Abstract
Background
China experienced a surge of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variants after adjusting its zero-coronavirus disease 2019 (COVID-19) policy. Although infections with Omicron variants are generally less severe than infections with previous SARS-CoV-2 variants, the clinical characteristics, persistent symptoms, and antibody responses in solid carcinoma patients (SCPs) with COVID-19 during the Omicron wave are unclear.
Methods
We conducted a cross-sectional study in April 2023, recruiting healthy controls (HCs) from the community and SCPs from Zhejiang Provincial People’s Hospital. Serum samples were collected, and a questionnaire was used to assess SARS-CoV-2 infection status, including demographic characteristics, clinical manifestations, and “long COVID” symptoms. Humoral immune responses were analyzed by enzyme-linked immunosorbent assays (ELISAs) targeting immunoglobulin G (IgG) antibodies against the receptor-binding domain (RBD; Omicron BA.4/5) protein and cell culture-based neutralization assays against Omicron variants (BA.4/5, BF.7, XBB.1.5, and EG.5).
Results
In total, 298 SCPs and 258 HCs were enrolled. Self-reported COVID-19 case rates were significantly lower in SCPs than in HCs (78.5% vs. 93.8%, P<0.001). Common COVID-19 symptoms were similar between the two groups, primarily comprising general (92.6% vs. 84.9%) and respiratory symptoms (51.9% vs. 48.2%) after acute infection. There was no significant difference in persistent symptoms at 1–3 months post-infection (P=0.353); fatigue was the most common symptom (45.0% vs. 44.8%). SCPs exhibited lower anti-RBD-IgG titers compared with HCs (1.061 vs. 1.978, P=0.001). The 50% pseudovirus neutralization titer (pVNT50) values for prevalent Omicron strains (BA.4/5 and BF.7) were lower in SCPs than in HCs (621.0 [288.8, 1333.0] vs. 894.1 [458.5, 1637.0] and 529.6 [215.3, 1264.5] vs. 463.1 [185.2, 914.0], respectively). Levels of antibodies against subsequent variants (XBB.1.5 and EG.5) also were reduced. There were no significant differences among carcinoma types in the levels of antibodies against Omicron variants. However, SCPs who received the SARS-CoV-2 vaccine or had COVID-19 during the Omicron wave displayed higher antibody levels.
Conclusions
This study elucidated the clinical and immunological characteristics of SCPs during the Omicron wave in China after the shift away from a zero-COVID-19 policy. Our findings provide insights regarding factors that influence COVID-19 symptoms and antibody levels in this population.
Keywords: COVID-19, Omicron, solid carcinoma, clinical characteristics, antibody response, long COVID
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has been a global health crisis involving widespread economic and social disruption, with over 760 million infections and 6.92 million deaths (1, 2). First detected in South Africa on November 9, 2021, the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rapidly spread worldwide due to its enhanced transmissibility and immune evasion capabilities (3). At the end of 2022, the Chinese government implemented a dynamic zero-COVID-19 strategy for COVID-19, although all Omicron variants have reduced the pathogenicity, but still a large number of patients infected with BA.5.2 and BF (4). In China, after the policy adjustments for COVID-19, there was a rapid increase in the number of individuals infected with the SARS-CoV-2 (5–7). In the meantime, China’s new ten policies (8) signify a societal reopening during the COVID-19 pandemic, aiming to mitigate its impact on the economy and society.
Among vulnerable populations, solid carcinoma patients have particularly high risks because of their compromised immune systems, which often result from the disease itself or associated treatments. The onset of COVID-19 can further exacerbate their existing conditions (9). Previous studies have shown that, compared with the general population, carcinoma patients exhibit higher risks of severe illness and mortality after contracting SARS-CoV-2 (10–13). A systematic meta-analysis of 14 studies, involving 62,000 COVID-19 patients in various countries, revealed a carcinoma incidence of 6%, significantly higher than the incidence of 0.2% observed in the general population (14). Another meta-analysis of 12,526 Chinese COVID-19 patients showed that carcinoma was a more prevalent comorbidity in those patients than in the general population (15).
Regarding symptoms after COVID-19, a study conducted in Italy revealed that common symptoms included cough, fever, dyspnea, musculoskeletal symptoms (e.g., myalgia, joint pain, and fatigue), gastrointestinal symptoms, and loss of appetite (16). In most cases, vaccinated carcinoma patients with COVID-19 experienced mild symptoms, primarily cough, sputum production, and fever (3, 17). In addition to acute symptoms, “long COVID” has received considerable attention; the reported prevalence of persistent symptoms after contracting COVID-19 ranges from 32.6% to 87% (18). Fatigue and post-exertional malaise are the most common symptoms in individuals with long COVID (19). Notably, antibody stability after contracting COVID-19 appears to be more robust than the stability induced by vaccination (20). Several studies have shown that the vast majority of carcinoma patients develop SARS-CoV-2-specific antibodies after infection, resulting in substantially elevated antibody levels (21). However, more recent findings indicate substantial immune escape for Omicron subvariants, resulting in decreased neutralizing titers associated with mutations in the spike protein (22).
Given the ongoing COVID-19 pandemic and the increased vulnerability of carcinoma patients to severe illness and mortality, it is essential to understand the clinical characteristics of these patients, as well as their immune responses to COVID-19. Specifically, there is a need to investigate differences in immune responses and clinical outcomes between solid carcinoma patients and healthy controls. Accordingly, this study analyzed clinical characteristics, immune responses, and long COVID symptoms in solid carcinoma patients, then compared the findings with data from healthy controls, to provide a comprehensive understanding of the impact of the Omicron wave on this vulnerable population.
2. Materials and methods
2.1. Study design and participants
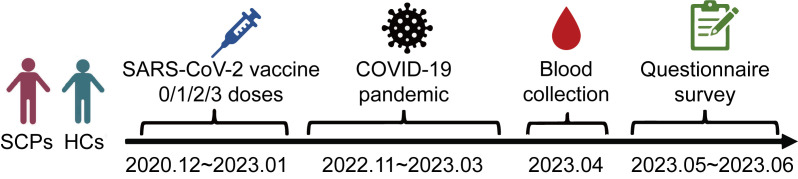
This cross-sectional study was conducted in Hangzhou, Zhejiang Province, China, from December 2022 to April 2023, after the wave of the COVID-19 pandemic within China. The study enrolled patients aged 18 and older who had been diagnosed with various types of solid carcinoma at Zhejiang Provincial People’s Hospital; the included carcinoma types were lung, digestive (colorectal, gastric, pancreatic, esophageal, and biliary tract), liver, breast, thyroid, prostate, and other (pituitary, head and neck, ovarian, bladder, renal, ureteral, acoustic neuroma, and uterine). Healthy controls undergoing health examinations at West Lake Community Health Center in Zhejiang Province were selected through sample matching. Blood samples were collected from all participants at both Zhejiang Provincial People’s Hospital and West Lake Community Health Center beginning in April 2023. Most participants had either received 1–3 doses of a COVID-19 vaccine or remained unvaccinated between December 2020 and January 2023 as part of a special population vaccination program. A telephone questionnaire survey was conducted from May to June 2023 to assess the participants’ symptoms after contracting COVID-19. Detailed demographic characteristics and relevant information were also collected from the hospital’s electronic medical record system ( Figure 1 ).
Figure 1.
Study design.
2.2. Immunological and antibody assessments
In this study, a pseudovirus assay was used to assess the neutralizing antibody potencies of serum samples against SARS-CoV-2 Omicron BA.4/5, BF.7, XBB.1.5, and EG.5 strains of pseudovirus (23). To prepare pseudoviruses, spike proteins with an 18-amino acid deletion from the C-terminus of SARS-CoV-2 PT (Wuhan-1 reference strain, GISAID: EPI_ISL_402119) and the spike protein of each Omicron subvariant were cloned into the pCAGGS vector. Codon optimization was performed to ensure plasmid compatibility with mammalian cell expression, and 30 μg of each construct were transfected into HEK-293T cells. VSV-ΔG-GFP pseudotyped virus was added 24 h after transfection. After 2 h of infection, the VSV-ΔG-GFP residues were removed by changing the medium to fresh complete DMEM medium. After 2 h of infection, the VSV-ΔG-GFP residues were removed by replacing the medium with fresh complete DMEM medium containing anti-VSV-G antibodies (I1-Hybridoma-CRL2700™, ATCC). After incubation for 30 h at 37°C, the supernatants were collected, filtered through a 0.45-μm filter (Cat#SLHP033RB, Millipore), aliquoted, and stored at -80°C. For the pseudovirus neutralization assay, serum samples were initially diluted to 1:8, then the samples were serially diluted two-fold on a 96-well plate. A SARS-CoV-2 pseudotyped virus was added and incubated with the diluted serum for 1 h at 37°C. The mixture was then added to a 96-well plate containing Vero cells. After an additional 20 h of incubation, the number of transducing units (TUs) was determined using the EVOS M7000 Automated Live Cell Fluorescence Imaging System (Thermo Fisher Scientific) in conjunction with ImageJ software. The median serum dilution that neutralized 50% of the virus (pVNT50) was calculated by nonlinear regression using GraphPad Prism software. The geometric mean titers (GMTs) of SARS-CoV-2 Omicron variant results are presented as pVNT50 titers.
Serum IgG antibodies (from convalescent serum samples) that bind to the SARS-CoV-2 receptor-binding domain (RBD; Omicron BA.4/5) protein were measured using an enzyme-linked immunosorbent assay kit (24). Recombinant RBD (Omicron BA.4/5) protein, identical in amino acid sequence to the target antigen, was coated onto the wells of an enzyme plate. One well served as a blank control (no reagents added), whereas 100 μl of positive and negative controls and diluted samples were added to their respective wells. The plate was sealed and placed in a constant temperature incubator at 37°C for 1 h. If IgG antibodies were present in the sample, they would bind to the pre-coated antigen. Each well was then washed five times with at least 300 μl of washing solution for 30 s each time to remove unbound substances. One hundred microliters of enzyme reagent were added to each well; the plate was then sealed and placed in a constant temperature incubator at 37°C for 30 min. After an additional washing step, a color development solution containing tetramethylbenzidine (TMB) was added to each well, and the plate was placed in a constant temperature incubator at 37°C in the dark for 15 min to allow color development. Finally, 50 μl of stop solution was added to each well to terminate the reaction, and the absorbance (OD450-630) values were read at wavelengths of 450 nm and 630 nm. The anti-RBD-IgG (Omicron BA.4/5) titer results are presented as OD450-OD630.
2.3. Statistical analysis
Demographic characteristics and symptoms after SARS-CoV-2 infection were collected for both solid carcinoma patients and healthy controls. Categorical variables, such as sex and age, are presented as numbers (percentages); they were analyzed using Pearson’s Chi-squared test or Fisher’s exact test. Continuous variables, such as pVNT50, are expressed as the median (interquartile range) [M (P25, P75)] for non-normally distributed data. Multiple independent groups meeting normality and homogeneity of variance assumptions were compared using one-way analysis of variance (ANOVA). Non-parametric Kruskal–Wallis and Mann–Whitney U tests were used for continuous variables that did not follow a normal distribution. Binary logistic regression and multiple linear regression were utilized for multifactorial analysis. All statistical tests were two-sided, and P-values <0.05 were considered statistically significant. All analyses were conducted using R version 4.3.1 (R Foundation for Statistical Computing) and GraphPad Prism 9 software.
3. Results
3.1. Demographic characteristics
This study included 298 solid carcinoma patients and 258 healthy controls. The participants’ demographic characteristics are presented in Table 1 . Among the solid carcinoma patients, there were 138 men (46.3%) and 160 women (53.7%), whereas the healthy controls comprised 114 men (44.2%) and 144 women (55.8%). Regarding age, 117 solid carcinoma patients (39.3%) were aged 18–59 years, and 181 (60.7%) were aged ≥60 years. Among the healthy controls, 76 (29.5%) were aged 18–59 years and 182 (70.5%) were aged ≥60 years. The proportion of participants aged 18–59 years was significantly greater among solid carcinoma patients than among healthy controls (P=0.015). In terms of body mass index (BMI), 66 carcinoma patients (22.1%) and 188 healthy controls (72.9%) had a BMI <25 kg/m2; 19 carcinoma patients (6.4%) and 69 healthy controls (26.7%) had a BMI ≥25 kg/m2; 213 carcinoma patients (71.5%) and 1 healthy controls (0.4%) had unknown BMI. Regarding COVID-19 vaccination status, 177 solid carcinoma patients (59.4%) had received the vaccine, whereas 121 (40.6%) had not. Among the healthy controls, 256 (99.2%) had received the vaccine and two (0.8%) had not. The proportion of unvaccinated individuals was significantly greater among solid carcinoma patients than among healthy controls (P<0.001). Among the solid carcinoma patients, 22 had lung carcinoma (7.4%), 83 had digestive carcinoma (27.9%), 21 had liver carcinoma (7.0%), 50 had breast carcinoma (16.8%), 21 had thyroid carcinoma (7.0%), 32 had prostate carcinoma (10.7%), and 70 had other carcinomas (23.5%).
Table 1.
Participant demographic characteristics.
| Variable | Solid carcinoma patients (N=298) |
Healthy controls (N=258) |
p |
|---|---|---|---|
| Sex | |||
| Male | 138 (46.3) | 114 (44.2) | 0.616 |
| Female | 160 (53.7) | 144 (55.8) | |
| Age, years | |||
| 18-59 | 117 (39.3) | 76 (29.5) | 0.015 |
| ≥60 | 181 (60.7) | 182 (70.5) | |
| Median (P25, P75 ) | 63 (53,71) | 67 (54,73) | |
| BMI (kg/m2) | |||
| <25.0 | 66 (22.1) | 188 (72.9) | 0.411 |
| ≥25.0 | 19 (6.4) | 69 (26.7) | |
| unknown | 213 (71.5) | 1 (0.4) | |
| Vaccine (s) administered | |||
| Yes | 177 (59.4) | 256 (99.2) | <0.001 |
| No | 121 (40.6) | 2 (0.8) | |
| COVID-19 infection | |||
| Yes | 234 (78.5) | 242 (93.8) | <0.001 |
| No | 64 (21.5) | 16 (6.2) | |
| Type of carcinoma | |||
| Lung carcinoma | 22 (7.4) | – | |
| Digestive carcinoma | 83 (27.9) | – | |
| Liver carcinoma | 21 (7.0) | – | |
| Breast carcinoma | 50 (16.8) | – | |
| Thyroid carcinoma | 21 (7.0) | – | |
| Prostate carcinoma | 32 (10.7) | – | |
| Other carcinomas | 70 (23.5) | – | |
Data are presented as number of participants (%) or median (P25, P75 ). aDigestive carcinoma (colorectal carcinoma, gastric carcinoma, pancreatic carcinoma, esophageal carcinoma, biliary tract carcinoma); bOther carcinomas (pituitary carcinoma; carcinomas of the mouth, nose, and throat; ovarian carcinomas; bladder carcinoma; renal carcinoma; ureter carcinoma; acoustic nerve carcinoma; uterine carcinoma). Statistical analyses were conducted using the Chi-squared test.
3.2. Analysis of COVID-19 clinical characteristics and associated factors
3.2.1. Clinical characteristics of COVID-19 in solid carcinoma patients and healthy controls
After the 2022.12-2023.4 wave of the COVID-19 pandemic in China, we conducted a questionnaire to assess COVID-19 status among the study participants ( Table 2 ). In terms of general symptoms after contracting COVID-19, 50 solid carcinoma patients (92.6%) and 169 healthy controls (84.9%) experienced systemic symptoms; the difference was not statistically significant (P=0.143). Respiratory symptoms were reported by 28 solid carcinoma patients (51.9%) and 96 healthy controls (48.2%); the difference was not statistically significant (P=0.638). Digestive tract symptoms were observed in five solid carcinoma patients (9.3%) and 10 healthy controls; this difference also was not statistically significant (P=0.399). However, 38 solid carcinoma patients (76.0%) and 95 healthy controls (54.6%) experienced fever with a body temperature ≥38°C (P=0.007). Regarding muscle soreness, 26 solid carcinoma patients (48.1%) reported this symptom; although this proportion was greater than that among healthy controls (73, 36.7%), the difference was not statistically significant (P=0.126). Concerning the timing of recovery after contracting COVID-19, nine solid carcinoma patients (16.7%) and 43 healthy controls (22.6%) experienced symptoms for ≥1 week; again, the difference was not statistically significant (P=0.354). In terms of medical visits after contracting COVID-19, 11 solid carcinoma patients (20.4%) and 34 healthy controls (17.8%) sought medical care; this difference was not statistically significant (P=0.667).
Table 2.
Symptoms of COVID-19.
| Variable | SCPs | HCs | P |
|---|---|---|---|
| General symptom | 0.143 | ||
| Yes | 50(92.6) | 169(84.9) | |
| No | 4(7.4) | 30(15.1) | |
| Respiratory symptom | 0.638 | ||
| Yes | 28(51.9) | 96(48.2) | |
| No | 26(48.1) | 103(51.8) | |
| Digestive tract symptom | 0.399 | ||
| Yes | 5(9.3) | 10(5.0) | |
| No | 40(90.7) | 189(95.0) | |
| Fever | 0.007 | ||
| <38°C | 12(24.0) | 79(45.4) | |
| ≥38°C | 38(76.0) | 95(54.6) | |
| Muscular soreness | 0.126 | ||
| Yes | 26(48.1) | 73(36.7) | |
| No | 28(51.9) | 126(63.3) | |
| Symptom recovery time | 0.354 | ||
| <1 weeks | 45(83.3) | 148(77.5) | |
| ≥1 weeks | 9(16.7) | 43(22.5) | |
| Medical visits after COVID-19 infection | 0.667 | ||
| Yes | 11(20.4) | 34(17.8) | |
| No | 43(79.6) | 157(82.2) | |
| Time of SARS-CoV-2 antigen or PCR turns to negative | 0.031* | ||
| <3 weeks | 36(92.3) | 143(99.3) | |
| ≥3 weeks | 3(7.7) | 1(0.7) | |
Data are presented as number of participants (%). Statistical analyses were conducted using the Chi-squared test and Fisher’s exact test*.
3.2.2. Factors influencing COVID-19 onset
In the single-factor analysis of solid carcinoma patients, 159 individuals (89.8%) who received the SARS-CoV-2 vaccine and 75 unvaccinated individuals (62.0%) contracted COVID-19; this difference was statistically significant (P<0.01) ( Table 3 ). The main independent variables in multifactorial analysis were the presence of COVID-19 and associated symptoms (general, respiratory, digestive, fever, and muscle soreness); the dependent variables were sex, age, body mass index, vaccination status, and malignancy type. The likelihood of contracting COVID-19 was significantly higher among participants who received the SARS-CoV-2 vaccine than among unvaccinated participants (odds ratio (25)=91.5; 95% confidence interval, 2.0–4086.5) ( Supplementary Table 1 ).
Table 3.
Associations between participant characteristics and COVID-19 symptoms in solid carcinoma patients.
| Variable | COVID-19 infection | General symptom | Respiratory symptom | Digestive tract symptom | Fever (≥38°C) | Muscular soreness | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | p | N (%) | p | N (%) | p | N (%) | p | N (%) | p | N (%) | p | |
| Sex | ||||||||||||
| Male | 112 (81.2) | 0.303 | 20 (95.2) | 0.953 | 8 (38.1) | 0.107 | 2 (9.5) | 1.000 | 15 (78.9) | 0.967 | 9 (42.9) | 0.535 |
| Female | 122 (76.3) | 30 (90.9) | 20 (60.6) | 3 (9.1) | 23 (74.2) | 17 (51.5) | ||||||
| Age, years | ||||||||||||
| 18-59 | 89 (76.1) | 0.407 | 23 (95.8) | 0.771 | 15 (62.5) | 0.161 | 2 (8.3) | 1.000 | 19 (82.6) | 0.313 | 12 (50.0) | 0.808 |
| ≥60 | 145 (80.1) | 27 (90.0) | 13 (43.3) | 3 (10.0) | 19 (70.4) | 14 (46.7) | ||||||
| BMI (kg/m2) | ||||||||||||
| <25.0 | 59 (89.4) | 0.831 | 41 (95.3) | 0.181* | 24 (55.8) | 0.249 | 5 (11.6) | 0.546 | 33 (82.5) | 0.082 | 21 (48.8) | 0.841 |
| ≥25.0 | 16 (84.2) | 9 (81.3) | 4 (36.4) | 0 (0.0) | 5 (50.0) | 5 (45.5) | ||||||
| Vaccine (s) administered | ||||||||||||
| Yes | 159 (89.8) | <0.001 | 48 (92.3) | 1.000* | 27 (51.9) | 1.000* | 5 (9.6) | 1.000* | 36 (75.0) | 1.000* | 25 (48.1) | 1.000* |
| No | 75 (62.0) | 2 (100.0) | 1 (50.0) | 0 (0.0) | 2 (100.0) | 1 (50.0) | ||||||
| Type of carcinoma | ||||||||||||
| Lung carcinoma | 15 (68.2) | 0.211 | 3 (75.0) | 0.634* | 2 (50.0) | 0.518* | 0 (0.0) | 0.758* | 1 (25.0) | 0.136* | 1 (25.0) | 0.297* |
| Digestive carcinoma | 66 (80.5) | 9 (100.0) | 3 (33.3) | 2 (22.2) | 7 (77.8) | 5 (55.6) | ||||||
| Liver carcinoma | 17 (85.0) | 1 (100.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) | ||||||
| Breast carcinoma | 34 (68.0) | 12 (92.3) | 9 (69.2) | 2 (15.4) | 8 (66.7) | 8 (61.5) | ||||||
| Thyroid carcinoma | 17 (81.0) | 5 (100.0) | 3 (60.0) | 0 (0.0) | 3 (75.0) | 4 (80.0) | ||||||
| Prostate carcinoma | 29 (90.6) | 6 (85.7) | 2 (28.6) | 0 (0.0) | 4 (80.0) | 2 (28.6) | ||||||
| Other carcinomas | 55 (78.6) | 14 (93.3) | 8 (53.3) | 1 (6.7) | 14 (93.3) | 5 (33.3) | ||||||
Data are presented as number of participants (%). Statistical analyses were conducted using the Chi-squared test and Fisher’s exact test*.
3.3. Analysis of immunogenicity
3.3.1. SARS-CoV-2 antibody levels in solid carcinoma patients and healthy controls
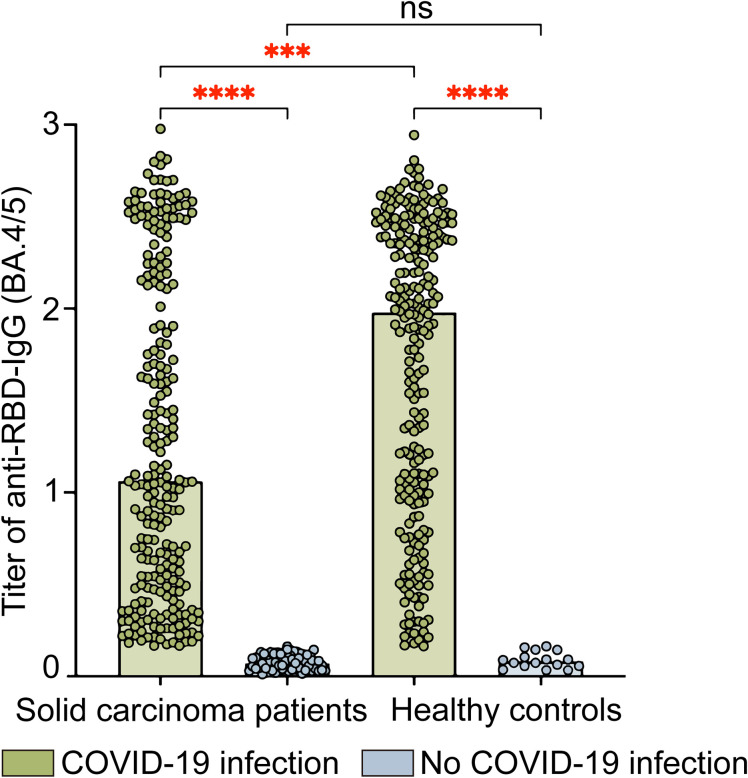
Regarding IgG antibody levels against the SARS-CoV-2 RBD (Omicron BA.4/5) protein, solid carcinoma patients who contracted COVID-19 had a value of 1.1 (0.5, 2.2), whereas patients who did not contract COVID-19 had a value of 0.1 (0.0, 0.1); this difference was statistically significant (P<0.001). Among healthy controls, the values for those who did and did not contract COVID-19 were 2.0 (1.0, 2.4) and 0.1 (0.1, 0.1), respectively; again, the difference was statistically significant (P<0.001). Among individuals with SARS-CoV-2 infection, neutralizing antibody levels were significantly lower in solid carcinoma patients than in healthy controls (P=0.0001) ( Figure 2 ).
Figure 2.
Levels of IgG antibodies against SARS-CoV-2 RBD (Omicron BA.4/5) protein in solid carcinoma patients and healthy controls. This figure compares the anti-RBD-IgG (BA.4/5) titers in SARS-CoV-2 infected and uninfected individuals for both solid carcinoma patients and healthy controls. The anti-RBD-IgG titers are presented as absorbance (OD450-630); the values were read at wavelengths of 450 nm and 630 nm. Statistical analyses were conducted using the Kruskal–Wallis test. ns, not significant; *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
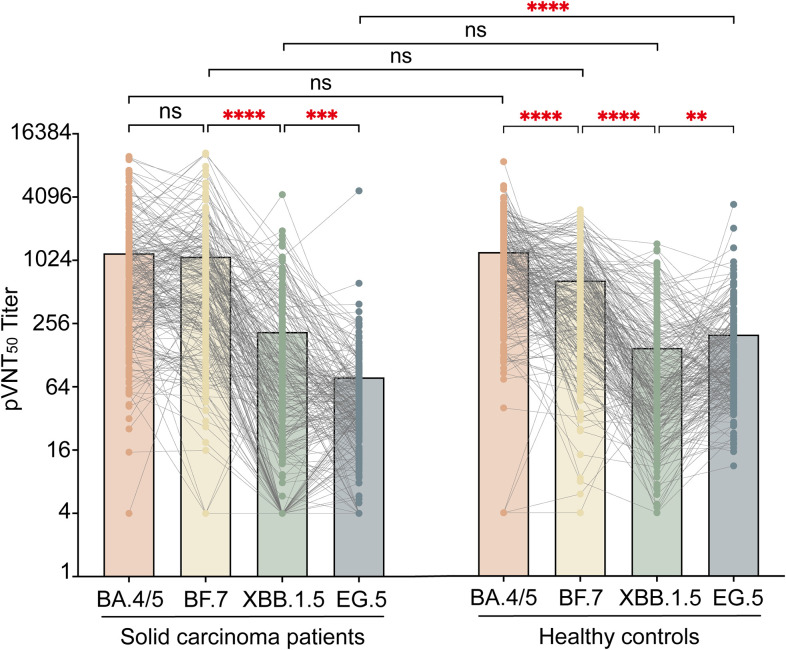
Neutralization testing against SARS-CoV-2 Omicron subvariants (BA.4/5, BF.7, XBB.1.5, and EG.5) in solid carcinoma patients and healthy controls with COVID-19 was conducted using pseudoviruses ( Figure 3 ). Among solid carcinoma patients, the GMTs of pVNT50 were 621.0 (288.8, 1333.0), 529.6 (215.3, 1264.5), 66.1 (17.8, 201.1), and 38.6 (21.7, 67.8) for BA.4/5, BF.7, XBB.1.5, and EG.5, respectively. Statistically significant differences were observed between BA.4/5 and XBB.1.5/EG.5 (P<0.001), BF.7 and XBB.1.5/EG.5 (P<0.001), and XBB.1.5 and EG.5 (P=0.0006). Among healthy controls, the GMTs of pVNT50 were 894.1 (458.5, 1637.0), 463.1 (185.2, 914.0), 59.3 (29.0, 163.6), and 106.8 (61.2, 217.3) for BA.4/5, BF.7, XBB.1.5, and EG.5, respectively. Significant differences were evident between BA.4/5 and XBB.1.5/EG.5 (P<0.001), BF.7 and XBB.1.5/EG.5 (P<0.001), and XBB.1.5 and EG.5 (P=0.0015). Among all individuals with COVID-19, solid carcinoma patients and healthy controls showed a significant difference in the GMT of pVNT50 for EG.5 (P<0.001).
Figure 3.
pVNT50 against SARS-CoV-2 variants in solid carcinoma patients and healthy controls. This figure presents the pVNT50 against Omicron variants (BA.4/5, BF.7, XBB.1.5, and EG.5) in SARS-CoV-2 infected solid carcinoma patients and healthy controls. Statistical analyses were conducted using the Kruskal–Wallis test. ns, not significant; *P<0.05;**P<0.01;***P<0.001; ****P<0.0001.
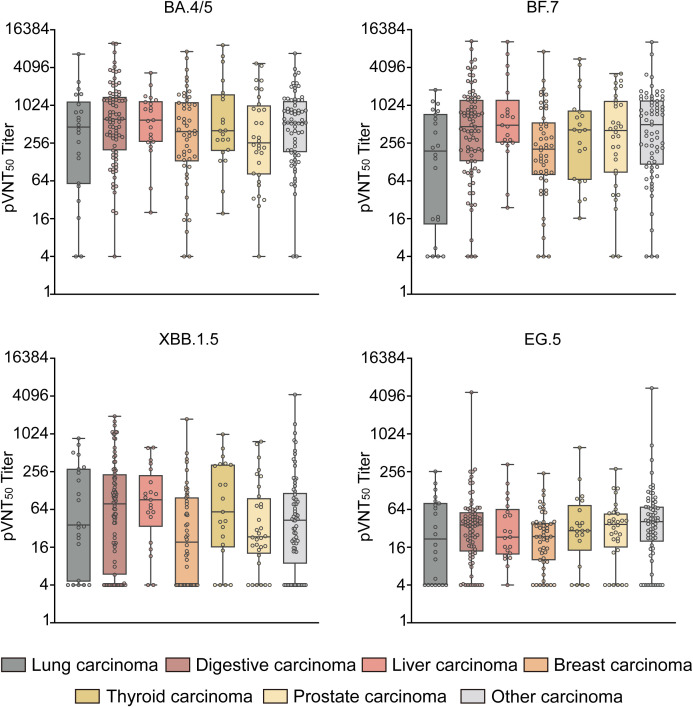
The GMTs of pVNT50 against Omicron BA.4/5, BF.7, XBB.1.5, and EG.5 variants were tested in healthy controls and patients with different types of solid carcinoma (e.g., lung, digestive, liver, breast, thyroid, prostate, and other) ( Figure 4 ). Regarding BA.4/5 antibody levels, digestive carcinoma showed the highest pVNT50 (614.6 [196.0, 1398.0]), followed by liver carcinoma (593.9 [266.7, 1194.5]); prostate carcinoma displayed the lowest pVNT50 (258.1 [80.9, 1023.8]). Concerning BF.7 antibody levels, other carcinomas showed the highest pVNT50 (503.9 [115.7, 1223.0]), followed by liver carcinoma (492.7 [258.3, 1254.6]); lung carcinoma displayed the lowest pVNT50 (191.3 [12.9, 747.2]). In terms of XBB.1.5 antibody levels, liver carcinoma showed the highest pVNT50 (91.2 [33.7, 226.5]), followed by digestive carcinoma (78.7 [5.8, 233.0]); breast carcinoma displayed the lowest pVNT50 (19.3 [4.0, 99.8]). With respect to EG.5 antibody levels, other carcinomas showed the highest pVNT50 (41.0 [19.6, 71.1]), followed by prostate carcinoma (37.0 [15.8, 55.3]); lung carcinoma displayed the lowest pVNT50 (21.7 [4.0, 81.6]) ( Figure 4 ).
Figure 4.
pVNT50 against Omicron variants in patients with different types of solid carcinoma. This figure illustrates the pVNT50 against Omicron variants (BA.4/5, BF.7, XBB.1.5, and EG.5) in patients with various types of solid carcinoma (lung, digestive, liver, breast, thyroid, prostate, and other). Statistical analyses were conducted using the Kruskal–Wallis test.
3.3.2. Factors influencing SARS-CoV-2 antibody levels
In the single-factor analysis of IgG antibody levels against the SARS-CoV-2 RBD (Omicron BA.4/5) protein in solid carcinoma patients, levels were significantly higher among vaccinated individuals (1.3 [0.5, 2.3]) than among unvaccinated individuals (0.3 [0.1, 0.7]) (P<0.001). Similarly, BA.4/5 antibody levels were significantly higher among vaccinated patients (708.9 [263.5, 1413.0]) than among unvaccinated patients (295.1 [97.8, 780.1]) (P<0.001). BF.7 antibody levels also were significantly higher among vaccinated patients (431.7 [151.9, 1010.5]) than among unvaccinated patients (273.9 [53.2, 1052.2]) (P=0.042). Furthermore, solid carcinoma patients who contracted COVID-19 had significantly higher IgG antibody levels against the SARS-CoV-2 RBD (Omicron BA.4/5) protein (1.1 [0.5, 2.2]) compared with the levels in patients who did not contract COVID-19 (0.1 [0.0, 0.1]) (P<0.001). BA.4/5 antibody levels were significantly higher among patients with COVID-19 (621.0 [288.8, 1333.0]) than among patients who did not develop COVID-19 (122.8 [33.7, 329.9]) (P<0.001). Similarly, BF.7 antibody levels were significantly higher among patients with COVID-19 (529.6 [215.3, 1264.5]) than among patients who did not develop COVID-19 (40.2 [11.0, 134.4]) (P<0.001) ( Table 4 ).
Table 4.
Associations between participant characteristics and antibody levels in solid carcinoma patients.
| Variable | Anti-RBD IgG | BA.4/5 | BF.7 | |||
|---|---|---|---|---|---|---|
| Titer | p | GMT | p | GMT | p | |
| Sex | ||||||
| Male | 0.9 (0.2,2.1) | 0.079 | 569.9 (206.1,1387.0) | 0.138 | 516.8 (137.0,1259.8) | 0.013 |
| Female | 0.6 (0.2,1.6) | 444.3 (146.9,1210.0) | 297.2 (87.5,836.5) | |||
| Age, years | ||||||
| 18-59 | 0.7 (0.2,1.6) | 0.195 | 420.9 (163.9,1207.0) | 0.634 | 312.6 (76.5,920.7) | 0.161 |
| ≥60 | 0.8 (0.2,1.9) | 538.1 (174.9,1248.8) | 404.8 (125.0,1096.0) | |||
| BMI (kg/m2) | ||||||
| <25.0 | 1.3 (0.5,2.4) | 0.906 | 627.5 (255.7,1405.3) | 0.756 | 294.7 (112.5,923.4) | 0.681 |
| ≥25.0 | 1.6 (0.5,2.2) | 833.7 (178.3,1243.0) | 622.1 (84.0,1100.0) | |||
| Vaccine (s) administered | ||||||
| Yes | 1.3 (0.5,2.3) | <0.001 | 708.9 (263.5,1412.5) | <0.001 | 431. (151.9,1010.5) | 0.042 |
| No | 0.3 (0.1,0.7) | 295.1 (97.8,780.1) | 273.9 (53.2,1052.2) | |||
| COVID-19 infection | ||||||
| Yes | 1.1 (0.5,2.2) | <0.001 | 621.0 (288.8,1333.0) | <0.001 | 529.6 (215.3,1264.5) | <0.001 |
| No | 0.1 (0.0,0.1) | 122.8 (33.7,329.9) | 40.2 (11.0,134.4) | |||
| Type of carcinoma | ||||||
| Lung carcinoma | 1.0 (0.1,2.4) | 0.734 | 461.4 (56.9,1177.3) | 0.496 | 191.3 (12.9,747.2) | 0.041 |
| Digestive carcinoma | 0.8 (0.3,1.6) | 614.6 (196.0,1398.0) | 467.2 (131.4,1250.0) | |||
| Liver carcinoma | 0.7 (0.2,1.3) | 593.9 (266.7,1194.5) | 492.7 (258.3,1254.6) | |||
| Breast carcinoma | 0.6 (0.1,1.4) | 390.6 (130.8,1149.8) | 205.1 (79.2,548.6) | |||
| Thyroid carcinoma | 0.7 (0.3,1.6) | 402.8 (191.4,1536.5) | 415.5 (66.2,845.9) | |||
| Prostate carcinoma | 0.8 (0.3,2.1) | 258.1 (80.9,1023.8) | 406.8 (86.3,1208.0) | |||
| Other carcinomas | 1.1 (0.2,2.2) | 542.2 (183.0,1197.5) | 503.9 (115.7,1223.0) | |||
Data are presented as median (P25, P75). Statistical analyses were conducted using the non-parametric Mann–Whitney test.
In the multifactorial analysis, the main independent variables were IgG antibody levels against the SARS-CoV-2 RBD (Omicron BA.4/5) protein, BA.4/5 antibody levels, and BF.7 antibody levels. The dependent variables included sex, age, body mass index, vaccination status, and malignancy type. Compared with unvaccinated participants, vaccinated participants had higher IgG antibody levels against the SARS-CoV-2 RBD (Omicron BA.4/5) protein (B=1.4; 95% confidence interval, 0.2–2.5) ( Supplementary Table 2 ).
3.4. Symptoms of long COVID
The proportions of participants with long COVID symptoms were 37.0% (20 individuals) among solid carcinoma patients and 30.4% (58 individuals) among healthy controls; the difference was not statistically significant (P=0.353). Among solid carcinoma patients, the most common symptom of long COVID symptom was fatigue (45.0%, nine cases), followed by dyspnea (20.0%, four cases). Among healthy controls, fatigue also was the most common symptom of long COVID (44.8%, 26 cases), followed by throat discomfort (15.5%, nine cases) ( Table 5 ).
Table 5.
Symptoms of long COVID.
| Variable | Solid carcinoma patients | Healthy controls | p |
|---|---|---|---|
| Persistent symptoms | 0.353 | ||
| Yes | 20(37.0) | 58(30.4) | |
| No | 34(63.0) | 133(69.6) | |
| Symptoms | |||
| Fatigue | 9(45.0) | 26(44.8) | 0.989 |
| Sleepy | 3(15.0) | 6(10.3) | 0.574 |
| Headache/dizziness/migraine | 1(5.0) | 4(6.9) | 1.000 |
| Cough | 2(10.0) | 7(12.1) | 1.000 |
| Discomfort in the pharynx | 1(5.0) | 9(15.5) | 0.409 |
| Taste/smell change or loss | 3(15.0) | 6(10.3) | 0.876 |
| Gastrointestinal discomfort | 1(5.0) | 0(0.0) | 0.256* |
| Breath/shortness of breath | 4(20.0) | 4(6.9) | 0.216 |
| Decreased exercise capacity | 2(10.0) | 2(3.5) | 0.577 |
| Arrhythmia/palpitations | 0(0.0) | 3(5.2) | 0.565* |
| Chest pain | 0(0.0) | 1(1.7) | 1.000* |
| Sleep disorder | 1(5.0) | 2(3.4) | 1.000* |
| Tinnitus/earache | 0(0.0) | 2(3.4) | 1.000* |
Data are presented as number of participants (%). Statistical analyses were conducted using the Chi-squared test and Fisher’s exact test*.
4. Discussion
As the COVID-19 pandemic persists, the virus continues to rapidly evolve, leading to the emergence of several Omicron variants that have become dominant worldwide, hitherto these types of mutant strains have caused extensive breakthrough infections both in China and around the world (26, 27). Some of these variants exhibit the ability to evade immunity conferred by prior infections or vaccinations, posing a renewed threat to public health (28–30).
In the present study, the proportion of vaccinated solid carcinoma patients was lower than that of healthy controls. This discrepancy may be attributed to various factors influencing vaccine hesitancy and uptake among carcinoma patients. These findings are consistent with the results of previous studies, which have frequently linked vaccine hesitancy in this population to concerns about vaccine-related side effects, uncertainty about vaccine efficacy and safety, and ongoing aggressive cancer treatments (31, 32). Intriguingly, we observed a higher rate of COVID-19 cases among vaccinated solid carcinoma patients than among unvaccinated patients. An online questionnaire in China revealed that COVID-19 vaccination may exacerbate some upper respiratory symptoms, such as sore throat, nasal congestion, and runny nose (33). This phenomenon may have occurred because vaccinated solid carcinoma patients engaged in more social activities and had increased opportunities for exposure, whereas unvaccinated individuals were more cautious about potential infection.
Regarding symptoms of COVID-19, solid carcinoma patients were more likely than healthy controls to experience fever with a maximum temperature of ≥38°C, suggesting that fever is more severe in carcinoma patients. However, the symptoms observed in this study were manageable. Multiple studies have linked SARS-CoV-2 infection to cough, sputum production, fever, and dyspnea in carcinoma patients (3, 34). Another study focusing on children and young adults with cancer revealed that all participants experienced mild to moderate symptoms after contracting COVID-19 (35). Additionally, the proportion of solid carcinoma patients who required ≥3 weeks to achieve negative results in a SARS-CoV-2 antigen or PCR was higher than the corresponding proportion of healthy controls, suggesting a longer recovery time in carcinoma patients.
Regarding the immune response to the Omicron variant of SARS-CoV-2, individuals with COVID-19 generally exhibit higher immunogenicity than those who do not contract COVID-19. However, solid carcinoma patients show lower antibody levels compared with healthy controls. A study by Donze et al. revealed that antibody levels, particularly anti-SARS-CoV-2 IgG antibodies targeting the S1 domain of the Spike protein, were significantly elevated among carcinoma patients with COVID-19 (35). Other studies have shown that antibody levels remain stable for 12 months after the initial infection (36, 37). In our study, vaccinated solid carcinoma patients with COVID-19 demonstrated stronger immunogenicity than their unvaccinated counterparts. Across different Omicron strains, both BA.4/5 and BF.7 elicited higher antibody levels than XBB.1.5 or EG.5 in both solid carcinoma patients and healthy controls. A study conducted in Beijing showed that neutralizing antibody levels against the prototypic (PT), BA.2, BA.4/5, and BF.7 strains were significantly elevated among individuals with COVID-19. Conversely, the subvariants BM.1.1.1, CH.1.1, XBB, XBB.1.5, and XBB.1.16 showed the lowest neutralizing antibody titers (38). Concerning the BF.7 strain tested in our study, male solid carcinoma patients exhibited stronger immunogenicity than female patients. Interestingly, our study revealed significant differences in the immune response to XBB.1.5 and EG.5 between solid carcinoma patients and healthy controls. Data from a study in South Korea showed lower levels of antibodies to the EG.5 variant than to the XBB.1.5 variant, similar to results in our solid carcinoma patients (39). In addition, another study pointed out that EG.5 is more infectious than XBB.1.5 in some areas, and 20% of the infection rate in the United States is related to EG.5, which may cause a part of the population to be more susceptible to SARS-CoV-2 EG.5 variant, resulting in higher antibody levels after infection EG.5 than XBB.1.5. This is consistent with what we have observed in healthy controls (40). At the same time, these differences may also be related to immunosuppression, prior immune response, and the effect of immunotherapy in solid carcinoma patients. Among different types of solid carcinomas, patients with other carcinomas displayed the highest BF.7 antibody levels, followed by liver carcinoma; patients with lung carcinoma exhibited the lowest levels. In terms of long COVID symptoms, the incidences did not significantly differ between solid carcinoma patients and healthy controls; fatigue was the most common symptom in both groups. A study by Soriano et al. showed that 10%–30% of individuals with COVID-19 experience persistent symptoms, including fatigue, cognitive impairment, shortness of breath, and mental disorders (41). A meta-analysis of 81 studies revealed that the proportion of individuals experiencing fatigue at ≥12 weeks after COVID-19 diagnosis was 0.32, whereas the proportion with cognitive impairment was 0.22 (42).
Our study had some limitations. First, the sample size was small, limiting the generalizability of the findings. Additionally, there was a low number of patients with each type of solid carcinoma. Second, because this was a cross-sectional survey, we could not confirm Omicron infection status through laboratory testing. Third, some areas of data collection were incomplete; we lacked key clinical records, such as patient treatment methods and specific dates of tumor diagnosis. These gaps in data may have introduced bias in our analysis of the impact of SARS-CoV-2 on solid carcinoma patients. Additionally, our research conclusions are drawn from the Omicron wave in China, and thus may not be fully generalizable to other countries with different healthcare systems or COVID-19 policies, and the variant studied is Omicron, which suggests that the findings might not apply to other variants of concern.
In conclusion, we found that solid carcinoma patients exhibited hesitancy and concerns regarding COVID-19 vaccination. They were more likely to develop fever after contracting COVID-19 and tended to exhibit slower recovery times. Both solid carcinoma patients and healthy controls demonstrated increased immunogenicity after contracting COVID-19, although solid carcinoma patients displayed lower immunogenicity compared with healthy controls. Vaccinated individuals in both groups showed higher immunogenicity after contracting COVID-19, compared with unvaccinated individuals. Antibody levels varied across Omicron strains; the levels were highest for antibodies against BA.4/5 and lowest for antibodies against EG.5. Antibody levels against Omicron BF.7 also varied according to solid carcinoma type; the levels were highest in patients with other carcinomas and lowest in patients with lung carcinoma. Additionally, we found that male solid carcinoma patients had higher antibody levels against Omicron BF.7. These findings suggest that factors such as sex, disease severity, and treatment regimens influence the immune responses of solid carcinoma patients to vaccines and viruses. Further research is needed to fully understand the impacts of these differences on clinical outcomes.
Acknowledgments
We would like to thank the Zhejiang Provincial People’s Hospital and Xihu District Center for Disease Control and Prevention for their help with the survey.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key Research and Development Program of Zhejiang Province (2021C03200); the Key Program of Health Commission of Zhejiang Province/Science Foundation of National Health Commission (WKJ-ZJ-2221); the Major Program of Zhejiang Municipal Natural Science Foundation (LD22H190001); the Explorer Program of Zhejiang Municipal Natural Science Foundation (LQ23H100001); Zhejiang Provincial Medical and Health Science and Technology Plan (2023RC143); Zhejiang Pioneer Goose Leading Program (2024C03216).
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Zhejiang Provincial Center of Disease Control and Prevention (ethics code number: KT2023025). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RD: Writing – original draft, Data curation, Formal Analysis, Writing – review & editing. WP: Conceptualization, Supervision, Writing – review & editing. NX: Data curation, Supervision, Writing – review & editing. PQ: Project administration, Supervision, Writing – review & editing. LD: Project administration, Supervision, Writing – review & editing. QH: Formal Analysis, Validation, Writing – review & editing. JJ: Resources, Conceptualization, Formal Analysis, Project administration, Writing – review & editing. FH: Conceptualization, Data curation, Resources, Investigation, Writing – review & editing. HZ: Writing – original draft, Writing – review & editing, Data curation, Formal Analysis, Investigation, Project administration, Resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1476186/full#supplementary-material
References
- 1. Xie D, Choi HK, Dalbeth N, Wallace ZS, Sparks JA, Lu N, et al. Gout and excess risk of severe SARS-coV-2 infection among vaccinated individuals: A general population study. Arthritis Rheumatol. (2023) 75:122–32. doi: 10.1002/art.42339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruhl L, Kühne JF, Beushausen K, Keil J, Christoph S, Sauer J, et al. Third SARS-coV-2 vaccination and breakthrough infections enhance humoral and cellular immunity against variants of concern. Front Immunol. (2023) 14:1120010. doi: 10.3389/fimmu.2023.1120010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu L, Liao Y, Yu X, Rong L, Chen B, Chen G, et al. Clinical characteristics and prognostic factors of COVID-19 infection among cancer patients during the december 2022 - february 2023 omicron variant outbreak. Front In Med. (2024) 11:1401439. doi: 10.3389/fmed.2024.1401439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang S, Gao Z, Wang S. China's COVID-19 reopening measures-warriors and weapons. Lancet. (2023) 401:643–4. doi: 10.1016/S0140-6736(23)00213-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang W, Wang R, Jin P, Yu X, Wang W, Zhang Y, et al. Clinical Characteristics and Outcomes of Liver Transplant Recipients Infected by Omicron During the Opening up of the Dynamic Zero-Coronavirus Disease Policy in China: A prospective, Observational Study. Am J Transplant. (2024) 24:631–40. doi: 10.1016/j.ajt.2023.09.022 [DOI] [PubMed] [Google Scholar]
- 6. Who COVID-19 Dashboard . Available online at: https://Data.Who.Int/Dashboards/COVID-19/Cases?M49=156 (Accessed October 15th, 2024).
- 7. Chen Y, Lin Y, Lu H, Wu X, Pan Y, Xia A, et al. Real-World Effectiveness of Molnupiravir, Azvudine and Paxlovid against Mortality and Viral Clearance among Hospitalized Patients with COVID-19 Infection During the Omicron Wave in China: A Retrospective Cohort Study. Diagn Microbiol Infect Dis. (2024) 109:116353. doi: 10.1016/j.diagmicrobio.2024.116353 [DOI] [PubMed] [Google Scholar]
- 8. Zero-COVID Policy Keeps Pandemic under Control . Available online at: https://english.www.gov.cn/news/topnews/202201/10/content_WS61db8e5cc6d09c94e48a3610.html (Accessed October 15th, 2024).
- 9. Wekking D, Senevirathne TH, Pearce JL, Aiello M, Scartozzi M, Lambertini M, et al. The impact of COVID-19 on cancer patients. Cytokine Growth Factor Rev. (2024) 75:110–8. doi: 10.1016/j.cytogfr.2023.11.004 [DOI] [PubMed] [Google Scholar]
- 10. Song Q, Bates B, Shao YR, Hsu FC, Liu F, Madhira V, et al. Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-world evidence from the national COVID cohort collaborative. J Clin Oncol. (2022) 40:1414–27. doi: 10.1200/jco.21.02419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song NJ, Chakravarthy KB, Jeon H, Bolyard C, Reynolds K, Weller KP, et al. Mrna vaccines against SARS-coV-2 induce divergent antigen-specific T-cell responses in patients with lung cancer. J Immunother Cancer. (2024) 12(1):e007922. doi: 10.1136/jitc-2023-007922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bytyci J, Ying Y, Lee LYW. Immunocompromised individuals are at increased risk of COVID-19 breakthrough infection, hospitalization, and death in the post-vaccination era: A systematic review. Immun Inflammation Dis. (2024) 12:e1259. doi: 10.1002/iid3.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-coV-2 infection: A nationwide analysis in China. Lancet Oncol. (2020) 21:335–7. doi: 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alaeddini M, Etemad-Moghadam S. SARS-COV-2 infection in cancer patients, susceptibility, outcome and care. Am J Med Sci. (2022) 364:511–20. doi: 10.1016/j.amjms.2022.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yin T, Li Y, Ying Y, Luo Z. Prevalence of comorbidity in chinese patients with COVID-19: systematic review and meta-analysis of risk factors. BMC Infect Dis. (2021) 21:200. doi: 10.1186/s12879-021-05915-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. (2020) 324:603–5. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seegers V, Rousseau G, Zhou K, Blanc-Lapierre A, Bigot F, Mahammedi H, et al. COVID-19 infection despite previous vaccination in cancer patients and healthcare workers: results from a french prospective multicenter cohort (Papesco-19). Cancers (Basel). (2023) 15(19):4777. doi: 10.3390/cancers15194777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bell ML, Catalfamo CJ, Farland LV, Ernst KC, Jacobs ET, Klimentidis YC, et al. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: results from the arizona covhort. PloS One. (2021) 16:e0254347. doi: 10.1371/journal.pone.0254347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klein J, Wood J, Jaycox JR, Dhodapkar RM, Lu P, Gehlhausen JR, et al. Distinguishing features of long COVID identified through immune profiling. Nature. (2023) 623:139–48. doi: 10.1038/s41586-023-06651-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brehm J, Spaeth A, Dreßler L, Masetto T, Dannenberg R, Peter C, et al. SARS-COV-2 antibody progression and neutralizing potential in mild symptomatic COVID-19 patients - a comparative long term post-infection study. Front Immunol. (2022) 13:915338. doi: 10.3389/fimmu.2022.915338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen I, Campisi-Pfinto S, Rozenberg O, Colodner R, Bar-Sela G. The humoral response of patients with cancer to breakthrough COVID-19 infection or the fourth bnt162b2 vaccine dose. Oncologist. (2023) 28:e225–e7. doi: 10.1093/oncolo/oyad003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao X, Zhang R, Qiao S, Wang X, Zhang W, Ruan W, et al. Omicron SARS-COV-2 neutralization from inactivated and zf2001 vaccines. N Engl J Med. (2022) 387:277–80. doi: 10.1056/NEJMc2206900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peng W, Ma X, Tan K, Wang H, Cong M, Zhang Y, et al. Evaluation of cross-neutralizing antibodies in children infected with omicron sub-variants. Lancet Reg Health West Pac. (2023) 40:100939. doi: 10.1016/j.lanwpc.2023.100939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang H, Xu N, Xu Y, Qin P, Dai R, Xu B, et al. Safety and immunogenicity of ad5-ncov immunization after three-dose priming with inactivated SARS-COV-2 vaccine in chinese adults. Nat Commun. (2023) 14:4757. doi: 10.1038/s41467-023-40489-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cervia-Hasler C, Brüningk SC, Hoch T, Fan B, Muzio G, Thompson RC, et al. Persistent complement dysregulation with signs of thromboinflammation in active long COVID. Science. (2024) 383:eadg7942. doi: 10.1126/science.adg7942 [DOI] [PubMed] [Google Scholar]
- 26. Parsons RJ, Acharya P. Evolution of the SARS-COV-2 omicron spike. Cell Rep. (2023) 42:113444. doi: 10.1016/j.celrep.2023.113444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spiteri G, D'Agostini M, Abedini M, Ditano G, Collatuzzo G, Boffetta P, et al. Protective Role of SARS-COV-2 Anti-S Igg against Breakthrough Infections among European Healthcare Workers During Pre and Post-Omicron Surge-Orchestra Project. Infection. (2024) 52(4):1347–56. doi: 10.1007/s15010-024-02189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Y, Guo L, Yuan J, Xu Z, Gu Y, Zhang J, et al. Viral and antibody dynamics of acute infection with SARS-COV-2 omicron variant (B.1.1.529): A prospective cohort study from shenzhen, China. Lancet Microbe. (2023) 4:e632–e41. doi: 10.1016/s2666-5247(23)00139-8 [DOI] [PubMed] [Google Scholar]
- 29. Regenhardt E, Kirsten H, Weiss M, Lübbert C, Stehr SN, Remane Y, et al. SARS-COV-2 vaccine breakthrough infections of omicron and delta variants in healthcare workers. Vaccines (Basel). (2023) 11(5):958. doi: 10.3390/vaccines11050958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Søraas A, Grødeland G, Granerud BK, Ueland T, Lind A, Fevang B, et al. Breakthrough infections with the omicron and delta variants of SARS-COV-2 result in similar re-activation of vaccine-induced immunity. Front Immunol. (2022) 13:964525. doi: 10.3389/fimmu.2022.964525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prabani KIP, Weerasekara I, Damayanthi HDWT. COVID-19 vaccine acceptance and hesitancy among patients with cancer: A systematic review and meta-analysis. Public Health. (2022) 212:66–75. doi: 10.1016/j.puhe.2022.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poghosyan H, Ni Z, Vlahov D, Nelson L, Nam S. COVID-19 Vaccine Hesitancy among Medicare Beneficiaries with and without Cancer History: A Us Population-Based Study. J Community Health. (2023) 48:315–24. doi: 10.1007/s10900-022-01174-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qin S, Li Y, Wang L, Zhao X, Ma X, Gao GF. Assessment of vaccinations and breakthrough infections after adjustment of the dynamic zero-COVID-19 strategy in China: an online survey. Emerg Microbes Infect. (2023) 12:2258232. doi: 10.1080/22221751.2023.2258232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Passaro A, Peters S, Mok TSK, Attili I, Mitsudomi T, de Marinis F. Testing for COVID-19 in lung cancer patients. Ann Oncol. (2020) 31:832–4. doi: 10.1016/j.annonc.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donze C, Min V, Ninove L, de Lamballerie X, Revon Rivière G, Verschuur A, et al. Bnt162b2 COVID-19 vaccines in children, adolescents and young adults with cancer-a 1-year follow-up. Vaccines. (2023) 11(5):989. doi: 10.3390/vaccines11050989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guo L, Wang G, Wang Y, Zhang Q, Ren L, Gu X, et al. SARS-COV-2 -specific antibody and T-cell responses 1 year after infection in people recovered from COVID-19: A longitudinal cohort study. Lancet Microbe. (2022) 3:e348–e56. doi: 10.1016/S2666-5247(22)00036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guo L, Zhang Q, Gu X, Ren L, Huang T, Li Y, et al. Durability and cross-reactive immune memory to SARS-COV-2 in individuals 2 years after recovery from COVID-19: A longitudinal cohort study. Lancet Microbe. (2024) 5:e24–33. doi: 10.1016/S2666-5247(23)00255-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Y, Qiao S, Dong L, Zhang R, Li R, Qin S, et al. Antibody response assessment of immediate breakthrough infections after zero-COVID policy adjustment in China. Lancet Reg Health West Pac. (2023) 40:100945. doi: 10.1016/j.lanwpc.2023.100945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hyun H, Nham E, Seong H, Yoon JG, Noh JY, Cheong HJ, et al. Long-Term Humoral and Cellular Immunity against Vaccine Strains and Omicron Subvariants (Bq.1.1, Bn.1, Xbb.1, and Eg.5) after Bivalent COVID-19 Vaccination. Front Immunol. (2024) 15:1385135. doi: 10.3389/fimmu.2024.1385135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sil D, Gautam S, Saxena S, Joshi S, Kumar D, Mehta A, et al. Comprehensive analysis of omicron subvariants: eg.5 rise, vaccination strategies, and global impact. Curr Drug Targets. (2024) 25:517–25. doi: 10.2174/0113894501296586240430061915 [DOI] [PubMed] [Google Scholar]
- 41. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a delphi consensus. Lancet Infect Dis. (2022) 22:e102–e7. doi: 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: A systematic review and meta-analysis. Brain Behav Immun. (2022) 101:93–135. doi: 10.1016/j.bbi.2021.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.