Abstract
Background
Concern about fetus radiation dose and assumed health effects remains a barrier for women considering a career in invasive cardiology. However, there is a lack of real-world fetus exposure data that can be used to support career decisions. The purpose of this work was to measure radiation exposure to invasive cardiologists which would contribute to dose to the fetus during pregnancy.
Methods
Radiation exposure to 42 female and male interventional cardiologists and electrophysiologists was monitored during their clinical routine. Multivariate analysis was used to assess the influences of patient radiation exposure, radioprotective garment material thickness (0.25, 0.35, 0.5 mm Pb equivalent), and cardiologist sex, height, and clinical role on occupational radiation exposure to the abdomen.
Results
Exposure to the abdomen of invasive cardiologists increased proportional to patient exposure and decreased predictably with increasing radioprotective material thickness. The median abdomen exposure when covered with a 0.5 mm Pb equivalent radioprotective material over a 40-week period was 0.22 mGy (95th percentile, 0.8 mGy). Physician sex, height, and clinical role did not influence occupational exposure.
Conclusions
The use of a 0.5 mm Pb equivalent radioprotective garment covering the abdomen, combined with appropriate radiation safety practices, can ensure that fetus radiation dose is below both US and international limits. Assumed fetus risk due to very low occupational radiation exposure is likely inconsequential in light of other known pregnancy risks.
Keywords: fetus radiation exposure, occupational health, pregnancy, radiation safety
Introduction
X-ray fluoroscopy and angiography are used to diagnose and guide treatment of a wide variety of cardiac diseases in both adult and pediatric patients. These x-ray–guided procedures contribute substantially to patient well-being and quality of life. The use of x-ray imaging also results in radiation doses to the patient and staff. For patients, the very low risk of adverse health effects due to radiation is justified by the medical benefit of the procedure. Physicians and staff performing procedures are subject to occupational exposure resulting from x-rays which scatter from within the x-ray tube assembly and the patient and are redirected toward personnel.1,2 For pregnant cardiologists, scatter exposure also contributes to fetus radiation dose.
Throughout, the term “exposure” (units mGy) will be used to describe radiation intensity outside of the body. The term “ dose” (units mSv) will be used to describe radiation burden to humans, including the fetus. In the US, the effective dose equivalent of x-ray workers is regulated such that no worker may receive an annual effective dose (E) >50 mSv (5000 mrem).3 In countries that have adopted International Commission on Radiological Protection (ICRP) recommendations, E is limited to 20 mSv annually.4 For interventional cardiologists and electrophysiologists, appropriate radiation safety practices, including utilization of low patient radiation dose rate protocols, limiting x-ray on-time, using accessory protective shields, and requisite use of radioprotective garments all mitigate against occupational radiation exposure and dose.5, 6, 7
Both the US National Council on Radiation Protection and Measurements (NCRP) and the ICRP recommend maximum permissible radiation dose to the fetus of pregnant workers. In the US, the fetus dose is limited to 0.5 mSv (50 mrem) per month, thus ensuring <5 mSv per term. In ICRP countries, fetus dose is limited to 1 mSv per term. These fetus dose limits were set to be conservatively low to minimize the risk of adverse effects known to occur following very high (>100 mSv) fetus dose levels.3,4,8 In the US, the natural risks of early pregnancy loss (10%),9 congenital anomalies (3%),10 and childhood cancer (0.02%)11 affect approximately 13% of known pregnancies or childhoods. By comparison, there is consensus in the literature that combined incremental risk of birth defects and cancer to an occupationally exposed fetus is extremely low, <0.01% per mSv.12, 13, 14, 15
Although multiple reports and publications conclude that the radiation risk to the fetus of an invasive cardiologist is extremely low, concern about radiation exposure during pregnancy remains one of the barriers to women considering a career in invasive cardiology and other specialties that use x-ray fluoroscopy.7,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 However, there is a general lack of real-world data evaluating exposure and potential for fetus dose which female trainees can use to support career decisions.13,16,18,29, 30, 31, 32, 33 In routine practice, fetus dose monitoring is accomplished by wearing a dedicated radiation monitor at the waist level and under the protective garment. These fetal radiation monitors are exchanged monthly from the time of pregnancy declaration until delivery. Due to the shielded location and short wearing duration, fetus exposure monitors frequently do not realize exposure values above the minimum sensitivity of the dosimeter. Given the relative infrequency of pregnancy among invasive cardiologists and imprecision of fetus monitors, the lack of relevant data is unlikely to be resolved without dedicated effort.
The primary purpose of this work was to measure scatter radiation exposure which transmits through the protective garment material of invasive cardiologists, and which would contribute to fetus dose during pregnancy. Secondary motivations included assessment of the influence of radioprotective garment thickness, cardiovascular specialty, clinical role, and physician sex and height on abdominal radiation exposure.
Methods
Routine occupational exposure monitoring demonstrated that exposure to cardiologists was greater than that to nurses or cardiovascular technologists participating in invasive cardiology procedures. Therefore, all female and male cardiologists working as staff consultants or advanced fellows in the cardiac catheterization (Cath) and heart rhythm services (HRS) practices at Mayo Clinic (Rochester, Minnesota) were invited to participate in this IRB-approved study. Participants from Cath included cardiologists who specialize in both adult and pediatric disease and those from HRS performed adult cardiac ablation and pacemaker implant procedures. Enrollee sex and height were documented. Participants were not asked whether they were pregnant; however, they were made aware of the primary purpose of this study. Enrollees participated in this study during their routine patient care responsibilities and were not subject to radiation exposure other than that received during their clinical routine.
Physician enrollees were not requested to modify clinical administration of x-ray radiation to patients or modify their occupational radiation protection habits. As part of routine practice, patient radiation exposure rates were established through collaboration between cardiologists, a medical physicist, and x-ray equipment representatives. Fourteen x-ray fluoroscopy systems from 2 vendors were used to treat patients. Patient procedure-specific radiation exposure metrics were stored in the patient medical record and population statistical summaries were reviewed monthly as part of our radiation safety continuous quality improvement initiative.
Mitigation of occupational exposure was provided by table-mounted lower body shields (which were routinely attached to the patient table), ceiling-mounted upper body shields, and radioprotective garments.34 The upper body shields were available in most procedure rooms (excluding pacemaker implant labs) and used routinely (but at the discretion of the cardiologist). In our practice, most procedures are performed by a team, including a supervising staff cardiologist and a fellow. A minority of cases are performed by a staff cardiologist and scrub technologist. The use of x-ray imaging to guide procedures is directly controlled by the cardiologist or fellow nearest the head end of the patient table.
Many US states require a minimum 0.25 mm Pb radioprotective garment and a minority of states (including ours) require a thicker 0.5 mm Pb garment to be worn while participating in fluoroscopically guided procedures. In our practice, occupational radiation exposure is routinely monitored by a single occupational exposure badge worn at the left collar and outside of the radioprotective garment. Patient and occupational dose reports are provided to the cardiologists annually as part of our radiation safety continuous quality improvement initiative.
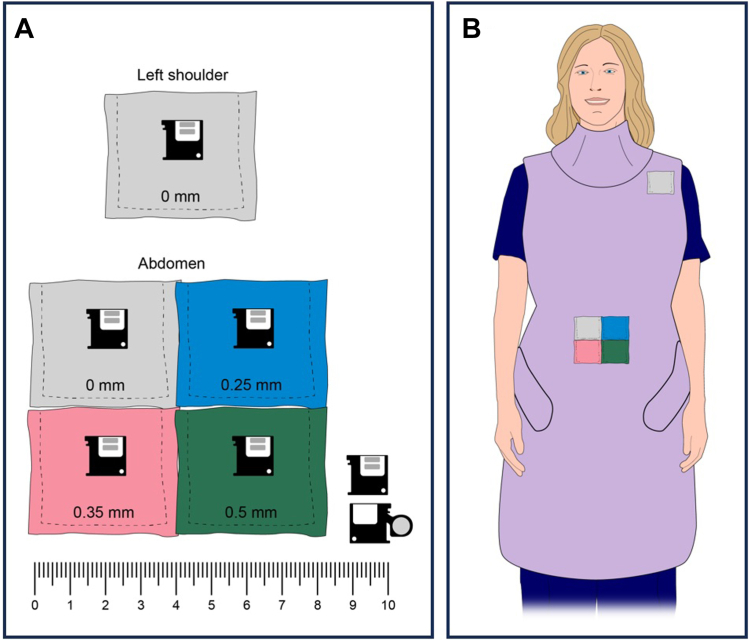
For this study, 5 small exposure monitors (nanoDot, Landauer Inc) (Figure 1, lower right) were fixed to the exterior of the physician’s radioprotective garment.35 One of these was positioned at the left shoulder and was intended to mimic the exposure to the occupational exposure monitoring badge worn at the left collar. The exposure monitor at the shoulder was encapsulated within a 4 × 4 cm2 pocket composed of only common fabric. The other 4 exposure monitors were placed inside 4 × 4 cm2 pockets made from fabric (1) or radioprotective material (3). These pockets were then secured to the outside of the standard 0.5 mm Pb equivalent radioprotective garment using tape. The pockets with monitors were positioned along the midline of the abdomen, at the approximate location of the navel. The outward-facing surface of the pockets was covered with 1 of 4 different materials including fabric only (0 mm) or 0.25, 0.35, and 0.5 mm Pb equivalent radioprotective material (Figure 1). These material thicknesses were studied because they represent the range of minimum garment thickness required by various states and are commonly available from manufacturers. Two types of lead-free radioprotective materials were used (Xenolite, Burlington Medical; and KIARMOR, Infab).
Figure 1.
Construction and placement of radioprotective material pockets and exposure monitors. (A) Pockets in which the exposure monitors were placed were made of fabric only (0 mm) or 0.25, 0.35, and 0.5 mm Pb-equivalent radioprotective material. Lower-right: exposure monitor, including extended arm to show the exposure absorbing element. (B) The pockets were secured to the outside of the radioprotective garment at the shoulder (1) and the abdomen (4). Note that the illustration does not include the radioprotective glasses which were routinely worn.
The exposure monitors were fixed to the protective garments and were exposed to radiation during routine clinical work for a nominal 6 month period. At the end of that period, the 5 monitors were recovered, and their accumulated exposure (mGy) was read using a microSTAR ii medical dosimetry system (Landauer Inc). The readout was calibrated using controlled exposure conditions which mimicked occupational radiation exposure conditions. Exposure measurements were extrapolated to a nominal 40-week gestation period for reporting herein.
In our practice, patient air-kerma area product (KAP, units Gy ⋅ cm2) values are electronically transferred from the x-ray systems and stored in a database. Patient KAP is influenced by several factors including patient size, procedure complexity, x-ray field size, and frame rate. For each study participant, the sum of the patient KAP administered over the study period was calculated and treated as an independent variable.
Discrete variables were presented as frequency (percentage). Continuous variables were presented as median (IQR) unless otherwise indicated and tested using the Kruskal-Wallis rank sum test. All P values were 2-sided and values of 0.05 or less were considered significant. Analysis was conducted using R version 4.2.2 (R Foundation for Statistical Computing). A multivariable mixed linear model was used to assess the influence of protective material thickness and manufacturer on exposure transmitting through the material. Physicians were treated as a random effect with an unstructured variance-covariance structure. The analysis also considered the influence of patient KAP, exposure monitor location (abdomen vs shoulder), physician role (fellow vs supervising cardiologist), clinical specialty (Cath vs HRS), sex, and physician height on exposure.
Both cumulative patient KAP and exposure were log10 transformed to mitigate data distribution skewness before applying the multivariable model. After model coefficients were calculated, they were back-transformed by exponentiating 10 to the power of the estimate prior to reporting herein. In this manner, the parameter estimates reported describe the multiplicative change in exposure attributed to each variable.
Results
Of the 45 cardiologists enrolled in this study, 3 were lost to follow-up due to loss of the abdomen exposure monitors. Of the 42 remaining participants, 27 (64) were from Cath practice and 15 (36%) from HRS; 11 (26%) were female and 31 (74%) were male; 32 (76%) were supervising staff cardiologists and 10 (24%) were advanced fellows (Table 1). The median height of female participants was 15 cm less than that of males (P ≤ .001).
Table 1.
Summary of participants who completed the study.
| Characteristic | N = 42 |
|---|---|
| Role | |
| Staff | 32 (76) |
| Fellow | 10 (24) |
| Sex | |
| Female | 11 (26) |
| Male | 31 (74) |
| Specialty | |
| Heart rhythm services | 15 (36) |
| Cardiac catheterization | 27 (64) |
| Height, cma | |
| Female | 165.1 (161.3-170.2) |
| Male | 180.3 (171.5-184.2) |
| Accrual period, d | 181.5 (178.8-183.0) |
Values are n (%) or median (IQR).
P < .001
Participant-specific results are included in the Supplementary Appendix. The median 40-week number of procedures performed was 149 (90% CI, 61-406) and the median 40-week accumulated patient KAP was 4911 Gy ⋅ cm2 (90% CI, 943-17,494). A statistical summary of 40-week exposure values at the left shoulder and the abdomen is provided in Table 2.
Table 2.
Summary of 40-week exposure values at the left shoulder and abdomen.
| Pb equivalent radioprotective material thickness, mm | Exposure, mGy |
|---|---|
| Left shoulder | |
| 0 | 2.44 (0.38-9.93) |
| Abdomen | |
| 0 | 5.15 (0.79-23.21) |
| 0.25 | 0.53 (0.07-2.00) |
| 0.35 | 0.35 (0.04-1.25) |
| 0.5 | 0.22 (0.05-0.83) |
Values are median (90% CI).
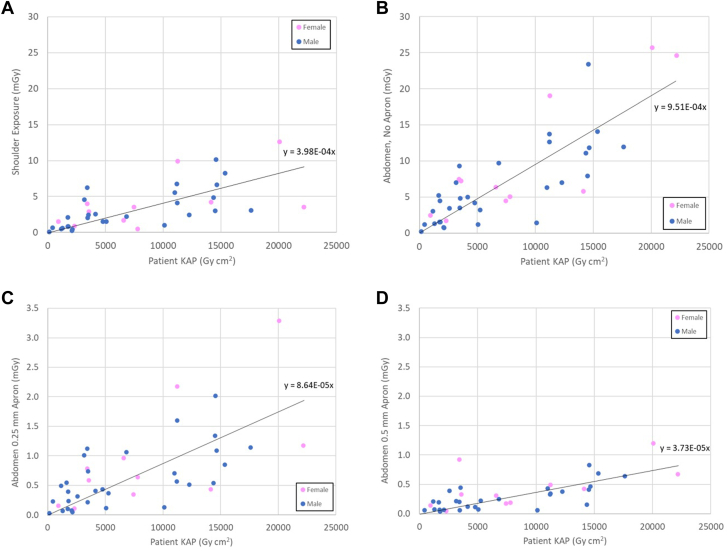
Individual participant 40-week exposure values (mGy), plotted versus the 40-week accumulated patient KAP (Gy ⋅ cm2) of procedures for which the physician participated, are shown in Figure 2. Exposure measurements from the shoulder and for 3 of the 4 exposure monitors at the abdomen are shown in Figure 2 (data for the 0.35 mm protective material not displayed for brevity). Data were plotted separately for female versus male participants. The fit lines in Figure 2 highlight the anticipated linear relationship between measured exposure and cumulative patient KAP. As demonstrated in Table 2 and Figure 2, there was substantial variability of measured exposure values, which will be further described in the Discussion.
Figure 2.
Measured exposure values versus physician-specific patient air-kerma area product (KAP) administered over the study period. All data extrapolated to a 40-week term. (A) Uncovered at left shoulder; (B) uncovered at abdomen; (C) 0.25 mm Pb equivalent material at abdomen; (D) 0.5 mm Pb equivalent material at abdomen.
Results of the multivariable analysis are shown in Table 3, with a value of 1.0 indicating a null result. The parameter estimates in Table 3 should be interpreted as multiplicative coefficients, with exposure at the left shoulder as the reference condition. That exposure at the abdomen (0 mm) was 2.21× greater than at the shoulder because the abdomen is closer to the x-ray tube and patient. That the model fit parameter estimates decrease with increasing material thickness is the expected result. The fraction of radiation exposure that transmits through the radioprotective material can be estimated by dividing the material-specific estimate by the 0 mm estimate. That there was a difference between the protective materials from the 2 manufacturers may be representative of lack of standards in protective garment manufacturing. Although there was a difference between protective materials from the 2 vendors when all 3 material thicknesses were considered, there was no difference between the 2 products for material thickness 0.5 mm Pb equivalent (P > .999). Finally, the following variates did not significantly influence occupational exposure: role, consultant vs fellow; clinical specialty, HRS vs Cath; sex, female vs male; and physician height.
Table 3.
Influence of studied variates on exposure measured at the abdomen, with shoulder exposure as the reference. The parameter estimates represent multiplicative effects.
| Parameter estimate | 95% CI | Pr (>|t|) | |
|---|---|---|---|
| Effect of abdomen and Pb equivalent material thickness vs shoulder | |||
| 0 mm | 2.21 | 1.85-2.63 | P < .001 |
| 0.25 mm | 0.20 | 0.17-0.24 | P < .001 |
| 0.35 mm | 0.13 | 0.11-0.16 | P < .001 |
| 0.5 mm | 0.09 | 0.08-0.11 | P < .001 |
| Material, Xenolite vs KIARMOR | |||
| All thicknesses | 1.69 | 1.15-2.50 | P = .01 |
| 0.5 mma | 0.88 | 0.49-1.58 | P > .999 |
| Role, fellow vs consultant | 1.34 | 0.79-2.27 | P = .28 |
| Clinical specialty, HRS vs Cath | 0.92 | 0.59-1.43 | P = .71 |
| Physician sex, female vs male | 1.16 | 0.68-2.00 | P = .57 |
| Physician height, per 10 cm | 0.90 | 0.72-1.13 | P = .36 |
Univariate test.
Discussion
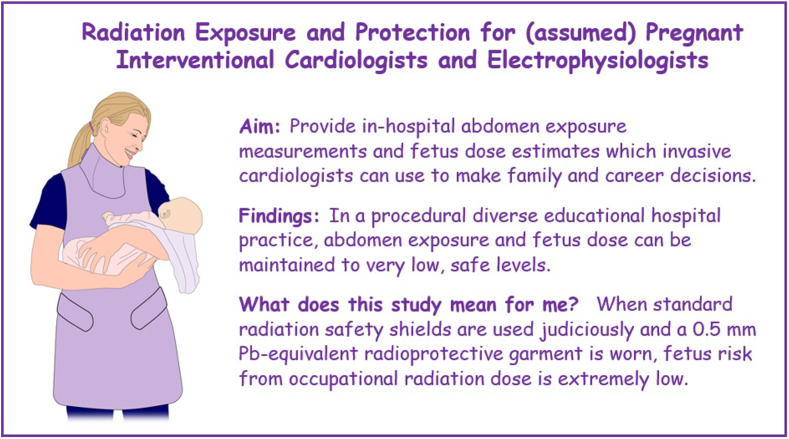
The major finding of this human experimental model study was that abdomen radiation exposure, as a surrogate for fetal radiation exposure, can be effectively managed using standard radiation safety measures (Central Illustration). That the study had 42 participants including females and males, fellows and supervising cardiologists, and interventional and electrophysiology specialists helps ensure broad applicability of the findings.
Central Illustration.
This study of 42 cardiac catheterization and heart rhythm services cardiologists demonstrated that fetus dose of cardiologists performing invasive procedures can be maintained at very low levels.
Other reports have suggested that a still conservatively high fetus dose can be estimated by dividing the exposure (mGy) at the abdomen by 2 and assigning human effective dose units (mSv)7,12,36,37If such a method is applied to the data presented herein, then typical fetus dose when covered with a 0.25 mm radioprotective garment is estimated to be 0.26 mSv (95th % 1.0 mSv) and that when covered with a 0.5 mm garment is estimated to be 0.11 mSv (95th % 0.42 mSv). These fetus dose estimates compare favorably with both NCRP and ICRP limits of 5 mSv and 1 mSv, respectively (Table 4). Importantly, these median dose estimates are lower than the 40-week dose expected from US natural sources (0.71-1.43 mSv), to which the fetus would also be exposed. Finally, they are also lower than the difference between living on the US Colorado Plateau versus along the Gulf or Atlantic Coasts (difference = 0.72 mSv).38
Table 4.
Radiation dose reference levels.
| Radiation dose (mSv) | |
|---|---|
| US NCRP occupational dose limit, annuala | 50 |
| US NCRP fetus dose limit, term, month | 5 (0.5) |
| ICRP adult occupational dose limit, annuala | 20 |
| ICRP fetus dose limit, term | 1 |
| US natural sources, annual (40 week), Gulf, Atlantic Coastsb | 0.92 (0.71) |
| US natural sources, annual (40 week), Colorado Plateaub | 1.86 (1.43) |
| Airplane flight (1000 miles)b | 0.01 |
Adult whole body effective dose equivalent.
US EPA (epa.gov/radiation/calculate-your-radiation-dose), includes terrestrial, cosmic, and internal radiation sources, and does not include lung dose contribution from radon gas.
As shown herein, occupational exposure is proportional to patient KAP. Our practice has made patient and staff radiation safety part of our culture. We work actively with our x-ray system providers to ensure an appropriate balance between image quality and patient radiation exposure rate. Because x-ray scatter is directly proportional to patient KAP, efforts to manage patient exposure inherently aid in the management of occupational exposure. Further, judicious use of accessory shields mitigates against occupational exposure, including at the abdomen.34
Although this was a single-center study, we are hopeful that the findings herein can be of use to others. To this end, individual occupational exposure monitoring values (the uncorrected exposure at the left collar) can be multiplied by the parameter estimates in Table 3 to estimate exposure to the abdomen, when covered with the selected radioprotective material thickness. Although this method is appropriate for estimating abdominal exposure, it should not be used in place of a dedicated fetus dose monitoring badge during pregnancy.
Multivariate analysis did not demonstrate that role, consultant vs fellow; clinical specialty, HRS vs Cath; sex, female vs male; or physician height affected exposure (Table 3). Any potential effect that these factors may have had was small in comparison to variability of the measured exposure data. Intercardiologist exposure values were highly variable, even after accounting for cumulative patient KAP (Table 2, Figure 2). This variability is likely due to a combination of factors including variable use of accessory shields and cardiologist position in the procedure room. The lower body shield was routinely attached to the patient table. Although the upper body shield was used for most procedures, for others it was unavailable (HRS, pacemaker implants) or impeded patient access (Cath, pediatric procedures) and not used ad hoc. Further, cardiologists’ proximity to and orientation with respect to the x-ray tube and patient scatter sources was variable, including whether the participant was positioned toward the head of the table and performing or toward the foot of the table and assisting the procedures. These factors represent typical variations in practice and were not controlled in this study.
The primary purpose of this work was to measure radiation exposure which could contribute to fetus radiation dose. A limitation of this work is that at least most of the study participants were unlikely to be pregnant during the study period. However, we also suppose that the radiation safety habits of a pregnant cardiologist would be at least as good as those of their nonpregnant colleagues. In this regard, the exposure data reported herein are unlikely to underestimate exposure to a pregnant cardiologist in our practice.
As previously noted, one of the motivations of this work was to acquire abdomen radiation exposure measurements under real-world conditions relevant to a pregnant cardiologist. That real data for pregnant cardiologists is scant is due at least in part to standard methods to monitor fetus exposure. In particular, typical monthly fetus exposure monitoring devices have a minimum threshold of 0.1 mGy. That is for real-world exposures below 0.1 mGy, no exposure value is reported. Dividing the 40-week exposure values reported herein by 10 provides an estimate of the exposure to a monthly fetus exposure monitoring badge. It is straightforward to predict that most such monthly estimates would be below the 0.1 mGy threshold of the monitoring badge and that a “below threshold” result would be reported. The positive interpretation of such nonresult is that radiation exposure was <0.1 mGy. A limitation of such approach is that the lack of data provides opportunity for uncertainty and concern.
Conclusion
This work demonstrates that abdomen exposure and fetus radiation dose to a pregnant invasive cardiologist can be maintained to very low levels provided that appropriate radiation safety practices are followed. Accessory shields should be used routinely, a 0.5 mm Pb equivalent radioprotective garment should be worn, and abdominal exposure when covered with the garment should be monitored. For 42 study participants including staff consultants and fellows and, women and men, typical 40-week abdomen exposure when covered with a 0.5 mm Pb equivalent radioprotective material was 0.22 mGy (90% CI, 0.05-0.83). A pregnant invasive cardiologist who maintains good radiation safety practices can take assurance that occupational radiation dose is unlikely to affect her fetus.
Declaration of competing interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding sources
This work was supported by Ischemic Heart Disease Research Program, Cardiovascular Diseases, Mayo Clinic, Rochester, MN.
Ethics statement and patient consent
This study was approved by and conducted in accordance with guidelines of the Mayo Clinic Institutional Review Board.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at https://doi.org/10.1016/j.jscai.2024.102239.
Supplementary material
References
- 1.Gutierrez-Barrios A., Cañadas-Pruaño D., Noval-Morillas I., Gheorghe L., Zayas-Rueda R., Calle-Perez G. Radiation protection for the interventional cardiologist: practical approach and innovations. World J Cardiol. 2022;14(1):1–12. doi: 10.4330/wjc.v14.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao A.T., Miller D., Hindal M., Fetterly K.A. Technical Note: Assessment of scatter originating from the x-ray tube collimator assembly of modern angiography systems. Med Phys. 2019;46(10):4371–4380. doi: 10.1002/mp.13720. [DOI] [PubMed] [Google Scholar]
- 3.National Council on Radiation Protection and Measurements Report No. 116 – Limitation of exposure to ionizing radiation. 1993. https://ncrponline.org/shop/reports/report-no-116-limitation-of-exposure-to-ionizing-radiation-supersedes-ncrp-report-no-91-1993/
- 4.ICRP The 2007 recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP. 2007;37(2-4) doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Writing Committee Members. Hirshfeld JW Jr, Ferrari V.A., et al. 2018 ACC/HRS/NASCI/SCAI/SCCT expert consensus document on optimal use of ionizing radiation in cardiovascular imaging: best practices for safety and effectiveness. Catheter Cardiovasc Interv. 2018;92(2):E35–E97. doi: 10.1002/ccd.27659. [DOI] [PubMed] [Google Scholar]
- 6.Roguin A., Wu P., Cohoon T., et al. Update on radiation safety in the cath lab – moving toward a “lead-free” environment. J Soc Cardiovasc Angiogr Interv. 2023;2(4) doi: 10.1016/j.jscai.2023.101040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dauer L.T., Miller D.L., Schueler B., et al. Occupational radiation protection of pregnant or potentially pregnant workers in IR: a joint guideline of the Society of Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2015;26(2):171–181. doi: 10.1016/j.jvir.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Ikenoue T., Ikeda T., Ibara S., Otake M., Schull W.J. Effects of environmental factors on perinatal outcome: neurological development in cases of intrauterine growth retardation and school performance of children perinatally exposed to ionizing radiation. Environ Health Perspect. 1993;101(Suppl 2):53–57. doi: 10.1289/ehp.93101s253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NICHD - Eunice Kennedy Shriver National Institute of Child Health and Human Development . National Institutes of Health; 2023. Pregnancy loss (before 20 weeks of pregnancy)https://www.nichd.nih.gov/health/topics/factsheets/pregnancyloss [Google Scholar]
- 10.U.S. Centers for Disease Control and Prevention. Birth defects; 2023. Accessed July 24, 2024. https://www.cdc.gov/birth-defects/about/?CDC_AAref_Val=https://www.cdc.gov/ncbddd/birthdefects/facts.html; 2023
- 11.U.S. Department of Health and Human Services. National Institutes of Health. National Cancer Institute. Surveillance, Epidemiology, and End Results Program; 2023. Accessed July 24, 2024. https://seer.cancer.gov/statistics-network/explorer; 2023
- 12.International Commission on Radiological Protection Pregnancy and medical radiation. ICRP Publication 84. Ann ICRP. 2000;30(1) [Google Scholar]
- 13.Best P.J.M., Skelding K.A., Mehran R., et al. SCAI consensus document on occupational radiation exposure to the pregnant cardiologist and technical personnel. Catheter Cardiovasc Interv. 2011;77(2):232–241. doi: 10.1002/ccd.22877. [DOI] [PubMed] [Google Scholar]
- 14.National Council on Radiation Protection and Measurements . Report No. 174 – Preconception and prenatal radiation exposure: health effects and protective guidance. 2013. https://ncrponline.org/publications/reports/ncrp-report-174/ [Google Scholar]
- 15.Shaw P., Duncan A., Vouyouka A., Ozsvath K. Radiation exposure and pregnancy. J Vasc Surg. 2011;53(1 Suppl):28S–34S. doi: 10.1016/j.jvs.2010.05.140. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan G.L., Chieffo A., Mehilli J., et al. The occupational effects of interventional cardiology: results from the WIN for safety survey. EuroIntervention. 2012;8(6):658–663. doi: 10.4244/EIJV8I6A103. [DOI] [PubMed] [Google Scholar]
- 17.Vu C.T., Elder D.H. Pregnancy and the working interventional radiologist. Semin Intervent Radiol. 2013;30(4):403–407. doi: 10.1055/s-0033-1359735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandra V., Dorsey C., Reed A., Shaw P., Banghart D. Monitoring of fetal radiation exposure during pregnancy. Soc Vasc Surg. 2013;58(3):710–714. doi: 10.1016/j.jvs.2013.01.052. [DOI] [PubMed] [Google Scholar]
- 19.Ghatan C.E., Fassiotto M., Jacobsen J.P., Sze D.Y., Kothary N. Occupational radiation exposure during pregnancy: a survey of attitudes and practices among interventional radiologists. J Vasc Interv Radiol. 2016;27(7):1013–1020.e3. doi: 10.1016/j.jvir.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Marx M.V. Baby on board: managing occupational radiation dose during pregnancy. Tech Vasc Interv Radiol. 2018;21(1):32–36. doi: 10.1053/j.tvir.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Wah T.M., Belli A.M. The interventional radiology (IR) gender gap: a prospective online survey by the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Cardiovasc Intervent Radiol. 2018;41(8):1241–1253. doi: 10.1007/s00270-018-1967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Englander M.J., Ghatan C. Radiation and the pregnant IR: myth versus fact. Cardiovasc Intervent Radiol. 2021;44(6):877–882. doi: 10.1007/s00270-020-02704-1. [DOI] [PubMed] [Google Scholar]
- 23.Grines C., Voeltz M., Dupont A., Tukaye D. Society for Cardiovascular Angiography and Interventions Women in Innovations. A paucity of female interventional cardiologists: what are the issues and how can we increase recruitment and retention of women? J Am Heart Assoc. 2021;10(5) doi: 10.1161/JAHA.120.019431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheney A.E., Vincent L.L., McCabe J.M., Kearney K.E. Pregnancy in the cardiac catheterization laboratory. Circ Cardiovasc Interv. 2021;14(4) doi: 10.1161/CIRCINTERVENTIONS.120.009636. [DOI] [PubMed] [Google Scholar]
- 25.Oliveros E., Burgess S., Nadella N., et al. Becoming a parent during cardiovascular training. J Am Coll Cardiol. 2022;79(21):2119–2126. doi: 10.1016/j.jacc.2022.03.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adeliño R., Malaczynska-Rajpold K., Perrotta L., et al. Occupational radiation exposure of electrophysiology staff with reproductive potential and during pregnancy: an EHRA survey. Europace. 2023;25(9) doi: 10.1093/europace/euad216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manzo-Silberman S., Velàzquez M., Burgess S., et al. Radiation protection for healthcare professionals working in catheterisation laboratories during pregnancy: a statement of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) in collaboration with the European Heart Rhythm Association (EHRA), the European Association of Cardiovascular Imaging (EACVI), the ESC Regulatory Affairs Committee and Women as One. EuroIntervention. 2023;19(1):53–62. doi: 10.4244/EIJ-D-22-00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saada M., Sanchez-Jimenez E., Roguin A. Risk of ionizing radiation in pregnancy: just a myth or a real concern? Europace. 2023;25(2):270–276. doi: 10.1093/europace/euac158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meek M., Chang M., Lensing S., Deloney L. Radiation exposure in pregnant and nonpregnant female interventional radiology workers. Radiol Technol. 2016;87(5):574–578. [PubMed] [Google Scholar]
- 30.Chu B., Miodownik D., Williamson M.J., Gao Y., St Germain J., Dauer L.T. Radiological protection for pregnant women at a large academic medical cancer center. Phys Med. 2017;43:186–189. doi: 10.1016/j.ejmp.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkozy A., De Potter T., Heidbuchel H., et al. Occupational radiation exposure in the electrophysiology laboratory with a focus on personnel with reproductive potential and during pregnancy: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS) Europace. 2017;19(12):1909–1922. doi: 10.1093/europace/eux252. [DOI] [PubMed] [Google Scholar]
- 32.Velázquez M., Pombo M., Unzué L., Bastante T., Mejía E., Albarrán A. Radiation exposure to the pregnant interventional cardiologist. Does is really pose a risk to the fetus? Rev Esp Cardiol (Engl Ed) 2017;70(7):606–608. doi: 10.1016/j.rec.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 33.Chen S.H., Brunet M.C. Fetal radiation exposure risk in the pregnant neurointerventionalist. J Neurointerv Surg. 2020;12(10):1014–1017. doi: 10.1136/neurintsurg-2019-015727. [DOI] [PubMed] [Google Scholar]
- 34.Fetterly K.A., Magnuson D.J., Tannahill G.M., Hindal J.D., Mathew V. Effective use of radiation shields to mimimize operator dose during invasive cardiology procedures. JACC Cardiovasc Interv. 2011;4(10):1133–1139. doi: 10.1016/j.jcin.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 35.Al-Senan R.M., Hatab M.R. Characteristics of an OSLD in the diagnostic energy range. Med Phys. 2011;38(7):4396–4405. doi: 10.1118/1.3602456. [DOI] [PubMed] [Google Scholar]
- 36.Faulkner K., Marshall N.W. Personal monitoring of pregnant staff in diagnostic radiology. J Radiol Prot. 1993;13(4):259–265. doi: 10.1088/0952-4746/13/4/003. [DOI] [Google Scholar]
- 37.Osei E.K., Kotre C.J. Equivalent dose to the fetus from occupational exposure of pregnant staff in diagnostic radiology. Br J Radiol. 2001;74(883):629–637. doi: 10.1259/bjr.74.883.740629. [DOI] [PubMed] [Google Scholar]
- 38.United States Environmental Protection Agency. Calculate your radiation dose. 2023. Accessed November 1, 2023. https://www.epa.gov/radiation/calculateyour-radiation-dose
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.