Abstract
Purpose
To analyze the outcomes of the iStent inject in a real-world clinical setting as a standalone procedure to lower intraocular pressure (IOP) in open-angle glaucoma.
Materials and methods
Patients with open-angle glaucoma having undergone iStent inject insertion without concurrent cataract extraction were included in this multicenter observational real-world study in Australia. Patient data was entered into the Fight Glaucoma Blindness! Registry. Assessments through 12 months included glaucoma subtype, IOP, medications, best-corrected visual acuity (BCVA), secondary surgical procedures, and adverse events. Kaplan–Meier survival curves for outcomes were reported according to the World Glaucoma Association (WGA).
Results
Sixty-one eyes from 44 patients with a mean age of 76 ± 11.4 underwent standalone iStent inject implantation. The mean ± SD preoperative IOP was 17.5 ± 7.5 mm Hg, and the mean preoperative number of topical medications was 2.5 ± 1.5. At 12 months postoperatively, there was no statistically significant IOP reduction, while the number of glaucoma medications used was reduced to 1.4 ± 1.5 (p < 0.001). Fourteen point one percent of eyes required a secondary pressure-lowering procedure within the 12-month follow-up window.
Conclusion
This assessment of standalone iStent inject implantation did not show any significant reduction in IOP, but there was a significant decrease in medication use in the real-world clinical setting. The procedure is safe with minimal adverse outcomes; however, a subset of patients required secondary procedures within 12 months of follow-up.
How to cite this article
Huynh B, Clement C, Nguyen V, et al. 12-month Safety and Efficacy Outcomes of a Standalone Trabecular Bypass Device. J Curr Glaucoma Pract 2024;18(3):103–109.
Keywords: Glaucoma, iStent inject, Minimally invasive glaucoma surgery, Real world data
Introduction
Glaucoma is a progressive optic neuropathy that is the leading cause of irreversible blindness globally,1,2 with over 110 million expected to have the condition by 2040.2 Lowering intraocular pressure (IOP), the only known modifiable risk factor, remains the only demonstrated management to slow or prevent disease progression.3,4 Topical medications or laser trabeculoplasty are usually offered as first-line therapy because of their efficacy and relatively good safety profile5; however, medical therapy can be complicated by side effects, including ocular surface disease, as well as poor adherence to treatment.6 Reasons for poor adherence are multifactorial and include a limited understanding of treatment aims, ineffective instillation techniques, and cost.7,8
Traditionally, patients receive incisional procedures when the maximum medical therapy and/or laser procedures fail to lower IOP adequately or there is evidence of progression. Incisional surgery can achieve large pressure reductions but has significant potential side effects. The Tube Versus Trabeculectomy (TVT) Study found IOP reductions at 5 years of 41.4% with the Baerveldt glaucoma implant and 49.5% for trabeculectomy with mitomycin C.9 Early postoperative adverse events arose in 21% of patients in the tube group and 37% of patients in the trabeculectomy cohort, most of which were transient and self-limited.10 Late postoperative complications occurred in 34% of the tube cohort and 36% of the trabeculectomy cohort over 5 years of follow-up.10
Minimally invasive glaucoma surgery (MIGS) comprises a heterogeneous group of glaucoma surgeries developed with the broad aim of bridging the relatively large safety gap between medical and laser glaucoma treatment and traditional glaucoma filtration surgery. The goal of the newer procedures is to maintain efficacy while providing a better risk profile and less intense postoperative care compared to traditional glaucoma surgery.11 Transtrabecular devices are a subgroup of MIGS that achieve IOP reduction by creating a bypass into Schlemm's canal.11 Schlemm's canal-based procedures have the safety advantage of avoiding complications associated with filtration surgeries, such as hypotony, wound leak, and choroidal effusions, although there is a likely trade-off of lesser effectiveness.12,13
The iStent inject is a second-generation trabecular micro-bypass stent that has shown success in lowering IOP and medication usage in association with cataract extraction.12,14-16 In contrast, less data is available regarding iStent inject as a standalone surgery.17-25 This study describes real-world outcomes of efficacy and safety of iStent inject as a standalone treatment for open-angle glaucoma.
Materials and Methods
Study Design
This retrospective multicenter, multisurgeon observational study analyzed data from eyes that underwent iStent inject implantation without concurrent cataract surgery. Data were entered into the Fight Glaucoma Blindness! (FGB!) registry,26 an audit and research tool that ensures accurate, comprehensive data collection of routine clinical care. Data extraction from the registry included eyes that were diagnosed with open-angle glaucoma (primary or secondary) and had undergone iStent inject implantation as a standalone procedure. Patients who had any prior incisional glaucoma surgery were excluded.
Efficacy outcomes consisted of changes in IOP and the number of topical IOP-lowering medications. Baseline IOP, best-corrected visual acuity (BCVA), visual field mean deviation (VFMD), and the number of topical glaucoma medications were based on the mean of the two visits (where available) immediately preceding iStent inject implantation. Adverse events included subsequent glaucoma procedures, loss of BCVA greater than 2 lines, hypotony with choroidal effusion, hyphema with greater than 2 lines BCVA loss, hyphema without vision loss, and infection (blebitis with or without endophthalmitis).
Fight Glaucoma Blindness is a quality assurance tool that has ethics approval for research through the Royal Australian and New Zealand College of Ophthalmology Human Research Ethics Committee. This study followed the principles of the Declaration of Helsinki.
Data Analyses
Data were extracted from the FGB! registry. The number of eyes and percentages were reported for categorical data, and means with standard deviations or medians with first and third quartiles were reported for continuous data. Data were reported using the World Glaucoma Association (WGA) guidelines.27 The primary outcome measure was the percentage IOP change from preoperative to 12 months postoperative. The secondary outcome measures were the percentage reduction of the mean number of glaucoma medications used and the complete and qualified success rates of greater than 20% IOP reduction at three IOP levels: 15, 18, and 21 mm Hg. Complete success was defined as patients meeting the IOP criteria and not requiring any topical glaucoma medications, while qualified success was defined as patients meeting the IOP criteria but still requiring medical therapy. Any patient requiring a further surgical or laser procedure within the follow-up window was considered a failure. Visits after the second procedure were censored with the ‘last observation carried forward’ until 12 months used for IOP and medication values. All analyses were performed using R software version 4.0.0 with the survival package (V3.1-12) for Kaplan–Meier survival analysis.28,29
Results
The analysis included 61 eyes from 44 patients, with a mean age of 76 ± 11.4. Baseline data are presented in Table 1. Glaucoma subtypes included primary open-angle glaucoma (POAG) (n = 47), secondary OAG (n = 5), normal tension glaucoma (NTG) (n = 7), and POAG/NTG suspect (n = 2). Twelve (19.7%) eyes were phakic, and 49 (80.3%) eyes were pseudophakic. Sixteen (26%) eyes had previously undergone selective laser trabeculoplasty (SLT). The mean preoperative IOP was 17.5 ± 7.5 mm Hg, and the mean preoperative number of topical agents was 2.5 ± 1.5.
Table 1.
Demographic and preoperative clinical characteristics for eligible eyes receiving standalone iStent inject with a 12-month visit or were censored prior to the 12-month visit
| Eyes | 61 |
| Patients | 44 |
| Procedures | 64 |
| Gender, % female patients | 65.9% |
| Eye side, n left eyes (%) | 32 (52.5%) |
| Age, mean years ± SD | 76 ± 11.4 |
| VA, mean letters ± SD | 78.6 ± 16.1 |
| IOP, mean mm Hg ± SD | 17.5 ± 7.5 |
| Medications, mean ± SD | 2.5 ± 1.5 |
| Visual field MD, mean ± SD* | −8.5 ± 6.1 |
| CCT, mean ± SD** | 531.9 ± 40.7 |
| Global RNFL thickness, mean ± SD*** | 72 ± 15.8 |
| Lens status, n (%) | |
| Phakic | 12 (19.7%) |
| Pseudophakic | 49 (80.3%) |
| Glaucoma subtype, n (%) | |
| Primary open angle glaucoma | 47 (77%) |
| Secondary open angle glaucoma | 5 (8.2%) |
| NTG | 7 (11.5%) |
| Ocular hypertension | 0 (0%) |
| Primary open angle/NTG suspect | 2 (3.3%) |
| Previous SLT, n (%) | 16 (26.2%) |
*Data available for 51 procedures; **Data available for 63 procedures; ***Data available for 50 procedures
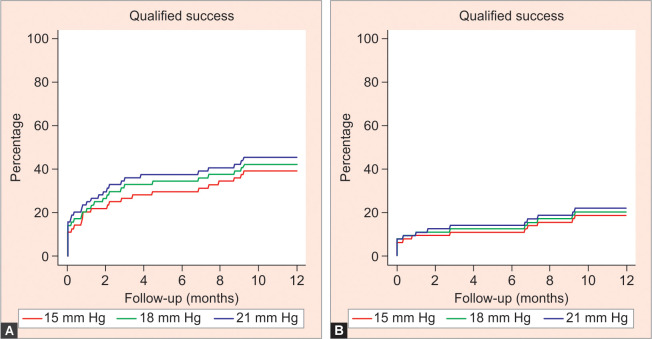
At 12 months, IOP reduced from 17.5 mm Hg to 17.0 mm Hg (5.3% IOP reduction), which was not statistically significant. The mean number of glaucoma medications used reduced from 2.5 to 1.4 (p < 0.001). Complete and qualified success rates at both 21 mm Hg and 18 mm Hg were 9.4 and 26.6%, respectively. At the 15 mm Hg threshold, complete success was 4.7% and qualified success was 15.6%. Table 2 shows the preoperative and postoperative IOP measurements, including the difference in IOP-lowering drops after 12 months. Figure 1 depicts the comparison between pre- and postoperative IOP following 12 months after standalone iStent inject, showing complete and qualified successes. Figure 2 illustrates the time to qualified and complete success using Kaplan–Meier survival analysis.
Table 2.
IOP and medication outcomes 12 months after standalone iStent inject. Censored procedures were included using the last observation carried forward. p-values are from paired t-tests for change in IOP and medications from preoperative levels
| IOP outcomes | ||
| Preoperative, mean ± SD | 17.5 ± 7.5 | |
| Final, mean ± SD | 17 ± 6.8 | |
| Change, mean (95% CI) | −0.5 (–2.5, 1.4) | 0.583 |
| % change, median (Q1, Q3) | 5.3% (–20, 27.8) | |
| Medication outcomes | ||
| Preoperative, mean ± SD | 2.5 ± 1.5 | |
| Final, mean ± SD | 1.4 ± 1.5 | |
| Change, mean (95% CI) | −1.1 (–1.4, –0.8) | < 0.001 |
| Complete success, n (%)* | ||
| IOP 15 mm Hg | 3 (4.7%) | |
| IOP 18 mm Hg | 6 (9.4%) | |
| IOP 21 mm Hg | 6 (9.4%) | |
| Qualified success, n (%)** | ||
| IOP 15 mm Hg | 10 (15.6%) | |
| IOP 18 mm Hg | 17 (26.6%) | |
| IOP 21 mm Hg | 17 (26.6%) |
*Complete success defined as a ≥20% reduction in IOP with final IOP below 15, 18, or 21 mm Hg without the use of medications; **Qualified success defined as a ≥20% reduction in IOP with final IOP below 15, 18, or 21 mm Hg; p-value < 0.001
Fig. 1.
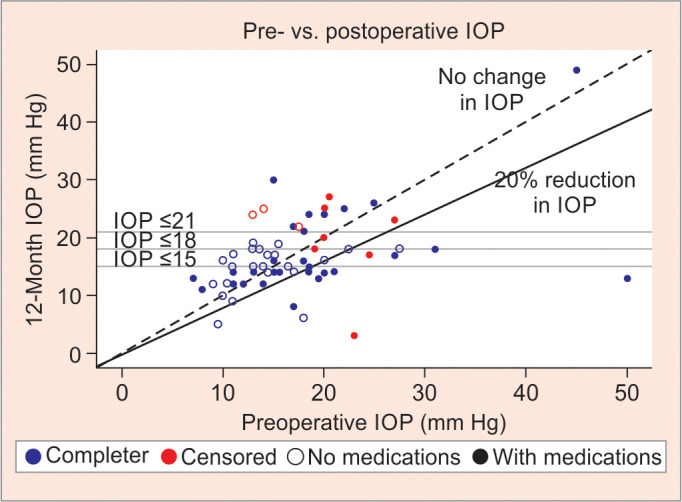
Pre- vs postoperative IOP 12 months after standalone iStent, showing complete (no medications) and qualified (with medications) successes depending on the threshold for IOP reduction (below 15, 18, or 21 mm Hg)
Figs 2A and B.
Kaplan–Meier survival curves for time to qualified and complete success
Table 3 shows the frequency of subsequent procedures and adverse events. Nine eyes underwent secondary interventions during the first 12 months of follow-up. The interventions included 3 trabeculectomies, 3 further standalone iStent inject implantations, 3 SLT treatments, 1 Cypass implantation, and 1 posterior vitrectomy for a rhegmatogenous retinal detachment. Some eyes received multiple secondary procedures; for example, 3 eyes underwent a repeat standalone iStent inject procedure, and two of these also had SLT treatment. The baseline characteristics of the 9 eyes requiring secondary procedures matched those of the rest of the cohort. One eye was phakic (11%), the average IOP was 19.2 ± 4.6 mm Hg, the mean number of topical medications was 3 ± 1.6, and 2 eyes had previous SLT (22%).
Table 3.
Frequency and percentage of procedures with an adverse event recorded at any time up until the 12-month visit
| Frequency (%) | |
|---|---|
| Eyes receiving subsequent procedure | 9 (14.1%) |
| Total secondary procedures | 11 |
| Loss of BCVA ≥2 lines, 0–3 months | 20 (31.2%) |
| Loss of BCVA ≥2 lines, 3–12 months | 14 (21.9%) |
| Early numerical hypotonya | 2 (3.1%) |
| Late numerical hypotonyb | 2 (3.1%) |
| Glaucoma device malposition | 0 (0%) |
| Hyphema with ≥2 line BCVA loss | 1 (1.6%) |
| Hyphema without vision loss | 2 (3.1%) |
| Blebitis with or without endophthalmitis | 0 (0%) |
aIOP <5 mm Hg within 3 months of procedure; bIOP <5 mm Hg between 3 and 12 months after procedure
The median (Q1, Q3) time to the second procedure was 143 (50, 194) days. The median (Q1, Q3) IOP at the visit immediately prior to the second procedure was 22 (18, 24.5) mm Hg. There were no reports of glaucoma device malposition, and no phakic eyes required cataract surgery within the study follow-up period.
Three cases of hypotony with visual compromise were recorded. In two cases, this was transient with complete resolution of acuity. In the third case, the low pressure was the result of a rhegmatogenous retinal detachment occurring 5 months after iStent inject surgery.
The data comparing eyes by preoperative IOP is displayed in Table 4. Eyes with preoperative IOP greater than 21 (n = 12) had a median IOP reduction of 26.2% from baseline, with IOP decreasing from 29 ± 9.1 mm Hg to 20.4 ± 10.8 mm Hg postoperatively (p = 0.024).
Table 4.
IOP and medication outcomes 12 months after standalone iStent inject by preoperative IOP
| IOP ≤21 | IOP >21 | |
|---|---|---|
| Procedures | 52 | 12 |
| IOP outcomes | ||
| Preoperative, mean (SD) | 14.9 ± 3.7 | 29 ± 9.1 |
| Final, mean (SD) | 16.2 ± 5.3 | 20.4 ± 10.8 |
| Change, mean (95% CI) | 1.3 (–0.1, 2.8) | −8.5 (–15.7, –1.4) |
| % change, median (Q1, Q3) | 11.2% (–11.9, 32.1) | −26.2% (–38.3, –10.1) |
| Medication outcomes | ||
| Preoperative, mean (SD) | 2.3 ± 1.3 | 3.2 ± 2 |
| Final, mean (SD) | 1.1 ± 1.3 | 2.7 ± 1.6 |
| Change, mean (95% CI) | −1.2 (–1.6, –0.9) | −0.5 (–1.1, 0.1) |
| % change, median (Q1, Q3) | −63.3% (–100, 0) | −14.3% (–22.5, 0) |
| Qualified success, n (%)* | ||
| IOP 15 mm Hg | 8 (15.4%) | 2 (16.7%) |
| IOP 18 mm Hg | 9 (17.3%) | 8 (66.7%) |
| IOP 21 mm Hg | 9 (17.3%) | 8 (66.7%) |
| Complete success, n (%)** | ||
| IOP 15 mm Hg | 3 (5.8%) | 0 (0%) |
| IOP 18 mm Hg | 4 (7.7%) | 2 (16.7%) |
| IOP 21 mm Hg | 4 (7.7%) | 2 (16.7%) |
*Complete success defined as a ≥20% reduction in IOP with final IOP below 15, 18, or 21 mm Hg without the use of medications; **Qualified success defined as a ≥20% reduction in IOP with final IOP below 15, 18, or 21 mm Hg
Discussion
This retrospective multicenter study provides real-world data on the use of iStent inject implantation without phacoemulsification. This cohort, as a whole, did not have a significant reduction in IOP at 12 months but did have a significant decrease in medication usage from 2.5 ± 1.5 medications preoperatively to 1.4 ± 1.5. The smaller subgroup of patients with a preoperative IOP greater than 21 had a significant IOP decrease of 26%, whereas 14% of eyes required a secondary glaucoma procedure within the 12-month follow-up. To date, there have only been two other publications looking at real-world data of standalone iStent inject implantation, both limited to single-center and single-surgeon studies.17,19 Our study fills a gap in the literature by reporting real-world multicenter and multisurgeon outcomes of a heterogeneous patient population outside of the clinical trial setting out to 12 months.
The finding of no significant reduction in IOP is somewhat different from other reports, which found IOP reductions of between 31% and 48% (Table 5).17-22 This discrepancy may, in part, be explained by differences in the baseline characteristics between studies. In this study, the cohort had relatively advanced glaucoma with an average mean deviation of –8.5 dB, whereas other studies have tended to recruit patients with mild to moderate glaucoma. Furthermore, the overall mean pretreatment IOP in the current study was 17.5 mm Hg, significantly lower than in other studies, which reported ranges of 24.4–26.3 mm Hg after medication washout and 21.2–25.3 mm Hg where no washout was performed.17-22
Table 5.
Comparison between the present study with published outcomes
| Follow-up period | Glaucoma subtype | Preop IOP (mean and SD) | Preop meds (mean and SD) | Postop IOP (mean and SD) | Postop meds (mean and SD) | % reduction IOP | % reduction meds | Secondary procedures (number of eyes and %) | Washout | |
|---|---|---|---|---|---|---|---|---|---|---|
| Current study | 12 months | TOTAL—64 | 17.5 ± 7.5 | 2.5 ± 1.5 | 17 ± 6.8 | 1.4 ± 1.5 | 2.8 | 44.0 | 9 (14.1) | No washout |
| Fea et al. 201418 | 12 months | TOTAL—94 | 21.1 ± 1.7 (pre-washout) 25.2 ± 1.4 (post-washout) | Not reported | 13.0 ± 2.3 | Not reported | 48.4 | Not reported | 1 (1.1) | Washout |
| Voskanyan et al. 201420 | 12 months | TOTAL—88 | 22.1 ± 3.3 (pre-washout) 26.3 ± 3.5 (post-washout) | 2.21 ± 0.44 | 15.7 ± 3.7 | Not reported | 39.7 | Not reported—15.2% reduced medications by 1, 71.7% reduced medications by 2 or more | 6 (6.1) | Washout |
| Klamann et al. 201517 | 6 months | POAG—17 | 21.19 ± 2.56 | 2.19 ± 0.91 | 14.19 ± 1.38 | 0.88 ± 0.62 | 33 | 59.8 | 0 (0) | No washout |
| PEX—15 | 23.75 ± 3.28 | 2.33 ± 1.23 | 15.33 ± 1.07 | 1.04 ± 0.30 | 35.5 | 55.4 | 0 (0) | No washout | ||
| Lindstrom et al. 201622 | 18 months | POAG—57 | 19.5 ± 1.5 (pre-washout) 24.4 ± 1.3 (post-washout) | 1 ± 0 | 14.4 ± 2.1 | 0.02 (1 patient on 1 medication) | 41 | 98.0 | 0 (0) | Washout |
| Berdahl et al. 201721 | 18 months | TOTAL—53 | 19.7 ± 1.5 (pre-washout) 24.9 ± 1.1 (post-washout) | 2 ± 0 | 12.9 ± 2.1 | 1 ± 0 | 34.5 | 50.0 | 0 (0) | Washout |
| Hengerer et al. 201919 | 36 months | TOTAL—44 | 25.3 ± 6.0 | 2.98 ± 0.88 | 14.6 ± 2.0 | 0.55 ± 0.79 | 42.3 | 81.5 | 2 (4.5) | No washout |
There is some suggestion that higher pretreatment IOPs are more likely to lead to larger IOP reductions.30 In a small subgroup analysis of eyes with baseline IOP greater than 21 (n = 12), there was a significant IOP decrease of 26.2% (p = 0.024), from baseline 29 ± 9.1 mm Hg to 20.4 ± 10.8 mm Hg at 12 months. However, this finding was matched by a lesser medication reduction in this small subgroup; eyes with baseline IOP ≤21 had a significant reduction in medication use from 2.3 ± 1.3 to 1.1 ± 1.3 at 12 months, while eyes with baseline IOP greater than 21 had a smaller, nonsignificant reduction in medication use from 3.2 ± 2 to 2.7 ± 1.6. Although the relationship between IOP and medication use is confounded by a number of variables, the tendency for patients with higher IOPs to remain on their topical medications may well have contributed to the increased reduction in IOP observed in this cohort.
A second potential explanation for the nonsignificant IOP reduction relates to the real-world nature of the study. In some cases, the clinician's motive for treatment may not have been solely to lower IOP but rather to reduce glaucoma medication usage. In certain clinical scenarios, clinicians may have been willing to tolerate a higher (or unchanged) IOP if it meant a reduction in medication usage. We examined the change in medication usage for the cohort whose IOP increased postoperatively vs the cohort whose IOP decreased and found no change in medications between them. This suggests that the reduction of medication usage was unlikely to be a major explanatory factor in clinician decision-making.
In this study, 9 (14%) eyes had secondary glaucoma interventions within the first 12 months, with 2 patients having 2 secondary procedures each (Table 3). The secondary procedures included trabeculectomy in 3 eyes, repeat insertion of iStent inject as a standalone procedure in 3 eyes, and SLT in 2 eyes. Other similar studies have reported secondary procedures performed in 0–6% of eyes.17-22 No cases of hypotony have been reported previously in standalone iStent inject implantation.17-22 This study recorded 3 cases of hypotony, with two cases having transient hypotony with a reduction in visual acuity but no long-term visual changes, and one case associated with a rhegmatogenous retinal detachment. The retinal detachment occurred 5 months after iStent inject surgery, suggesting it was not related to the device implantation.
The particular role of different MIGS procedures in the glaucoma treatment algorithm is still being determined. One possibility is that trabecular bypass stents could serve as a replacement for trabeculectomy, at least in some patients.31 Part of the challenge in comparing outcomes is that they vary widely depending on the definition of success, the surgical techniques employed, the patient population, and the length of follow-up. The Victorian Trabeculectomy Audit found that after 2 years, the qualified success rate (IOP ≤18 mm Hg ± medications) was 91.4%.32 Similar data from 9 glaucoma units across the UK found a 2-year qualified success rate (IOP <21 mm Hg and 20% IOP reduction) of 87%.33 The trabeculectomy arm of the TVT study, with longer outcomes of 5 years, reported a qualified success rate (IOP <21 mm Hg and 20% IOP reduction) of 50%.9
For comparison, this study found a 1-year qualified success rate (IOP <21 mm Hg and 20% IOP reduction) of 27% and a complete success rate of 9.4%. These outcomes therefore compare relatively poorly to the trabeculectomy outcomes noted above.32-34 Based on this real-world data, patients who are not at target pressure and need significant IOP reduction do not appear to achieve high success rates with standalone iStent inject. Such patients are more likely to achieve the required pressure reduction with standard filtration surgery.
For a MIGS procedure potentially aiming to bridge the gap between medical/laser treatment and filtration surgery, another relevant comparator is SLT, which has an established safety and efficacy profile.5 A 2015 systematic review and meta-analysis found that SLT lowered IOP between 7 and 36% in patients with POAG.35 However, many studies report only the percentage medication reduction for the entire cohort, making comparison of success rates more challenging. For studies using standardized reporting, one found a 1-year qualified success rate (IOP <21 mm Hg and > 20% reduction) of 61%,36 while another reported a less impressive 1-year success rate (>20% reduction) of 29%.37 The results from this study place standalone iStent inject toward the bottom of this range. Further studies with consistent reporting measures are required to better delineate these findings.
The nature of this data comes with some inherent limitations. We identified all cases of standalone iStent inject for each participating surgeon, thereby minimizing the chance of any bias in case selection. However, a 12-month follow-up is relatively short in the context of the long-term management of glaucoma, and some patients required secondary interventions within this period. The mean preoperative IOP was reasonably low (17.5 mm Hg) with no medication washout, reflecting the real-world nature of the study where the clinicians’ motive for treatment may have been to reduce medication usage rather than solely reducing IOP. Future MIGS studies should also consider using patient-reported outcomes, as this is the real-world metric that interventions ultimately aim to maximize. Additionally, investigating the cost of standalone MIGS procedures in comparison to other procedures such as combined phacoemulsification, trabeculectomy, and SLT would also be valuable.38,39
Conclusion
This retrospective multicenter study suggests that standalone iStent inject implantation provides a statistically significant reduction in medication use after 12 months but no significant reduction in IOP in the real-world clinical setting. The procedure is safe with minimal adverse events. Cost-effectiveness assessments will help determine the role of this procedure within the glaucoma surgical treatment paradigm.
Clinical Significance
iStent inject insertion without concurrent phacoemulsification is a procedure with a good safety profile. A reduction in medications, but not IOP, was achieved in this real-world study.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Sihota R, Angmo D, Ramaswamy D, et al. Simplifying “target” intraocular pressure for different stages of primary open-angle glaucoma and primary angle-closure glaucoma. Indian J Ophthalmol. 2018;66(4):495–505. doi: 10.4103/ijo.IJO_1130_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 5.Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505–1516. doi: 10.1016/S0140-6736(18)32213-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lusthaus J, Goldberg I. Current management of glaucoma. Med J Aust. 2019;210(4):180–187. doi: 10.5694/mja2.50020. [DOI] [PubMed] [Google Scholar]
- 7.Friedman DS, Okeke CO, Jampel HD, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology. 2009;116(6):1097–1105. doi: 10.1016/j.ophtha.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Kosoko O, Quigley HA, Vitale S, et al. Risk factors for noncompliance with glaucoma follow-up visits in a residents’ eye clinic. Ophthalmology. 1998;105(11):2105–2111. doi: 10.1016/S0161-6420(98)91134-4. [DOI] [PubMed] [Google Scholar]
- 9.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789–803e2. doi: 10.1016/j.ajo.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–814e1. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam K, Lawlor M. Minimally Invasive Glaucoma Surgery [Internet] Springer; Singapore: 2021. Anatomy of the aqueous outflow drainage pathways. pp. 1–216. p. [Google Scholar]
- 12.Samuelson TW, Sarkisian SR, Jr,, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811–821. doi: 10.1016/j.ophtha.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology. 2019;126(1):29–37. doi: 10.1016/j.ophtha.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, et al. Glaukos iStent inject® trabecular micro-bypass implantation associated with cataract surgery in patients with coexisting cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmol. 2016;2016:1056573. doi: 10.1155/2016/1056573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clement CI, Howes F, Ioannidis AS, et al. One-year outcomes following implantation of second-generation trabecular micro-bypass stents in conjunction with cataract surgery for various types of glaucoma or ocular hypertension: multicenter, multi-surgeon study. Clin Ophthalmol. 2019;13:491–499. doi: 10.2147/OPTH.S187272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalaby WS, Jia J, Katz LJ, et al. iStent inject: comprehensive review. J Cataract Refract Surg. 2021;47(3):385–399. doi: 10.1097/j.jcrs.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 17.Klamann MK, Gonnermann J, Pahlitzsch M, et al. iStent inject in phakic open angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2015;253(6):941–947. doi: 10.1007/s00417-015-3014-2. [DOI] [PubMed] [Google Scholar]
- 18.Fea AM, Belda JI, Rekas M, et al. Prospective unmasked randomized evaluation of the iStent inject (®) versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014;8:875–882. doi: 10.2147/OPTH.S59932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengerer FH, Auffarth GU, Riffel C, et al. Second-generation trabecular micro-bypass stents as standalone treatment for glaucoma: a 36-month prospective study. Adv Ther. 2019;36(7):1606–1617. doi: 10.1007/s12325-019-00984-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voskanyan L, Garcia-Feijoo J, Belda JI, et al. Prospective, unmasked evaluation of the iStent® inject system for open-angle glaucoma: synergy trial. Adv Ther. 2014;31(2):189–201. doi: 10.1007/s12325-014-0095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berdahl J, Voskanyan L, Myers JS, et al. Implantation of two second-generation trabecular micro-bypass stents and topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 18-month follow-up. Clin Exp Ophthalmol. 2017;45(8):797–802. doi: 10.1111/ceo.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindstrom R, Lewis R, Hornbeak DM, et al. Outcomes following implantation of two second-generation trabecular micro-bypass stents in patients with open-angle glaucoma on one medication: 18-month follow-up. Adv Ther. 2016;33(11):2082–2090. doi: 10.1007/s12325-016-0420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macher T, Haberle H, Wachter J, et al. Trabecular microbypass stents as minimally invasive approach after conventional glaucoma filtration surgery. J Cataract Refract Surg. 2018;44(1):50–55. doi: 10.1016/j.jcrs.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 24.Davids AM, Pahlitzsch M, Boeker A, et al. iStent inject as a reasonable alternative procedure following failed trabeculectomy? Eur J Ophthalmol. 2018;28(6):735–740. doi: 10.1177/1120672117747010. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed IIK, Fea A, Au L, et al. A prospective randomized trial comparing hydrus and iStent microinvasive glaucoma surgery implants for standalone treatment of open-angle glaucoma: the COMPARE study. Ophthalmology. 2020;127(1):52–61. doi: 10.1016/j.ophtha.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 26.Lawlor M, Nguyen V, Brooks A, et al. Efficient capture of high-quality real-world data on treatments for glaucoma: the Fight Glaucoma Blindness! Registry. BMJ Open Ophthalmol. 2021;6(1):e000903. doi: 10.1136/bmjophth-2021-000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaarawy T, Sherwood M, Grehn F. Guidelines on Design and Reporting of Glaucoma Surgical Trials. Kugler Publications; 2009. [Google Scholar]
- 28.Team RC. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2018. [Google Scholar]
- 29.Therneau TM. https://CRAN.R-project.org/package=survival A package for survival analysis in R. 2020 [cited 2024 Sep 14]. Available from:
- 30.Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the ocular hypertension treatment study. Ophthalmology. 2012;119(9):1826–1831. doi: 10.1016/j.ophtha.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bloom P, Au L. “Minimally invasive glaucoma surgery (MIGS) is a poor substitute for trabeculectomy”-the great debate. Ophthalmol Ther. 2018;7(2):203–210. doi: 10.1007/s40123-018-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan Gaskin JC, Sandhu SS, Walland MJ. Victorian trabeculectomy audit. Clin Exp Ophthalmol. 2017;45(7):695–700. doi: 10.1111/ceo.12948. [DOI] [PubMed] [Google Scholar]
- 33.Kirwan JF, Lockwood AJ, Shah P, et al. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology. 2013;120(12):2532–2539. doi: 10.1016/j.ophtha.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 34.Edmunds B, Thompson JR, Salmon JF, et al. The National Survey of Trabeculectomy. II. Variations in operative technique and outcome. Eye (Lond) 2001;15(Pt 4):441–448. doi: 10.1038/eye.2001.152. [DOI] [PubMed] [Google Scholar]
- 35.Wong MO, Lee JW, Choy BN, et al. Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in open-angle glaucoma. Surv Ophthalmol. 2015;60(1):36–50. doi: 10.1016/j.survophthal.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Schlote T, Kynigopoulos M. Selective laser trabeculoplasty (SLT): 1-year results in early and advanced open angle glaucoma. Int Ophthalmol. 2016;36(1):55–61. doi: 10.1007/s10792-015-0079-1. [DOI] [PubMed] [Google Scholar]
- 37.Miraftabi A, Nilforushan N, Nassiri N, et al. Selective laser trabeculoplasty in patients with pseudoexfoliative glaucoma vs primary open angle glaucoma: a one-year comparative study. Int J Ophthalmol. 2016;9(3):406–410. doi: 10.18240/ijo.2016.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berdahl JP, Khatana AK, Katz LJ, et al. Cost-comparison of two trabecular micro-bypass stents versus selective laser trabeculoplasty or medications only for intraocular pressure control for patients with open-angle glaucoma. J Med Econ. 2017;20(7):760–766. doi: 10.1080/13696998.2017.1327439. [DOI] [PubMed] [Google Scholar]
- 39.Ontario H. iStent for adults with glaucoma: a health technology assessment. Ont Health Technol Assess Ser. 2021;21(10):1–42. 34422143 [PMC free article] [PubMed] [Google Scholar]