–13) | 23.3 (21.9–26.4) | 0.013 |
| pCO 2 (mmHg), 5 | 10 (8–11) | 17.5 (15–17.8) | 0.008 |
| p-value | 0.021 | 0.010 | |
| Glucose(mg/dL), 1 | 88 (84–94) | 276 (257–291) | 0.009 |
| Glucose(mg/dL), 3 | 27 (20–31) | 216 (195.5–221) | 0.014 |
| Glucose(mg/dL), 5 | 0 (0–0) | 118 (93–136) | 0.005 |
| p-value | 0.002 | 0.003 | |
| Lactate(mmol/L), 1 | 1.4 (1.3–1.4) | 2.4 (1.8–2.5) | 0.058 |
| Lactate(mmol/L), 3 | 8.1 (8.1–10.4) | 10.7 (10.5–11.0) | 0.14 |
| Lactate(mmol/L), 5 | 12.6 (12.2–12.9) | 17.8 (17.7–18.0) | 0.009 |
| p-value | 0.003 | 0.003 | |
| LDH(IU/L), 1 | 44 (36–61) | 75 (59–88) | 0.29 |
| LDH(IU/L), 3 | 59 (55–78) | 73 (71–129) | 0.29 |
| LDH(IU/L), 5 | 85 (78–290) | 116 (103–153) | 0.65 |
| p-value | 0.09 | 0.17 | |
p-values in bold stand for statistically significant results (p<0.05). IQR: interquartile range; LDH: lactate dehydrogenase.
Table IV.
Hemostatic parameters on days 1, 3 and 5 in Mirasol-treated platelets stored in PAS and plasma
| Parameter/Day | Mirasol-median (IQR) | p-value | |
|---|---|---|---|
| PAS | Plasma | ||
| LTA ADP (%), 1 | 21 (20–36) | 27 (27–47) | 0.40 |
| LTA ADP (%), 3 | 7 (5–8) | 19 (13–25.5) | 0.10 |
| LTA ADP (%), 5 | 3 (0–4) | 11 (11–13) | 0.045 |
| p-value | 0.008 | 0.015 | |
| CT EXTEM (sec), 1 | 77 (69–85) | 53 (47–61) | 0.07 |
| CT EXTEM (sec), 3 | 65 (63–77) | 65.5 (50.5–68.5) | 0.71 |
| CT EXTEM (sec), 5 | 70 (54–79) | 60 (43–70) | 0.29 |
| p-value | 0.62 | 0.95 | |
| CFT EXTEM (sec), 1 | 63 (48–84) | 49 (47–51) | 0.20 |
| CFT EXTEM (sec), 3 | 69 (58–113) | 53.5 (51–64) | 0.14 |
| CFT EXTEM (sec), 5 | 109 (107–1094) | 57 (53–57) | 0.046 |
| p-value | 0.08 | 0.08 | |
| A10 EXTEM (mm), 1 | 56 (47–57) | 62 (60–63) | 0.021 |
| A10 EXTEM (mm), 3 | 31 (29–36) | 38.5 (33.5–40.5) | 0.38 |
| A10 EXTEM (mm), 5 | 20 (19–25) | 27 (23–31) | 0.17 |
| p-value | 0.004 | 0.005 | |
| MCF EXTEM (mm), 1 | 61 (50–61) | 66 (64–67) | 0.011 |
| MCF EXTEM (mm), 3 | 35 (30–41) | 41 (38.5–42.5) | 0.21 |
| MCF EXTEM (mm), 5 | 20 (19–26) | 34 (30–37) | 0.11 |
| p-value | 0.004 | 0.007 | |
| MCE EXTEM, 1 | 156 (102–157) | 191 (178–200) | 0.016 |
| MCE EXTEM, 3 | 53 (43–70) | 70.5 (62.5–75) | 0.17 |
| MCE EXTEM, 5 | 26 (24–35) | 52 (43–58) | 0.11 |
| p-value | 0.004 | 0.007 | |
p-values in bold stand for statistically significant results (p<0.05). IQR: interquartile range; LTA: light transmission aggregometry; EPI: epinephrine; ADP: adenosine diphosphate; CT: clotting time; CFT: clot formation time; A10: amplitude 10 min after CT; A20: amplitude 20 min after CT; A30: amplitude 30 min after CT; MCF: maximum clot firmness; LI60:lysis index at 60 minutes; MCE: maximum clot elasticity.
Figure 1. The most critical platelet parameters on days 1, 3 and 5 in control and Mirasol-treated platelets stored in PAS and plasma.
PAS: platelet additive solution; ADP: adenosine diphosphate; LTA: light transmission aggregometry; MCF: maximum clot firmness.
DISCUSSION
The time-dependent effect of PRTs on PCs has been previously documented in relevant literature, both for plasma-PCs and PAS-PCs. In plasma-PCs, PRT enhances storage lesions by means of increased metabolism and enhanced platelet activation, compared to untreated PCs19,23. A time-depended decrease in clot strength and in thrombin generation capacity has been also observed indicating a probably impaired hemostatic capacity after PRT process19. In some studies, the impact of PRTs appears even from day 3 of storage11,19,23–26. Thus, a reasonable question arises whether the use of another suspension medium can preserve the metabolic and hemostatic capacity of treated platelets more adequately throughout storage period.
Indeed, several studies report that the use of PAS retains more sufficiently the metabolic properties of PRT treated-PCs and pH values reduce to a smaller extent compared to plasma, due to acetate and the higher buffer capacity which is provided by the suspension medium. Nevertheless, the hypermetabolic pattern still occurs4,15,16,26,27–31. As a result, glucose levels seem to be exhausted in a disproportionate manner compared to pH levels, indicating that glucose could be a more appropriate quality marker regarding a longer than the 5-day storage period20,32. The hemostatic profile of PRT-PCs, as it is assessed by viscoelastic methods, is also affected compared to untreated-PCs. This observation has been made for both plasma and PAS platelets19,20.
To our knowledge there are no available studies investigating the effect of plasma and PAS on metabolic and hemostatic profile of Mirasol-treated PCs, by comparing the same platelet donors, thus ensuring almost zero heterogeneity. In our plasma versus PAS comparative study, we evaluated the metabolic and hemostatic profile of both PRT-treated and untreated-PCs derived from the same donor, stored in PAS and plasma.
The evaluation of metabolic profile revealed decreased pH levels during the 5-days storage period, both in treated and control-PCs, in both storage mediums. Untreated-PCs in PAS maintained their pH levels more efficiently compared to untreated-PCs in plasma, as they exhibited improved values on day 5 (p=0.008), even though on day 3 both PAS and plasma untreated-PCs demonstrated identical pH values. Beyond day 3, PAS managed to preserve better pH values. On the other hand, Mirasol-treatment combined with time, had a more profound, time-dependent effect on pH values regardless the storage medium, but treated-PCs in PAS had a significantly less exaggerated pH reduction on day 5, as compared to controls. Nevertheless, all units conformed to the established standards for the use of the product at the end of the 5-day storage period33,34. Compared to our study, van Der Meer et al27 reported even better maintained values in PAS-PCs. Although they observed decreased pH levels in Mirasol-treated split-PLT units both in plasma and PAS, they reported stable pH levels in control-PCs stored in PAS throughout storage. Furthermore, they reported that on day 8 of storage, control-PCs in plasma exhibited similar pH values with treated-PCs in PAS on the same day, indicating a protective role of PAS.
Regarding lactate levels, we documented better preserved values in untreated-PCs stored in PAS compared to untreated-PCs stored in plasma, a finding that was time-dependent. Even though the baseline was different between the two groups and the rate of increase similar, it seems that PAS could preserve lower lactate values, compared to plasma, from the first day of storage. In treated-PCs, a time-dependent increase in lactate values was observed in both storage mediums, which was significantly higher in plasma PCs on day 5, as compared to those suspended in PAS. The milder increase of lactate from the beginning of storage, in PAS-PCs both in treated and control units, is probably responsible for the better maintained pH levels in these groups.
Glucose levels exhibited a time-dependent and significant decrease in untreated-PCs in both storage mediums. Moreover, the difference among them was significant from the first day of storage and remained significant until the last day. Even though the reduction rate was significant in both groups, it is of note that on the last day of storage, glucose levels in PAS-PCs were extremely lower compared to plasma-PCs. PRT had an additional impact on glucose levels, resulting in complete exhaustion on day 5 on treated-PCs in PAS, but glucose reserves were still present on treated-PCs in plasma. Since glucose was exhausted, further production of lactate as a by-product of aerobic metabolism was halted. Janetzko et al.15 suggested a protective role of PAS due to the presence of acetate in PAS which counterbalanced the increased ATP demand after PRT, resulting in reduced acceleration of glucose consumption. This observation was opposite to their previous findings23 regarding PCs stored in plasma, where glucose consumption and lactate production were more profound. Platelets use glucose as their main energy source to form ATP. The use of PAS offers an additional endogenous fuel, because acetate constitutes an extra energy source used for the needs of oxidative phosphorylation. As a result, it can counterbalance the increased ATP demand after PRT-treatment resulting in reduced glucose consumption15. Nevertheless, we could not confirm a protective role of PAS in preserving glucose levels both in treated and untreated-PCs in our study.
Untreated-PCs demonstrated similar reduction levels in aggregating capacity regardless of the storage medium. This finding is not in line with current literature35,36, as the in vitro aggregation capacity has been reported to be more severely affected when PAS is used as compared to plasma, attributed to the lack of fibrinogen and vWF due to the minimum plasma content. Additionally, the rapid desensitization of ADP receptors after the release of granular ADP during storage, contributes to the reduced in vitro aggregation capacity, while PRT itself reduces platelet aggregation in a time dependent way27. Thus, this controversy is probably resulting from the relatively small number of samples examined in our study and needs to be further investigated. Nevertheless, we made an interesting observation involving platelet aggregation after stimulation with ADP, which was more profoundly affected by Mirasol-treatment in PCs stored in PAS, compared to treated-PCs stored in plasma. This finding was also time-dependent and statistically important on day 5. It can be assumed that the combination of PRT with PAS, as a storage medium, had a negative impact on platelet aggregation. Similar results were reported by Van der Meer et al27 for both ADP and collagen agonists. The decreased aggregation pattern was also observed and once more, the results were more profound for Mirasol-treated PCs in PAS.
Finally, in our study, the hemostatic profile, as reflected by ROTEM variables, exhibited only time-dependent alterations regardless of the storage medium in untreated-PCs. It seems that storage medium does not influence clot formation and stability, as similar findings were obtained for the two mediums. There are limited studies in the literature evaluating the hemostatic profile of treated-PCs using viscoelastic methods5. ROTEM has been used in one study19, whereas there is also only one study comparing both viscoelastic methods20. In accordance to our findings the treatment itself influences ROTEM measurements having a negative impact on the hemostatic profile, regardless of the storage medium20.
To our knowledge this is the first study comparing the hemostatic profile of Mirasol-treated and untreated PCs collected from the same donors and stored in PAS and plasma. It seems that the use of PAS both in PRT treated and untreated-PCs cannot establish a favorable role regarding platelet functionality and hemostatic capacity in vitro. According to our results its superiority is restricted to metabolic activity mainly at the late storage time in treated-PCs. However, several limitations of our study have to be acknowledged. A small sample size has been used while all observations are derived from in vitro measurements. Another limitation is considered the 5-days in vitro evaluation, instead of a 7-day period that is proposed for PRT-treated PCs. Furthermore, no specific instructions regarding dietary conditions prior to the donation were given to the donors included in our study, resulting in possible variations. Large research, strictly controlled studies as well as studies including parameters37 affecting hemostatic results and patients’ outcome, should be undertaken in order to confirm our findings.
CONCLUSIONS
The use of PASs is a promising alternative to plasma, aiming to reduce allergic reactions and prolong storage in transfusion medicine especially for PRT-treated PCs. We concluded that extension in storage time is not an easy or safe option, because even though the metabolic profile is better preserved with the use of PAS, this does not apply to the hemostatic profile of PRT-treated and untreated PCs. Well-designed clinical trials are required in order to estimate whether the in vivo efficacy of these PC products depends on the time of transfusion during the proposed extended storage period.
Footnotes
AUTHORSHIP CONTRIBUTIONS: EP, AET, AGT and ST conceptualized the concept. EP, AET, SK, ST and AGK designed the methodology. AGT, AGK, EL, SM, RS, SPF, ER, GS, FF, PD and EK were involved in data collection, analysis and interpretation. EP, AET and ST wrote the manuscript. All the co-Authors critically revised and approved the final version of the manuscript.
Authors declare no conflicts of interest.
REFERENCES
- 1.Goodrich RP, Edrich RA, Li J, Seghatchian J. The Mirasol PRT system for pathogen reduction of platelets and plasma: an overview of current status and future trends. Transfus Apher Sci. 2006;35:5–17. doi: 10.1016/j.transci.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Asano H, Lee CY, Fox-Talbot K, Koh CM, Erdinc MM, Marschner S, et al. Treatment with riboflavin and ultraviolet light prevents alloimmunization to platelet transfusions and cardiac transplants. Transplantation. 2007;84:1174–1182. doi: 10.1097/01.tp.0000287318.94088.d7. [DOI] [PubMed] [Google Scholar]
- 3.Cardo LJ, Salata J, Mendez J, Reddy H, Goodrich R. Pathogen inactivation of Trypanosoma cruzi in plasma and platelet concentrates using riboflavin and ultraviolet light. Transfus Apher Sci. 2007;37:131–137. doi: 10.1016/j.transci.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Picker SM, Steisel A, Gathof BS. Effects of Mirasol PRT treatment on storage lesion development in plasma-stored apheresis-derived platelets compared to untreated and irradiated units. Transfusion. 2008;48:1685–1692. doi: 10.1111/j.1537-2995.2008.01778.x. [DOI] [PubMed] [Google Scholar]
- 5.Tsalas S, Petrou E, Tsantes AG, Sokou R, Loukopoulou E, Houhoula D, et al. Pathogen Reduction Technologies and Their Impact on Metabolic and Functional Properties of Treated Platelet Concentrates: A Systematic Review. Semin Thromb Hemost. 2023;49:523–541. doi: 10.1055/s-0042-1757897. [DOI] [PubMed] [Google Scholar]
- 6.George JN, Pickett EB, Heinz R. Platelet membrane glycoprotein changes during the preparation and storage of platelet concentrates. Transfusion. 1988;28:123–126. doi: 10.1046/j.1537-2995.1988.28288179014.x. [DOI] [PubMed] [Google Scholar]
- 7.Kwon SY, Kim IS, Bae JE, Kang JW, Cho YJ, Cho NS, et al. Pathogen inactivation efficacy of Mirasol PRT System and Intercept Blood System for non-leucoreduced platelet-rich plasma-derived platelets suspended in plasma. Vox Sang. 2014;107:254–260. doi: 10.1111/vox.12158. [DOI] [PubMed] [Google Scholar]
- 8.Reddy HL, Dayan AD, Cavagnaro J, Gad S, Li J, Goodrich RP. Toxicity testing of a novel riboflavin-based technology for pathogen reduction and white blood cell inactivation. Transfus Med Rev. 2008;22:133–153. doi: 10.1016/j.tmrv.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Goodrich RP, Li J, Pieters H, Crookes R, Roodt J, Heyns Adu P. Correlation of in vitro platelet quality measurements with in vivo platelet viability in human subjects. Vox Sang. 2006;90:279–285. doi: 10.1111/j.1423-0410.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 10.Shrivastava M. The platelet storage lesion. Transfus Apher Sci. 2009;41:105–113. doi: 10.1016/j.transci.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 11.AuBuchon JP, Herschel L, Roger J, Taylor H, Whitley P, Li J, et al. Efficacy of apheresis platelets treated with riboflavin and ultraviolet light for pathogen reduction. Transfusion. 2005;45:1335–1341. doi: 10.1111/j.1537-2995.2005.00202.x. [DOI] [PubMed] [Google Scholar]
- 12.Basu D, Basu S, Radhakrishnan VS, Bhattacharya S, Chakraborty S, Sinha S, et al. Comparison of quality and efficacy of apheresis platelets stored in platelet additive solution vis a vis plasma. Indian J Hematol Blood Transfus. 2021;37:648–657. doi: 10.1007/s12288-021-01408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bashir S, Mohsin S, Amin H, Rehman M, Hussain S, Saeed T. Comparison of changes in platelet count, mean platelet volume and swirling in stored platelet concentrates with and without platelet additive solution. J Appl Hematol. 2014;5:10–14. doi: 10.4103/1658-5127.131819. [DOI] [Google Scholar]
- 14.Das S, Kumar MLH. Comparative Evaluation of Quality Parameters of Platelet Stored in Additive Solution Versus Plasma. AHB. 2022;12:163–167. doi: 10.4103/aihb.aihb_124_21. [DOI] [Google Scholar]
- 15.Janetzko K, Hinz K, Marschner S, Goodrich R, Klüter H. Pathogen reduction technology (Mirasol) treated single-donor platelets resuspended in a mixture of autologous plasma and PAS. Vox Sang. 2009;97:234–239. doi: 10.1111/j.1423-0410.2009.01193.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson L, Winter KM, Reid S, Hartkopf-Theis T, Marschner S, Goodrich RP, et al. The effect of pathogen reduction technology (Mirasol) on platelet quality when treated in additive solution with low plasma carryover. Vox Sang. 2011;101:208–214. doi: 10.1111/j.1423-0410.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- 17.van der Meer PF. PAS or plasma for storage of platelets? A concise review. Transfus Med. 2016;26:339–342. doi: 10.1111/tme.12325. [DOI] [PubMed] [Google Scholar]
- 18.Chandra T, Gupta A, Kumar A, Afreen S. Morphological and functional changes in random donor platelets stored for seven days in platelet additive solution. IJBT. 2011;1:20–25. doi: 10.5348/ijbti-2011-5-OA-5. [DOI] [Google Scholar]
- 19.Petrou E, Nikolopoulos GK, Kriebardis AG, Pantavou K, Loukopoulou E, Tsantes AG, et al. Haemostatic profile of riboflavin-treated apheresis platelet concentrates. Blood Transfus. 2022;20:223–234. doi: 10.2450/2021.0089-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsalas S, Tsantes AG, Petrou E, Mellou S, Sokou R, Loukopoulou E, et al. The effects of pathogen reduction technology on apheresis platelet concentrates stored in PAS. Blood Transfus. 2024;22:405–414. doi: 10.2450/BloodTransfus.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsantes AE, Mantzios G, Giannopoulou V, Tsirigotis P, Bonovas S, Rapti E, et al. Monitoring aspirin treatment in patients with thrombocytosis: comparison of the platelet function analyzer (PFA)-100 with optical aggregometry. Thromb Res. 2008;123:100–107. doi: 10.1016/j.thromres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. doi: 10.1111/j.1365-2257.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 23.Janetzko K, Hinz K, Marschner S, Goodrich R, Klüter H. Evaluation of different preparation procedures of pathogen reduction technology (Mirasol®)-treated platelets collected by plateletpheresis. Transfus Med Hemother. 2009;36:309–315. doi: 10.1159/000230038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, de Korte D, Woolum MD, Ruane PH, Keil SD, Lockerbie O, et al. Pathogen reduction of buffy coat platelet concentrates using riboflavin and light: comparisons with pathogen-reduction technology-treated apheresis platelet products. Vox Sang. 2004;87:82–90. doi: 10.1111/j.1423-0410.2004.00548.x. [DOI] [PubMed] [Google Scholar]
- 25.Ruane PH, Edrich R, Gampp D, Keil SD, Leonard RL, Goodrich RP. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion. 2004;44:877–885. doi: 10.1111/j.1537-2995.2004.03355.x. [DOI] [PubMed] [Google Scholar]
- 26.Ostrowski SR, Bochsen L, Windeløv NA, Salado-Jimena JA, Reynaerts I, Goodrich RP, et al. Hemostatic function of buffy coat platelets in additive solution treated with pathogen reduction technology. Transfusion. 51:344–356. doi: 10.1111/j.1537-2995.2010.02821.x. 201. [DOI] [PubMed] [Google Scholar]
- 27.van der Meer PF, Bontekoe IJ, Daal BB, de Korte D. Riboflavin and UV light treatment of platelets: a protective effect of platelet additive solution? Transfusion. 2015;55:1900–1908. doi: 10.1111/trf.13033. [DOI] [PubMed] [Google Scholar]
- 28.Van Aelst B, Feys HB, Devloo R, Vanhoorelbeke K, Vandekerckhove P, Compernolle V. Riboflavin and amotosalen photochemical treatments of platelet concentrates reduce thrombus formation kinetics in vitro. Vox Sang. 2015;108:328–339. doi: 10.1111/vox.12231. [DOI] [PubMed] [Google Scholar]
- 29.Ballester-Servera C, Jimenez-Marco T, Morell-Garcia D, Quetglas-Oliver M, Bautista-Gili AM, Girona-Llobera E. Haemostatic function measured by thromboelastography and metabolic activity of platelets treated with riboflavin and UV light. Blood Transfus. 2020;18:280–289. doi: 10.2450/2020.0314-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galan AM, Lozano M, Molina P, Navalon F, Marschner S, Goodrich R, et al. Impact of pathogen reduction technology and storage in platelet additive solutions on platelet function. Transfusion. 2011;51:808–815. doi: 10.1111/j.1537-2995.2010.02914.x. [DOI] [PubMed] [Google Scholar]
- 31.Castrillo A, Cardoso M, Rouse L. Treatment of buffy coat platelets in platelet additive solution with the mirasol(®) pathogen reduction technology system. Transfus Med Hemother. 2013;40:44–48. doi: 10.1159/000345679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reikvam H, Marschner S, Apelseth TO, Goodrich R, Hervig T. The Mirasol pathogen reduction technology system and quality of platelets stored in platelet additive solution. Blood Transfus. 2010;8:186–192. doi: 10.2450/2010.0141-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Korte D. Richtlijn bloedproducten [Guideline blood products] 6th ed. Amsterdam: Sanquin Blood Supply; 2013. [in Dutch.]] [Google Scholar]
- 34.Fung MK, Grossman BJ, Hillyer C, Westhoff CM, editors. Technical manual. 18th ed. Bethesda (MD): American Association of Blood Banks; 2014. [Google Scholar]
- 35.Murphy S, Gardner FH. Platelet storage at 22 degrees C; metabolic, morphologic, and functional studies. J Clin Invest. 1971;50:370–377. doi: 10.1172/JCI106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escolar G, McCullough J. Platelet in vitro assays: their correspondence with their in vivo hemostatic potential. Transfusion. 2019;59:3783–3793. doi: 10.1111/trf.15559. [DOI] [PubMed] [Google Scholar]
- 37.Valsami S, Grouzi E, Mochandreou D, Pouliakis A, Piroula-Godoy M, Kokori S, et al. Effect of mirasol pathogen reduction technology system on immunomodulatory molecules of apheresis platelets. Transfus Apher Sci. 2023;62:103523. doi: 10.1016/j.transci.2022.103523. [DOI] [PubMed] [Google Scholar]