Carotid artery stenting (CAS) has been established as an effective and safe procedural treatment for carotid stenosis, with similar rates of efficacy compared to carotid endarterectomy.1 However, CAS confers a higher risk of periprocedural stroke.1 To mitigate this risk, along with adequate patient selection, embolic protection devices (EPD) were introduced, reducing periprocedural stroke risk by ∼ 40% in observational studies.2 Despite guideline recommendations for the use of EPD,3 CAS without EPD still occurs.4 However, variability and factors associated with these practices have yet to be explored. We aim to examine the variability across operators and centers and factors associated with CAS without EPD.
We included patients aged ≥18 years who underwent CAS between 2015 to 2019 from the Vascular Quality Initiative registry. Patients were excluded if: (1) procedure was performed at center with high missing rates (defined as third quartile + 1.5 × interquartile); (2) missing identifier for providers or center; (3) procedure was unable to proceed as planned; (4) undergoing CAS as part of intracranial treatment; (5) missing data for EPD use; (6) any of the covariates missing among those included in modeling, and (7) undergoing CAS with transcarotid artery revascularization, as EPD is intrinsic of the procedure. Approval for the study was granted by the Vascular Quality Initiative Research Advisory Committee and the Yale Institutional Review Board.
No EPD use by the operator and center was calculated as the number of CAS without EPD used divided by total CAS procedures. The volume of CAS procedures was assessed by dividing into quartiles operators and centers. A multilevel mixed-effects logistic regression adjusted for demographics, medical comorbidities, preoperative evaluation, and lesion characteristics was performed and the median odds ratio (MOR) was calculated. The MOR measures the median odds of not using EPD during CAS when comparing patients with similar traits treated by randomly chosen operators and centers. It reflects the probability of not using EPD associated with unmeasured operator and center characteristics. We included 3 multilevel logistic regression models, each varying by the specific random effect applied: one model included a random effect for the operator, another included a random effect for the center, and a third model included 2 random effects for the operator nested within the center. In a separate analysis, the model with the operator nested within the center as a random effect was replicated to explore the association of the volume of operators and centers and CAS without EPD. All analyses were performed on 8 imputed data sets by chained equations and results were pooled using Rubin’s rule.5 All tests were 2-tailed, with a significance level set at a P value <.05.
A total of 19,476 patients, 1581 providers, and 414 centers were included (Supplemental Table S1). EPD were used in 88.7% of CAS procedures. Patients in whom CAS without EPD was used were more likely to be younger (66.0 ± 13.0 vs 70.1 ± 9.7 years; standard difference [SDiff], –0.360), female (41.9% vs 35.5%; SDiff, 0.132), non-White (86.7% vs 89.3%; SDiff, 0.132), not insured by Medicare or Medicaid (45.0% vs 36.7%; SDiff, 0.206), have fewer medical comorbidities, and undergoing urgent or emergent procedures (39.6% vs 22.4%; SDiff, 0.38). Patients in the CAS without EPD group were also less likely to have atherosclerotic (67.6% vs 80.3%; SDiff, 0.306) and restenotic (11.7% vs 17.3%; SDiff, 0.158) lesions, while dissection and trauma/fibromuscular dysplasia/other etiologies were more common (12.1% vs 1.1%; SDiff, 0.453 and 10.2% vs 1.5%; SDiff, 0.380, respectively). All differences were statistically significant (P < .001).
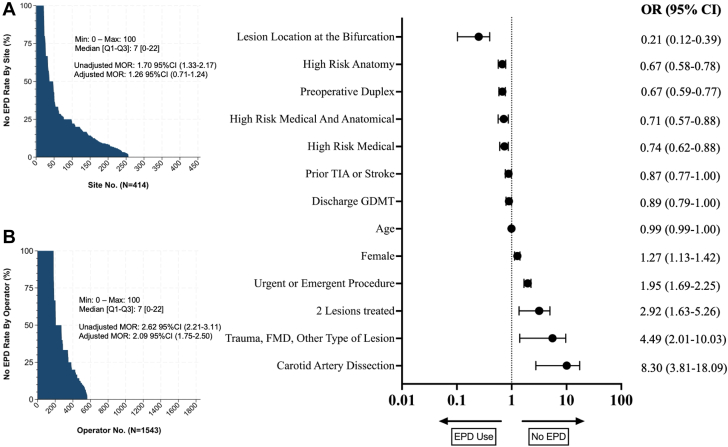
The variability for centers and operators was minimal (Figure 1). Adjusted MOR for operator and center were 2.09 (95% CI, 1.75-2.50) and 1.25 (95% CI, 0.92-1.72), respectively. The MOR in the model including the operator nested within the center as random effect were 1.25 (95% CI, 0.91-1.72) for the center and 0.94 (95% CI, 0.71-1.24) for the operator nested within the center (Supplemental Table S2). Factors significantly associated without EPD used are shown in Figure 1. When assessing operator volume (using the fourth quartile as a reference of high-volume operators), procedures by operators in the third, second, and first quartile were associated without EPD used, with an OR of 1.27 (95% CI, 1.02-1.59; P = .035), 2.08 (95% CI, 1.60-2.72; P < .001), and 3.86 (95% CI, 2.79-5.33; P < .001), respectively (Supplemental Table S3). Similarly, when assessing center volume quartiles in a separate model and using the fourth quartile as reference (high-volume centers), the first quartile was significantly associated with CAS without EPD used, with an OR of 2.50 (95% CI, 1.48-4.21; P = .001) (Supplemental Table S4).
Figure 1.
Variability of carotid artery stenting (CAS) without the use of embolic protection devices (EPD) by operator (A). (B) Shows factors significantly associated with EPD use using the multilevel mixed-effects logistic regression with operator nested within the center as random effects. The model was adjusted for age, race, ethnicity, primary insurer, living at home, body mass index, hypertension, diabetes, smoking, chronic obstructive pulmonary disease, coronary artery disease, previous stroke or TIA, prior carotid endarterectomy or stenting, GDMT, high-risk carotid endarterectomy, preoperative duplex, preoperative CT or MRI, preoperative angiography, the urgency of the procedure, number of lesions, atherosclerotic lesion, restenotic lesion, dissection, other types of lesions, side of lesion, lesion at the bifurcation, lesion at the common carotid artery, and year of surgery. FMD, fibromuscular dysplasia; GDMT, guideline-directed medical therapy; mOR, median odds ratio; Q1-Q3; interquartile range; TIA, transient ischemic attack.
CAS without EPD is still performed in modern practice,4 and in this observational data set, 1 in 10 patients underwent CAS without having an EPD attempted. In addition, CAS without EPD was associated with younger, female, and healthier patients, as well as with urgent or emergent scenarios and lower-volume centers and operators. All precautions should be taken to mitigate the risk of periprocedural stroke. Because a clinical trial for EPD seems unlikely, it is necessary to adhere to current guidelines and available data. Moreover, with the recent Medicare expansion for CAS, routine use of EPD in CAS should be a priority. Future work to understand potential explanations as to why certain subpopulations may be at risk of not receiving these devices should be integrated into national quality improvement efforts targeted at improving the quality of overall CAS care.
Acknowledgments
Declaration of competing interest
Carlos Mena-Hurtado reports unrestricted research grants from Philips and Shockwave Medical and is a consultant for Abbott Vascular, Cook, and Optum Labs. Kim G. Smolderen reports unrestricted research grants from Philips, Merck, Shockwave Medical, and Johnson & Johnson; she is a consultant for Optum Labs, Cook, Tegus, Twill Inc, and Abbott Vascular. All other authors report no conflicts of interest.
Funding sources
This work was not supported by funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement and patient consent
The Vascular Quality Initiative (VQI) was used for the analysis, which is a deidentified national database. Approval for the study was granted by the VQI Research Advisory Committee and the Yale Institutional Review Board.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2024.102170.
Supplementary material
References
- 1.Brott T.G., Hobson R.W., 2nd, Howard G., et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363(1):11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg N., Karagiorgos N., Pisimisis G.T., et al. Cerebral protection devices reduce periprocedural strokes during carotid angioplasty and stenting: a systematic review of the current literature. J Endovasc Ther. 2009;16(4):412–427. doi: 10.1583/09-2713.1. [DOI] [PubMed] [Google Scholar]
- 3.Naylor R., Rantner B., Ancetti S., et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur J Vasc Endovasc Surg. 2023;65(1):7–111. doi: 10.1016/j.ejvs.2022.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Wang S.X., Marcaccio C.L., Patel P.B., et al. Distal embolic protection use during transfemoral carotid artery stenting is associated with improved in-hospital outcomes. J Vasc Surg. 2023;77(6):1710–1719.e6. doi: 10.1016/j.jvs.2023.01.210. [DOI] [PubMed] [Google Scholar]
- 5.Rubin D.B. John Wiley & Sons, Inc.; 2004. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.