Abstract
Background
GNL2, a nuclear protein, is involved in ribosome production and cell cycle regulation. However, its expression and function in different types of tumors are not well understood. Comprehensive studies across multiple cancer types are needed to assess the potential of GNL2 as a diagnostic, prognostic, and immunological marker.
Methods
mRNA expression data, copy number alteration threshold data, masked copy number segmentation data, and DNA methylation 450 K data from The Cancer Genome Atlas (TCGA) pan-cancer cohort were obtained from the Firehose database. Additional data, including miRNA, The Cancer Proteome Atlas (TCPA), mutation data, and clinical information, were sourced from the University of California Santa Cruz (UCSC) Xena database. The cBioPortal database facilitates the examination of GNL2 mutation frequency, location, and 3D structure in the TCGA database. Gene Expression Omnibus (GEO) data verified the transcriptome level expression in the TCGA cohort. Protein expression levels were analyzed via the Human Protein Atlas (HPA) database and the Clinical Proteomic Tumor Analysis Consortium (CPTAC) database. Gene set enrichment analysis (GSEA) was employed to investigate the biological role of GNL2 across cancers. Multiple immune infiltration algorithms from the TIMER2.0 database were utilized to examine the correlation between GNL2 expression and the tumor immune microenvironment. The transcriptome-wide immune infiltration results were validated using 72 single-cell datasets from the Tumor Immune Single-cell Hub (TISCH) database. Pan-cancer survival maps were constructed, and GNL2 expression in different molecular subtypes across cancers was examined. The relationship between GNL2 and drug resistance was investigated using data from CellMiner, GDSC, and CTRP. The Comparative Toxicogenomics Database (CTD) was used to identify chemicals affecting GNL2 expression.
Results
GNL2 is located primarily in the nucleus, and its expression is regulated mainly through somatic copy number alteration (SCNA) and aberrant DNA methylation, according to TCGA data. Database analysis and immunohistochemical results from clinical samples revealed high GNL2 expression in most tumors, which was correlated with diagnostic significance. High GNL2 expression often indicates a poor prognosis with pan-cancer prognostic value. Gene set enrichment analysis (GSEA) suggested that GNL2 is involved in tumor development through cell proliferation-related pathways. GNL2 expression is correlated with the expression of immune-related genes and the infiltration levels of multiple immune cells. The relationships between GNL2 and various drugs and chemicals were examined, revealing its influence on drug sensitivity and identifying five chemicals countering GNL2-mediated pro-cancer effects.
Conclusion
Comprehensive bioinformatics analysis of GNL2 in pan-cancer tissues, combined with experimental validation, elucidated the pan-cancer expression pattern of GNL2, determined its diagnostic and prognostic value, and explored the biological functions of GNL2. GNL2 may be involved in the regulation of cell cycle progression and remodeling of the tumor microenvironment and is associated with poor prognosis as a risk factor in most tumors. The potential of GNL2-based cancer therapies is emphasized, assisting in predicting the response to chemotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-024-01516-w.
Keywords: GNL2, Pan-cancer, Biomarkers, Survival, Diagnosis, Prognosis, TME
Introduction
Cancers represent a significant public health challenge worldwide, with the number of cases and related fatalities increasing annually [1]. Recent advancements in immunotherapy and antibody-drug conjugates (ADCs) have led to a significant shift in tumor treatment, leading to notable improvements in patient survival. However, these treatments do not benefit all patients equally, a phenomenon closely linked to tumor heterogeneity [2]. The ongoing exploration of tumors has deepened with the advent of high-throughput sequencing technology and the integration of genomics and bioinformatics. These advancements have systematically elucidated the molecular mechanisms driving tumor development and the biological basis of tumor heterogeneity, thereby enhancing the understanding of tumors. Investigations into the entire tumor ecosystem, particularly the tumor microenvironment, have broadened research perspectives in tumor treatment and provided a foundation for optimizing patient stratification, thereby facilitating personalized and precise treatment [3].
The complexity and heterogeneity of carcinogenesis necessitate pan-cancer level analysis. By integrating genomic data from large-scale, multi-omics measurements and examining the expression patterns of single genes in pan-cancer tissues, a more comprehensive understanding of their molecular functions and regulatory networks can be achieved. This approach not only aids in determining the diagnostic and prognostic value of genes at the pan-cancer level but also provides strategies for identifying new therapeutic targets [4, 5]. Nucleolar GTP-binding protein 2 (GNL2), also known as NGP-1 and nucleostemin 2, is a member of the Nucleostemin (NS) family and plays crucial roles in stem cell growth and development, ribosome biogenesis, and protein synthesis [6]. GNL2 is involved in nucleolar signaling pathways, particularly in cell cycle regulation [7, 8]. Dysregulation of GNL2 expression has been observed in various cancers, prompting investigations into its biological function in relation to cancer development. In the RNA-metasome network for macromolecular biosynthesis in human cells described by Shiro Iuchi et al., GNL2 is a central member involved in pre-60 S ribosome maturation and chromatin organization and is associated mainly with cell proliferation. Knockdown of GNL2 hinders cell proliferation [9, 10]. Dong et al. [11] demonstrated that genes co-expressed with GNL2 are involved mainly in ribosome biosynthesis and that GNL2 knockdown in HCC cells significantly reduces cell proliferation, migration, and invasive ability, suggesting a pro-carcinogenic role for GNL2 in HCC. In a study on high-grade plasmacytoid ovarian cancer, GNL2 was found to be highly expressed in ovarian cancer, with its silencing reducing xenograft tumor formation and inhibiting the growth, invasion, and migration of ovarian cancer cells [12]. This study hypothesizes that GNL2 may act as an oncogene in cancer development, providing a new theoretical framework for understanding tumor biology and potentially leading to new diagnostic and therapeutic avenues. However, the current understanding of the role of GNL2 in cancer is limited to specific cancer types, and its pan-cancer diagnostic and prognostic value remains unclear.
In this study, GNL2 dysregulation across various cancers was systematically studied through the integration of multi-genomics and bioinformatics. This study comprehensively analyzed the GNL2 profile in human cancers, including its expression patterns and mutation status in pan-cancer, and explored its relationship with the clinical features of pan-cancer and potential mechanisms of action [13]. Dysregulation of GNL2 expression was found to be correlated with various cancer characteristics, the tumor microenvironment, drug resistance, and clinical prognosis, highlighting the critical role of GNL2 in cancer. This study, therefore, provides a foundation for further research on GNL2-associated molecular mechanisms and therapeutic development. All data for this study were sourced from reputable open databases, and analyses were conducted using web tools and the R language.
Materials and methods
Datasets and source
mRNA expression data, copy number alteration threshold data, masked copy number segmentation data, and DNA methylation 450 K data for The Cancer Genome Atlas (TCGA) pan-cancer cohort were downloaded from the Firehose databases (http://gdac.broadinstitute.org). These datasets included tumor tissue samples and normal tissue samples. miRNA, The Cancer Proteome Atlas (TCPA), mutation data, molecular subtyping, and clinically relevant data were obtained from the University of California Santa Cruz (UCSC) Xena database (https://xenabrowser.net/datapages/). External validation data at the mRNA level were sourced from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/), and protein-level mass spectrometry data were obtained from the Clinical Proteomic Tumor Analysis Consortium (CPTAC) database (https://proteomics.cancer.gov/programs/cptac). Immunohistochemistry and immunofluorescence data were sourced from the Human Protein Atlas (HPA) database. Pan-cancer immune infiltration results, assessed by multiple immune infiltration algorithms, were obtained from the TIMER2.0 database (http://timer.cistrome.org/). Importantly, these public databases are freely accessible, and the study adhered strictly to their data extraction policies, negating the need for ethical review and approval from an ethics committee.
Expression profiling analysis of GNL2
Three-dimensional difference analysis was performed to validate the dysregulation of GNL2 expression between tumors and normal tissues. Initially, to compensate for the limited number of normal tissues in the TCGA dataset, normal tissue expression data from the Genotype-Tissue Expression (GTEx) database were incorporated, expanding the sample size and enhancing confidence levels. The Wilcoxon test was employed to detect differences, with P < 0.05 deemed significant. The ‘magnetogram’ package facilitated visualization of the GNL2 expression distribution across various organs. Additionally, differences in GNL2 mRNA expression between tumors and adjacent normal tissues in the TCGA cohort were compared via Wilcoxon analysis. Paired samples grouped by cancer type in the TCGA cohort were subjected to Wilcoxon tests, with significance thresholds set at *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001. Furthermore, the diagnostic value of GNL2 in pan-cancer was evaluated using receiver operating characteristic (ROC) curves generated by the ‘pROC’ package. The area under the curve (AUC) values ranged from 0.5 to 1, with values closer to 1 indicating superior diagnostic performance. Generally, AUC values of 0.5–0.7, 0.7–0.9, and > 0.9 signify low precision, definite precision, and high precision, respectively.
External and experimental validation
External validation at the transcriptional level was conducted using the GEO database. Protein level validation of GNL2 expression was based on mass spectrometry data from CPTAC and immunohistochemistry data from the HPA database. Experimental validation was implemented for liver hepatocellular carcinoma (LIHC). Tumor tissue samples and corresponding peritumoral tissue samples from 40 LIHC patients (2016–2022) were collected from the General Surgery and Hepatobiliary Surgery Departments of the First Affiliated Hospital of Anhui Medical University. None of the patients had undergone radiotherapy or chemotherapy prior to surgery. Tissue samples were collected with informed consent, and the study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Anhui Medical University (No. 20040158). Tissues were fixed in paraformaldehyde and sent to Wuhan Xavier Company for immunohistochemistry (IHC) using a rabbit polyclonal anti-GNL2 antibody (NBP1–8164, NOVUS). The staining intensity of the sections was analyzed via ImageJ software. The subcellular localization of GNL2 was assessed via the HPA database, with immunofluorescence images of GNL2 cell sub-localization in various human cell lines sourced from the HPA database.
Somatic copy-number alteration (SCNA), mutation and DNA methylation analysis
The cBioPortal website (http://www.cbioportal.org) was utilized to access, download, analyze, and visualize cancer genomics data, including somatic mutation, DNA copy number alteration (CNA), and DNA methylation data [14]. Selection of the “TCGA Pan Cancer Atlas Study” and GNL2 genetic alteration search were conducted at the site [15]. Mutation frequency, type, and CNA data for all TCGA cancers were obtained from the website’s “Cancer Type Summary” module. GNL2 mutation positions were visualized in a 3D protein structure schematic using the site’s “mutation” tool. SCNA and mutation analysis considered heterozygosity and purity of amplifications and deletions, with over 5% frequency deemed high-frequency SCNAs. The association between SCNAs and expression was evaluated through Spearman’s correlation between the expression values and the copy number fragment values of each gene. The library R package “ChAMPdata” was used to annotate methylation probes of each gene, and differential methylation in tumor and normal samples was tested using the Wilcoxon rank test. Significantly hypomethylated or hypermethylated genes were identified with a P value cut-off of 0.05. Spearman’s correlation between GNL2 transcript expression and promoter DNA methylation beta values was also calculated and considered significant if P < 0.05.
Pathways and functional mechanisms analysis
To elucidate the molecular pathways associated with GNL2, tumor samples were divided into two groups based on GNL2 expression levels: the top 30% and the bottom 30%. Gene set enrichment analysis (GSEA) was conducted to examine the activation or suppression of 50 hallmark gene sets and 74 metabolic gene sets in different tumors within the high-expression group compared to the low-expression group. The CancerSEA website processed single-cell analyses from datasets such as HCMDB, Cyclebase, and StemMapper for functional analyses, redefining 14 functional states [16]. The z score is an algorithm proposed by Lee et al. that reflects the activity of a given pathway by integrating characteristic gene expression [17]. We used the z-score algorithm in the R package GSVA for 14 functional state gene sets. The values of each gene set were enumerated separately as z scores. The statistical correlation between GNL2 and gene-based z scores was calculated using Pearson’s correlation analysis. Additionally, the relationship between GNL2 expression and the cell cycle was observed using the HPA database. The Compartmentalized Protein-Protein Interaction (ComPPI) database (https://comppi.linkgroup.hu/) was employed to filter out biologically improbable protein-protein interactions on the basis of subcellular localization. Proteins interacting with GNL2 were identified using ComPPI’s localization and interaction scores [18]. Gene effect scores from clustered regularly interspersed short palindromic repeats (CRISPR) knockout screens published by Broad’s Achilles and Sanger’s SCORE projects were analyzed. Negative scores implied the inhibition of cell growth or death post-knockdown. Scores are normalized such that nonessential genes have a median score of 0 and independently identified common essentials have a median score of − 1 [19]. Finally, systematic identification of proteins and microRNAs associated with GNL2 was conducted using Spearman’s correlation analysis.
Correlation between GNL2 expression and tumor immunity
Microenvironment
The continuous interaction between tumor cells and the tumor microenvironment (TME) is critical in tumorigenesis, progression, metastasis, and response to therapy. This study investigated the correlation between GNL2 expression and the expression of immune-related genes, including immune-activating, immune-suppressing, chemokine, chemokine receptor, and major histocompatibility complex (MHC) genes. TIMER 2.0 provides a more reliable assessment of immune infiltration in the TCGA tumor profile using seven state-of-the-art algorithms (CIBERSORT, CIBERSORT_ABS, EPIC, MCPCOUNTER, QUANTISEQ, TIMER, and XCELL) [20–23]. The Tumor Immune Single-cell Hub (TISCH) database was used to obtain the expression landscape of GNL2 in 72 single-cell datasets, validating that the tumor microenvironment results from single-cell resolution. This study offers a comprehensive analysis and visualization of the tumor microenvironment and immune infiltration of GNL2 in a pan-cancer cohort.
Correlation of GNL2 expression with clinical features in pan-cancer
The correlation of GNL2 expression with clinical features in pan-cancer was also examined. The Kruskal-Wallis test was used to measure the differences in the expression of GNL2 across different stages and molecular subtypes [24]. Five pan-cancer immune subtypes, namely, C1 (wound healing), C2 (IFN-γ dominant), C3 (inflammation), C4 (lymphocyte depletion), C5 (immune quieting), and C6 (TGF-β dominant), were obtained from the USCS Xena database. The Kruskal-Wallis test was used to examine GNL2 expression in different molecular subtypes. The chi-square test was used to compare the differences in molecular subtypes and clinical features between the high- and low-GNL2 expression groups.
Survival data from the TCGA database were analyzed using the “survival” and “survminer” R packages to identify correlations between GNL2 expression and prognostic indicators, including overall survival (OS), disease-specific survival (DSS), the disease-free progression interval (PFI) and the disease-free interval (DFI). Kaplan-Meier and univariate Cox analyses were used to determine whether GNL2 was a risk or protective factor, resulting in a high-confidence GNL2 survival map. The optimal cut-off values for the GNL2 mRNA high- and low-expression cohorts were determined using the R package “survminer”, with a log-rank test used to assess the significance of both groups via the survfit function. The “forestplot” package was used to visualize the results of the Cox analysis of survival data.
Identification of chemicals interacting with GNL2
The Comparative Toxicogenomics Database (CTD) is a large, publicly available repository linking toxicological information on chemicals, genes, phenotypes, diseases, and exposures [25]. The CTD was utilized to explore chemicals that interact with target genes. Gene expression and drug sensitivity were analyzed using the Gene Set Cancer Analysis (GSCA) database (http://bioinfo.life.hust.edu.cn/web/GSCALite/) [26]. GSCALite offers data on 750 small-molecule drugs from the Genomics of Drug Sensitivity in Cancer (GDSC) database and the Cancer Therapeutics Response Portal (CTRP) database and facilitates the exploration of valuable small-molecule drugs related to gene expression. Additionally, the cancer cell line platform established by the National Cancer Institute (NCI) is extensively used for drug screening based on gene expression. NCI-60 expression data, including data from 60 human cancer cell lines from nine different cancer types (leukemia, colon, lung, central nervous system, kidney, melanoma, ovarian, breast, and prostate), were obtained from CellMiner. The relationship between mRNA expression and the drug sensitivity z score was analyzed, and Spearman’s correlation coefficients were calculated [27]. Differentially expressed genes in GNL2 high- and low-expression samples across various cancer types were identified. The top 150 up- or downregulated genes were compiled as GNL2-associated marker genes. The CMAP_gene_signatures.rdata file, containing 1288 compound-associated signatures, was downloaded from (https://www.pmgenomics.ca/bhklab/sites/default/files/downloads) for match score calculations. The methodology used was consistent with that used in previous publications [28, 29]. The results for 32 cancer types were summarized and presented graphically via R language.
Statistical analysis
All the data were statistically analyzed via webtools and R software (V.4.3.0) (Institute of Statistics and Mathematics, Vienna, Austria) [30]. Pearson’s correlation analysis was used for normally distributed data, whereas Spearman’s correlation analysis was used otherwise. The Kruskal-Wallis rank sum test and Wilcoxon rank sum and signed rank tests detected differences between multiple and two variables, respectively [24]. Cox and Kaplan-Meier survival analyses were performed using the “survival” package, with the log-rank test employed in the KM method for significance testing [31]. The “survminer” package was used to perform Kaplan-Meier analyses [32]. Hazard ratios (HR) and 95% confidence intervals (CI) were used to describe the relative risk. The “pROC” package facilitated ROC curve analysis for genetic diagnostic performance evaluation. All the statistical tests were two-sided. P values < 0.05 and < 0.0001 were considered statistically significant and extremely significant, respectively. Statistical significance was reported at *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Results
Aberrant expression of GNL2 among pan-cancer
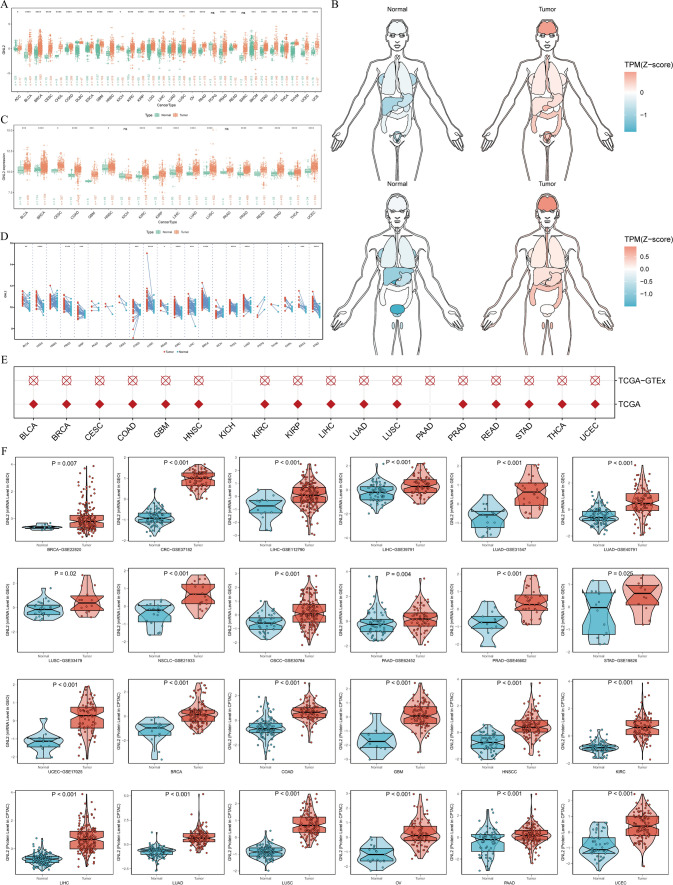
To determine the pattern of GNL2 dysregulation across cancers, the normal sample size was expanded using the TCGA and GTEx databases, yielding GNL2 expression levels from a pan-cancer perspective (Fig. 1A). GNL2 expression distribution was visualized via an organogram (Fig. 1B), revealing differential expression in most cancer types with a consistent pattern of significant upregulation in cross-cancer analysis. Difference analysis (Fig. 1C) and paired difference analysis (Fig. 1D) were performed for GNL2 in TCGA samples, and logistic regression analysis (Fig. 1E) validated these results. ROC curve estimation (Supplementary Fig. 1) demonstrated that GNL2 mRNA expression had satisfactory sensitivity and specificity for diagnosing 19 tumors, with an area under the curve > 0.7. These findings remained robust after enlarging the normal group sample size with GTEx database data (Supplementary Fig. 2). mRNA expression was validated via 13 datasets from the GEO database (Fig. 1F). The mass spectrometry data from the CPTAC database revealed the phenotypic similarity of GNL2 at the protein and transcriptional levels (Fig. 1F). In general, GNL2 was more highly expressed in tumor tissues (Fig. 2A). Experimental validation of LIHC aligned with HPA database findings (Fig. 2B). Immunofluorescence in the three cell lines revealed the nuclear localization of GNL2 (Fig. 2C), which was corroborated by siRNA labeling for subcellular localization (Fig. 2D). The reproducibility and consistency across databases, cancer types, methods, and histologies indicate that GNL2 dysregulation likely plays a role in various cancers, which is unlikely to be attributable to technical artifacts, chance, or bias in the TCGA sample criteria.
Fig. 1.
Expression landscape of GNL2 across pan-cancer. A The expression levels of GNL2 in different tumor tissues compared to the corresponding normal tissues from TCGA and GTEx datasets. B Expression and distribution of GNL2s in various organs. C The Y-axis represents the GNL2 mRNA expression in TCGA. Boxplots exhibit the median, quartiles, minimum, and maximum values, with each point representing a single sample. p-values are based on the Wilcox test. D Similar to (C), but in paired samples are grouped by cancer types from the TCGA. Each point represents a single sample. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001) (E) Logistic regression analysis of both TCGA and TCGA-GTEx datasets. Red means OR is greater than 1, while blue represents an OR value between 0 and 1. F GNL2 mRNA and protein expression were validated in GEO and CPTAC databases respectively
Fig. 2.
Immunohistochemical and immunofluorescence validation. A Visualization of GNL2s staining within tumor tissues using HPA. B Carried out experimental validation specifically in LIHC, and the results are consistent with HPA. C Identification of GNL2 expression is localized to nucleus. D The siRNA was labeled, enabling subcellular localization to be conducted and confirming the results of immunofluorescence
Genetic alterations of GNL2 in cancers
Mutliomics data, including genetic variant, somatic copy number alteration (SCNA), mRNA expression, and DNA methylation data, from tumors and normal tissues in the TCGA pan-cancer cohort were examined. The 2D and 3D structures of the GNL2 mutation sites were also systematically analyzed (Fig. 3A, B). The cBioPortal database indicated that the frequency of GNL2 genetic alterations was generally low in almost all cancers, with mutations being the most common type of genetic alteration and amplification predominating in OV (Fig. 3C). SCNA plays a key role in regulating gene expression (Fig. 3D). The percentage of SCNA in GNL2 in cancer was further investigated. Generally, SCNAs appeared at a high frequency in most cancer types (more than 5% of all samples) and at a lower frequency only in a very small number of tumors (Fig. 3E). The effect of SCNA on GNL2 mRNA expression was assessed by calculating Spearman’s correlation between gene expression and copy number in the masked copy number segment of TCGA. The results revealed a significant correlation between GNL2 mRNA expression and SCNA in most tumors (Fig. 3F), suggesting that GNL2 copy number abnormalities, which are common in most cancers, affect gene expression. Abnormal DNA methylation is associated with tumorigenesis. We analyzed the average β-values of methylation sites in promoter, super-enhancer, and CpG island regions in the GNL2 pan-cancer genome. We performed differential comparisons between cancer and normal tissues as well as correlation analyses with GNL2 expression. GNL2 showed a complex methylation pattern in a pan-cancer cohort (Fig. 3G, H), with most tumor tissues being hypomethylated compared with normal tissues. Despite the differences in the methylation pattern of GNL2, a negative correlation between GNL2 mRNA expression and DNA methylation was generally observed, indicating the complexity and cancer specificity of GNL2 gene expression regulation.
Fig. 3.
Genetic alterations of GNL2 in cancers. A Representation of GNL2 genetic alteration sites and the corresponding case counts across pan-cancer data from cBioPortal. B 3D structure of GNL2 mutation sites. C Analysis displaying the frequency of GNL2 mutations in different tumor types. D Relationship between GNL2 mRNA expression and genetic alterations. E Histogram shows the frequency of somatic copy number alterations for GNL2 in each cancer type. F The Spearman’s correlation depicting the relationship between somatic copy number alterations and GNL2 expression levels. G Heatmap shows the differential methylation patterns (promoter, CpG, super enhancer) of GNL2 in cancers; hypermethylated and hypomethylated GNL2s are marked in red and blue, respectively (Wilcoxon rank-sum test). H Spearman’s correlation assessing the relationship between GNL2 transcriptional expression and the differential methylation patterns (promoter, CpG, super enhancer). Red and blue represent positive and negative correlations, respectively
Associations between GNL2 and pathways in cancer
The relationships between 14 cancer marker scores and GNL2 were analyzed, with DNA repair and cell cycle-related scores showing higher correlation coefficients than other scores and being positively correlated (Fig. 4A). This finding is consistent with subsequent pathway analyses. The transcriptomes of two tumor subgroups with the top and bottom 30% of GNL2 genes were used to investigate relevant cell signaling pathways in cancer by GSEA for each cancer. Metabolism-related pathways were systematically analyzed, revealing good concordance between cancer types and suggesting conserved GNL2 function. Cell cycle-related pathways were usually enriched in tumors with higher GNL2 levels (Fig. 4B). The HPA database indicated that GNL2 has a cell cycle-dependent nature, and changes in transcript expression correlate with the cell cycle (Fig. 4C–M). miRNAs and proteins associated with GNL2 mRNA expression were further systematically analyzed (Supplementary Table 2). comPPI identified genes that may have reciprocal relationships with GNL2 (Supplementary Fig. 3).
Fig. 4.
Pathways and functional mechanisms analysis. A The GNL2 mRNA expression exhibited a high correlation with 14 malignant features across all tumors. Cell cycle scoring and DNA repair scoring were generally positively correlated with GNL2 mRNA expression. B Enrichment differences of GNL2 within 50 HALLMARK and 74 metabolism gene sets. NES is the normalized enrichment score in the GSEA algorithm. C–M Gene effect scores sourced from CRISPR knockout screens conducted by Broad’s Achilles and Sanger’s SCORE projects. Negative scores indicate cell growth inhibition and/or death subsequent to gene knockout. These scores are normalized, assigning a median score of 0 to nonessential genes and a median score of -1 to independently identified common essentials
High GNL2 expression correlates with immune infiltration in cancer
The involvement of GNL2 in the process of immune infiltration in pan-cancer was explored by assessing the correlation of GNL2 with MHC molecules, immunosuppressive genes, immunoactivating genes, and chemokines (Fig. 5A). A complex cancer-specific pattern emerged, with GNL2 positively correlated with immune genes in some tumors, e.g., KIRC, LGG, and THCA, but negatively associated in others, e.g., BLCA, KICH, LUAD, LUSC, PRAD, SKCM, and STA. The specific cell types regulated by GNL2 in the tumor microenvironment (TME) were explored using the TIMER2.0 database. Overall, GNL2 expression was negatively correlated with the number of infiltrating immune cells, including neutrophils and endothelial cells, in various cancers. However, a positive correlation was observed between GNL2 expression and CD4 + Th2 cells (Supplementary Fig. 4). These findings suggest that GNL2 is involved in the process of immune infiltration inhibition and the formation of an immune desert-type microenvironment, playing a crucial role in tumor immune interactions. The trend of this correlation varied slightly across different cancers due to different proportions of immune infiltration and unique tumor microenvironments, with some variation across different tumors. For example, GNL2 was positively correlated with macrophage infiltration in KICH and LGG but was mostly negatively correlated with infiltration in other tumors. However, the seven software programs (assessment methods) corroborated each other and clarified the accuracy of the analysis (Fig. 5B). Additionally, the 72 single-cell datasets of the TISCH database described the expression landscape of GNL2 in the tumor microenvironment more consistently, including 31 cancer types, suggesting that GNL2 is expressed primarily in malignant cells, fibroblasts, CD8Tex cells, and monocytes (Fig. 5C).
Fig. 5.
Association of GNL2 expression with immune infiltration. A The heatmap showed correlations between SUV39H2 mRNA expressions and chemokine, chemokine receptor, immune-inhibitor, immune-stimulatory, and MHC genes. B Seven software were used to assess the correlation between GNL2 expression and cancer immune infiltration. (*P < 0.05, **P < 0.01, ***P < 0.001) C Cellular sources of GNL2 in pan-cancer at the single-cell level. The heatmap showed expression distributions of GNL2 in different cells among 24 types of cancer at the single-cell level
Correlation of GNL2 with clinical features of pan-cancer
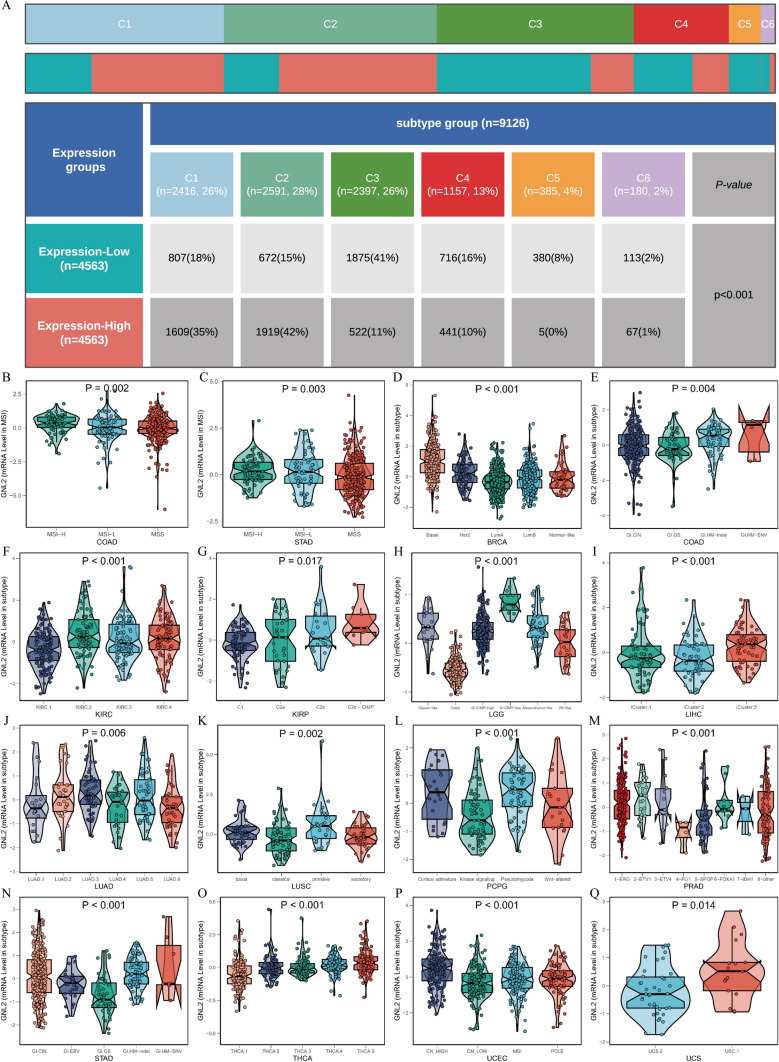
The relationship between GNL2 and immune typing was analyzed based on the immune subtypes of five pan-cancers obtained from the USCS Xena database. The chi-square test indicated a predominance of patients with C1 and C2 subtypes in the group with high GNL2 expression, whereas the group with low GNL2 expression contained more patients with C3 subtypes (Fig. 6A). The Kruskal-Wallis test revealed that GNL2 expression was relatively high in the C1 and C2 subtypes and relatively low in the C3 subtype (Supplementary Fig. 5). The relationships between GNL2 and its molecular phenotypes in 16 cancer types were further investigated, revealing significant differences in GNL2 expression across various molecular phenotypes of different cancer types (Fig. 6B–Q).
Fig. 6.
Analysis of clinical variables and molecular subtypes. A The chi-square test confirmed higher occurrences of C1 and C2 subtypes in the GNL2 high-expression group and more C3 subtypes in the GNL2 low-expression group. B–Q The Kruskal-Wallis Rank Sum Test and Wilcoxon Rank Sum and Signed Rank Tests detected differences between multiple and two variables, Kruskal-Wallis examined the differences in GNL2 expression across different molecular subtypes.(C1 (wound healing); C2 (IFN-gamma dominant); C3 (inflammatory); C4 (lymphocyte depleted); C5 (immunologically quiet); C6 (TGF-b dominant))
GNL2 expression was positively correlated with tumor stage in five cancer types, suggesting its association with the progression of these tumors (Supplementary Fig. 6). The correlation between GNL2 expression and clinical survival was analyzed to determine the ability of GNL2 to predict cancer survival. Pan-cancer survival analysis revealed that GNL2 was correlated with survival in multiple cancer types (Fig. 7A), with relatively homogeneous correlations. In most cases, GNL2 acts as a risk factor for different cancers, whereas in a few tumors, such as OV, PCPG, and TGCT, higher GNL2 expression indicates better survival. These findings indicate the varied roles of GNL2 in different cancers and the need for further exploration of its functional role in cancer survival. Forest plots were used to present the results of Cox survival analysis for the four types of survival (Fig. 7B–E). Kaplan-Meier curves were generated for the results of the log-rank test for four types of survival for KIRP and LIHC, where both the KM and Cox results for these two tumors were significant (Fig. 7F–M).
Fig. 7.
Survival landscape of pan-cancer. A Summary of the correlation between expression of GNL2 with overall survival (OS), disease-specific survival (DSS), disease-free interval (DFI) and progression-free interval (PFI) using univariate Cox regression and Kaplan-Meier models. Red indicates GNL2 as a risk factor impacting cancer patient prognosis, while green represents a protective factor. Only p values < 0.05 are displayed. B–E The forest plot exhibited the prognostic role of GNL2 in cancers by univariate Cox regression method. F–M Kaplan-Meier survival analysis and log-rank testing were performed using “survival” and “survminer” packages
Correlation of GNL2 with chemotherapeutic agents and various small molecule compounds
The potential correlation between drug sensitivity and GNL2 expression was investigated using three different databases (CTRP, GDSC, and CellMiner). In the CellMiner database, GNL2 was positively correlated with the sensitivity z score for many drugs (Fig. 8A). In the CTRP and GDSC databases, a significant negative correlation with the IC50 of many drugs was observed (Fig. 7B-C), suggesting that GNL2 is a potential chemotherapy-sensitive gene. Chemical substances that can alter GNL2 expression were identified on the basis of the CTD database (Supplementary Table 1), with 22 substances decreasing GNL2 expression and 40 substances promoting GNL2 expression. A CMap analysis was performed to explore potential therapeutic options that could counteract GNL2-mediated tumor promotion. A signature of GNL2-associated genes, including the 150 genes whose expression was most significantly upregulated and the 150 genes whose expression was most significantly downregulated, was constructed by comparing patients with high and low GNL2 expression in each cancer type. GNL2-related features were compared with CMap gene features via the XSum method to obtain similarity scores for 1288 compounds. Among them, AH.6809, Fasudil, Exisulin, X4.5. dianilinophthalimide, and mercaptopurine exhibited significantly lower scores in most cancer types, suggesting their potential in suppressing GNL2-mediated pro-carcinogenic effects (Supplementary Fig. 7).
Fig. 8.
Drug resistance analysis. Drug sensitivity analysis based on GNL2 expression using three different databases: Cellminer (A), CTRP (B), GDSC (C). P < 0.05 was considered statistically significant
Discussion
GNL2 is highly expressed in several tumors, such as hepatocellular carcinoma, ovarian carcinoma, colorectal carcinoma, and breast cancer, and not only regulates the proliferation, invasiveness, and tumorigenicity of tumor cells but also correlates with poor prognosis, indicating its potential as a prognostic marker and therapeutic target [8, 11, 12]. The understanding of the biological function of GNL2 and its relationship with tumor development remains incomplete. This study revealed the expression profile, mutation characteristics, and correlation with the clinical features of GNL2 in a pan-cancer dataset for the first time, identifying its diagnostic value and predictive potential. Furthermore, the correlations between GNL2 and the malignant features of tumors and the immune microenvironment were explored, providing a basis for further in-depth studies of the biological functions of GNL2. Finally, the relationships between GNL2 expression and immunotherapy and chemotherapy efficacy were analyzed, expanding the scope for developing more effective treatment strategies.
The analysis revealed that, compared with normal or paired paracancerous tissues, GNL2 expression was generally upregulated in several cancer types, including hepatocellular carcinoma, breast carcinoma, uroepithelial carcinoma, lung carcinoma, gastric adenocarcinoma, and colorectal carcinoma. The absence of a corresponding trend in a few tumors might be attributed to the small sample size of normal tissues in the database. This finding was consistently validated at both the mRNA and protein levels. Immunohistochemistry results from the HPA database also revealed high expression of GNL2 in tumor tissues. For further validation, tumor tissue samples and corresponding peritumoral tissue samples from 40 HCC patients were collected. The protein level of GNL2 was greater in HCC tumor tissues than in peritumoral tissues. The ROC curves drawn for 19 tumors all showed excellent AUC values, suggesting that GNL2 could be a novel biomarker with high diagnostic value across cancers. The predictive performance of GNL2 for survival across cancers was further analyzed. Survival analysis revealed that GNL2, as a risk factor, was associated with poor prognosis in most tumors. However, in some tumors, e.g., OV, PCPG, and TGCT, patients with high expression of GNL2 have a better survival prognosis. The biological function of GNL2 may be regulated differently in these tumors.
The molecular typing of tumors, which is based on key features, holds great clinical significance. Thorsson et al. comprehensively evaluated the immunogenomic features of 33 tumors and defined six immune subtypes: C1-wound healing, C2-IFN-γ dominant, C3-inflammatory, C4-lymphocyte depleted, C5-immunologically quiet, and C6-TGF-β dominant [33]. Patients with the C1 and C2 subtypes predominated in the GNL2 high-expression group, and GNL2 expression was relatively high in patients with these subtypes, whereas more patients with the C3 subtypes were in the GNL2 low-expression group, with relatively low GNL2 expression in these patients. Thorsson et al. demonstrated that the C1 and C2 subtypes have high proliferation rates and relatively poor prognoses, and the C3 subtype has low to moderate tumor cell proliferation characteristics and a relatively good prognosis, which aligns with the finding that high GNL2 expression promotes cell proliferation and predicts poor prognosis. Moreover, significant differences in GNL2 expression were noted in various molecular types of different cancer types. These results suggest that GNL2 could be a reliable biomarker for assisting in the molecular typing of patients, facilitating the development of precise treatment strategies.
Genomic instability, a hallmark of cancer, can be caused by gene mutations and abnormal epigenetic modifications [34]. The characteristics of GNL2 gene expression across cancers were comprehensively analyzed, from genetic variants to epigenetic alterations. In most tumors, gene mutation is the main type of genetic alteration in GNL2, forming the basis for malignant behavior acquisition and tumor progression. A significant correlation between GNL2 mRNA expression and SCNA was observed in most tumors, suggesting that SCNA was the major modality regulating GNL2 gene expression. Copy number variation, an important form of human genetic variation and a key feature of the cancer genome, leads to structural changes in chromosomes, affecting gene expression levels and playing a crucial role in oncogene activation and antioncogene inactivation. The hypothesis is that increased copy number mutation events promote the expression of GNL2, which then plays an oncogenic role [35, 36]. DNA methylation, a key epigenetic mechanism, regulates gene expression by altering chromatin structure, DNA conformation, and stability, as well as DNA-protein interactions [37]. Genome-wide DNA methylation levels are commonly reduced in tumor cells, leading to increased genomic instability and mutation rates [38]. During carcinogenesis, CpG islands in the promoter region of antioncogenes transition from a hypomethylated to a hypermethylated state, and vice versa for oncogenes, thus inactivating antioncogenes and activating oncogenes, a hallmark of cancer progression and deterioration [39, 40]. This epigenetic regulation is complex and varies across different tumor types [41]. The DNA methylation level of GNL2 varies in different tumor tissues. However, in most tumor tissues, the DNA methylation level of GNL2 is lower than that in normal tissues, and GNL2 mRNA expression is negatively correlated with DNA methylation. These findings suggest that GNL2 is activated as an oncogene in most tumors, thereby promoting tumor progression.
Gene set enrichment analysis revealed that cell proliferation-related pathways, including E2F targets, the G2M checkpoint, Myc targets v1, and Myc targets v2, which are involved mainly in the cell cycle, metabolism, and cell proliferation and differentiation regulation, were enriched in tumors with high GNL2 expression [42, 43]. This finding is consistent with previous reports highlighting the key role of GNL2 in active cell proliferation and cell cycle progression [12]. Abnormal cell cycle regulation, which leads to uncontrolled malignant proliferation of tumor cells, is a fundamental characteristic of malignant tumors [44]. The pro-carcinogenic effect of GNL2 is closely linked to its role in cell cycle regulation. Debduti Datta et al. demonstrated that GNL2 can promote cell cycle progression through the activation of the p53/p21Cip-1/Waf1 pathway and stimulate cell proliferation [8]. This study revealed that GNL2 may also be involved in cell cycle regulation through additional signaling pathways, such as E2F targets, the G2M checkpoint, Myc targets v1, and Myc targets v2. Given the clinical efficacy of CDK4/6 inhibitors targeting cell cycle mechanisms, it is reasonable to consider GNL2, which is closely associated with the cell cycle, as a potential therapeutic target.
With respect to the tumor microenvironment (TME), GNL2 expression appears to influence immune cell infiltration differently across cancer types. In most tumors, GNL2 was negatively correlated with most immune genes. This finding implies that high GNL2 expression may contribute to the formation of an immunosuppressive tumor microenvironment, potentially leading to insensitivity to immune checkpoint inhibitor therapy [13]. In most tumors, GNL2 expression was negatively correlated with the infiltration of multiple immune cell types but was positively correlated with CD4 + Th2 cells. Significant cancer type differences were also observed, with GNL2 significantly positively correlated with macrophage infiltration in KICH and LGG but mostly negatively correlated with infiltration in other tumors. Previous studies have confirmed that the majority of macrophages in the tumor microenvironment exhibit M2-type characteristics, which play a role in suppressing the immune response of the tumor microenvironment, often predicting a poor prognosis and a lack of efficacy for immunotherapy [45]. Thus, GNL2 could serve as a key marker for predicting the efficacy of immunotherapy in KICH and LGG patients. The expression landscape of GNL2 in the tumor microenvironment revealed that it was expressed mainly in malignant cells, CD8Tex cells, fibroblasts, and monocyte macrophages. Fibroblasts can convert the TME to an immune-rejecting phenotype through the transforming growth factor-β (TGF-β) signaling pathway [46]. CD8Tex cells gradually lose the production and proliferation potential of effector cytokines, increase the expression of inhibitory receptors, and decrease the effect of TCR stimulation, resulting in poor immunotherapy outcomes [47]. Therefore, these cell types, including mononuclear macrophages, can promote the development of an immunosuppressive TME, leading to immune escape and a poor immunotherapy response [48, 49]. This finding suggests that GNL2 may participate in the formation of the immunosuppressive tumor microenvironment and could be a marker for predicting the characteristics of the tumor immune microenvironment and the efficacy of immunotherapy.
The correlation between GNL2 and the efficacy of various drugs was analyzed, revealing that patients with high GNL2 expression were more sensitive to drugs such as docetaxel, doxorubicin, vincristine, etoposide, camptothecin, cyclophosphamide, isocyclophosphamide, and acitretinib. Interestingly, this group of patients appeared to be less responsive to anti-EGFR-targeted drugs (afatinib, gefitinib, smetinib, darafinil, trametinib, etc.). CMap analysis identified potential compounds that target GNL2 across cancers; AH.6809, Fasudil, Exisulin, X4.5.dianilinophthalimide, and mercaptopurine are recognized as potential inhibitors of the pro-oncogenicity of GNL2, potentially counteracting its pro-oncogenic effects.
Conclusions
In conclusion, this study revealed that GNL2 is significantly upregulated in most cancer tissues compared with normal tissues and has been experimentally validated in LIHC. It plays a pro-cancer role by regulating cell cycle progression, is associated with poor prognosis, and can be used as a potential pan-cancer diagnostic and prognostic marker. The analysis revealed that GNL2 may be involved in remodeling the tumor microenvironment, mediating the formation of an immunosuppressive tumor microenvironment. As a potential therapeutic target, GNL2 is capable of predicting immunotherapy efficacy and sensitivity to chemotherapeutic agents. However, the potential molecular mechanisms underlying these observations need to be further explored and validated through additional basic and clinical studies.
Supplementary Information
Acknowledgements
We express our appreciation for all the open public databases mentioned in this article.
Abbreviations
- TCGA
The Cancer Genome Atlas
- ICGC
International cancer genome consortium
- GEO
Gene Expression Omnibus
- TCPA
The Cancer Proteome Atlas
- CPTAC
Clinical Proteomic Tumor Analysis Consortium
- HPA
Human Protein Atlas
- GTEx
Genotype tissue-expression
- TISCH
The tumor immune single-cell hub
- CTD
Comparative Toxicogenomics Database
- GDSC
Genomics of drug sensitivity in cancer
- CTRP
Cancer Therapeutics Response Portal
- GSCA
Gene Set Cancer Analysis
- SCNA
Somatic Copy-number Alteration
- CNAs
DNA copy number alterations
- GSVA
Gene set variation analysis
- GSEA
Gene set enrichment analysis
- TME
Tumor microenvironment
- AUC
Area under the curve
- KM
Kaplan-Meier
- DSS
Disease-specific survival
- DFI
Disease-free interval
- PFI
Progress-free interval
- PPI
Protein-protein interaction
- CRISPR
Clustered Regularly Interspersed Short Palindromic Repeats
- BLCA
Bladder urothelial carcinoma
- BRCA
Breast invasive carcinoma
- CESC
Cervical squamous cell carcinoma and endocervical adenocarcinoma
- CHOL
Cholangiocarcinoma
- CCLE
Cancer cell line encyclopedia
- COAD
Colon adenocarcinoma
- COAD/READ
Colon adenocarcinoma/rectum adenocarcinoma esophageal carcinoma
- ESCA
Esophageal carcinoma
- GBM
Glioblastoma multiforme
- GBMLGG
Glioma
- HNSC
Head and neck squamous cell carcinoma
- KICH
Kidney chromophobe
- KIRC
Kidney renal clear cell carcinoma
- KIRP
Kidney renal papillary cell carcinoma
- LGG
Brain lower grade glioma
- LIHC
Liver hepatocellular carcinoma
- LUAD
Lung adenocarcinoma
- LUSC
Lung squamous cell carcinoma
- OV
Ovarian serous cystadenocarcinoma
- PAAD
Pancreatic adenocarcinoma
- PCPG
Pheochromocytoma and paraganglioma
- PRAD
Prostate adenocarcinoma
- ROC
Receiver operating characteristic curve
- SARC
Sarcoma
- SKCM
Skin cutaneous melanoma
- STAD
Stomach adenocarcinoma
- STES
Stomach and esophageal carcinoma
- TGCT
Testicular Germ Cell Tumors
- THCA
Thyroid carcinoma
- THYM
Thymoma
- UCEC
Uterine corpus endometrial carcinoma
- UCS
Uterine carcinosarcoma
- UVM
Uveal melanoma
Author contributions
SG was responsible for the design of the study, collecting and processing data and generating images as well as the writing and submission of the paper. LZ contributed technical assistance. Corresponding author GS supervised this study throughout and reviewed the article.
Funding
This research was supported by the Major Natural Science Research Project of Universities in Anhui Province (2024AH040192); Natural Science Youth Foundation of the First Affiliated Hospital of USTC (2023YJQN002).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
Tissue samples were collected with informed consent, and the study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Anhui Medical University (No. 20040158). All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA A Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Liu B, Zhang Z. Accelerating the understanding of cancer biology through the lens of genomics. Cell. 2023;186(8):1755–71. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Xiao J, Bai J, Tian Y, Qu Y, Chen X, Wang Q, Li X, Zhang Y, Xu J. Molecular characterization and clinical relevance of m 6 a regulators across 33 cancer types. Mol Cancer. 2019;18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng H, Wang M, Zhang S, Hu D, Yang Q, Chen M, Zhang X, Zhang Y, Dai J, Liou YC. Comprehensive pan-cancer analysis reveals NUSAP1 is a novel predictive biomarker for prognosis and immunotherapy response. Int J Biol Sci. 2023;19(14):4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Essers PB, Pereboom TC, Goos YJ, Paridaen JT, MacInnes AW. A comparative study of nucleostemin family members in zebrafish reveals specific roles in ribosome biogenesis. Dev Biol. 2014;385(2):304–15. [DOI] [PubMed] [Google Scholar]
- 7.Paridaen JTML, Janson E, Utami KH, Pereboom TC, Essers PB, van Rooijen C, Zivkovic D, MacInnes AW. The nucleolar GTP-binding proteins Gnl2 and nucleostemin are required for retinal neurogenesis in developing zebrafish. Dev Biol. 2011;355(2):286–301. [DOI] [PubMed] [Google Scholar]
- 8.Datta D, Anbarasu K, Rajabather S, Priya RS, Desai P, Mahalingam S. Nucleolar GTP-binding protein-1 (NGP-1) promotes G1 to S phase transition by activating cyclin-dependent kinase inhibitor p21Cip1/Waf1. J Biol Chem. 2015;290(35):21536–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iuchi S, Paulo JA. RNAmetasome network for macromolecule biogenesis in human cells. Commun Biol. 2021;4(1):1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Angelis PM, Svendsrud DH, Kravik KL, Stokke T. Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery. Mol Cancer. 2006;5(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong Y, Cai Q, Fu L, Liu H, Ma M, Wu X. Study of the G protein nucleolar 2 value in liver hepatocellular carcinoma treatment and prognosis. Biomed Res Int. 2021;2021:4873678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura K, Reid BM, Chen A, Chen Z, Goode EL, Permuth JB, Teer JK, Tyrer J, Yu X, Kanetsky PA. Functional analysis of the 1p34. 3 risk locus implicates GNL2 in high-grade serous ovarian cancer. Am J Hum Genet. 2022;109(1):116–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei S, Lu K, Xing J, Yu W. A multidimensional pan-cancer analysis of DCAF13 and its protumorigenic effect in lung adenocarcinoma. FASEB J. 2023;37(4):e22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan H, Yan M, Zhang G, Liu W, Deng C, Liao G, Xu L, Luo T, Yan H, Long Z. CancerSEA: a cancer single-cell state atlas. Nucl Acid Res. 2019;47(D1):D900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee E, Chuang HY, Kim JW, Ideker T, Lee D. Inferring pathway activity toward precise disease classification. PLoS Comput Biol. 2008;4(11):e1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veres DV, Gyurkó DM, Thaler B, Szalay KZ, Fazekas D, Korcsmáros T, Csermely P. ComPPI: a cellular compartment-specific database for protein–protein interaction network analysis. Nucl Acid Res. 2015;43(D1):D485-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dempster JM, Boyle I, Vazquez F, Root DE, Boehm JS, Hahn WC, Tsherniak A, McFarland JM. Chronos: a cell population dynamics model of CRISPR experiments that improves inference of gene fitness effects. Genome Biol. 2021;22:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2. 0 for analysis of tumor-infiltrating immune cells. Nucl Acid Res. 2020;48(W1):W509-14. [DOI] [PMC free article] [PubMed]
- 21.Qiu C, Shi W, Wu H, Zou S, Li J, Wang D, Liu G, Song Z, Xu X, Hu J, Geng H. Identification of molecular subtypes and a prognostic signature based on inflammation-related genes in colon adenocarcinoma. Front Immunol. 2021;12:769685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng D, Zhu W, Shi X, Wang Z, Wei W, Wei Q, Yang L, Han P. Immune-related gene index predicts metastasis for prostate cancer patients undergoing radical radiotherapy. Exp Hematol Oncol. 2023;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi Q, Pu Y, Chao F, Bian P, Lv L. ACAP1 deficiency predicts inferior immunotherapy response in solid tumors. Cancers (Basel). 2022;14(23). [DOI] [PMC free article] [PubMed]
- 24.Hasan S. Effects of plyometric vs. strength training on strength, sprint, and functional performance in soccer players: a randomized controlled trial. Sci Rep. 2023;13(1):4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis AP, Grondin CJ, Johnson RJ, Sciaky D, Wiegers J, Wiegers TC, Mattingly CJ. Comparative toxicogenomics database (CTD): update 2021. Nucl Acid Res. 2021;49(D1):D1138-43. [DOI] [PMC free article] [PubMed]
- 26.Liu CJ, Fe H, Xie GY, Miao YR, Li XW, Zeng Y, Guo AY. GSCA: an integrated platform for gene set cancer analysis at genomic, pharmacogenomic and immunogenomic levels. Brief Bioinform. 2023;24(1):bbac558. [DOI] [PubMed] [Google Scholar]
- 27.Reinhold WC, Wilson K, Elloumi F, Bradwell KR, Ceribelli M, Varma S, Wang Y, Duveau D, Menon N, Trepel J. CellMinerCDB: NCATS is a web-based portal integrating public cancer cell line databases for pharmacogenomic explorations. Cancer Res. 2023;83(12):1941–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN, Kamińska B, Huelsken J, Omberg L, Gevaert O. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell. 2018;173(2):338–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang C, Zhang H, Chen M, Wang S, Qian R, Zhang L, Huang X, Wang J, Liu Z, Qin W. A survey of optimal strategy for signature-based drug repositioning and an application to liver cancer. Elife. 2022;11:e71880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sepulveda JL. Using R and bioconductor in clinical genomics and transcriptomics. J Mol Diagn. 2020;22(1):3–20. [DOI] [PubMed] [Google Scholar]
- 31.Schober P, Vetter TR. Kaplan-Meier curves, log-rank tests, and cox regression for time-to-event data. Anesth Analgesia. 2021;132(4):969–70. [DOI] [PubMed] [Google Scholar]
- 32.Duan J, Zhu L, Shi Y, Wang W, Wang T, Ning T, Zhang L, Bai M, Li H, Liu R. Chemotherapy re-use versus anti-angiogenic monotherapy as the third-line treatment of patients with metastatic colorectal cancer: a real-world cohort study. BMC Cancer. 2024;24(1):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Yang T-HO, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA. The immune landscape of cancer. Immunity. 2018;48(4):812–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez-Jiménez F, Muiños F, Sentís I, Deu-Pons J, Reyes-Salazar I, Arnedo-Pac C, Mularoni L, Pich O, Bonet J, Kranas H. A compendium of mutational cancer driver genes. Nat Rev Cancer. 2020;20(10):555–72. [DOI] [PubMed] [Google Scholar]
- 35.Steele CD, Abbasi A, Islam SMA, Bowes AL, Khandekar A, Haase K, Hames-Fathi S, Ajayi D, Verfaillie A, Dhami P. Signatures of copy number alterations in human cancer. Nature. 2022;606(7916):984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao Z, Wang S, Wu C, Wu T, Zhao X, Ning W, Wang G, Wang J, Chen J, Diao K. The repertoire of copy number alteration signatures in human cancer. Brief Bioinform. 2023;24(2):bbad053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattei AL, Bailly N, Meissner A. DNA methylation: a historical perspective. Trends Genet. 2022;38(7):676–707. [DOI] [PubMed] [Google Scholar]
- 38.Mazloumi Z, Farahzadi R, Rafat A, Asl KD, Karimipour M, Montazer M, Movassaghpour AA, Dehnad A, Charoudeh HN. Effect of aberrant DNA methylation on cancer stem cell properties. Exp Mol Pathol. 2022;125:104757. [DOI] [PubMed] [Google Scholar]
- 39.Baylin SB, Jones PA. A decade of exploring the cancer epigenome—biological and translational implications. Nat Rev Cancer. 2011;11(10):726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349(21):2042–54. [DOI] [PubMed] [Google Scholar]
- 41.Li R, Di L, Li J, Fan W, Liu Y, Guo W, Liu W, Liu L, Li Q, Chen L. A body map of somatic mutagenesis in morphologically normal human tissues. Nature. 2021;597(7876):398–403. [DOI] [PubMed] [Google Scholar]
- 42.Chida K, Oshi M, Roy AM, Yachi T, Nara M, Yamada K, Matsuura O, Hashizume T, Endo I, Takabe K. E2F target score is associated with cell proliferation and survival of patients with hepatocellular carcinoma. Surgery. 2023;174(2):307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oshi M, Patel A, Le L, Tokumaru Y, Yan L, Matsuyama R, Endo I, Takabe K. G2M checkpoint pathway alone is associated with drug response and survival among cell proliferation-related pathways in pancreatic cancer. Am J Cancer Res. 2021;11(6):3070–84. [PMC free article] [PubMed] [Google Scholar]
- 44.Suski JM, Braun M, Strmiska V, Sicinski P. Targeting cell-cycle machinery in cancer. Cancer Cell. 2021;39(6):759–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Therapy. 2021;6(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salmon H, Remark R, Gnjatic S, Merad M. Host tissue determinants of tumour immunity. Nat Rev Cancer. 2019;19(4):215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franco F, Jaccard A, Romero P, Yu YR, Ho PC. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. 2020;2(10):1001–12. [DOI] [PubMed] [Google Scholar]
- 48.Chen W, Song T, Zou F, Xia Y, Xing J, Yu W, Rao T, Zhou X, Li C, Ning J. Prognostic and immunological roles of IL18RAP in human cancers. Aging. 2023;15(17):9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17(12):887–904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.