Abstract
Background
Acute kidney injury (AKI) is a common complication among patients requiring cardiopulmonary bypass (CPB) during paediatric cardiac surgery. Plasma-free haemoglobin (PFH) produced by haemolysis during CPB contributes to AKI. This study aimed to determine the association between PFH and postoperative AKI during paediatric cardiac surgery requiring CPB.
Methods
This prospective, single-centre, observational study included children aged <5 yr who underwent cardiac surgery requiring CPB. PFH was measured pre-CPB, every 30 min during CPB, after modified ultrafiltration, on ICU admission, and once a day at 1–3 days after surgery. The study outcome included the relationship between peak PFH concentrations and the development of postoperative AKI up to 3 days after surgery. Additionally, multivariable analysis was performed to determine the risk factors for AKI.
Results
Of 179 patients, 74 (41%) developed postoperative AKI. Patients who developed AKI had significantly higher peak PFH concentrations (80 mg dl−1 [inter-quartile range, 50–132.5] vs 60 mg dl−1 [40–100]; P=0.006). Multivariable analysis did not identify peak PFH concentrations as an independent risk factor for postoperative AKI (odds ratio [OR] 1.00; 95% confidence interval [CI]: 0.99–1.00; P=0.268). Factors associated with postoperative AKI were age in months (OR 0.96; 95% CI: 0.94–0.99; P=0.007) and CPB duration (OR 1.02; 95% CI: 1.01–1.02; P<0.001).
Conclusions
There was an association between postoperative AKI and CPB time rather than PFH. Perioperative peak PFH concentrations were significantly higher in patients with postoperative AKI after paediatric cardiac surgery requiring CPB.
Keywords: acute kidney injury, cardiopulmonary bypass, observational study, paediatric cardiac surgery, plasma-free haemoglobin
Acute kidney injury (AKI) is a common complication among patients requiring cardiopulmonary bypass (CPB) for paediatric cardiac surgery.1 The incidence of AKI varies according to diagnostic criteria and is reportedly as high as 30–60%.2, 3, 4, 5 AKI is associated with longer hospital stays, prolonged length of mechanical ventilation, and increased risk for mortality.2, 3, 4, 5 Prediction and prevention of AKI is of great importance to improve outcomes of patients.
The pathophysiology of AKI after cardiac surgery requiring CPB is complex and multifactorial. Contributing factors may include hypoperfusion, ischaemia–reperfusion injury, and inflammation. The presence of haemolysis induced by CPB may be one potential mechanism, adding to these contributing factors.6,7 Haemolysis releases plasma-free haemoglobin (PFH) from red blood cells. PFH may cause AKI by mechanisms, such as tubular epithelial damage owing to reactive oxygen species generation, renal ischaemia via microcirculatory vasoconstriction owing to endothelial nitric oxide depletion, and tubular obstruction owing to PFH precipitation and haeme cast formation.8
Haemolysis during extracorporeal membrane oxygenation (ECMO) is reportedly associated with AKI development in paediatric patients.9,10 However, to our knowledge, there are only a few reports on the association between haemolysis and postoperative AKI in patients who underwent cardiac surgery and required CPB. Studies on the paediatric population include patients who range from neonate to adolescent.11, 12, 13 A target population up to adolescents would include an age group that is physiologically close to that of adults. Conversely, studies on neonates and infants are inevitably smaller in size, and previous reports have been small in size.12,13
This study aimed to determine the association between PFH and postoperative AKI in a particularly young age group of patients with physiological characteristics different from those of adults. We hypothesised that there is an association between postoperative AKI and haemolysis, and that the degree of haemolysis is highly severe among patients who develop postoperative AKI.
Methods
Study design
This prospective observational study was approved by the institutional review board of Okayama University Hospital (No. 1807-017). All children younger than 5 yr of age who underwent paediatric cardiac surgery under CPB, either corrective or palliative, from November 2018 to March 2021, were prospectively enrolled. All patient representatives gave written informed consent before enrolment. Patients with pre-existing renal dysfunction, defined as preoperative serum creatinine (sCr) concentrations met chronic kidney disease stage 3 of the Japanese survey,14 were ineligible from the study population. Patients aged less than 3 months used the criteria for patients aged 3–5 months. Emergency surgery and cases where parental consent was not obtained were also considered ineligible. Patients requiring postoperative ECMO, receiving haptoglobin administration for macroscopic haematuria during the study period, and those with unavailable data were excluded from statistical analysis after enrolment.
Anaesthesia and cardiopulmonary bypass management
Anaesthetic induction included fentanyl 10 μg kg−1 and rocuronium 1 mg kg−1. It was then maintained by fentanyl 30–100 μg kg−1 as appropriate for all patients. Sevoflurane was used at 1–3% if needed, although it is usually used at low or very low concentration. Additionally, midazolam 10 mg and rocuronium 30 mg were initiated in CPB circuit for anaesthesia management during CPB. All patients received methylprednisolone 30 mg kg−1 before CPB.
All CPB involved the use of a roller pump (MERA HAS II, Senko Medical Instrument Mfg. Co., Ltd., Tokyo, Japan). CPB flow was based on a cardiac index of 2.4 L min−1 m−2 in patients weighing over 10 kg or flow of 150 ml kg−1 min−1 in patients under 10 kg. Perfusion pressure, which is monitored by an arterial line placed at the upper limb, lower limb, or both limbs, was maintained at 30–40 mm Hg for neonates, 35–45 mm Hg for infants, and 40–50 mm Hg for patients older than 1 yr. Perfusion pressure was maintained by administering vasoconstrictor or vasodilators as needed at the discretion of the perfusionist. Core temperatures were managed at 25–35°C for each patient. Oxygenator type and size were decided based on patient weight. An oxygenator (Terumo RX05, Terumo Cardiovascular Systems, Tokyo, Japan) was used for patients weighing <10 kg, and another oxygenator (Terumo RX15, Terumo Cardiovascular System) for patients weighing >10 kg. Left atrial venting was performed in all patients with aortic cross-clamp (ACC) and cardiac arrest. Cell saver and sucker bypass were used as appropriate.
A circuit blood prime with packed red blood cells was used for patients weighing <7 kg or when the expected diluted haematocrit was <25%. All patients received furosemide 20 mg in the CPB circuit. All patients underwent modified ultrafiltration after CPB.
Plasma-free haemoglobin measurements
Blood samples were collected pre-CPB, every 30 min during CPB, after modified ultrafiltration, at ICU admission, and once a day for 1–3 days after surgery. Blood samples were collected from the venous side of the CPB circuit or a central venous or arterial catheter. Blood samples were centrifuged at 3000 rpm for 3 min. Plasma was immediately aliquoted and PFH was measured using HemoCue® Plasma/Low Hb system (Radiometer Medical, Tokyo, Japan). PFH was measured using the azide methaemoglobin method, which involves measuring absorbance of the sample at 570 and 880 nm. The HemoCue® Plasma/Low Hb system had a sensitivity to detect and quantify PFH in the range of 30–3000 mg dl−1.
Data collection and statistical analysis
Collected patient characteristics and clinical data included age, sex, weight, surgical procedure, the Risk-Adjusted classification for Congenital Heart Surgery (RACHS-1) score,15 preoperative haemoglobin concentration, preoperative sCr concentration, CPB duration, ACC duration, presence or absence of CPB blood priming, peak PFH concentration, and Kidney Disease: Improving Global Outcomes (KDIGO) score.16 The peak PFH was defined as the maximum value of PFH detected during the observation period.
The primary outcome was the development of AKI within 3 days after surgery. AKI was defined as an increase in sCr concentration of ≥1.5 times baseline or increased >0.3 mg dl−1 (26.5 μM) at any point during the study period as described in the KDIGO guidelines. sCr measurements were taken on ICU admission and on the morning of postoperative day (POD) 1–3. Urine output was not used in our definition owing to postoperative variable physician-dependent diuretic use. The latest value of sCr before surgery was used as a baseline measurement.
We assumed that the probability of AKI was 40% and 20% among patients with PFH higher and lower than the median value, respectively. It was necessary to enrol 162 participants to achieve 80% power at the two-sided significance level of 0.05. Considering a dropout rate of 20%, the study required the enrolment of approximately 200 patients. Considering the nature of the study, multivariable regression analysis was considered suitable to statistically analyse the data. With the sample size of 200, the regression analysis can include five predictors while assuming the incidence of AKI for the entire cohort to be 30% using the 10 event-per-variable rules17 without over fitting. Thus, our analysis could have an adequate power.
Categorical values were presented as frequencies and compared using the χ2 test or Fisher exact test when appropriate. Continuous variables were presented as the median and inter-quartile range (IQR); they were compared using the Wilcoxon rank sum test.
Multivariable analysis for AKI was performed to determine independent risk factors. Risk factors with a P-value of ≤0.1 in univariate analysis were included in a multivariable logistic regression model.
Additionally, patients were divided into the following groups, and subgroup analysis was performed concerning peak PFH: (1) CPB duration of <120 min or >120 min; (2) RACHS-1 scores of 1, 2, and 3 or larger; (3) neonates, infants, 1–4 yr of age, or non-neonates; and (4) cyanosis or non-cyanosis.
All statistical tests were two-sided, and a P-value of <0.05 was considered statistically significant. All statistical analyses were performed with JMP version 14.0 (SAS, Cary, NC, USA) and STATA version 18.0 (Stata Corp LLC, College Station, TX, USA). Data are reported following the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.18
Results
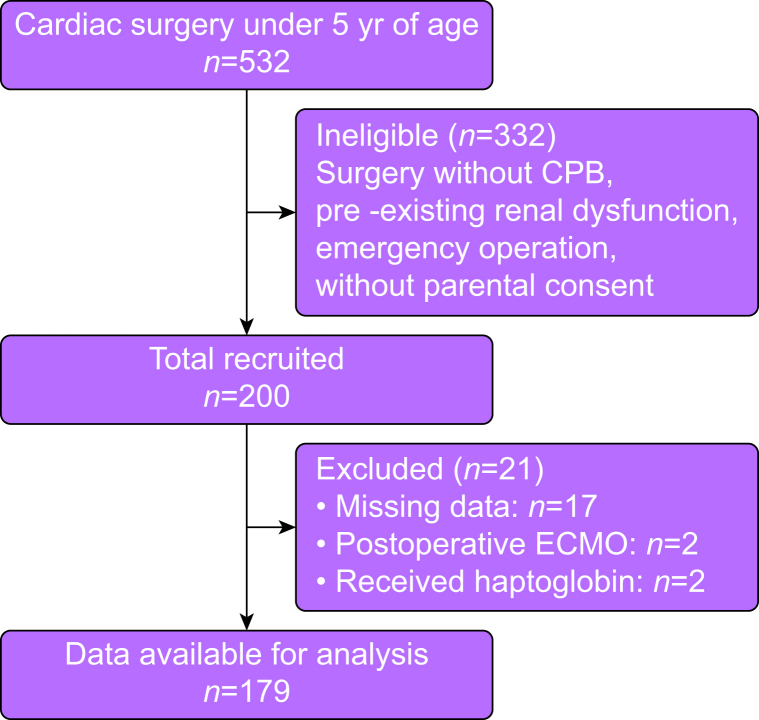
Of the 200 patients enrolled, 179 (89.5%) were eligible for analysis, excluding two patients who required postoperative ECMO, two patients who received haptoglobin at the discretion of the anaesthetist in charge for macroscopic haematuria, and 17 patients with unavailable data (Fig 1). Of the 179 patients, 26 (14.5%) were neonates, 84 (46.9%) were infants, and 69 (38.5%) were older than 1 yr.
Fig 1.
Study flow chart. CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation
Demographic and clinical characteristics of 179 patients are shown in Table 1, Table 2. The median (IQR) age and body weight were 9 (3–22) months and 7 (4.1–9.45) kg, respectively. AKI assessed by KDIGO criteria was identified in 74 (41%) patients, including 48 (27%) for stage 1, 14 (8%) for stage 2, and 12 (7%) for stage 3. All participants in AKI stage 3 required peritoneal dialysis (PD). Indication for PD was decreased urine output, at the discretion of intensivist. Criteria for AKI were met in 23 patients at ICU admission, 47 on POD 1, three on POD 2, and one on POD 3. The incidence of AKI in patients <10 kg (managed with CPB flow of 150 ml kg−1 min−1) and patients >10 kg (managed with flow of 2.4 L min−1 m−2) was 64/152 (42%) and 10/27 (37%), respectively. All patients had recovered from AKI at the time of ICU discharge. Compared with participants without AKI, those with AKI were younger (7 [2–14] vs 10 [3–28]; P=0.05) and had lower body weight (6.28 [3.65–8.42] vs 7.56 [4.94–10.8]; P=0.043), lower preoperative sCr (0.32 [0.24–0.36] vs 0.33 [0.25–0.35]; P=0.024), longer CPB duration (134.5 [101.5–187.5] vs 99 [74.5–136]; P<0.001), and longer ACC duration (76.5 [42.25–102.25] vs 44 [33–77]; P<0.001) (Table 1, Table 2).
Table 1.
Patient's baseline characteristics by acute kidney injury. Continuous data described as median (inter-quartile range). All other data described as proportion (percentage). AKI, acute kidney injury; RACHS-1, Risk-Adjusted classification for Congenital Heart Surgery.
| All patients (n=179) | Non-AKI (105/179, 59%) | AKI (74/179, 41%) | P-value | |
|---|---|---|---|---|
| Age (months) | 9 (3–22) | 10 (3–28) | 7 (2–14) | 0.05 |
| Male sex, n (%) | 101 (56) | 61 (58) | 40 (54) | 0.591 |
| Weight (kg) | 7 (4.1–9.45) | 7.56 (4.94–10.8) | 6.28 (3.65–8.42) | 0.043 |
| RACHS-1, n (%) | 0.051 | |||
| Risk category 1 | 4 (2) | 4 (3.81) | 0 | |
| Risk category 2 | 68 (38) | 43 (41.0) | 25 (33.8) | |
| Risk category 3 | 88 (49) | 50 (47.6) | 38 (51.4) | |
| Risk category 4 | 15 (8) | 7 (6.67) | 8 (10.8) | |
| Risk category 5 | 0 | 0 | 0 | |
| Risk category 6 | 4 (2) | 1 (0.95) | 3 (4.05) | |
| Preoperative haemoglobin (g dl−1) | 14.6 (12.9–16.1) | 14.7 (12.8–16.35) | 14.3 (13.2–15.9) | 0.815 |
| Preoperative serum creatinine (mg dl−1) | 0.33 (0.25–0.35) | 0.33 (0.27–0.35) | 0.32 (0.24–0.36) | 0.024 |
Table 2.
Patients' intraoperative data by acute kidney injury. Continuous data described as median (inter-quartile range). ACC, aortic cross-clamp; AKI, acute kidney injury; CPB, cardiopulmonary bypass; PFH, plasma-free haemoglobin.
| All patients (n=179) | Non-AKI (105/179, 59%) | AKI (74/179, 41%) | P-value | |
|---|---|---|---|---|
| CPB duration (min) | 117 (81–148) | 99 (74.5–136) | 134.5 (101.5–187.5) | <0.001 |
| ACC duration (min) | 54 (34–90) | 44 (33–77) | 76.5 (42.25–102.25) | <0.001 |
| Blood priming of CPB, n (%) | 117 (65) | 64 (60.95) | 53 (71.62) | 0.14 |
| Peak PFH (mg dl−1) | 70 (40–110) | 60 (40–100) | 80 (50–132.5) | 0.006 |
Concentrations of peak plasma-free haemoglobin
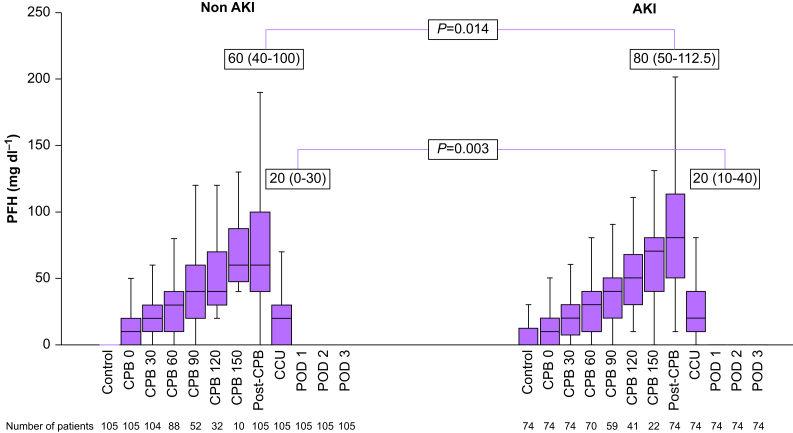
A significant difference was found in peak PFH between AKI and non-AKI groups (80 mg dl−1 [50–132.5] vs 60 mg dl−1 [40–100]; P=0.006) (Table 2). PFH concentrations increased in proportion to the CPB duration (r=0.99), peaked after the end of CPB, and then declined rapidly to below detection sensitivity on the POD 1 in both AKI and non-AKI groups (Fig 2). Significant differences were found between PFH concentrations of AKI and non-AKI groups at post-CPB (80 mg dl−1 [50–112.5] vs 60 mg dl−1 [40–100]; P=0.01) and ICU admission (20 mg dl−1 [10–40] vs 20 mg dl−1 [0–30]; P<0.01). However, there was no significant difference in PFH concentrations during CPB. Patients with blood primed CPB had relatively high baseline PFH concentrations (10 mg dl−1 [0–30] vs 0 mg dl−1 [0–10], P<0.001). However, there was no significant difference in the rate of development of AKI between patients with or without blood priming of CPB (P=0.14; Table 2).
Fig 2.
Changes in plasma-free haemoglobin (PFH). AKI, acute kidney injury; CPB, cardiopulmonary bypass; POD, postoperative day
Multivariable analysis
Factors with P-values of ≤0.1 in the univariate analysis included age in months, weight, RACHS-1 score, preoperative sCr, CPB duration, ACC duration, and peak PFH. Age and CPB duration were selected as explanatory variables because a high correlation was found between age and weight (r=0.91) and between CPB duration and ACC duration (r=0.70). Age in months (odds ratio [OR] 0.96; 95% confidence interval [CI]: 0.94–0.99; P=0.007) and CPB duration (OR 1.02; 95% CI: 1.01–1.02; P<0.001) were identified as independent risk factors for postoperative AKI; however, peak PFH (OR 1.00; 95% CI: 0.99–1.00; P=0.268) was not identified as an independent risk factor for postoperative AKI (Table 3).
Table 3.
Independent risk factors associated with acute kidney injury. CI, confidence interval; CPB, cardiopulmonary bypass; OR, odds ratio; PFH, plasma-free haemoglobin; RACHS-1, Risk-Adjusted classification for Congenital Heart Surgery.
| Risk factor | OR (95% CI) | P-value |
|---|---|---|
| Age (months) | 0.964 (0.939–0.99) | 0.007 |
| RACHS-1 | 1.26 (0.76–2.10) | 0.368 |
| Preoperative serum creatinine | 0.088 (0.0055–1.41) | 0.086 |
| CPB duration | 1.02 (1.01–1.02) | <0.001 |
| Peak PFH | 1.00 (0.999–1.01) | 0.268 |
Subgroup analysis
The patients were divided into following groups to analyse for peak PFH: (1) CPB time of <120 min and >120 min; (2) RACHS-1 scores of 1, 2, and 3 or more; (3) neonates, infants, 1–4 yr, and non-neonates; and (4) cyanosis or non-cyanosis. Patients with RACHS-1 score of >3 (90 mg dl−1 [55–150] vs 70 mg dl−1 [50–100]; P=0.02), infants (90 mg dl−1 [60–130] vs 60 mg dl−1 [40–120]; P=0.045), non-neonates (80 mg dl−1 [50–130] vs 60 mg dl−1 [40–90]; P=0.001), and non-cyanotic patients (80 mg dl−1 [50–110] vs 40 mg dl−1 [30–60]; P=0.001) who developed AKI had higher peak PFH concentrations (Table 4).
Table 4.
Subgroup analysis: comparing peak PFH between non-AKI and AKI. AKI, acute kidney injury; CPB, cardiopulmonary bypass; PFH, plasma-free haemoglobin; RACHS-1, Risk-Adjusted classification for Congenital Heart Surgery.
| Non-AKI | AKI | P-value | |
|---|---|---|---|
| CPB of <120 (n=95) | 50 (32.5–70), n=64 | 60 (40–80), n=31 | 0.339 |
| CPB of >120 (n=84) | 100 (60–120), n=41 | 100 (80–160), n=43 | 0.145 |
| RACHS-1 1, 2 (n=72) | 50 (40–110), n=47 | 70 (45–90), n=25 | 0.185 |
| RACHS-1 of >3 (n=107) | 70 (50–100), n=58 | 90 (55–150), n=49 | 0.014 |
| Neonates (n=26) | 110 (60–150), n=15 | 110 (40–140), n=11 | 0.787 |
| Infants (n=84) | 60 (40–120), n=43 | 90 (60–130), n=41 | 0.045 |
| 1–4 yr of age (n=69) | 50 (40–70), n=47 | 70 (47.5–105), n=22 | 0.111 |
| Non-neonates (n=153) | 60 (40–90), n=90 | 80 (50–130), n=63 | 0.001 |
| Cyanosis (n=111) | 75 (57.5–120), n=62 | 80 (55–145), n=49 | 0.548 |
| Non-cyanosis (n=68) | 40 (30–60), n=43 | 80 (50–110), n=25 | 0.001 |
Discussion
This study demonstrates that PFH was significantly higher in patients who developed postoperative AKI in paediatric cardiac surgery requiring CPB. However, multivariable analysis demonstrates that PFH concentrations were not identified as independent risk factors for postoperative AKI. To our knowledge, this is the largest study to investigate the association between postoperative AKI and PFH in paediatric cardiac surgery.
Consistent with previous studies, changes in PFH concentrations, particularly peak PFH concentrations, were higher in paediatric patients who developed postoperative AKI.11, 12, 13 This study revealed a peak PFH in the AKI group of 80 mg dl−1, which was considered reasonable compared with previous reports.11, 12, 13 PFH was measured every 30 min during CPB, allowing detailed observation of PFH changes. Previous reports have not measured PFH as frequently as this study, and the ability to observe PFH trends is the strength of this study. PFH increases in proportion to CPB time and peaks soon after CPB weaning. Additionally, PFH is significantly higher in the AKI group at the end of CPB as the AKI group tends to have a longer CPB time. The fact that PFH concentrations showed a rapid decline after the end of CPB was similar to previous reports.11, 12, 13 The rapid decline in PFH after separation from the CPB circuit may be, in part, as a result of the cessation of CPB-induced haemolysis. In addition, haemolysis might enhance haptoglobin production, which in turn enhances the haptoglobin–haemoglobin complex formation.
In contrast to our study, several studies revealed an association between PFH and postoperative AKI in paediatric cardiac surgery. Kim-Campbell and colleagues11 conducted a prospective study on 60 patients aged from 6 months to approximately 12 yr. They reported that ΔPFH at the end of CPB was associated with postoperative AKI in low-risk paediatric cardiac surgery. Their multivariable analysis revealed PFH changes and male sex as risk factors for AKI, especially in patients older than 2 yr. Additionally, Mamikonian and colleagues12 revealed an association between PFH elevation and postoperative AKI in paediatric cardiac surgery. They enrolled 40 patients aged 4 days to 4.8 yr. One difference between these two studies and ours is the age of the patients. The median age of the patients in each study was 2.5 yr and 15.5 months, respectively, whereas the median age of our patients was 9 months. A retrospective study by Ricci and colleagues13 included 22 neonates younger than our study, with a median age of 111 days, and revealed that PFH was not associated with renal dysfunction. Additionally, the relationship between AKI and PFH changes is lost in the patient population younger than 2 yr in the study by Kim-Campbell and colleagues.11 Several studies have reported an association between PFH and AKI during ECMO or CPB in adults.19,20 Physiological differences between adults and infants and differences in CPB techniques might have contributed to the different results obtained in this study. We believe that factors other than haemolysis might have a stronger effect in young patients or those with high disease severity.
The subgroup analysis revealed that patients with higher RACHS-1 scores, infants, non-neonates, and non-cyanotic patients who developed AKI had significantly higher PFH concentrations. Patients with higher RACHS-1 scores may have more complex surgeries and a longer CPB duration leading to AKI development. The cases with lower RACHS-1 score group have more simple cases and less variation in CPB duration, which may account for the lack of difference in peak PFH between groups with and without AKI. In neonates, 110 mg dl−1 of peak PFH was higher than in other age groups, probably because of more complex surgeries performed in the neonatal period and the longer CPB duration. Additionally, technical factors, such as smaller cannulas, may have affected to greater risk of haemolysis in this population. High haemoglobin concentrations have been associated with haemolysis in paediatric patients on ECMO.21 Similarly, patients requiring CPB with high haemoglobin concentrations, such as those with cyanotic heart disease, might be more susceptible to haemolysis than patients without cyanosis. Therefore, peak PFH was as high as 75 mg dl−1 even in the non-AKI group.
Limitations
Our study has several limitations. Firstly, the age difference among the patients included in the study altered CPB priming, and also the size of the oxygenator, cell saver, and tubing size. Aside from size-based differences, CPB management strategy was uniform as this is a single-centre study, and this should be considered when interpreting the study results. A multicentre study is needed to refute or confirm our findings. Secondly, the low measurement sensitivity of the HemoCue® Plasma/Low Hb system used to measure PFH is another limitation of our study. The absorbance principle used in HemoCue® Plasma/Low Hb system may not differentiate between haeme and free haemoglobin.22 Furthermore, the relationship between PFH and haptoglobin has not been investigated. Thirdly, perfusion pressure, cardiac function, use of inotropic and vasoconstrictor agents, mean arterial pressure, transfusion data, amount of diuretics use in ICU, and other factors that may contribute to the production of PFH and development of AKI were not evaluated. Also, clinical outcomes associated with AKI such as duration of mechanical ventilation and length of stay were not evaluated. In addition, the impact of CPB duration raises the question of whether, rather than peak PFH concentrations, the more important component may be the duration of time that PFH concentrations remain elevated. This was not measured in our study. Fourthly, sCr may not be an appropriate indicator to assess renal function in this population. As glomerular filtration rate does not reach adult concentrations until 2 yr of age,23 the maturity of renal function is not taken into account in this study. Finally, the sample size was calculated assuming a factor of two difference in the incidence of AKI, resulting in an overestimation of the effect of PFH. The results of this study suggest that the impact of PFH is likely to be smaller and the possibility of insufficient sample size should be considered.
In conclusion, there was an association between postoperative AKI and CPB time rather than PFH. Perioperative peak PFH concentrations were significantly higher in patients with postoperative AKI in cardiac surgery requiring CPB in relatively young paediatric patients.
Author's contributions
Study conception: T Sakura, TK
Data collection: all authors
Conducted statistical analyses: T Sakura, TK
Manuscript writing: T Sakura
Manuscript review: T Shimizu, KS, TI, HM
Manuscript revision: TK
Declarations of interest
The authors declare that they have no conflict of interest.
Handling editor: Susan M. Goobie
References
- 1.Pedersen K. Acute kidney injury in children undergoing surgery for congenital heart disease. Eur J Pediatr Surg. 2012;22:426–433. doi: 10.1055/s-0032-1322540. [DOI] [PubMed] [Google Scholar]
- 2.Li S., Krawczeski C.D., Zappitelli M., et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39:1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LoBasso M., Schneider J., Sanchez-Pinto L.N., et al. Acute kidney injury and kidney recovery after cardiopulmonary bypass in children. Pediatr Nephrol. 2022;37:659–665. doi: 10.1007/s00467-021-05179-5. [DOI] [PubMed] [Google Scholar]
- 4.Aydin S.I., Seiden H.S., Blaufox A.D., et al. Acute kidney injury after surgery for congenital heart disease. Ann Thorac Surg. 2012;94:1589–1595. doi: 10.1016/j.athoracsur.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 5.Morgan C.J., Zappitelli M., Robertson C.M.T., et al. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr. 2013;162:120–127.e1. doi: 10.1016/j.jpeds.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 6.O’Neal J.B., Shaw A.D., Billings F.T.I.V. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care. 2016;20:187. doi: 10.1186/s13054-016-1352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neumayr T.M., Alge J.L., Afonso N.S., Akcan-Arikan A. Acute kidney injury after pediatric cardiac surgery. Pediatr Crit Care Med. 2022;23:e249–e256. doi: 10.1097/PCC.0000000000002933. [DOI] [PubMed] [Google Scholar]
- 8.Billings F.T.I.V., Ball S.K., Roberts L.J.I.I., Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. 2011;50:1480–1487. doi: 10.1016/j.freeradbiomed.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borasino S., Kalra Y., Elam A.R., et al. Impact of hemolysis on acute kidney injury and mortality in children supported with cardiac extracorporeal membrane oxygenation. J Extra Corpor Technol. 2018;50:217–224. [PMC free article] [PubMed] [Google Scholar]
- 10.Liberio B.M., Brinton J.T., Gist K.M., Soranno D.E., Kirkley M.J., Gien J. Risk factors for acute kidney injury in neonates with congenital diaphragmatic hernia. J Perinatol. 2021;41:1901–1909. doi: 10.1038/s41372-021-01119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim-Campbell N., Gretchen C., Callaway C., et al. Cell-free plasma hemoglobin and male gender are risk factors for acute kidney injury in low risk children undergoing cardiopulmonary bypass. Crit Care Med. 2017;45:e1123–e1130. doi: 10.1097/CCM.0000000000002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamikonian L.S., Mamo L.B., Smith P.B., Koo J., Lodge A.J., Turi J.L. Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants and children. Pediatr Crit Care Med. 2014;15:e111–e119. doi: 10.1097/PCC.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricci Z., Pezzella C., Romagnoli S., et al. High levels of free haemoglobin in neonates and infants undergoing surgery on cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2014;19:183–187. doi: 10.1093/icvts/ivu129. [DOI] [PubMed] [Google Scholar]
- 14.Ishikura K., Uemura O., Wada N., et al. Pre-dialysis chronic kidney disease in children: results of a nationwide survey in Japan. Nephrol Dial Transplant. 2013;28:2345–2355. doi: 10.1093/ndt/gfs611. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins K.J., Gauvreau K., Newburger J.W., Spray T.L., Moller J.H., Iezzoni L.I. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 16.Kellum J.A., Lameire N., KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 19.Hu J., Rezoagli E., Zadek F., Bittner E.A., Lei C., Berra L. Free hemoglobin ratio as a novel biomarker of acute kidney injury after on-pump cardiac surgery: secondary analysis of a randomized controlled trial. Anesth Analg. 2021;132:1548–1558. doi: 10.1213/ANE.0000000000005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graw J.A., Hildebrandt P., Krannich A., et al. The role of cell-free hemoglobin and haptoglobin in acute kidney injury in critically ill adults with ARDS and therapy with VV ECMO. Crit Care. 2022;26:50. doi: 10.1186/s13054-022-03894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalton H.J., Cashen K., Reeder R.W., et al. Hemolysis during pediatric extracorporeal membrane oxygenation: associations with circuitry, complications, and mortality. Pediatr Crit Care Med. 2018;19:1067–1076. doi: 10.1097/PCC.0000000000001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh J.Y., Hamm J., Xu X., et al. Absorbance and redox based approaches for measuring free heme and free hemoglobin in biological matrices. Redox Biol. 2016;9:167–177. doi: 10.1016/j.redox.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz G.J., Furth S.L. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol. 2007;22:1839–1848. doi: 10.1007/s00467-006-0358-1. Handling editor. [DOI] [PubMed] [Google Scholar]