Abstract
Background
Hematological disorders are often treated with blood transfusions. Many blood group antigens and variants are population-specific, and for patients with rare blood types, extensive donor screening is required to find suitable matches for transfusion. There is a scarcity of knowledge regarding blood group variants in Aboriginal Australian populations, despite a higher need for transfusion due to the higher prevalence of renal diseases and anemia.
Materials and methods
In this study, we applied next-generation sequencing and analysis to 245 samples obtained from Aboriginal Australians from South-East Queensland, to predict antigen phenotypes for 36 blood group systems.
Results
We report potential weak antigens in blood group systems RH, FY and JR that have potential clinical implications in transfusion and pregnancy settings. These include partial DIII type 4, weak D type 33, and Del RHD (IVS2-2delA). The rare Rh phenotypes D+ C+ E+ c− e+ and D+ C+ E+ c+ e− were also detected.
Discussion
The comprehensive analyses of blood group genetic variant profiles identified in this study will provide insight and an opportunity to improve Aboriginal health by aiding in the identification of appropriate blood products for population-specific transfusion needs.
Keywords: blood transfusion, population genetics, rare blood types, next-generation sequencing
INTRODUCTION
Blood group antigens are the primary indicator to assess the compatibility between blood donor and recipient in transfusion medicine. Pre-transfusion testing of patients with a transfusion requirement (obstetric, surgery, hematological disorders) is of vital importance to facilitate blood group antigen matching in order to provide safe and compatible blood and blood products for transfusion. Blood group antigen typing methods are also employed in the diagnosis, treatment and management of other disorders of antigen incompatibility, including hemolytic disease of the fetus and newborn (HDFN), and solid organ transplantation1,2. Extended blood group genotyping and matching between donors and patients reduces the risk of alloimmunization and, for already alloimmunised patients, it is critical to meet ongoing transfusion needs3. Interestingly with regard to donor screening, Gleadall et al. identified 2–6 times more compatible donors for patients with multiple red cell (RBC) alloantibodies, including hard-to-transfuse patients, by employing a dense donor-typing approach4.
Aboriginal Australians experience a higher incidence of renal disease and anemia than the general Australian population5 which results in an increased dependency and frequency of blood transfusions. Most blood donors in Australia have European ancestry6; therefore understanding the diverse nature of blood group antigens and their potential for adverse transfusion reactions in Aboriginal people is vital for the provision of safe and equitable transfusion approaches and hemovigilance. Cultural considerations relating to blood, historical disadvantage and previous inappropriate research approaches have led to genuine concerns from the Aboriginal Australian population7 about participation in medical research. As a result, there are relatively few studies investigating diversity in blood groups within the Aboriginal Australian population.
Between the 1940’s and the early 1970’s, approximately 19 blood group antigens from a small number of Aboriginal Australian populations were studied using serology8–14. Relatively few blood group systems had been described since this period, whereas by 2023 the International Society of Blood Transfusion (ISBT) recognises 360 blood group antigens and over 1,800 allele within 45 blood group systems. NGS technologies have the potential to detect all polymorphisms in each individual’s genome, including null alleles, novel variants, complex gene rearrangements, and copy number variations15,16. NGS-based blood group profiling studies in Western desert8 and Tiwi Island populations17 have also shown that the prevalence of various blood group alleles varies between Aboriginal Australians and other population groups worldwide. Previous research has emphasized the significance of blood group characterization in diverse ethnic and cultural groups as antigen prevalence differs amongst global populations18,19. Up to date blood group frequency data is important to guide blood donor recruitment to support patients requiring blood transfusion and minimise hemolytic transfusion reactions (HTR’s) in diverse populations18–20.
Harnessing NGS technology to minimise risk and improve health outcomes for Aboriginal Australian patients requiring frequent transfusions is essential, provided culturally acceptable, respectful processes are addressed. The study was designed and conducted in collaboration with Aboriginal organizations and their representatives, and Aboriginal governance was integrated throughout the project. In this report, we present the NGS findings for the most clinically significant groups, the ABO (ISBT 001), RH (ISBT004) and KEL (ISBT 006) systems and discuss predictions for weakened or rare antigens in systems including FY (ISBT 008), JR (ISBT 032). Overall findings are compared with previous population studies.
MATERIALS AND METHODS
Study design and recruitment of participants in the South East Queensland Aboriginal (SEQA) population cohort
An Aboriginal Advisory Committee (AAC) was established to maintain Aboriginal governance and culturally safe research practices throughout this study. The AAC consisted of Aboriginal researchers, stakeholders, consumers, and community members. Respectful consultation and community engagement was conducted prior to any sample collection. Any feedback or suggestions for improvement that emerged from this consultation process were incorporated into the final study design and protocol. Potentially eligible participants were invited to one-on-one consultations with Aboriginal research staff at the participating Aboriginal Community Controlled Health Organisation, Carbal Aboriginal Medical Services, Queensland. As part of the consenting process, it was ensured that participants understood this study and had the opportunity to ask any questions or raise their concerns prior to providing informed consent. If they agreed, all participants provided written informed consent. In collaboration with Australian Red Cross Lifeblood, sample receipt, transport, serological testing, nucleic acid extraction and blood group targeted sequencing was conducted under the governance of the AAC. Safe storage and respectful handling of samples was also ensured, under the leadership of the AAC and according to appropriate guiding principles21.
Data collection
SEQA population serological phenotyping and Targeted Exome Sequencing (TES)
ABO, and Rh (D, C, E, c,e) phenotyping was undertaken for all samples using monoclonal typing reagents (Immulab, Parkville, Australia) by tube direct hemagglutination technique. 27/127 red cell samples that failed to react with Epiclone 2 anti-D (IgM DVI−) by tube direct hemagglutination were tested using an anti-D monoclonal blend (IgG/IgM, Immucor [Norcross, GA, USA]) by tube indirect antiglobulin test at 37°C (IAT). Additional phenotyping for the K antigen was undertaken for 127 samples using by direct hemagglutination technique at 37°C using monoclonal anti-K (IgM clone MS-56, Immulab). Samples that typed positive for the K antigen (No.=7) were tested for the k antigen using a polyclonal typing reagent by IAT at 37°C (Immulab)22,23. Genomic DNA was extracted for all samples and submitted for targeted blood group exome (TES) sequencing on the Illumina MiSeq (Illumina Inc., San Diego, CA, USA) as previously described by Roulis et al., 202022.
The 1000 genomes project phase 3
The 1KGP3 project includes 2,504 whole genome sequencing (WGS) samples from 26 population groups classified into five super populations. WGS binary alignment map (BAM) files (a compressed binary format with reference genome alignment information) were accessed through the 1KGP3 project FTP server24.
Blood group profiling and population genomic analyses
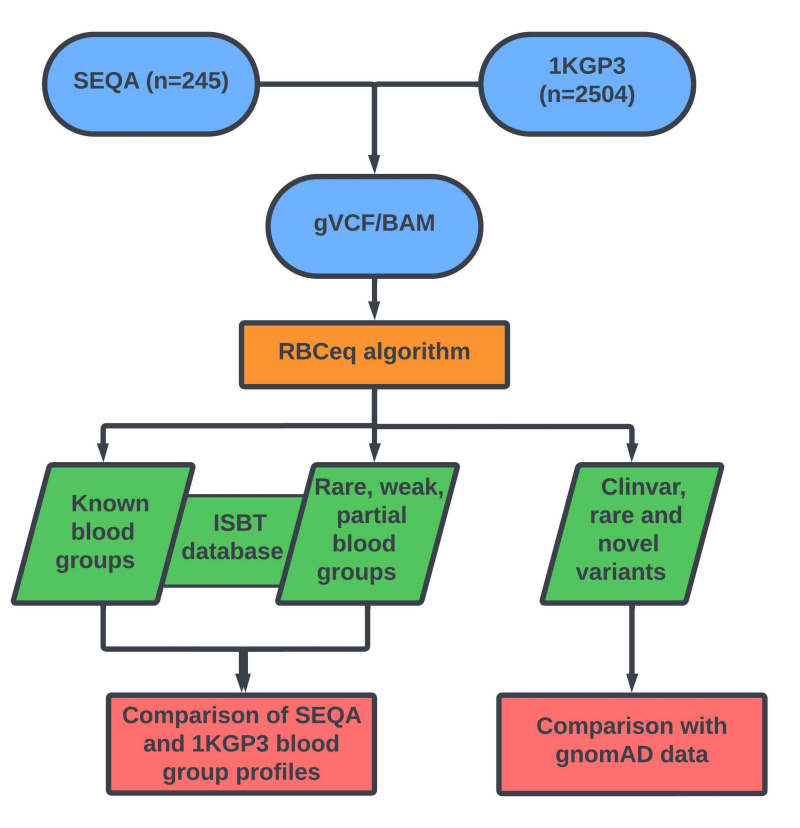
BAM and VCF files for each individual SEQA sample, and individuals in the 1KGP3 cohort were submitted for blood group genotype and phenotype prediction using the first iteration of an automated interpretative algorithm, RBCeq version V1.0.0.125. RBCeq V1.0.0.1 considers 36 blood group systems and two transcription factors encoded by 44 genes (Online Supplementary Table SI; Figure 1)25. We also compared the blood group variant profiles of the SEQA population with those reported in other Aboriginal Australian populations9–14,17 including Western Desert Aboriginal Australians (WD)8 Tiwi Islanders (TI)17 and Northern Territory (NT)26 where predicted genotyping profiles or NGS data was available.
Figure 1.
An overview of the workflow used to comprehensively characterise population-specific blood group variants and predicted phenotypes
RESULTS
Overview of comparative data for ABO (ISBT 001), RH (004), LU (005), KEL (006) and SC (013)
Phenotypes predicted by RBCeq were concordant with serology for ABO, Rh and KEL, except in two cases where the predicted ABO phenotype did not match serology. Manual analysis of the VCF for these two samples suggests that the RBCeq prediction is correct.
For ABO (ISBT 001), the group O phenotype prevalence is >50% in all Aboriginal populations except in WD, where it is 14%. The prevalence of groups B (7.76%) and AB (1.22%) was higher in SEQA as compared to other Aboriginal populations, while these groups were absent in the WD population. Three individuals had predicted B1 (ABO*B.01/ABO*O.01.02) or B3 (ABO*B3.02/ABO*O.01.35) alleles. The A antigen was predicted to have a >2 fold higher prevalence in the WD (86%) in comparison with SEQA and other Aboriginal populations. Additionally, 17 individuals were predicted to have the A2 phenotype in SEQA and one in the TI population which is not reported in WD and NT (Table I). For the Rh system, the predicted RhD negative phenotype was 9.38% which was >3 fold higher compared to other Aboriginal populations (0–3%).
Table I.
Comparison of predicted blood group antigens in SEQA population with other Aboriginal Australian populations
| Blood group antigens | SEQA prediction % (No.=245) | Western desert %8 (No.=72) | Tiwi islanders %17 (No.=457) | Northern Territory %26 (No.=2,130) | Literature % (1940–1970 serology)9–14 | Comment9–14 |
|---|---|---|---|---|---|---|
| O | 52.24 (No.=128) | 14 (No.=10) | 81.18 (No.=371) | 51.9 (No.=1,191) | NA | - |
| A | 38.77 (No.=95) | 86 (No.=62) | 18.59 (No.=85) | 14.1 (No.= 876) | NA | - |
| B | 7.76 (No.=19) | 0 | 0.21 (No.=1) | 2.4 (No.=52) | NA | - |
| AB | 1.22 (No.=3) | 0 | 0 | 0.5 (No.=11) | 2 | - |
| D+ | 90.61 (No.=222) | 97 (No.=70) | 100 (No.=457) | 98 (No.=2,104) | - | - |
| D− | 9.38 (No.=23) | 3 (No.=2) | 0 | 1.2 (No.=26) | - | - |
| Lu(a+b+) | 4.08 (No.=10) | 0 | 0 | - | 0 | 7.5% in European50 |
| K+k+ | 8.57 (No.=21) | 0 | 0 | - | NA | - |
In contrast, previous studies have reported a much lower prevalence of D− in the NT Aboriginal population at 1.2%27, whilst the frequency in the WD population was reported at 3%8 and D− was completely absent in the TI population17 (Table I).
With respect to the LU system, the predicted Lu(a+b+) phenotype was only present in the SEQA population and absent in other Aboriginal populations (Table I). In comparison with 1KGP3, the predicted phenotype Au(a−b+) (LU*02.19) in the SEQA population is 6.53%, which was lower than that observed in African and European populations (Table II). Alloantibodies formed against the K antigen are the second major cause of HDFN in Australia after anti-D. Alloantibodies against KEL antigens have been reported in Aboriginal Australian ICU patients26, as well as in thalassemic patients of non-Indigenous Australian heritage28.
Table II.
Distribution of predicted genotypes for LU, KEL and SC, and its comparison with 1KGP3
| Blood group system and ISBT number | Predicted phenotype | RBCeq prediction (%) | |||||
|---|---|---|---|---|---|---|---|
| SEQA % (No.=245) | AFR % (No.=661) | AMR % (No.=347) | EAS % (No.=504) | EUR % (No.=503) | SAS % (No.=489) | ||
|
LU
005 |
LU2, LU18−, LU19 or Au(a−b+) | 6.53 (No.=16) | 19.21 (No.=127) | 6.91 (No.=24) | 0.99 (No.=5) | 10.34 (No.=52) | 4.91 (No.=24) |
|
KEL
006 |
KEL1, KEL2 or K+k+ | 8.57 (No.=21) | 0.45 (No.=3) | 4.32 (No.=15) | - | 7.55 (No.=38) | 1.23 (No.=6) |
|
SC
013 |
Sc1+, Sc2+ | 0.82 (No.=2) | - | - | - | 0.2 (No.=1) | 0.41 (No.=2) |
AFR: African; AMR: American; EAS: East Asian; EUR: European; SAS: South Asian.
The KEL*01/02 genotype predicting the K+k+ phenotype was 8.57% among the SEQA population, and was absent in all other Aboriginal populations (Table I). When comparing this genotype in population groups in 1KGP3, the prevalence is 7.55% in European, whereas it is absent in the East Asian population. The K+k+ phenotype has been reported at a prevalence of 2% amongst the African population29 (Table II).
We also detected two individuals in the SEQA cohort, one in the European population and two in the South Asian population carrying heterozygous SC*02 (Sc2+) alleles. The ERMAP c.169G>A variant encoding for the SC*02 variant was not detected in either the WD or TI population datasets8,17 (Table II).
Investigation of Rh predictive phenotype
Our investigation revealed that 90.61% (No.=222) of the SEQA population were homozygous for the RHD gene, with two individuals predicted as hemizygous for RHD gene encoding the alleles weak D Type3: RHD*01W.03 and Del RHD (IVS2-2delA):RHD*53, which were phenotyped as altered D antigen and D− by serology. The SEQA cohort exhibited the second-highest proportion of D− resulting from complete deletion of the RHD gene (9.38%, No.=23), after the EUR cohort (16.10%, No.=81), while the lowest prevalence of this type was observed in the EAS population (0.2%, No.=1) (Table III).
Table III.
Distribution of the RhD positive (D or RH:1) and negative (D−) phenotype
| Blood group system and ISBT number | Predicted phenotype | RBCeq prediction | Literature29,31,33 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SEQA % (No.=245) | AFR % (No.=661) | AMR % (No.=347) | EAS % (No.=504) | EUR % (No.=503) | SAS % (No.=489) | European % | Asian % | AFR % | ||
|
RHD
004 |
D RH:1 |
90.61 (No.=222) | 96.52 (No.=628) | 93.95 (No.=326) | 99.80 (No.=503) | 83.90 (No.=422) | 94.48 (No.=462) | 85 | 99 | 92 |
|
RHD
004 |
D− RH:−1 |
9.38 (No.=23) | 3.48 (No.=23) | 6.05 (No.=21) | 0.2 (No.=1) | 16.10 (No.=81) | 5.52 (No.=27) | 15 | 1 | 8 |
AFR: African; AMR: American; EAS: East Asian; EUR: European; SAS: South Asian.
The RHCE gene encodes for C/c and E/e antigens, which are inherited together, separately from RhD antigens, however are considered together in 8 distinct Rh haplotypes30. Rare Rh phenotypes predicted in the SEQA cohort were compared with previously published reports31. The D+ C+ c+ E+ e− (0.41%) and D+ C+ E+ c− e+ (2.86%) phenotypes which were predicted in the SEQA population, both containing the Rz haplotype, are also rare in other populations (Table IV) and were absent in the 1KGP3 dataset. The D+ C− E− c+ e+ phenotype, containing the R0 haplotype, is common in the African population (45.8%) and has a lower frequency in the SEQA cohort (0.41%) and Asian populations (0.3%) (Table IV). The SEQA cohort exhibited a higher prevalence of predicted D+ C− E+ c+ e− (4.48%) phenotype than any other population, while the D+ C+ E− c+ e+ phenotype prevalence was highest in European (34.9%) and SEQA cohorts (30.61%) respectively (Table IV)32,33.
Table IV.
RH blood group predictive phenotype prevalence in SEQA and other population datasets from literature31
| Observed and predicted phenotype | RBCeq prediction | Literature31 | ||
|---|---|---|---|---|
| SEQA % | European % | Asian % | African % | |
| D+ C+ E+ c+ e− | 0.41 (No.=1) | 0.1 | 0.4 | Rare/− |
| D+ C+ E+ c− e+ | 2.86 (No.=7) | 0.2 | 1.4 | Rare/− |
| D+ C− E− c+ e+ | 0.41 (No.=1) | 2.1 | 0.3 | 45.8 |
| D+ C− E+ c+ e− | 4.48 (No.=11) | 2.3 | 4.4 | 0.2 |
| D+ C+ E− c+ e+ | 30.61 (No.=75) | 34.9 | 8.5 | 21 |
Predicted distribution of weak and partial antigens in the SEQA population
The predicted RhD partial DIII type 4 (RHD*03.04) was observed in two individuals (No.=2) and predicted Weak D Type3 (RHD*01W.3) was observed in one SEQA and European individual. The RHD*53 allele, associated with clinically significant Del RHD (IVS2-2delA) phenotype was predicted in one individual of the SEQA population and has been previously reported in Australia34 but was not observed in any population of the 1KGP3 dataset. Weak D Type33(RHD*01W.33) has been associated with anti-D formation35, and here we report for the first time the presence of weak D Type33 at a frequency of 0.82% (No.=2), not only in SEQA but in any Australian aboriginal population (Table V).
Table V.
Distributions of predicted weak and partial antigens
| Blood group system and ISBT number | Predicted phenotype | RBCeq prediction | |||||
|---|---|---|---|---|---|---|---|
| SEQA % (No.=245) | AFR % (No.=661) | AMR % (No.=347) | EAS % (No.=504) | EUR % (No.=503) | SAS % (No.=489) | ||
|
RHD
004 |
Partial DIII type 4 | 0.82 (No.=2) | 0.60 (No.=4) | - | - | - | - |
|
RHD
004 |
Del RHD (IVS2-2delA) | 0.41 (No.=1) | - | - | - | - | - |
|
RHD
004 |
Weak D Type 33 | 0.82 (No.=2) | 0.45 (No.=3) | - | - | - | - |
|
RHD
004 |
Weak D Type3 | 0.41 (No.=1) | - | - | - | 0.19 (No.=1) | - |
|
FY
008 |
Fy(a−b+w), Fy. | 1.63 (No.=4) | - | 1.44 (No.=5) | - | 1.19 (No.=6) | 0.2 (No.=1) |
|
JR
032 |
Jr(a+w) | 1.22 (No.=3) | - | 1.73 (No.=6) | 7.94 (No.=40) | 1.19 (No.=6) | 0.61 (No.=3) |
AFR: African; AMR: American; EAS: East Asian; EUR: European; SAS: South Asian.
In comparing the SEQA and IKGP3 datasets, we detected the FY*02W.01 allele that encodes the Fy(a−b+W) FYX phenotype in 1.63% (No.=4) of the SEQA population, 1.44% (No.=5) of the American population (Colombia and Puerto Rico), 0.2% (No.=1) of the South Asian population, while it was missing from African population (Table V). The FYX gene frequency has previously been reported as 0.015% in a small proportion of Europeans36 and was absent in the WD population dataset8.
While HTRs due to Anti-Jr(a) are rare, cases of severe and sometimes fatal HDFN have been reported37,38. The ABCG2*01W.01 allele, which encodes the Jr(a+w) predicted phenotype, was higher in the East Asian population (7%, No.=40) compared to other populations, including SEQA, where the prevalence was in the range of 1–2%.
Comparison of SEQA blood groups with those of other Australian Aboriginal populations
Historically, the Fy(a+) phenotype in the FY system was reported to be 100% prevalence in the Aboriginal population, with the presence of Fy(b+) unreported9,39. In comparing SEQA data to WD and TI populations, we predicted a 20% prevalence of Fy(a−b+) in the SEQA population, compared to 1.39% in the WD and 0.88% in TI populations respectively (Table VI).
Table VI.
Comparison of predicted blood group antigens in SEQA population with other Aboriginal Australian populations
| Blood group antigens | SEQA prediction % (No.=245) | Western desert %8 (No.=72) | Tiwi Islanders %17 (No.=457) | Northern territory %26,27 (No.=2,130) | Literature (1940–1970 serology)9–14 | Comment9–14 |
|---|---|---|---|---|---|---|
| Fy(a−b+) | 20 (No.=49) | 1.39 (No.=1) | 0.88 (No.=3) | - | 0 | 100% Fy(a+) in Bentinck, Mornington and Forsyth Islanders39 |
| Yk(a−) | 8.16 (No.=20) | 37.5 (No.=27) | 10.07 (No.=46) | - | NA | Clinically significant |
The Knops blood group (KN) alloantibodies can be difficult to identify, as they often mimic or mask the presence of other antibodies and therefore need to be correctly identified before dismissing their significance. 8.16% (No.=20) of samples in this dataset were homozygous for the variant c.4223C>T encoding the KN*01.-05 allele, thereby resulting in a prediction of the Yk(a–) phenotype. The Yk(a−) phenotype is common among most global populations and was detected at a frequency of 37.5% in the WD population and 10.07% in the TI population.
RBCeq blood group profiling of SEQA population reveals rare and novel variants
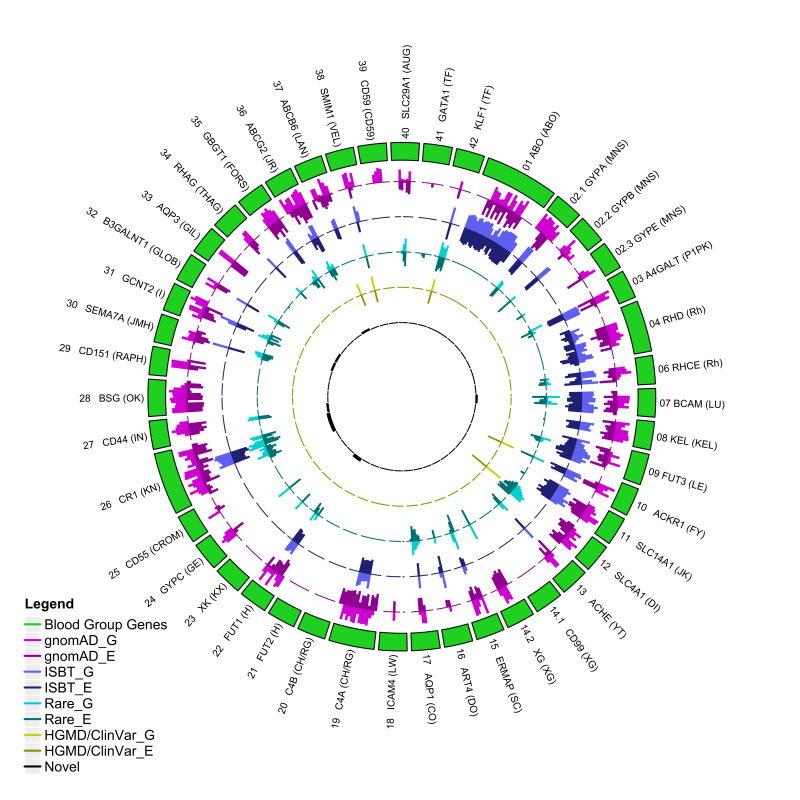
We additionally characterised variants that have not been formally identified as blood group alleles by the ISBT but were predicted to potentially impact structural formation (Figure 2). We detected four variants with disease-associated polymorphism with functional evidence (DFP) and functional polymorphism (FP) related to blood group variation and reduced protein expression: rs117351327 (KLF1), rs5036 (DI - ISBT 010), rs150221689 (LAN - 033), and rs12721643 (JR - 032). rs5036 is a Band 3 Memphis electrophoretic variant in SLC4A1 in the Diego system40. rs150221689 has been associated with reduced LAN expression41 and rs12721643 was associated with reduced JR protein expression as reported by HGMD. We also detected one variant associated with the FY blood group system (ACKR1:rs118062001) reported in ClinVar (ClinvarID: 1174942), however the impact of this variant is unknown. RBCeq identified nine computationally predicted novel and potentially antigenic non-synonymous variants present in exonic regions for the KN (022), GLOB (028), LAN (033), I (027), IN (023), LU (005) and H (018) blood group systems (Figure 2; Online Supplementary Table SII).
Figure 2.
Circos plot showing the distribution of genetic variants in RBC antigen-encoding genes and their frequencies
The outer ring (green) represents the RBC encoding genes. Box length represents the number of variants observed. G denotes gnomAD genome frequency, while E denotes gnomAD exome frequency. The outer purple (light/dark) circle indicates the distribution of variant frequencies across different blood group genes from the gnomAD data. The blue (light/dark) circle indicates the number of variants with ISBT allele designations relative to all gnomAD variants. The aqua (light/dark) circle indicates the distribution of gnomAD rare non-ISBT variants. The dark yellow circle indicates the number of non-ISBT variants annotated by the ClinVar/HGMD database. The black circle shows the distribution of the number of novel variants.
DISCUSSION
Aboriginal Australians are 15–30 times more likely to develop end-stage renal disease requiring kidney transplantation42,43, with associated increased transfusion requirements. Blood group profiling at the population level provides insight into the frequency of blood group alleles and antigens represented in the population and can help in sourcing the most appropriate antigen-matched blood for transfusion. In this study, we utilised RBCeq25 to provide a comprehensive predicted genotype and phenotype for 36 blood groups, as well as identifying rare and novel blood group variants in the SEQA cohort. The findings of the study demonstrate that rare blood group genotypes of the SEQA population are not only distinct from European populations, which make up the majority of blood donors, but also illustrate the diversity of blood group antigen prevalence among different Aboriginal Australian populations.
The ABO system is the prime determinant for transfusion matching, and in comparing the ABO group variant profile of the SEQA population with that of other Aboriginal Australian populations we found the highest prevalence of B (7.76%) phenotypes in SEQA, as compared to WD/TI/NT populations8,17,26. Universal plasma donors are AB phenotype, and this is the rarest of the four ABO phenotypes with prevalence of 3–5% in all populations33. The AB phenotype was reported at 1.22% in the SEQA population, higher than reported in NT but lower than previous reports in Indigenous Australians from Queensland (2%)9.
The RhD blood group is the second most clinically significant blood group after ABO, and extremely immunogenic. Partial D proteins lack certain RhD epitopes and individuals identified as partial D should ideally receive D− blood products. However, these individuals can be mistyped as being D+, which places them at risk of alloimmunization44. Additionally, anti-D alloantibodies from a mother with a partial D phenotype can place an RhD-positive foetus at risk of HDFN. The RhD typing strategies used in Germany and other European countries protect carriers of Partial D categories IV and VI from anti-D immunization but not carriers of other partial D antigens45. In our analysis, we detected variants in two individuals from the SEQA population encoding for the Partial D Category III type 4 phenotype, Weak D Type 33, and also one individual with a clinically significant Del RHD(IVS2-2delA) and one with Weak D Type3 phenotype. Earlier studies have identified these phenotypes in the Australian population but they have not been found in the 1KGP3 dataset except in African and European populations34. Additionally, typing for C/c and E/e antigens in the RhCE should be considered for neonates at risk of HDFN29, and for individuals with regular transfusion needs, as antibodies towards these antigens, particularly anti-c, are often encountered29. We reported the population-scale prevalence of the rare phenotypes D+ C+ E+ c− e+ and D+ C+ E+ c+ e− in the SEQA cohort. The importance of accurately determining RH system phenotypes has been particularly illustrated in a study examining the prevalence of Rhc alloimmunization in a retrospective study of Aboriginal and Torres Strait Islander peoples admitted to intensive care in the Northern Territory26.
Insights into the prevalence of these rarer Rh phenotypes in the SEQA cohort reveals transfusion management for the Aboriginal population requires a different approach to prevent alloimmunization. The provision of rare blood requires further attention and culturally safe engagement with Aboriginal communities. Australia’s population is increasingly more diverse because of migration and the blood group landscape is reflective of this change. Recent reports have highlighted a decrease in D− blood donors but an increased demand for D− blood, within a population that is becoming increasing D+27,46. With respect to the SEQA cohort, we observed higher prevalence D− (9.38%, No.=23), in contrast to recent reports from the Northern Territory where 1.2% of Australian Aboriginal peoples were found to be D−. This disparity reiterates that blood group profiles vary between geographically diverse Australian Aboriginal peoples and more comprehensive blood group characterization studies are imperative to understand blood group prevalence, facilitating blood supply planning and effective hospital blood inventory management.
In examining our dataset for rare variants, we observed the variant for the Scianna blood group antigen Sc2+ as heterozygous in two individuals in the SEQA cohort, whilst the 1KGP3 dataset had one in the European population, and two in the South Asian population. The Scianna blood group is comprised of two high frequency (Sc1+ and Sc3+) and two low frequency (Sc2+ and Sc4+) antigens32. As only 0.82% of the SEQA cohort carry the Sc2+ antigen, there is a risk of alloimmunization to individuals without this low frequency antigen. Anti-Sc2 has been shown to cause HDFN on rare occasions, and the first case of a severe HTR to anti-Sc2 has also recently been reported32. Additionally, we report the presence of the Duffy FYX allele in the SEQA population, as well as European and South Asian populations. The FYX allele encodes a weakly-expressed Fyb antigen, which is not always detected by monoclonal anti-Fyb47, and mistyping of this antigen may lead to delayed HTRs47. The SEQA population had higher predicted prevalence of clinically significant K+k+, Fy(a−b+), and Lu(a+b+) phenotypes as compared to other populations, while the variant encoding KEL:31(KYO+) antigen was detected in the Western Desert population but was absent in the SEQA population. A major limitation of the study is that additional red cell serology to confirm the presence of low incidence antigens predicted by RBCeq was not undertaken, and investigations of hybrid Rh and MNS changes were not performed.
The HGMD professional database, a manually curated database for locating disease-causing mutations, was used to explore the functional polymorphism of variants. The findings demonstrated that the HGMD-reported variations rs5036 (DI), rs150221689 (LAN), and rs12721643 (JR) are linked to reduced gene and protein expression. Further investigation is needed to explore the antigenic effect of these functional variants. Additionally, we identified nine novel non-synonymous variants using in silico predictive techniques.
Understanding the unique blood group profiles of Aboriginal populations is an essential step forward for equitable transfusion research, to inform transfusion professionals with the knowledge to provide safe transfusion support for First Nations peoples. This genomic research highlights the importance of study co-design with Aboriginal peoples, and that further steps forward are required to translate the findings to ensure transfusion care is delivered in a culturally safe and supported environment. The proper management and care of Aboriginal patients requiring blood transfusions is a delicate yet essential procedure, that aims to improve their health, wellbeing and quality of life7. Advancement in genomic technologies is increasingly transforming disease diagnosis, treatment, and prevention. The benefit of this progress, however, is inequitably provided for Aboriginal populations, who continue to experience poor health outcomes48. By establishing Aboriginal governance, co-design and culturally appropriate methodologies, this study highlights the importance of ethically appropriate and scientifically relevant research. The findings from this study provide clinically significant and valuable information that can potentially benefit transfusion-dependent Aboriginal Australians and elevate further research work in the field to assist in developing holistic transfusion approaches mindful of the thoughts, feelings and beliefs of Aboriginal peoples with respect to blood49.
Supplementary Information
ACKNOWLEDGEMENTS
This study was conducted in collaboration with the Carbal Medical Services in Toowoomba, Queensland, in consultation with the Indigenous Advisory Committee and under the approval of the Australian Red Cross Lifeblood Human Research Ethics Committee (HREC 2018#17). We acknowledge and pay respects to all Aboriginal and Torres Strait Islander peoples. We are grateful for the opportunity to work together and for the trust placed in us to undertake research in a respectful and collaborative manner. We thank all the community members of the Carbal Medical Services who placed their trust in all aspects of the study and in the ongoing storage of samples until completion of the study. All samples will be disposed of in a culturally appropriate manner or will be returned to those members as detailed in the consent form.
Footnotes
ETHICS APPROVAL: The study was co-designed and approved by the AAC who established culturally safe and ethical practices. The study was approved by the Australian Red Cross Lifeblood Ethics Committee (Reference number: 2018#17), as well as the Queensland University of Technology Human Research Ethics Committee (2021-3941-4347).
FUNDING: This work was supported in part by Advance Queensland Research Fellowship, MRFF Genomics Health Futures Mission (76,757), and the Australian Red Cross Lifeblood. The Australian governments fund the Australian Red Cross Lifeblood for the provision of blood, blood products and services to the Australian community.
AUTHORS’ CONTRIBUTIONS: Concept and design: SHN, RF, DI, ER, CH, SJ, CD. Data Analysis and validation SJ, CD, ER, CH. Visualization: SL, SJ. DNA sample collection and processing: TC, RG, ER, CD. First draft of the manuscript: SJ, CD, ER, CH, RF, SHN. Manuscript revision: SJ, ER, CD, CH, RF, SHN, MT, AB, MP, DI, BN. All authors have read and approved the final manuscript.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Delaney M, Matthews DC. Hemolytic disease of the fetus and newborn: managing the mother, fetus, and newborn. Hematology Am Soc Hematol Educ Program. 2015;2015:146–151. doi: 10.1182/asheducation-2015.1.146. [DOI] [PubMed] [Google Scholar]
- 2.Ng MSY, Ullah S, Wilson G, McDonald S, Sypek M, Mallett AJ. ABO blood group relationships to kidney transplant recipient and graft outcomes. PLoS One. 2020;15:e0236396. doi: 10.1371/journal.pone.0236396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin GY, Du XL, Shan JJ, Zhang YN, Zhang YQ, Wang QH. MNS, Duffy, and Kell blood groups among the Uygur population of Xinjiang, China. Genet Mol Res. 2017;16 doi: 10.4238/gmr16019176. [DOI] [PubMed] [Google Scholar]
- 4.Gleadall NS, Veldhuisen B, Gollub J, Butterworth AS, Ord J, Penkett CJ, et al. Development and validation of a universal blood donor genotyping platform: a multinational prospective study. Blood Adv. 2020;4:3495–3506. doi: 10.1182/bloodadvances.2020001894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majoni SW, Lawton PD, Rathnayake G, Barzi F, Hughes JT, Cass A. Narrative review of hyperferritinemia, iron deficiency, and the challenges of managing anemia in Aboriginal and Torres Strait Islander Australians with CKD. Kidney Int Rep. 2020;6:501–512. doi: 10.1016/j.ekir.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanya Davison JD, Flower R. Australia’s ethnic face is changing, and so are our blood types. [Accessed on 12/07/2023]. Available at: https://theconversation.com/australiasethnic-face-is-changing-and-so-are-our-blood-types-113454.
- 7.Kowal E, Greenwood A, McWhirter RE. All in the blood: a review of Aboriginal Australians’ cultural beliefs about blood and implications for biospecimen research. J Empir Res Hum Res Ethics. 2015;10:347–359. doi: 10.1177/1556264615604521. [DOI] [PubMed] [Google Scholar]
- 8.Schoeman EM, Roulis EV, Perry MA, Flower RL, Hyland CA. Comprehensive blood group antigen profile predictions for Western Desert Indigenous Australians from whole exome sequence data. Transfusion. 2019;59:768–778. doi: 10.1111/trf.15047. [DOI] [PubMed] [Google Scholar]
- 9.Erber WN, Buck AM, Threlfall TJ. The haematology of indigenous Australians. Hematology. 2004;9:339–350. doi: 10.1080/10245330410001714176. [DOI] [PubMed] [Google Scholar]
- 10.Scott ID, Scott MK. Blood groups of some Australian Aborigines of the Western Desert. Nature. 1966;212:545. doi: 10.1038/212545a0. [DOI] [PubMed] [Google Scholar]
- 11.Vos GH, Kirk RL. A “naturally-occurring” anti-E which distinguishes a variant of the E antigen in Australian aborigines. Vox Sang. 1962;7:22–32. doi: 10.1111/j.1423-0410.1962.tb03225.x. [DOI] [PubMed] [Google Scholar]
- 12.Simmons RT, Semple NM, Cleland JB, Casley-Smith JR. A blood group genetical survey in Australian aborigines at Haast’s Bluff, Central Australia. Am J Phys Anthropol. 1957;15:547–553. doi: 10.1002/ajpa.1330150406. [DOI] [PubMed] [Google Scholar]
- 13.Sanger R, Walsh RJ, Kay MP. Blood types of natives of Australia and New Guinea. Am J Phys Anthropol. 1951;9:71–78. doi: 10.1002/ajpa.1330090106. [DOI] [PubMed] [Google Scholar]
- 14.Birdsell JB, Boyd WC. Blood groups in the Australian Aborigines. AJBA. 1940;27:69–90. doi: 10.1002/ajpa.1330270128. [DOI] [Google Scholar]
- 15.McBean R, Liew YW, Wilson B, Kupatawintu P, Emthip M, Hyland C, Flower R. Genotyping confirms inheritance of the rare At(a−) type in a case of haemolytic disease of the newborn. J Pathol Clin Res. 2015;2:53–55. doi: 10.1002/cjp2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane WJ, Westhoff CM, Gleadall NS, Aguad M, Smeland-Wagman R, Vege S, et al. Automated typing of red blood cell and platelet antigens: a whole-genome sequencing study. Lancet Haematol. 2018;5:e241–e251. doi: 10.1016/S2352-3026(18)30053-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadhao S, Hoy W, Lee S, Patel HR, McMorran BJ, Flower RL, et al. The genomic landscape of blood groups in Indigenous Australians in remote communities. Transfusion. 2022;62:1110–1120. doi: 10.1111/trf.16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puobon U, Intharanut K, Mitundee S, Nathalang O. Determining of JK*A and JK*B Allele Frequency Distribution among Muslim Blood Donors from Southern Thailand. Malays J Med Sci. 2019;26:58–65. doi: 10.21315/mjms2019.26.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langer IBV, Visentainer JEL, Zacarias JMV, Grilo KTM, Hatschbach PR, Zimmermann RS, et al. Genotyping of Dombrock and Lutheran blood group systems in blood donors from the southwestern region of the state of Paraná, Southern Brazil. Hematol Transfus Cell Ther. 2019;41:25–30. doi: 10.1016/j.htct.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler MM, Lannert KW, Huston H, Fletcher SN, Harris S, Teramura G, et al. Genomic characterization of the RH locus detects complex and novel structural variation in multi-ethnic cohorts. Genet Med. 2019;21:477–486. doi: 10.1038/s41436-018-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratt G, Vidgen M, Kaladharan S, Pearson J, Whiteman D, Waddell N. Genomic Partnerships: Guidelines for genomic research with Aboriginal and Torres Strait Islander peoples of Queensland. QIMR Berghofer Medical Research Institute; [Accesed on 13/04/2023]. Available at: https://www.qimrberghofer.edu.au/wp-content/uploads/2020/09/2019-Indigenous-Health-Genomics-Guide-v9-WEB.pdf. [Google Scholar]
- 22.Roulis E, Schoeman E, Hobbs M, Jones G, Burton M, Pahn G, et al. Targeted exome sequencing designed for blood group, platelet, and neutrophil antigen investigations: proof-of-principle study for a customized single-test system. Transfusion. 2020;60:2108–2120. doi: 10.1111/trf.15945. [DOI] [PubMed] [Google Scholar]
- 23.Moulds JM, Nowicki S, Moulds JJ, Nowicki BJ. Human blood groups: incidental receptors for viruses and bacteria. Transfusion. 1996;36:362–374. doi: 10.1046/j.1537-2995.1996.36496226154.x. [DOI] [PubMed] [Google Scholar]
- 24.1000 Genomes Project Consortium. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jadhao S, Davison CL, Roulis EV, Schoeman EM, Divate M, Haring M, et al. RBCeq: a robust and scalable algorithm for accurate genetic blood typing. EBioMedicine. 2022;76:103759. doi: 10.1016/j.ebiom.2021.103759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noutsos T, Perry MA, Secombe PJ, Roxby DJ, Sinha R, Campbell LT. A retrospective cohort study of red cell alloimmunisation in rural, remote, and Aboriginal and Torres Strait Islander peoples admitted to intensive care in the Northern Territory, Australia. J Clin Med. 2023;12:1606. doi: 10.3390/jcm12041606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLean A, Szabo F, Wang Z. ABO and Rhesus D blood groups in the Northern Territory of Australia. Intern Med J. 2021;51:1485–1489. doi: 10.1111/imj.15199. [DOI] [PubMed] [Google Scholar]
- 28.Mangwana S, Gangwar V. Anti Kpa alloantibody: development of a rare alloantibody in a non-Hodgkin›s lymphoma patient of Indian origin. Asian J Transfus Sci. 2018;12:81–84. doi: 10.4103/ajts.AJTS_23_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dean L. Blood Groups and Red Cell Antigens [Internet] Chapter 9. Bethesda (MD): National Center for Biotechnology Information (US); The Duffy blood group; 2005. [Accessed on 12/07/2023]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK2271/ [Google Scholar]
- 30.Avent ND, Reid ME. The Rh blood group system: a review. Blood. 2000;95:375–387. doi: 10.1182/blood.V95.2.375. [DOI] [PubMed] [Google Scholar]
- 31.Reid ME, Lomas-Francis C. Blood group antigens & antibodies: a guide to clinical relevance & technical tips. 2nd ed. Cambridge, MA, USA: Starbright Books; 2020. [Google Scholar]
- 32.Daniels G. Human blood groups. 3rd ed. Oxford: Wiley-Blackwell; 2013. [Google Scholar]
- 33.Reid M, Lomas F, Olsson M. The blood group antigen factsbook. Vol. 3. London: Elsevier; 2012. [Google Scholar]
- 34.Scott SA, Nagl L, Tilley L, Liew YW, Condon J, Flower R, et al. The RHD(1227G>A) DEL-associated allele is the most prevalent DEL allele in Australian D− blood donors with C+ and/or E+ phenotypes. Transfusion. 2014;54:2931–2940. doi: 10.1111/trf.12701. [DOI] [PubMed] [Google Scholar]
- 35.Floch A. Molecular genetics of the Rh blood group system: alleles and antibodies—a narrative review. Annals of Blood. 2021:6. doi: 10.21037/aob-20-84. [DOI] [Google Scholar]
- 36.Lewis M, Kaita H, Chown B. The Duffy blood group system in Caucasians. a further population sample. Vox Sang. 1972;23:523–527. doi: 10.1111/j.1423-0410.1972.tb03845.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim H, Park MJ, Sung TJ, Choi JS, Hyun J, Park KU, et al. Hemolytic disease of the newborn associated with anti-Jra alloimmunization in a twin pregnancy: the first case report in Korea. Korean J Lab Med. 2010;30:511–515. doi: 10.3343/kjlm.2010.30.5.511. [DOI] [PubMed] [Google Scholar]
- 38.Peyrard T, Pham BN, Arnaud L, Fleutiaux S, Brossard Y, Guerin B, et al. Fatal hemolytic disease of the fetus and newborn associated with anti-Jr. Transfusion. 2008;48:1906–1911. doi: 10.1111/j.1537-2995.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- 39.Simmons RT, Tindale NB, Birdsell JB. A blood group genetical survey in Australian aborigines of Bentinck, Mornington and Forsyth Islands, Gulf of Carpentaria. Am J Phys Anthropol. 1962;20:303–320. doi: 10.1002/ajpa.1330200314. [DOI] [PubMed] [Google Scholar]
- 40.Ranney HM, Rosenberg GH, Morrison M, Mueller TJ. Frequencies of Band 3 variants of human red cell membranes in some different populations. Br J Haematol. 1990;75:262–267. doi: 10.1111/j.1365-2141.1990.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 41.Koszarska M, Kucsma N, Kiss K, Varady G, Gera M, Antalffy G, et al. Screening the expression of ABCB6 in erythrocytes reveals an unexpectedly high frequency of Lan mutations in healthy individuals. PLoS One. 2014;9:e111590. doi: 10.1371/journal.pone.0111590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoy WE. Kidney disease in Aboriginal Australians: a perspective from the Northern Territory. Clin Kidney J. 2014;7:524–530. doi: 10.1093/ckj/sfu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kidney Health Australia [internet] Health Professional Resources. Aboriginal and Torres Strait Islander people. [Accessed on 23/11/2022]. Available at: https://kidney.org.au/health-professionals/health-professional-resources/aboriginal-and-torres-strait-islander-peoples.
- 44.Sandler SG, Flegel WA, Westhoff CM, Denomme GA, Delaney M, Keller MA, et al. It’s time to phase in RHD genotyping for patients with a serologic weak D phenotype. College of American Pathologists Transfusion Medicine Resource Committee Work Group. Transfusion. 2015;55:680–689. doi: 10.1111/trf.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Zabern I, Wagner FF, Moulds JM, Moulds JJ, Flegel WA. D category IV: a group of clinically relevant and phylogenetically diverse partial D. Transfusion. 2013;53(11 Suppl 2):2960–2973. doi: 10.1111/trf.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirani R, Weinert N, Irving DO. The distribution of ABO RhD blood groups in Australia, based on blood donor and blood sample pathology data. Med J Aust. 2022;216:291–295. doi: 10.5694/mja2.51429. [DOI] [PubMed] [Google Scholar]
- 47.Olsson ML, Smythe JS, Hansson C, Poole J, Mallinson G, Jones J, Avent ND, Daniels G. The Fy(x) phenotype is associated with a missense mutation in the Fy(b) allele predicting Arg89Cys in the Duffy glycoprotein. Br J Haematol. 1998;103:1184–1191. doi: 10.1046/j.1365-2141.1998.01083.x. [DOI] [PubMed] [Google Scholar]
- 48.Luke J, Dalach P, Tuer L, Savarirayan R, Ferdinand A, McGaughran J, et al. Investigating disparity in access to Australian clinical genetic health services for Aboriginal and Torres Strait Islander people. Nat Commun. 2022;13:4966. doi: 10.1038/s41467-022-32707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perry M, Flower R, Hyland CA, Spiegel T, Keegan A, White K, et al. Culturally safe blood transfusion and blood donation for Aboriginal and/or Torres Strait Islander peoples. Transfus Med. 2022;32(Suppl 3):7. doi: 10.1111/tme.12933. [DOI] [Google Scholar]
- 50.Al-Riyami AZ, Al-Marhoobi A, Al-Hosni S, Al Mahrooqi S, Schmidt M, O’Brien S, et al. Prevalence of red blood cell major blood group antigens and phenotypes among Omani blood donors. Oman Med J. 2019;34:496–503. doi: 10.5001/omj.2019.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.