Abstract
Background
The effects of intraoperative permissive hypercapnia (PaCO2 of 45–55 mmHg) on the central nervous system remain unclear. Neurofilament light chain (NfL, a protein found in the axons and nerve fibers of neurons) has been associated with central nervous system disorders. This study investigated the effect of intraoperative permissive hypercapnia on plasma NfL concentration 1 day postoperatively, and in turn on the central nervous system, during laparoscopic surgery.
Methods
This investigation was a prospective, single-blind randomized controlled trial. Eighty-four individuals aged above 60 years were randomly allocated to either the normocapnia group with an PaCO2 of 35–45 mmHg (n=42) or the hypercapnia group with a PaCO2 of 45–55 mmHg (n=42). The primary outcome was the 1-day postoperative plasma NfL concentration. Secondary outcomes included the area under the curve (AUC) values for PaCO2 and regional cerebral oxygen saturation (rSO2). The Mann–Whitney U-test was mainly used to analyze the outcomes.
Results
The final analysis included 38 and 40 patients in the normocapnia and hypercapnia groups, respectively. There was no statistically significant difference observed between the groups regarding the preoperative and 1-day postoperative plasma NfL concentration (14.0 [11.1, 19.9] vs 16.3 [9.06, 19.9] pg/mL, P>0.05; 23.4 [16.8, 32.3] vs 21.5 [15.6, 29.9] pg/mL, P>0.05, respectively). However, in both groups, the postoperative plasma concentration of NfL showed a significant increase when compared with the preoperative levels (both P < 0.001). The AUCs of PaCO2 and rSO2 from the beginning to the end of the pneumoperitoneum were significantly higher in the hypercapnia group compared with the normocapnia group (both P<0.05).
Conclusion
Our results indicate that intraoperative permissive hypercapnia targeting a PaCO2 of 45–55 mmHg does not significantly influence postoperative early plasma NfL elevation levels in elderly patients undergoing laparoscopic surgery. During general anesthesia, intraoperative permissive hypercapnia might not significantly impact the central nervous system.
Keywords: hypercapnia, laparoscopic surgery, neurofilament light chain, general anesthesia
Introduction
Laparoscopic surgery is generally preferred because it causes less trauma compared with open surgery.1 However, there is a potential concern with laparoscopic surgery known as hypercapnia. It occurs due to the contrast in carbon dioxide (CO2) pressure between the abdominal cavity and the blood, along with the high CO2 solubility in the blood, which allows for rapid CO2 absorption through the peritoneum when creating a pneumoperitoneum.2 Concerning major abdominal surgeries, anesthetists have observed that maintaining the arterial partial pressure of carbon dioxide (PaCO2) within a normal range is challenging for certain patients. This has resulted in the practice of permissive hypercapnia, in which a higher-than-normal PaCO2 is tolerated intraoperatively.3
Permissive hypercapnia is a ventilation strategy that involves using low tidal volume ventilation, enabling a slight increase in PaCO2 concentration and permitting a certain acidosis level.4 CO2 plays a crucial role in regulating cerebral vascular tension. It has been found that a 1 mmHg increase in PaCO2 is typically accompanied by a 2%-4% increase in cerebral blood flow in healthy individuals.5 A continuous increase in CO2 concentration can cause brain swelling and intracranial pressure elevation.4,6 The maintenance of mild hypercapnia during surgical operations has demonstrated improved regional cerebral oxygen saturation (rSO2)7 and a lower incidence of postoperative cognitive impairment.8 While a study conducted by Wang et al revealed that the presence of hypercapnia during orthopedic surgery in elderly patients is associated with the emergence and development of postoperative cognitive impairment.9 Therefore, understanding the potential effects of permissive hypercapnia on the central nervous system intraoperatively is essential.
Neurofilament light chain (NfL), a protein found in the axons and nerve fibers of neurons, a protein found in the axons and nerve fibers of neurons, has been associated with a range of central nervous system disorders.10–12 An increase in NfL concentration has been observed in instances of axonal damage resulting from cerebral hypoperfusion.13 According to reports, the cerebrospinal fluid to plasma ratio of NfL is 40:1.14 With the development of the fourth-generation immunoassay technology Simoa, plasma NfL concentration can be measured in picograms, enabling even the slightest changes to be detected.15,16 An increase in NfL concentration can serve as a noninvasive biomarker for identifying patients with brain injuries.17 Observational studies have shown that NfL levels increase in cases of acute injuries (such as traumatic brain injury)18 and post-surgery,19 and are higher in patients with chronic neurological disorders.20,21
The influence of intraoperative permissive hypercapnia on the central nervous system remains uncertain. Therefore, we conducted a randomized controlled trial to investigate the effects of permissive hypercapnia on the central nervous system in elderly patients undergoing laparoscopic surgery by measuring alterations in postoperative plasma NfL levels.
Material and Methods
Trial Design
This single-center, prospective, randomized controlled trial was conducted at the Affiliated Hospital of Jiaxing University between April 2023 and January 2024. The study was approved by the ethics committee of the affiliated hospital of Jiaxing University, China, on February 14, 2023 (reference number 2023-KY-050) and registered with ClinicalTrials.gov (https://clinicaltrials.gov/study/ NCT05793437, principal investigator: Qing-he Zhou, date of registration: March 31, 2023). All participants enrolled in the trial signed written informed consent before enrollment.
Sample Size Calculation
The sample size was calculated using PASS software (Version 15.0). The preliminary study revealed that the plasma NfL concentrations were 21.1 ± 7.3 and 26.8 ± 8.2 pg/mL in the hypercapnia group (unpublished data, n = 14) and the normocapnia group (unpublished data, n = 14) within 24 h postoperatively, respectively. A sample size of 34 patients per group was needed to provide a power of 80% and a two-sided significance level of 0.05. Considering a dropout rate of approximately 20%, the sample size was increased to 84.
Participants and Baseline Data Collection
This study included participants aged 60 years or older who underwent laparoscopic surgery, with an expected pneumoperitoneum duration of > 1 h and required a hospital stay of at least 72 h postoperatively. The selection of the age criterion was based on the guidelines provided by the World Health Organization (https://www.who.int/health-topics/ageing) and recent research conducted on elderly individuals.22–24 Only patients with a body mass index (BMI) of 18–28 kg/m2, who were conscious and able to think independently, and an American Society of Anesthesiologists (ASA) physical status classification of II, or III were included in the study.
The exclusion criteria encompassed severe heart failure defined by New York Heart Association class III or IV, arrhythmia or atrial fibrillation; moderate or severe obstructive lung disease as determined by the preoperative lung function test; renal dysfunction with blood creatinine level > 177 μmol/L or active liver disease; evident neurological and psychiatric disorders; recent use of sedative-hypnotics, analgesics, and antidepressants; intracranial hypertension (including hydrocephalus, brain tumors, and brain-occupying lesions); severe hypertension or hypotension; severe preoperative metabolic acidosis and hypercapnic respiratory failure; undergoing an emergency surgical procedure or being required to convert to an open surgical approach because of unanticipated circumstances; refusal to follow-up postoperatively; serious complications during the procedure, including subcutaneous emphysema; transfer to the intensive care unit after surgery; current participation in other clinical studies that may potentially impact the outcomes of this study.
Following the acquisition of written informed consent, fundamental patient data was gathered. Patients completed the questionnaires at their own convenience, both preoperatively and 1 day postoperatively. The study employed the Confusion Assessment Method (CAM) as a questionnaire to assess delirium, with scores of ≤19 indicating the absence of delirium, scores ranging from 20 to 22 indicating the possibility of delirium, and scores of ≥22 indicating the presence of delirium. Another questionnaire utilized was the quality of recovery (QoR-40), which consisted of 40 questions and evaluated five areas of rehabilitation. Additionally, pain intensity was measured using an 11-point numerical rating scale (NRS) as the third questionnaire.
Randomization and Blinding
Patients were randomly assigned in a 1:1 ratio using random numbers generated from www.random.org and block randomization (block size of 4) to either the normocapnia or hypercapnia group. Assignments were contained in prepared opaque envelopes that were opened on the day of the operation. Each block had a random arrangement of two patients receiving the normocapnia intervention and two patients receiving the hypercapnia intervention. All participants involved with patient care and study data collection/data analysis, including the surgeons, operating room and postanesthesia care unit nurses, research assistants, and statisticians were blinded to the group assignment. Only the attending anesthesiologists performing the anesthesia procedure were not blinded to the study.
Intervention, Anesthesia, and Perioperative Care
Before the procedure, the end-expiratory carbon dioxide (EtCO2) was calibrated. Radial artery catheterization was performed to monitor arterial blood pressure. PaCO2 and arterial partial pressure of oxygen (PaO2) were assessed via arterial blood gas (ABG) analysis before preoxygenation (T0). Rapid anesthesia induction was achieved by intravenous injection of propofol (2.0–2.5 mg/kg), sufentanil (0.3–0.5 μg/kg), and cisatracurium (0.15 mg/kg). Endotracheal intubation and volume control ventilation mode (Carestation 650; General electric medical system Co., Ltd., Wuxi, China) were subsequently performed. The PaCO2 was maintained at 35 to 45 mmHg for the normocapnia group and at 45 to 55 mmHg for the hypercapnia group8 by adjusting respiratory parameters based on the values of PaCO2. The tidal volume (Vt, 6–8 mL/kg of predicted body weight) and respiratory rate (RR, 12–16 times/min) were set in the hypercapnia group; the Vt (8–10 mL/kg of predicted body weight) and RR (12–16 times/min) were set in the normocapnia group. The calculation of predicted body weight (PBW) was calculated as 50+0.91 × (height [cm]−152.4) for man and 45.5+0.91 × (height [cm]−152.4) for women. Remifentanil (0.1–0.3 μg·kg−1·min−1) was intravenously infused, and the anesthesiologist titrated sevoflurane to maintain the BIS within 40–60. ABG analysis was also measured at 30 min (T1), 60 min (T2), and 90 min (T3; in case the pneumoperitoneum time falls short of 90 min, there is no data for T3) after pneumoperitoneum. The intra-abdominal pressure used during surgery was standardized at 10–12 mmHg. ABG was measured again and determined if PaCO2 was within the target range before deflation (T4). The ventilation parameter settings were adjusted if the PaCO2 concentration deviated from the research requirements, and additional ABG samples were collected at the discretion of the anesthetist. Additionally, the lowest pH limit was set to 7.2 for the hypercapnia group. Mean arterial pressure (MAP), blood oxygen saturation, regional cerebral oxygen saturation (rSO2), included heart rate (HR), and PetCO2 were also recorded at five time points (T0-T4).
If the mean arterial pressure was less than 65 mmHg, or there was a decrease in systolic blood pressure of less than 20%, or the systolic blood pressure dropped below 100 mmHg,25 a single intravenous dose of 10–20 micrograms of norepinephrine would be administered. If the systolic blood pressure was above 140 mmHg and the diastolic blood pressure was greater than 90 mmHg, or if there was an increase of more than 20% from the baseline, 5–10 milligrams of urapidil would be given intravenously. If the heart rate was over 100 beats/min, 20–30 mg of esmolol would be administered. If the heart rate was below 50 beats/min, 0.5 mg of atropine would be given intravenously. In cases of oxygen saturation below 90%, respiratory parameters should be adjusted to increase oxygen concentration.
Outcomes Measurement
The primary outcome of this study was the plasma NfL concentration 1 day postoperatively. Furthermore, we observed the following outcomes: the abnormal rate of NfL concentration 1 day postoperatively (referring to the literature26), rSO2, QoR-40, CAM scores, and postoperative delirium (POD) incidence 1 day postoperatively. The area under the curve (AUC) values of PaCO2, rSO2, pH, and MAP from T0 to T4 for the participant were calculated.
Measurement of NfL
Before the surgical procedure, blood samples with a volume of 5 mL were collected. Similarly, blood samples were obtained once again 24 h after the operation. These samples were stored in vacutainer tubes produced by Kang Wei Shi Medical Device Co, LTD, which were equipped with the anticoagulant ethylenediaminetetraacetic acid (EDTA). Subsequently, the tubes were subjected to centrifugation at a speed of 3000 rpm for a duration of 10 minutes within an hour. Following this, the tubes were transferred into 500-μL aliquots using Eppendorf tubes (AXYGEN). The samples were initially preserved at a temperature of −80°C within the laboratory of the affiliated hospital of Jiaxing University. The samples were subsequently transported using dry ice for analysis. The evaluation of NfL concentration was performed using the ultrasensitive Simoa technology on the automated Simoa HD-X platform (Quanterix, MA, US) at GBIO Laboratory in Hangzhou, Zhejiang Province, China. Dilute blood samples at a ratio of 1:4. Measure calibrator and quality control samples in duplicate. All sample measurements should be carried out by laboratory technicians accredited by the committee in a single experiment using the same batch of reagents. The operator should have no knowledge of the participants’ disease status. The coefficients of variation of intra-assay were 5.78% for a quality control sample containing 3.940 pg/mL of NfL and 0.96% for a quality control sample with 177.241 pg/mL of NfL. The lower limit of quantification was recorded as 0.3080 pg/mL.
Measurement of PaCO2 and rSO2
PaCO2 was measured by the Roche Cobas b 123 (Roche Diagnostics, Shanghai, China) in whole blood. The use of exclusively sodium heparin as the anticoagulant for analysis is essential. Additionally, it is crucial to collect the blood sample for analysis from eligible individuals’ blood vessels and immediately send it for analysis, ideally within 15 minutes.
In order to collect rSO2, a FORE-SIGHT regional oximeter manufactured by Gloryway Medical, Beijing, China was used. NIRS sensors were installed on the bilateral regions of the patient’s forehead, facilitating the measurement of rSO2 in both hemispheres of the brain.
Statistical Analysis
The analysis was conducted using SPSS version 23.0 (IBM, Armonk, NY, USA). Continuous data were inspected and tested for distribution using the Shapiro–Wilk test. Normally distributed data were presented as mean (Standard deviation; SD) and analysed by unpaired Student’s t-test between the groups. Non-normally distributed data were expressed as median (25%–75% range) and analysed by the Mann–Whitney U-test between the groups. For intra-group comparisons, Wilcoxon-tests or paired Student’s t-test are used to analyze. The χ²-test, continuity correction χ²-test, or Fisher’s exact test was employed to analyze categorical variables, as appropriate. A significance level of P < 0.05 was considered statistically significant.
Results
Patient Characteristics
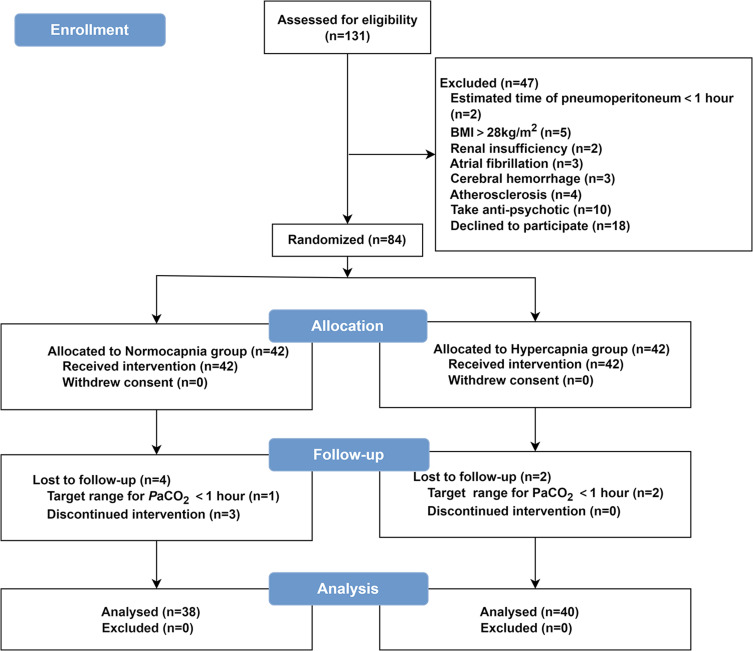
From April 2023 to January 2024, we enrolled 131 elderly patients undergoing laparoscopic surgery. Of whom 84 patients signed the informed consent form and were enrolled in the study. 6 patients were lost to follow-up because 3 had the duration of the target range for PaCO2 is <1 h and 3 were discontinued from the intervention (2 were converted to an open surgical approach because of unanticipated circumstances; 1 was transferred to the intensive care unit postoperatively). The final analysis included 78 individuals, including 38 and 40 in the normocapnia and hypercapnia group, respectively (Figure 1). The characteristics of the patients at baseline and peri-operation are shown in Table 1.
Figure 1.
Patient flow through the study.
Table 1.
Baseline and Perioperative Characteristics of the Included Patients
| Normocapnia (n=38) | Hypercapnia (n= 40) | P values | |
|---|---|---|---|
| Age (years) | 69.4 ± 5.2 | 69.1 ± 5.9 | 0.816 |
| Male sex, n (%) | 19 (50.0%) | 27 (67.5%) | 0.116 |
| BMI (kg/m2) | 22.8 ± 2.6 | 22.8 ± 2.4 | 0.978 |
| Education (years) | 6 (0, 9) | 6 (6, 9) | 0.491 |
| ASA physical status, n (%) | 0.115 | ||
| II | 20 (52.6%) | 28 (70.0%) | |
| III | 18 (47.4%) | 12 (30.0%) | |
| Comorbidities | |||
| Hypertension, n (%) | 20 (52.6%) | 25 (62.5%) | 0.378 |
| Diabetes, n (%) | 5 (13.2%) | 5 (12.5%) | 0.931 |
| Heart disease, n (%) | 5 (13.2%) | 3 (7.5%) | 0.653 |
| Respiratory disease, n (%) | 4 (10.5%) | 5 (12.5%) | 1.000 |
| Perioperative characteristics | |||
| PaO2 (mmHg) | 85.0 (77.0, 91.0) | 81.0 (73.6, 87.4) | 0.218 |
| PaCO2 (mmHg) | 39.0 (36.3, 40.2) | 39.4 (36.0, 44.0) | 0.545 |
| pH | 7.43 (7.41, 7.45) | 7.42 (7.41, 7.43) | 0.287 |
| Hb (g/L) | 126 ± 21.3 | 127 ± 18.4 | 0.838 |
| Scr (μmol/L) | 73.8 (68.5, 85.4) | 73.8 (66.3, 90.5) | 0.795 |
| BUN (mmol/L) | 5.08 (4.31, 6.26) | 5.47 (4.56, 6.71) | 0.263 |
| Surgery type, n (%) | 0.549 | ||
| Prostatic resection | 0 (0.0) | 2 (5.0%) | |
| Gastric resection | 4 (10.5%) | 3 (7.5%) | |
| Colorectal resection | 34 (89.5%) | 35 (87.5%) |
Note: Data are presented as the mean ± SD, median (interquartile range), or number of patients (%). P-value as a result of t-test, U Mann–Whitney or χ²-test.
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists; PaO2, partial pressures of oxygen in arterial blood; PaCO2, partial pressures of carbon dioxide in arterial blood; Hb, haemoglobin; Scr, serum creatinine; BUN, blood urea nitrogen.
Neurofilament Light
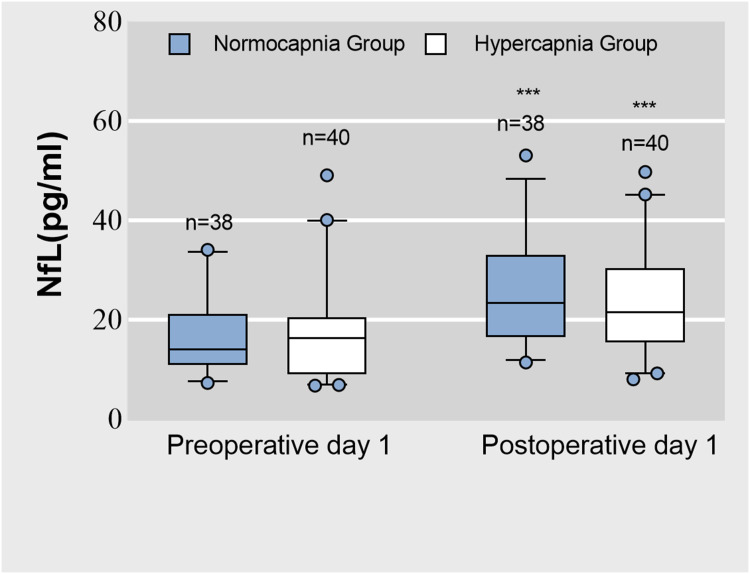
The normocapnia group exhibited a significant increase in plasma NfL concentration from preoperatively to postoperatively (14.0 [11.1, 19.9] vs 23.4 [16.8, 32.3] pg/mL, P < 0.001; Figure 2). Similarly, the hypercapnia group showed a significant increase in plasma NfL concentration from preoperatively to postoperatively (16.3 [9.1, 19.9] vs 21.5 [15.6, 29.9] pg/mL, P < 0.001; Figure 2). However, there were no significant differences in plasma NfL concentration between the groups at baseline and 1 day postoperatively (both P > 0.05). Additionally, the abnormal rate of NfL 1 day postoperatively did not differ between the two groups (hypercapnia group, 15.0%; normocapnia group, 26.3%; RR: 0.57; 95% CI: 0.23–1.42; P > 0.05).
Figure 2.
The plasma neurofilament light chain (NfL) concentration on preoperative and postoperative day 1. ***Compared with preoperative, postoperative plasma NfL concentration was significantly higher (P < 0.001, the Mann–Whitney U-test).
Postoperative Delirium
The CAM scores did not show a significant difference between the two groups (P > 0.05). Among the 78 analyzed patients, 3 (3.8%) developed delirium 1 day postoperatively. The association between the incidence of POD and the use of permissive hypercapnia was not significant, with the hypercapnia group showing an incidence of 5.0% compared to 2.6% in the normocapnia group (RR: 1.90; 95% CI: 0.18–20.1; P > 0.05; Table 2).
Table 2.
Postoperative Data
| Normocapnia (n=38) | Hypercapnia (n=40) | P value | |
|---|---|---|---|
| Extubation time (min) | 19.8 (18.0, 30.0) | 25.2 (16.5, 42.6) | 0.606 |
| PACU duration (min) | 87.6 (71.4, 109) | 95.0 (65.0, 110.0) | 0.947 |
| QoR-40 scores | 173 ± 7.9 | 181 ± 6.2 | 0.079 |
| NRS scores | 2 (2.0, 3.0) | 2 (2.0, 3.0) | 0.503 |
| Postoperative CAM | 17 (14.0, 17.0) | 17 (14.8, 20.8) | 0.156 |
| Presence of postoperative delirium, n (%) | 1 (2.6%) | 2 (5.0%) | 1.000 |
| Postoperative hospital duration (day) | 9 (8.0, 10.0) | 9 (8.0, 11.0) | 0.245 |
| Complications after surgery | |||
| Abdominal infection, n (%) | 7 (18.4%) | 5 (12.5%) | 0.469 |
| Pleural effusion, n (%) | 0 (0) | 2 (5.0%) | 0.241 |
| Feeble, n (%) | 5 (13.2%) | 4 (10.0%) | 0.935 |
| Nausea and vomiting, n (%) | 11 (28.9%) | 7 (17.5%) | 0.230 |
| PPCs, n (%) | 10 (26.3%) | 7 (17.5%) | 0.346 |
| Fever, n (%) | 10 (26.3%) | 9 (22.5%) | 0.695 |
Note: Data are presented as the mean ± SD, median (interquartile range), or number of patients (%). P-value as a result of t-test, U Mann–Whitney or χ²-test.
Abbreviations: PACU, postanesthesia care unit; QoR-40, the quality of recovery-40 Questionnaire; NRS, numerical rating scale; CAM, the Confusion Assessment Method; PPCs, postoperative pulmonary complications.
PaCO2, rSO2, pH, and MAP
The AUCs values of PaCO2 and rSO2 from the beginning to the end of the pneumoperitoneum were significantly higher in the hypercapnia group compared with the normocapnia group (P < 0.05). The two groups demonstrated no significant difference in the AUCs values of pH and MAP (both P > 0.05) (Table 3, supplementary Figure 1 and 2).
Table 3.
AUCs of PaCO2, rSO2, pH, MAP in the Two Groups
| Normocapnia (n=38) | Hypercapnia (n=40) | P value | |
|---|---|---|---|
| AUC of PaCO2 | 5888 (4390, 6754) | 6645 (4971, 7805) | 0.025 |
| AUC of rSO2 | 8775 (8415, 8948.0) | 11,625 (10,283.0, 13,875.0) | 0.012 |
| AUC of pH | 1069 (892.0, 1300.0) | 1027 (770.0, 1172.0) | 0.284 |
| AUC of MAP | 11955 (10,416.0, 15,108.0) | 11,490 (9175.0, 13,658.0) | 0.159 |
Note: Data are presented as median (interquartile range). P-value as a result of U Mann–Whitney.
Abbreviations: AUC, area under the curve; PaCO2, partial pressures of carbon dioxide in arterial blood; rSO2, regional cerebral oxygen saturation; MAP, mean arterial pressure.
Other Intraoperative Characteristics and Postoperative Outcomes
The two groups showed no significant difference in the NRS scores (P > 0.05), as well as QoR-40 scores postoperatively (P > 0.05; Table 2). However, the normocapnia group had a significantly higher incidence of hypotension compared to the hypercapnia group (P < 0.05, Table 4). Other results did not differ between the two groups (all P > 0.05).
Table 4.
Intraoperative Data
| Normocapnia (n= 38) | Hypercapnia (n= 40) | P values | |
|---|---|---|---|
| Duration of anesthesia (min) | 171 (150, 201) | 164 (135, 187) | 0.358 |
| Duration of surgery (min) | 149 (121,174) | 157 (135,187) | 0.358 |
| Duration of pneumoperitoneum (min) | 143 (120, 170) | 133 (105, 160) | 0.322 |
| Total fluid infusion | |||
| Crystalloid solution (mL) | 1000 (1000, 1300) | 1000 (1000, 1500) | 0.835 |
| Colloidal solution (mL) | 500 (500, 500) | 500 (250, 500) | 0.169 |
| Red blood cells | 0 | 0 | |
| Urine output (mL) | 275 (150, 400) | 200 (150, 300) | 0.556 |
| Estimated blood loss (mL) | 30 (30, 50) | 50 (30, 50) | 0.751 |
| Intraoperative drugs | |||
| Propofol (mg) | 150 (120, 200) | 150 (108, 200) | 0.460 |
| Sufentanil (μg) | 25 (25, 30) | 27 (20, 30) | 0.747 |
| Atracurium (mg) | 22.2 ± 7.3 | 24.4 ± 4.3 | 0.207 |
| Sevoflurane (mL/h) | 16.6 (14.0, 19.8) | 15.8 (12.3, 18.7) | 0.351 |
| Remifentanil (mg) | 1.2 (1.0, 1.6) | 1.2 (1.0, 1.4) | 0.135 |
| Atropine, n (%) | 8 (21.1%) | 13 (32.5%) | 0.255 |
| Vasopressor drugs, n (%) | 24 (63.2%) | 31 (77.5%) | 0.165 |
| Hypotensor, n (%) | 0 (0) | 0 (0) | |
| Adverse events, n (%) | |||
| Hypotension | 20 (52.6%) | 8 (20.0%) | 0.003 |
| Hypertension | 5 (13.2%) | 6 (15.0%) | 0.815 |
| Bradycardia | 15 (39.5%) | 11 (27.5%) | 0.262 |
| Tachycardia | 4 (10.5%) | 1 (2.5%) | 0.325 |
| Hypoxemia | 1 (2.6%) | 1 (2.5%) | 1.000 |
Note: Data are presented as the mean ± SD, median (interquartile range), or number of patients (%). P-value as a result of t-test, U Mann–Whitney or χ²-test.
Discussion
The study primarily revealed that postoperative plasma NfL concentrations were notably elevated compared to preoperative levels in all patients. Nevertheless, there was no variation in plasma NfL concentration on postoperative day 1 between patients who underwent permissive hypercapnia with a target PaCO2 of 45–55 mmHg and those who did not. Intraoperative permissive hypercapnia might not significantly impact the central nervous system during general anesthesia.
Our trial revealed that the NfL concentration 1 day postoperatively was associated with slightly and nonsignificantly lower postoperative plasma NfL elevation levels, indicating that permissive hypercapnia may not harm the central nervous system. The slightly lower postoperative plasma NfL levels during intraoperative permissive hypercapnia may be linked to the rise in PaCO2 levels, which in turn activated the sympathetic adrenal mechanism and sympathetic nervous tension.27 This ultimately led to an elevation in blood pressure and an enhancement in brain oxygen delivery caused by an increase in cerebral perfusion.28 Physiologically, NfL is more easily detected in the axons of the brain white matter,10 and radiological studies have shown that the brain white matter is particularly susceptible to ischemic damage.29 Cerebral ischemia, caused by hypotension, can be decreased by permissive hypercapnia with increasing in cerebral perfusion,30 which may be the anatomical basis for the above-mentioned results.
The AUC of PaCO2 from T0-T4 was calculated for participants, and the PaCO2 levels were significantly higher in patients with hypercapnia than with normocapnia during surgery. The current study revealed that higher rSO2 levels are associated with permissive hypercapnia, which is congruent with previous data.8 According to Wong et al,8 hypercapnia (PaCO2 45–55mmHg) resulted in a stable increase in rSO2 values in both hemispheres compared to the baseline, while the control group (PaCO2 35–40mmHg) showed a decrease in rSO2. In our study, no statistically significant difference was revealed in POD, which agrees with the primary outcome measure. The differences in result compared to Wang et al9 may be attributed to the varying range of control for intraoperative hypercapnia.4,6
On postoperative day 1, the plasma NfL concentration indicates a significant increase compared to the baseline level. This observation is associated with the results of a previous study, which demonstrated that anesthesia and surgery are associated with neuronal damage and a notable rise in postoperative plasma NfL concentration.19 Research revealed an independent correlation between the severity of delirium and NfL levels 1 day postoperatively.19,31
Our trial had several strengths. Previous studies have mainly focused on the effects of PetCO2 on the central nervous system.32–34 Our study continuously monitored changes in intraoperative blood gas parameters and adjusted the ventilator settings to maintain the target range of PaCO2, ensuring the accuracy of the experiment. NfL, a component of the neuronal cytoskeleton, is increasingly recognized as a specific biomarker in the clinical evaluation of neurological patients.10 Additionally, the high sensitivity of the Simoa technique allowed reliable quantification of NfL in blood, obviating the need for lumbar punctures and facilitating repeat assays in the perioperative period.19
The trial had some limitations. First, this study only set the range of PaCO2 with hypercapnia intervention to be less than 55 mmHg, which is the common scope of laparoscopic surgery, without considering the effect of a higher PaCO2 range on NfL. Second, blinding the procedure was not feasible in this trial as ventilation parameters had to be continuously adjusted according to PaCO2 levels, resulting in an open-label design. Third, the trial only assessed NfL levels preoperatively and 1 day postoperatively. Bias development due to the short follow-up period could not be ruled out.
Conclusion
In conclusion, anesthesia and surgery can cause a significant increase in postoperative plasma NfL levels among all patients; intraoperative permissive hypercapnia targeting a PaCO2 of 45–55 mmHg does not significantly influence postoperative early plasma NfL elevation levels in elderly patients undergoing laparoscopic surgery. These suggest that intraoperative permissive hypercapnia may not significantly affect the central nervous system, while anesthesia and surgery may have a certain influence.
Acknowledgments
The authors express their gratitude to Xiao-Ping Shen, Yang-Yang Chen, and their colleagues for helping to collect blood samples.
Funding Statement
The study was approved by the Zhejiang Medical and Health Science and Technology Plan Project (2024KY436), the Key Discipline Established with Zhejiang Provincial Traditional Chinese Medical Innovation Team (No. 2022-19), Zhejiang Provincial Key Clinical Specialty-Anesthesiology (2023-ZJZK-001) and Jiaxing Key Discipline of Medicine Anesthesiology (2023-ZC-001).
Abbreviation
PaCO2, arterial carbon dioxide partial pressure; CO2, carbon dioxide; BMI, body mass index; ASA, American Society of Anesthesiologists; NfL, Neurofilament light chain; POD, postoperative delirium; QoR-40, quality of recovery-40; NRS, numerical rating scale; AUC, area under the curve; rSO2, regional cerebral oxygen saturation; CAM, Confusion Assessment Method; EtCO2, end-expiratory carbon dioxide; PetCO2, end-expiratory carbon dioxide partial pressure; HR, heart rate; BIS, bispectral index; PaO2, arterial partial pressure of oxygen; ABG, arterial blood gas; Vt, tidal volume; RR, respiratory rate; MAP, Mean arterial pressure; POD, postoperative delirium; PBW, predicted body weight (PBW); EDTA, ethylenediaminetetraacetic acid; SD, standard deviation.
Data Sharing Statement
All the data and material generated during the current study are available from the corresponding author upon reasonable request (zqh10980@zjxu.edu.en).
Ethics Approval and Informed Consent
The study was approved by the ethics committee of the affiliated hospital of Jiaxing University, China, on February 14, 2023 (reference number 2023-KY-050). This study was registered with clinicaltrials.gov (https://clinicaltrials.gov/study/NCT05793437, principal investigator: Qing-he Zhou, date of registration: March 31, 2023) before patient enrollment. All enrolled participants in the trial signed written informed consent preoperatively. The research protocol complied with the Consolidated Standards of Reporting Trials (CONSORT) statement and the Helsinki Declaration.
Consent for Publication
The details of any images, videos, recordings, etc can be published.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Enomoto T, Saida Y, Takabayashi K, et al. Open surgery versus laparoscopic surgery after stent insertion for obstructive colorectal cancer. Surg Today. 2016;46(12):1383–1386. doi: 10.1007/s00595-016-1331-7 [DOI] [PubMed] [Google Scholar]
- 2.Yu T, Cheng Y, Wang X, et al. Gases for establishing pneumoperitoneum during laparoscopic abdominal surgery. Cochrane Database Syst Rev. 2017;6(6):CD009569. doi: 10.1002/14651858.CD009569.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joe YE, Lee CY, Kim N, et al. Effect of permissive hypercarbia on lung oxygenation during one-lung ventilation and postoperative pulmonary complications in patients undergoing thoracic surgery: a prospective randomised controlled trial. Eur J Anaesthesiol. 2023;40(9):691–698. doi: 10.1097/EJA.0000000000001873 [DOI] [PubMed] [Google Scholar]
- 4.Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 1990;16(6):372–377. doi: 10.1007/BF01735174 [DOI] [PubMed] [Google Scholar]
- 5.Ito H, Kanno I, Ibaraki M, et al. Changes in human cerebral blood flow and cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab. 2003;23(6):665–670. doi: 10.1097/01.WCB.0000067721.64998.F5 [DOI] [PubMed] [Google Scholar]
- 6.Dinsmore M, Han JS, Fisher JA, et al. Effects of acute controlled changes in end-tidal carbon dioxide on the diameter of the optic nerve sheath: a transorbital ultrasonographic study in healthy volunteers. Anaesthesia. 2017;72(5):618–623. doi: 10.1111/anae.13784 [DOI] [PubMed] [Google Scholar]
- 7.Park CG, Jung WS, Park HY, et al. Comparison of the effects of normocapnia and mild hypercapnia on the optic nerve sheath diameter and regional cerebral oxygen saturation in patients undergoing gynecological laparoscopy with total intravenous anesthesia. J Clin Med. 2021;10(20):4707. doi: 10.3390/jcm10204707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong C, Churilov L, Cowie D, et al. Randomised controlled trial to investigate the relationship between mild hypercapnia and cerebral oxygen saturation in patients undergoing major surgery. BMJ Open. 2020;10(2):e029159. doi: 10.1136/bmjopen-2019-029159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Li Z, Yu Y, et al. Risk factors contributing to postoperative delirium in geriatric patients postorthopedic surgery. Asia Pac Psychiatry. 2015;7(4):375–382. doi: 10.1111/appy.12193 [DOI] [PubMed] [Google Scholar]
- 10.Gaetani L, Blennow K, Calabresi P, et al. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870–881. doi: 10.1136/jnnp-2018-320106 [DOI] [PubMed] [Google Scholar]
- 11.Casey CP, Lindroth H, Mohanty R, et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143(1):47–54. doi: 10.1093/brain/awz354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fong TG, Vasunilashorn SM, Ngo L, et al. Association of plasma neurofilament light with postoperative delirium. Ann Neurol. 2020;88(5):984–994. doi: 10.1002/ana.25889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakimovski D, Gibney BL, Marr K, et al. Lower cerebral arterial blood flow is associated with greater serum neurofilament light chain levels in multiple sclerosis patients. Eur J Neurol. 2022;29(8):8):2299–2308. doi: 10.1111/ene.15374 [DOI] [PubMed] [Google Scholar]
- 14.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577–589. doi: 10.1038/s41582-018-0058-z [DOI] [PubMed] [Google Scholar]
- 15.Rohrer JD, Woollacott IO, Dick KM, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87(13):1329–1336. doi: 10.1212/WNL.0000000000003154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rissin DM, Kan CW, Campbell TG, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010;28(6):595–599. doi: 10.1038/nbt.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fohner AE, Bartz TM, Tracy RP, et al. Association of serum neurofilament light chain concentration and MRI findings in older adults: the cardiovascular health study. Neurology. 2022;98(9):e903–e911. doi: 10.1212/WNL.0000000000013229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahim P, Politis A, van der Merwe A, et al. Neurofilament light as a biomarker in traumatic brain injury. Neurology. 2020;95(6):e610–e622. doi: 10.1212/WNL.0000000000009983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evered L, Silbert B, Scott DA, et al. Association of changes in plasma neurofilament light and tau levels with anesthesia and surgery: results from the CAPACITY and ARCADIAN studies. JAMA Neurol. 2018;75(5):542–547. doi: 10.1001/jamaneurol.2017.4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattsson N, Andreasson U, Zetterberg H, et al. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74(5):557–566. doi: 10.1001/jamaneurol.2016.6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Disanto G, Barro C, Benkert P, et al. Serum Neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857–870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan MT, Cheng BC, Lee TM, et al. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25(1):33–42. doi: 10.1097/ANA.0b013e3182712fba [DOI] [PubMed] [Google Scholar]
- 23.Pan H, Liu C, Ma X, et al. Perioperative dexmedetomidine reduces delirium in elderly patients after non-cardiac surgery: a systematic review and meta-analysis of randomized-controlled trials. Can J Anesth. 2019;66(12):1489–1500. doi: 10.1007/s12630-019-01440-6 [DOI] [PubMed] [Google Scholar]
- 24.van Norden J, Spies CD, Borchers F, et al. The effect of peri-operative dexmedetomidine on the incidence of postoperative delirium in cardiac and non-cardiac surgical patients: a randomised, double‐blind placebo‐controlled trial. Anaesthesia. 2021;76(10):1342–1351. doi: 10.1111/anae.15469 [DOI] [PubMed] [Google Scholar]
- 25.Wickham AJ, Highton DT, Clark S, et al. Treatment threshold for intra-operative hypotension in clinical practice-a prospective cohort study in older patients in the UK. Anaesthesia. 2022;77(2):153–163. doi: 10.1111/anae.15535 [DOI] [PubMed] [Google Scholar]
- 26.Bornhorst JA, Figdore D, Campbell MR, et al. Plasma neurofilament light chain (NfL) reference interval determination in an Age-stratified cognitively unimpaired cohort. Clin Chim Acta. 2022;535:153–156. doi: 10.1016/j.cca.2022.08.017 [DOI] [PubMed] [Google Scholar]
- 27.Kiely DG, Cargill RI, Lipworth BJ. Effects of hypercapnia on hemodynamic, inotropic, lusitropic, and electrophysiologic indices in humans. Chest. 1996;109(5):1215–1221. doi: 10.1378/chest.109.5.1215 [DOI] [PubMed] [Google Scholar]
- 28.Glodzik L, Rusinek H, Tsui W, et al. Different relationship between systolic blood pressure and cerebral perfusion in subjects with and without hypertension [published correction appears in hypertension. Hypertension. 2020;75(6):e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luyt CE, Galanaud D, Perlbarg V, et al. Diffusion tensor imaging to predict long-term outcome after cardiac arrest: a bicentric pilot study. Anesthesiology. 2012;117(6):1311–1321. doi: 10.1097/ALN.0b013e318275148c [DOI] [PubMed] [Google Scholar]
- 30.Sobczyk O, Battisti-Charbonney A, Fierstra J, et al. A conceptual model for CO2-induced redistribution of cerebral blood flow with experimental confirmation using BOLD MRI. Neuroimage. 2014;92:56–68. doi: 10.1016/j.neuroimage.2014.01.051 [DOI] [PubMed] [Google Scholar]
- 31.Brown CH, Lewis A, Probert J, et al. Perioperative neurofilament light plasma concentrations and cognition before and after cardiac surgery: a prospective nested cohort study. Anesthesiology. 2022;137(3):303–314. doi: 10.1097/ALN.0000000000004327 [DOI] [PubMed] [Google Scholar]
- 32.Mutch WAC, El-Gabalawy R, Girling L, et al. End-tidal hypocapnia under anesthesia predicts postoperative delirium. Front Neurol. 2018;9:678. doi: 10.3389/fneur.2018.00678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahrens E, Tartler TM, Suleiman A, et al. Dose-dependent relationship between intra-procedural hypoxaemia or hypocapnia and postoperative delirium in older patients. Br J Anaesth. 2023;130(2):e298–e306. doi: 10.1016/j.bja.2022.08.032 [DOI] [PubMed] [Google Scholar]
- 34.Schopfer L, Habre W, Pichon I, et al. Effect of permissive mild hypercapnia on cerebral vasoreactivity in infants: a randomized controlled crossover trial. Anesth Analg. 2021;133(4):976–983. doi: 10.1213/ANE.0000000000005325 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data and material generated during the current study are available from the corresponding author upon reasonable request (zqh10980@zjxu.edu.en).