Abstract
Rhabdomyolysis (RM) is characterised by the breakdown of skeletal muscle tissue, releasing toxic intracellular components into circulation. It presents with dark urine, muscle weakness, myalgia, and elevated creatine phosphokinase levels (CPK). Drug-induced RM is aetiologically significant. This case report describes a 25-year-old male who developed severe RM and Acute Kidney Injury (AKI) after intramuscular (IM) heroin administration as a first time user. IM heroin use can induce higher CPK levels due to direct myocyte toxicity and mechanical trauma. The highly vascularised gluteal muscles with type 1 fibres at the injection site likely exacerbated the severity. Additional factors included lower mitochondrial density in males and alcohol exposure. Despite aggressive fluid resuscitation, renal replacement therapy (RRT) was required, and the patient responded well to haemodialysis. This case highlights AKI as a severe complication of IM heroin use, underscoring the need for further research into drug-induced RM.
Keywords: heroin, rhabdomyolysis, acute kidney injury, intramuscular administration
Introduction
Rhabdomyolysis is a pathological syndrome characterised by disruption of skeletal muscle integrity and the subsequent release of toxic intracellular components into the bloodstream as well as cell components such as myoglobin, creatine kinase, lactate dehydrogenase and potassium. RM manifests with dark urine, acute muscle weakness, myalgia and swelling with CPK >1000 U/l or CPK >5× upper normal limit [1]. RM can be categorised into traumatic and non-traumatic forms based on its aetiology [2]. One of the consequences of RM is AKI which is found in 15%–33% of cases of RM [3]. Drug-induced RM has an approximate incidence rate of 1 in 100 000 [4]. Psychoactive drug-induced RM, predominantly manifests in heroin users (57.2%), followed by amphetamines (30.5%) with cocaine accounting for the smallest proportion (26.6%) [5]. Although RM induced by heroin users is common [5], it rarely results in severe RM [6]. The phenomenon of first-time heroin use via IM administration remains an aspect that warrants further investigation. This report endeavours to elucidate the effects of IM heroin administration in a first-time user presenting with severe RM and AKI.
Case report
A 25 year old male was discovered recumbent by relatives after a night of partying, presenting with emesis, aggression and localised pain in the right lower limb and gluteal region. Initial evaluation in the emergency department revealed no traumatic injury, however, worsening symptoms led to re-evaluation the following day. Laboratory findings revealed unremarkable complete blood count, elevated levels of serum CPK (42 527 IU/l), serum creatinine (735 µmol/l), urea (44.3 mmol/l) and potassium (5.7 mmol/l). Urinalysis revealed red-brown coloured urine without red blood cells, proteinuria (+1), glucosuria (+1) and WBCs (9–10/hpf) (Fig. 1a). These results confirmed RM and AKI. Toxicology established heroin administration 1796 μg/l (> 5× the baseline) without detection of any other substances. Although he initially denied taking medication, after the toxicology analysis, the patient admitted that he administered heroin once intramuscularly in the right gluteal region on the night of the incident.
Figure 1.
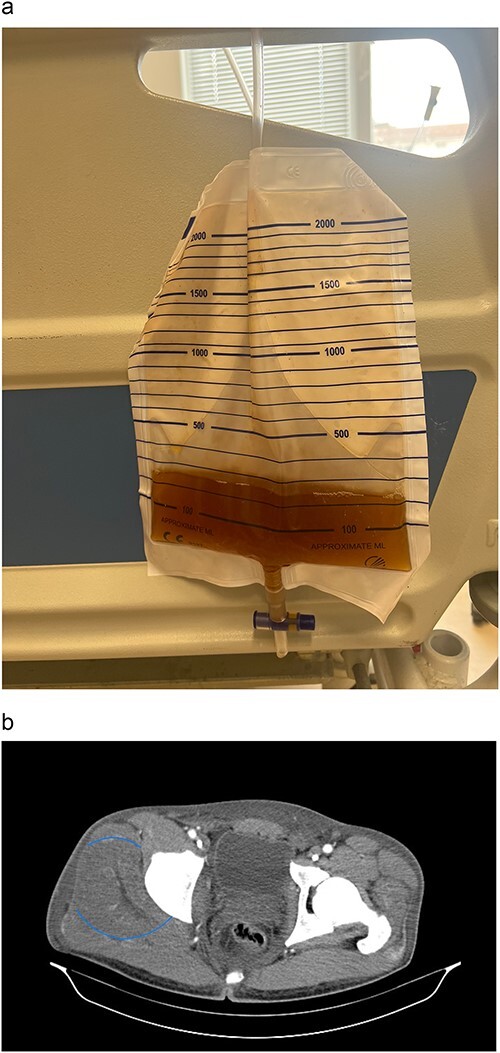
(a) Patient’s urine sample. (b) CT scan of pelvis, axial view showing regional oedema in the right gluteal muscle, indicated by the blue circle.
A CT scan demonstrated gluteal muscle oedema and myositis located in the right gluteal region (Fig. 1b). Upon commencement of conventional treatment with intravenous (IV) fluid resuscitation, AKI management failed, necessitating RRT with haemodialysis due to progression of azotaemia with serum creatinine rising to 1052 µmol/l. Renal function improved post-RRT cessation with a total length of stay (LOS) of 9 days. Subsequent patient follow-up was incomplete due to failure of attendance.
Discussion
The patient presented with classic RM symptoms following IM heroin injection, exhibiting markedly elevated levels of CPK (42 527 IU/l). Severe RM is delineated by a CPK level exceeding 10 000 IU, unequivocally placing our patient within this severe category [3]. Literature suggests that CPK levels >5000 increases the risk of AKI [3]. Meta Analysis reveals that 40.6% of heroin users developed RM while in the wards [5]. Comparative analysis between IM and IV route of heroin administration revealed that while IM administration had significantly lower peak plasma concentrations, heroin remained in circulation for a longer period [7]. This would suggest that the IM route of administration, in contrast to the IV route, potentiates the risk of severe drug-induced RM. Although the IM route of administration is less commonly employed due to inadequate venous access, individuals who abuse heroin are increasingly utilising this method. This practice is associated with complications such as, neuropathy, infections, muscle induration, thrombosis and pain. Compared to IV administration, IM injections incur greater direct myotoxicity and higher CPK levels with consequent necrosis, which can lead to RM and subsequent AKI [5, 8].
The mechanical trauma is postulated to be a significant contributing factor in the pathogenesis of focal nerve injury and localised RM observed in heroin abusers [5]. The pathophysiology involves sarcolemmal disruption from heroin toxins, intracellular calcium influx, and ischemia-induced ATP depletion, culminating in mitochondrial dysfunction and muscle damage [2]. This cascade results in the release of muscle enzymes, including CPK, into the bloodstream. Thus, the markedly elevated CPK levels observed underscore the severity of muscle injury in this patient. Subsequent rupture of muscle membranes also results in the release of myoglobin into the bloodstream initiating intrarenal AKI via nephrotoxic effects of myoglobin on the renal tubules [2].
Further validation of the severity of inflammation and ensuing RM in the right upper quadrant, the site of IM injection of heroine, is supported by data derived from heroin-induced myopathy in rat skeletal muscles. Evidence demonstrates that the vascularity and fibrous composition of the injected muscle influences the progression of RM. The rat model suggested that heroin-induced myopathy was not equal in all muscle groups, indicating that enhanced vascularity facilitates heightened heroin dispersion, with a predominance of type 1 muscle fibres exacerbating vulnerability to heroin-induced RM [9]. Notably, the patient’s point of entry for injecting heroin, the gluteus medius and maximus, are highly vascularised muscles with a predominance of type 1 muscle fibres [10]. This may explicate why the patient exhibited extremely elevated levels of CPK. To further support the severity of RM, it has been documented that males have a lower number of mitochondria in skeletal muscles in comparison to females, consequently, decreasing protective factors against RM due to decreased oxidative phosphorylation capacities, making males more susceptible to RM [5]. Additional factors contributing to RM include alcohol exposure with the severity of RM correlating to the LOS. Pooled incidence for ethanol induced RM was found to be a minute (3.0%) when compared to other psychoactive substances such as heroin (57.2%) [5]. The patient had a total LOS of 216 h, in which a median LOS of patients with severe RM was 108.98 h [6].
The suggested fundamentals of conservative treatment of RM induced AKI remains to be aggressive IV volume resuscitation and administration of diuretics [11]. When severe complications of RM induced AKI such as metabolic derangements (hyperkalaemia, hyperazotaemia and acidosis) ensue without response to conventional treatment, RRT must be initiated [11]. RRT demonstrated a favourable response in patients with AKI secondary to severe RM, with hemofiltration being the most common modality [11]. However, recent literature suggests that haemodialysis with high-permeability membranes play a more valuable role in removing myoglobin in the case of RM induced AKI [11]. Our patient showed poor response to conventional therapies, subsequently undergoing haemodialysis with a positive outcome. The refractoriness to conventional treatment modalities suggests the severity of RM in this case, potentially indicating a more profound manifestation compared to cases involving IV heroin injection.
Acknowledgements
Not applicable.
Contributor Information
Nikolay Dimov, Clinic of Nephrology, UMHAT “Sveti Georgi”, Plovdiv, 15 Vasil Aprilov Blvd., 4002, Bulgaria; Second Department of Internal Diseases, Section of Nephrology, Medical University of Plovdiv, Plovdiv, 15 Vasil Aprilov Blvd., 4002, Bulgaria.
Tahsin Sultana, Medical University of Plovdiv, Plovdiv, 15 Vasil Aprilov Blvd., 4002, Bulgaria.
Aishah Dafeeah, Medical University of Plovdiv, Plovdiv, 15 Vasil Aprilov Blvd., 4002, Bulgaria.
Hafsa Choudhury, Medical University of Plovdiv, Plovdiv, 15 Vasil Aprilov Blvd., 4002, Bulgaria.
Dimitar Nikolov, Clinic of Nephrology, UMHAT “Sveti Georgi”, Plovdiv, 15 Vasil Aprilov Blvd., 4002, Bulgaria; Second Department of Internal Diseases, Section of Nephrology, Medical University of Plovdiv, Plovdiv, 15 Vasil Aprilov Blvd., 4002, Bulgaria.
Conflict of interest
None declared.
Funding
Not applicable.
Ethical approval
Ethical approval was obtained by the hospital ethics committee.
Consent
A written informed patient consent was obtained.
Guarantor
Dr Nikolay Dimov.
References
- 1. Stahl K, Rastelli E, Schoser B. A systematic review on the definition of rhabdomyolysis. J Neurol 2020;267:877–82. [DOI] [PubMed] [Google Scholar]
- 2. Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: pathophysiology and diagnosis. Eur J Intern Med 2007;18:90–100. [DOI] [PubMed] [Google Scholar]
- 3. Ramos DA, Dorgo S. Rhabdomyolysis: considerations for recognition and prevention for practitioners. Strength Cond J 2014;36:56–61. [Google Scholar]
- 4. Wen Z, Liang Y, Hao Y. et al. Drug-induced rhabdomyolysis atlas (DIRA) for idiosyncratic adverse drug reaction management. Drug Discov Today 2019;24:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amanollahi A, Babeveynezhad T, Sedighi M. et al. Incidence of rhabdomyolysis occurrence in psychoactive substances intoxication: a systematic review and meta-analysis. Sci Rep 2023;13:17693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waldman W, Kabata PM, Dines AM. et al. Euro-DEN research group; Dargan PI, Sein Anand J. Rhabdomyolysis related to acute recreational drug toxicity-a euro-DEN study. PLoS One 2021;16:e0246297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rook EJ, Huitema AD, van den Brink W. et al. Pharmacokinetics and pharmacokinetic variability of heroin and its metabolites: review of the literature. Curr Clin Pharmacol 2006;1:109–18. [DOI] [PubMed] [Google Scholar]
- 8. Meyer M, Eichenberger R, Strasser J. et al. One prick and then it’s done: a mixed-methods exploratory study on intramuscular injection in heroin-assisted treatment. Harm Reduct J 2021;18:134. 10.1186/s12954-021-00584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peña J, Aranda C, Luque E. et al. Heroin-induced myopathy in rat skeletal muscle. Acta Neuropathol 1990;80:72–6. [DOI] [PubMed] [Google Scholar]
- 10. Lehecka BJ, Smith BS, Rundell T. et al. The reliability and validity of gluteal endurance measures (GEMs). Int J Sports Phys Ther 2021;16:1442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petejova N, Martinek A. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: a critical review. Crit Care 2014;18:224. [DOI] [PMC free article] [PubMed] [Google Scholar]


