Abstract
Zinc finger protein 384 (ZNF384) is a highly conserved transcribed gene associated with the development of multiple tumors, however, its role and mechanism in serous ovarian cancer (SOC) are unknown. We first confirmed that ZNF384 was abnormally highly expressed in SOC tissues by bioinformatics analysis and immunohistochemistry. We further used lentivirus packaging and transfection techniques to construct ZNF384 overexpression or knockdown cell lines, and through a series of cell function experiments, gradually verified that ZNF384 promoted a series of malignant behaviors of SOC cell proliferation, migration, and invasion. By establishing a xenotransplantation model in nude mice, it was confirmed that ZNF384 promoted the progress of SOC in vivo. Mechanistically, Overexpression of ZNF384 enhanced the transcriptional activity of Lin-28 homolog B (LIN28B), which promoted the malignant behavior of SOC cells. In addition, LIN28B could regulate the expression of the downstream factor ubiquitin D (UBD) in SOC cells, further promoting the development of SOC. This study shows that ZNF384 aggravates the malignant behavior of SOC cells through the LIN28B/UBD axis, which may be used as a diagnostic biomarker for patients with SOC.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10565-024-09938-6.
Keywords: Serous ovarian cancer, ZNF384, LIN28B, UBD
Introduction
Ovarian cancer comes in a variety of histological forms, with serous ovarian cancer (SOC) being the most prevalent morphologic subtype (Izar et al. 2020). HGSOC accounts for approximately 90% of SOC and is the most malignant pathologic type with the worst prognosis (Peres et al. 2019; Kurman 2013). Due to the deep location of the ovary in the pelvic cavity and there are no effective markers for early diagnosis of cancer, 75% of patients are found with advanced tumors (Dochez et al. 2019). Despite the continuous optimization of chemotherapy regimens, the effect of SOC treatment has been unsatisfactory, with 5-year survival rates still hovering at 30%-40% (Boehm et al. 2022). Therefore, further understanding of the pathogenesis of SOC and finding new therapeutic targets have become the key to prolonging the survival period and improving clinical outcomes of patients with SOC.
Zinc finger protein 384 (ZNF384) is a transcription factor, which contributes to the transcriptional regulation of extracellular matrix genes (Nakamoto et al. 2000). ZNF384 can be coupled with SYNRG, EWSR1, and other genes, thereby modulating downstream signaling factors, such as JAK/STAT3, and inducing the development of multiple leukemia subtypes (Yamamoto et al. 2019; Shago et al. 2016). Furthermore, studies suggested that the reason why ZNF384 promotes the malignant progression of cancer may be related to the enhancement of the proliferation and invasion ability of tumor cells (He et al. 2019; Yan et al. 2022). Although various evidence suggests that ZNF384 might be an oncogenic factor that promotes the occurrence of tumors, the research of ZNF384 in SOC is still lacking.
Ubiquitin D (UBD) is a ubiquitin-like protein involved in various biological processes including cellular immunity, apoptosis, and signal transduction (Song et al. 2021). In recent years, a growing number of studies have revealed an association between increased UBD expression and various malignant tumor progression (Yan et al. 2010; Chou et al. 2022; Yuan et al. 2014). Lee et al. found that UBD expression increased in ovarian cancer tissues (Lee et al. 2003). Lin-28 homolog B (LIN28B) is a functionally conserved RNA-binding protein that can maintain the proliferative capacity of pluripotent stem cells and control embryonic development (Tsanov et al. 2017; Zhang et al. 2018). LIN28B is considered to be an important oncogene in a variety of solid tumors and has been reported to predict poor prognosis (Helsmoortel et al. 2016; Xu et al. 2022; Ren et al. 2018). The expression of LIN28B in hypothalamic-pituitary-gonad tissues has been intensively studied over the past few decades, and interestingly, LIN28B is widely expressed in the ovary (Grieco et al. 2013). In addition, high expression of LIN28B can increase the risk of SOC and the malignancy of the disease (Yong et al. 2018).
Based on the above studies, our work aims to explore the biological role of ZNF384 in SOC and its potential mechanisms. We found that ZNF384 is overexpressed in SOC and is related to poor prognosis in patients with SOC. Functional experiments showed that ZNF384 can promote malignant behaviors of SOC cells, including migration and invasion. Mechanically, ZNF384 can enhance the transcriptional activity of LIN28B by binding to the LIN28B promoter, and then regulate the downstream target UBD to promote SOC progression. In conclusion, this study reveals that ZNF384 may be a potential therapeutic target for SOC.
Materials and methods
Patients and samples
One hundred cases of paraffin-fixed SOC tissues and thirty cases fresh SOC tissues and thirty cases non-tumor-ovarian tissues were collected from The Shengjing Hospital from March. 2023 to September. 2023, among which the non-tumor ovarian tissues were mainly derived from the ovarian tissues removed during the operation of patients with uterine fibroids or adenomyosis.
Online bioinformatic analysis tools
Raw data between SOC and normal ovarian tissue were obtained from the NCBI (http://www.ncbi.nlm.nih.gov/gds/) dataset and the gene expression profiling (GSE14407) was selected. DEGs were selected by |log2FC|> 1 and adj. P < 0.05 in the GSE14407 dataset. GO enrichment analysis and KEGG enrichment analysis software were R packages ggplot2. The R package clusterProfile and enrichplot were used to analyze GSEA results. Mutation mapping of ZNF384 was obtained from the SOC datasets using the cBioportal database (https://www.cbioportal.org/). The GSCA database (https://guolab.wchscu.cn/GSCA/) was used to analyze the effect of ZNF384 expression on the prognosis of ovarian cancer.
Cell lines
The SOC cell lines OVCAR-8, OVCAR-3, and SKOV3 were obtained from Saibaikang Company (China). OVCAR-8 and OVCAR-3 cells were cultured in RPMI-1640 medium (Solarbio, China) containing 10% FBS. SKOV3 cells were cultured in McCoy’s 5A medium (Procell, China) containing 10% FBS.
Lentivirus infection and transfection
Two shRNA fragments targeting the ZNF384 sequence: 5’-GAATCTCACTCAATTCTAAC-3’ or 5’-CAATGTTCATCAACAAGATGA-3’. The scramble shRNA targeting 5’-TTCTCCGAACGTGTCACGT-3’ was used as a control. These fragments were ligated into the lentiviral vector pLKO.1-EGFP-puro, and the enzymatic cleavage sites were AgeI and EcoRI sites. The coding sequence of ZNF384 (NM_001039920) was ligated into the lentiviral vector pLJM1-EGFP-puro, and the enzymatic cleavage sites were NheI and AgeI sites. The SOC cells were infected with lentivirus at the MOI of 20.
For lipofection transfection, cells in 6-well plates and transfected with Lipofectamine™ 3000 (Invitrogen, USA). Cells were maintained in a 37 °C and 5% CO2 incubator.
Western blotting
The protein extracted from the tissue or cell was quantitatively concentrated with a BCA protein assay kit (Beyotime, China). Proteins were separated via SDS-PAGE (Beyotime, China). Next, samples were transferred to PVDF membranes (Abcam, China). Samples were blocked with the specified sealing solution (Beyotime, China) for 1 h, followed by incubating with primary antibodies and secondary antibodies. The bands were visualized using ECL luminol (Beyotime, China). The antibodies are shown in Table 1.
Table 1.
Antibodies employed in this study
| Antibody | Product number | Company | Origin | Dilution |
|---|---|---|---|---|
| ZNF384 | cat# ab176689 | Abcam | UK | 1:3000 |
| LIN28B | cat# 24017–1-AP | Proteintech | China | 1:1000 |
| UBD | cat# 13003–2-AP | Proteintech | China | 1:500 |
| Goat anti-rabbit | cat# A0208 | Beyotime | China | 1:5000 |
| Goat anti-mouse | cat# A0216 | Beyotime | China | 1:5000 |
qPCR assay
RNA was extracted from SOC cell lines by Trizol reagent (BioTeke, China), and its concentration was determined. cDNA was synthesized using the BeyoRT II M-MLV Reverse Transcriptase (Beyotime, China). The PCR reactions were performed using the qPCR SYBR Green (Solarbio, China). Finally, quantitative fluorescence analysis was done with the Exicycler™ 96 fluorescence quantifier (Bioneer, Korea). The primers are shown in Table 2.
Table 2.
Primers employed in this study
| Gene | Forward sequence (from 5’ to 3’) | Reverse sequence (from 5’ to 3’) |
|---|---|---|
| qPCR ZNF384 | GGTAGCATCGACCCTAACCG | CATCCTCAGGGGAGAGGACA |
| qPCR LIN28B | CAGCCAAAGAAGTGCCA | AGCCTCCTGAGGAAACG |
| qPCR UBD | ATGCTTCCTGCCTCTGT | GGGTAAGGTGGATGGTC |
| qPCR β-actin | GGCACCCAGCACAATGAA | TAGAAGCATTTGCGGTGG |
| ChIP LIN28B-1 | ATGCCATCATTGAGATTACTT | AGGGCTACTTTCTCATTTTATT |
| ChIP LIN28B-2 | CCTTTTCTTTTCTCCCTAAC | CACTCCAGCACTGTTTCAC |
| RIP UBAP2L | GAAACTGGGAACAACCTCA | TTTCTTTGCCTTCCTCAT |
| RIP UBE2T | ATGCCAGACAGTGGACAGAG | CAGGTTTAAAAGATTTCAAAATAC |
| RIP UBD | TTGTATTGGAGGGTGAC | CTGGCTTTGAATGCTCT |
| RIP UBE3A | TTAGTTCAAGGACAGCA | GAAGATTTCCTCCACAA |
| RIP UBE3C | GGAGTTGTATCCCGCATTT | TATCCTCGTGGGTCTGG |
| RIP USP1 | TGTTATGGTGGTGGACT | CAATGGTTCTGGCTTAC |
| RIP SOX2 | GGGCAAAAGTTTTAGAC | AAATGGAAAGTTGGGAT |
CCK-8 assay
We seeded 5 × 103 SOC cells in 96-well plates. 10 μL of CCK-8 reagent (KeyGen Biotech, China) was added at the indicated times. The optical density value was determined at 450 nm by the microplate reader (BIOTEK, USA).
Wound healing assay
SOC cells were cultured in a serum-free medium and then treated with mitomycin C (SIGMA, USA) for 1 h. Subsequently, the cell monolayer was scratched to form a scratching wound using a sterile pipette tip. After 24 h of culture, the wound images of the cells were taken under the microscope at 0 h and 24 h.
Colony formation assay
Lentivirus-infected SOC cells were digested by trypsin and counted. About 400 cells were inoculated in each petri dish. Till incubation lasted for 2 weeks visible colonies were formed. Then each petri dish was fixed by 4% paraformaldehyde (Aladdin, China) for 25 min and stained with Giemsa (KeyGen Biotech, China) for 5 min.
Transwell assay
For cell invasion assays, SOC cells were cultured in a serum-free medium. The transwell chamber containing Matrigel-coated polycarbonate membrane filter (Corning, USA) was placed into the 24-well plates, 800 μL culture medium with 10% FBS was added to the lower chamber, the upper chamber was added with 200 μL cell suspension and fixed with 4% paraformaldehyde for 20 min. Finally, cells were stained with 0.4% crystal violet and counted using an inverted light microscope. Five visual field counting cells were selected for each sample and the number was averaged.
ELISA
The content of MMP-2 was quantified by Human MMP-2 ELISA Kits (Multisciences, USA). The culture supernatants were centrifuged at 300 × g for 10 min to remove sediment. The OD values were detected at 450 nm with the microplate reader.
Immunofluorescence
Each cell sheet was fixed with paraformaldehyde, permeabilized with 0.1% Triton X-100 (Beyotime, China) for 30 min, then incubated with BSA (Sangon, China) for 15 min at room temperature. The SOC cells were further incubated with primary antibodies including Vimentin (Affinity, China) at 4 °C. On the second day, the sections were followed by incubation with fluorescent secondary antibodies anti-rabbit IgG (CST, USA) for 1 h. Finally, the DAPI (Aladdin, China) was used to stain the nuclei and the anti-fluorescence quencher (Solarbio, China) was added. The fluorescence images were photographed by the fluorescence microscope.
Animal models
6–8 weeks female BALB/c nude mice were obtained from Changzhou Cavens Laboratory Animal Co. LTD (China) and subjected to xenograft tumor experiments. Mice were randomly grouped, and SKOV3 and OVCAR-8 cells (1 × 105) in the logarithmic growth phase were injected subcutaneously into the flanks of mice, respectively. The tumor volume was measured every 5 days, and the tumor tissue was collected after 25 days for subsequent testing. SKOV3 and OVCAR-8 cells (1 × 106) were intra-peritoneally injected into the nude mice according to groups, and the tumor metastasis in vivo was observed by IVScope8200 Small animal live imaging system (Clinx, China) after feeding for 4 weeks. All animal experiments were performed with consent from China Medical University (Approval No. CMU2023049).
Immunohistochemistry
Immunohistochemistry was performed using ZNF384 antibody (Bioss, China), Ki67 antibody (Affinity, China). In brief, the sections were subjected to dewaxing and rehydration. After the heat-induced antigen recovery, the sections were sequentially incubated with 3% hydrogen peroxide and 1% BSA for 15 min, respectively. The primary antibodies were added to the sections at 4 °C. On the second day, the secondary antibodies were added. Finally, sections were stained with diaminobenzidine (Solarbio, China) and re-stained with hematoxylin (Solarbio, China). The images were photographed by the microscope. The H-score method was used to quantify the ZNF384 and Ki67 expression. The staining intensity was into 4 categories: 0, no staining; 1 + , weak staining; 2 + , moderate staining; and 3 + , strong staining, and the percentage of cells at different staining intensities was determined by visual assessment. The formula for calculating the score is H-score = Σ (Pi x i), where the Pi is the proportion of positive cells with a certain intensity, and the i is the staining intensity. The H-score is based on the 200 score standard, more than 200 score is defined as high expression, less than 200 score is defined as low expression.
Dual-luciferase assay
OVCAR-8 cells were inoculated in 12-well plates to 90% confluency. Dual luciferase vectors containing different LIN28B promoter sequences were constructed and co-transfected with ZNF384 overexpression plasmid into OVCAR-8 cells. Cells were collected after 48 h to detect the promoter activity. The luciferase assay kit (KeyGen Biotech, China) was used to measure the luciferase activities. The ratio of luciferase was calculated according to the results of the multifunctional microplate reader (TECAN, Switzerland).
ChIP assay
For the ChIP experiment, the ChIP Assay Kit (Beyotime, China) was used. OVCAR-8 cells were fixed with 37% formaldehyde to crosslink the DNA and protein, followed by ultrasonic treatment to shear the genomic DNA. After centrifugation of the sample at 12,000–14,000 × g for 4 min, the samples were incubated with the target antibody or with the negative control anti-IgG overnight. Finally, the immunoprecipitated DNA was analyzed by PCR. The primers for ChIP detection are listed in Table 2.
RIP assay
For the RIP experiment, the EZ-Magna RIP Kit (Millipore, USA) was used. First, OVCAR-8 cells were lysed in the RIP lysis buffer. The suspension was centrifugated at 14,000 rpm for 10 min and cell supernatant was incubated with the RIP immunoprecipitation buffer which contained anti-LIN28B-conjugated beads. The above samples were further incubated with protease K for 30 min. Finally, the immunoprecipitated RNA was analyzed by PCR. The primers for RIP detection are listed in Table 2.
RNA pull-down assay
For the RNA pull-down experiment, the BersinBio™ RNA pulldown Kit (Bersinbio, China) was used. In brief, according to the length/mass ratio of 1 ug/1000 nt, biotin-labeled target RNA probes and NC probes with corresponding mass were respectively taken to form the RNA secondary structure. The RNA probes forming the RNA secondary structure were added to the beads and incubated with the cell lysate. Finally, the RNA complex bound to the beads was eluted and extracted for immunoblot analysis.
Statistical analysis
The GraphPad Prism 9.5 software was used to analyze data. All data were expressed as mean ± SD. For two-group comparisons, the statistical differences were assessed by Studen’s t-test. For multiple comparisons, the statistical differences were evaluated by ANOVA followed by Tukey’s test. The criterion for significance was p < 0.05 for all comparisons.
Results
Identification of DEGs and their functional annotation in serous ovarian cancer
We performed DEGs screening on the SOC dataset GSE14407 with |log2FC|> 1, adj. P < 0.05, and then performed GO and KEGG enrichment analysis of the up-regulated DEGs (Fig. 1a). We found that many up-regulated DEGs were enriched in molecular functions related to DNA transcriptional activity as well as in pathways associated with cell cycle and cell adhesion. Furthermore, to reduce the impact of DEGs screening on enrichment results, we further performed GSEA analysis on the GSE14407 dataset and annotated GO and KEGG (Fig. 1b-c). The results of the GSEA analysis indicated that genes under this gene set were significantly enriched in molecular functions related to DNA transcriptional activity and epithelial cell proliferation, and pathways related to the cell cycle. After excluding transcription factors known to have a role in ovarian cancer, we noted that Zinc finger protein 384 (ZNF384) is a common C2H2 type zinc finger protein transcription factor, which can bind to DNA, activate RNA polymerase, and promote the downstream gene expression (extracellular matrix genes, cell cycle regulatory proteins) (He et al. 2019; Zhu et al. 2023). In addition, ZNF384 has been extensively studied in gynecological tumors, which can promote the malignant progression of cervical cancer and breast cancer (Meng et al. 2022; Mori et al. 2015). Therefore, we chose ZNF384 for follow-up study.
Fig. 1.
Identification of DEGs and their functional annotation in serous ovarian cancer. a GO and KEGG enrichment analysis of up-regulated DEGs in GSE14407 serous ovarian cancer database. Raw data between serous ovarian cancer and normal ovarian tissue were obtained from the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/gds/) dataset and the gene expression profiling (GSE14407) was selected. Differentially expressed genes (DEGs) were selected by |log2FC|> 1 and adj. P < 0.05 in the GSE14407 dataset. Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis software were R packages ggplot2. b Enrichment fraction diagram of GO-GSEA in the GSE14407 dataset. c Enrichment fraction diagram of KEGG-GSEA in the GSE14407 dataset. Gene Set Enrichment Analysis (GSEA) analysis software was R package clusterProfiler + enrichplot. GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Gene and Genome; DEG, differentially expressed genes; GSEA, Gene Set Enrichment Analysis
ZNF384 is highly expressed in serous ovarian cancer
The ZNF384 expression level in normal ovarian epithelial tissues and SOC tissues of the GSE14407 database was further analyzed, and it can be seen that ZNF384 was overexpressed in SOC tissues. In addition, we demonstrated the location of the up-regulated DEGs in the dataset on the chromosome by circos plot, and the location of ZNF384 on the chromosome is shown in Fig. 2a. We further collected 30 cases fresh SOC tissues and 30 cases non-tumor-ovarian tissues to detect the expression of ZNF384 by qPCR. The results demonstrated that the ZNF384 expression was higher in SOC tissues compared to the non-tumor-ovarian tissues (Fig. 2b). In addition, in silico data analysis showed that ZNF384 was altered in 7% of SOC and the mutation type was predominantly amplification (Fig. 2c). We further analyzed whether ZNF384 expression affects the prognosis of SOC by Gene Set Cancer Analysis dataset, and the results demonstrated that patients with high ZNF384 expression had significantly lower OS and DSS than patients with low expression. In addition, there was a trend of decreased PFS and DFI in patients with high ZNF384 expression. The above analysis suggested that high expression of ZNF384 may indicate a poor disease prognosis (Fig. 2d). We further collected data from 100 clinical samples of SOC patients to analyze whether its expression affects clinicopathological features. The data indicated that ZNF384 expression was closely associated with the FIGO stage and Lymph node metastasis (Table 3). Figure 2e showed representative immunohistochemical staining maps of low and high expression of ZNF384 and the immunohistochemical score of the samples was shown in Supplementary Table 1. The above analysis indicated that ZNF384 was abnormally highly expressed in SOC and was related to poor tumor prognosis.
Fig. 2.
ZNF384 is highly expressed in serous ovarian cancer. a Circos plot showed the location of up-regulated genes in chromosomes of the GSE14407 dataset. The outermost track showed the chromosome information of these genes. The box plot in the middle of the Circos plot showed the expression of ZNF384 in normal and cancer tissue in the GSE14407 database. b Relative expression of ZNF384 in 30 serous ovarian cancer tissues and 30 normal ovarian tissues by qPCR. c The genomic profile of ZNF384 was obtained from the serous ovarian cancer data set (TCGA) using cBioportal database (https://www.cbioportal.org/). d The association between ZNF384 expression and OS, PFS, DFI, and DSS in serous ovarian cancer from the GSCA database (https://guolab.wchscu.cn/GSCA/). e Immunohistochemistry staining images of high and low expression of ZNF384. The scale bar indicates 50 µm. TCGA, The Cancer Genome Atlas; GSCA, Gene Set Cancer Analysis; OS, overall survival; PFS, progression-free survival; DFI, disease-free interval; DSS, disease-specific survival. ****p < 0.0001 as compared to the Normal group. Data are presented as mean ± SD. qPCR, quantitative real-time PCR
Table 3.
Correlation of ZNF384 expression and clinicopathological parameters in serious ovarian cancer tissues
| Characteristics | No | ZNF384 expression level | P value | |
|---|---|---|---|---|
| Low | High | |||
| Overall | ||||
| Patient age | ||||
| ≤ 55 | 58 | 23 | 35 | |
| > 55 | 42 | 12 | 30 | 0.25141 |
| Pathological grade | ||||
| Well differentiated | 13 | 4 | 9 | |
| Moderately differentiated | 3 | 1 | 2 | 0.093951 |
| Poorly differentiated | 84 | 30 | 54 | |
| FIGO stage | ||||
| I-II | 43 | 22 | 21 | |
| III-IV | 57 | 13 | 44 | 0.0325 |
| Lymph node metastasis | ||||
| Yes | 42 | 8 | 34 | |
| No | 58 | 27 | 31 | 0.00443 |
| Intestinal metastasis | ||||
| Yes | 39 | 12 | 27 | |
| No | 61 | 23 | 38 | 0.47817 |
| Diaphragmatic metastasis | ||||
| Yes | 9 | 2 | 7 | |
| No | 91 | 33 | 58 | 0.39951 |
Differences between groups were done by the Chi-square test
p < 0.05 was considered significantly different
ZNF384 promotes the growth of serous ovarian cancer cells in vitro
Next, we specifically reduced or increased endogenous expression of ZNF384 in SOC cell lines by lentiviral interference or overexpression techniques. We first detected the expression of ZNF384 in normal ovarian epithelial cells (IOSE-80) and 5 lines of serous ovarian cancer cells (OVCAR-8, HEY A8, OVCAR-3, HEY and SKOV3) by western blot, and found that ZNF384 expression was higher in OVCAR-8 and OVCAR-3 cells and lower in SKOV3, so we selected OVCAR-8 and OVCAR-3 cells to ZNF384 knockdown treatment and selected SKOV3 cells to overexpression treatment (Fig. 3a). As shown in Fig. 3b, protein and mRNA levels of ZNF384 were decreased in ZNF384-shRNA lentivirus-infected SOC cells. The above analyses confirmed that ZNF384 was specifically depleted by ZNF384 shRNA. Similarly, the ZNF384 expression was significantly increased in SKOV3 cells transfected with ZNF384 overexpression lentivirus (Fig. 3c). We next assessed the effects of ZNF384 knockdown or overexpression on SOC cell proliferation, including cell viability and colony-forming ability. The results demonstrated that compared with the shCon group, ZNF3B4 knockdown dramatically inhibited cell viability, while forced expression of ZNF384 in SOC cells enhanced cell viability (Fig. 3d). In clone formation assay, ZNF384 knockdown groups were observed to restrain SOC cells proliferation whereas ZNF384 overexpression groups showed the opposite phenotype (Fig. 3e). These data suggested that ZNF384 promoted the proliferation of SOC cells.
Fig. 3.
ZNF384 promotes the growth of serous ovarian cancer cells in vitro. a Western blot analysis of ZNF384 expression in normal ovarian epithelial cells (IOSE-80) and 5 lines of serous ovarian cancer cells (OVCAR-8, HEY A8, OVCAR-3, HEY and SKOV3). b Western blot and qPCR analysis of ZNF384 expression in OVCAR-8 and OVCAR-3 cells with ZNF384 knockdown. c Analysis of ZNF384 expression in SKOV3 cells with ZNF384 overexpression. ****p < 0.0001 as compared to the shCon or Vector group. d Cell proliferation of OVCAR-8, OVCAR-3, and SKOV3 cells after lentivirus infection was detected by CCK8 assay. *p < 0.05, **p < 0.01 as compared to the shCon or Vector group. e Colony formation assay of OVCAR-8, OVCAR-3, and SKOV3 cells was performed. The experiments were repeated three times. **p < 0.01, ***p < 0.001, ****p < 0.0001 as compared to the shCon or Vector group. Data are presented as mean ± SD. qPCR, quantitative real-time PCR
ZNF384 promotes the invasion and migration of serous ovarian cancer cells
Meanwhile, we also evaluated the effect of ZNF384 on SOC metastasis. The cells of ZNF384 knockdown groups had a greater migrating distance and slower wound healing rate compared to the cells of shCon group after 24 h, while up-regulation of ZNF384 significantly increased the migratory capacity of SOC cells (Fig. 4a). In addition, the transwell assay results showed similar trends in the invasion of SOC cells (Fig. 4b). The overexpression of MMP-2 in ovarian cancer is related to tumor invasion and metastasis (Vos et al. 2016). Therefore, we verified whether ZNF384 affects the production of MMP-2. As shown in Fig. 4c, the MMP-2 concentration in the shZNF384 groups decreased extremely compared to the shCon groups, whereas ZNF384 overexpression resulted in an increase in MMP-2 concentration. Past studies have suggested that vimentin overexpression may be associated with increased metastatic capacity of SOC cells through the epithelial to mesenchymal transformation (Psilopatis et al. 2023). On this basis, we further evaluated the regulatory effect of ZNF384 on vimentin protein expression. Immunofluorescence assay showed that ZNF384 knockdown inhibited vimentin expression compared with control cells, while ZNF384 overexpression showed the opposite results (Fig. 4d). These data strongly indicated that ZNF384 promotes SOC cell invasion, possibly in part through the influence of MMP-2.
Fig. 4.
ZNF384 promotes the invasion and migration of serous ovarian cancer cells. a The effects of ZNF384 on the migration of serous ovarian cancer cells by wound healing assay. Photographs showed cell migration before and after injury under the microscope at 100 × magnification field. Quantification of cell migration by measuring wound closure areas before and after injury. b Effect of ZNF384 on the invasion of serous ovarian cancer cells by transwell assay (crystal violet staining × 200). c MMP-2 levels in the supernatants of OVCAR-8, OVCAR-3, and SKOV3 cells were detected by ELISA assay. *p < 0.05, **p < 0.01, ****p < 0.0001 as compared to the shCon or Vector group. d Representative immunofluorescence images of vimentin expression (red) in OVCAR-8, OVCAR-3, and SHOV3 cells. Cell nuclei were stained by DAPI (blue). The scale bar indicates 50 µm. Data are presented as mean ± SD. DAPI, 4′6’-diamidino-2-phenylindole dihydrochloride
ZNF384 promotes tumor growth and metastasis in vivo
We further explored the effect of ZNF384 on the SOC cells by establishing a vivo xenograft animal model. The results indicated that the tumor volume and weight in the ZNF384 knockdown group were significantly reduced, indicating that the tumor growth in the shZNF384 group was slower. In contrast, overexpression of ZNF384 promoted the growth rate of tumors (Fig. 5a-c). In addition, we demonstrated that the expression levels of Ki67, a marker for tumor growth, were downregulated after ZNF384 knockdown using immunohistochemical staining, while the overexpression of ZNF384 was the opposite (Fig. 5d). Next, we further verified whether it affects the metastasis of SOC cells in vivo. ZNF384 knockdown or overexpressed SOC cells were intra-peritoneally injected into the nude mice, respectively, and the bioluminescence of the mice was observed in intravital imaging after 4 weeks. As shown in Fig. 5e, compared with the shCon group, the bioluminescence level of the shZNF384 group was reduced, while ZNF384 overexpression significantly enhanced the bioluminescence level in mice. In addition, the number of tumor nodules was detected and counted, and the results were consistent with the expectation that ZNF384 overexpression significantly increased the number of metastatic nodules (Fig. 5f). Taken together, our results demonstrated that ZNF384 may have a promoting effect on the development of SOC in vivo.
Fig. 5.
ZNF384 promotes tumor growth and metastasis in vivo. a-c Xenograft tumors were formed by subcutaneous injection of OVCAR-8 or SKOV3 cells into BALB/c nude mice. The tumors were stripped after 25 days, photographed, measured, and weighed. *p < 0.05, **p < 0.01, ****p < 0.0001 as compared to the shCon or Vector group. d Immunohistochemical staining images of ZNF384, Ki67 expression in tumor tissues. The scale bar indicates 50 µm. e Bioluminescence images of BALB/c nude mice after intraperitoneal injection of OVCAR-8 or SKOV3 cells. f Representative images of abdominal cavity metastasis of tumors in different groups of nude mice (left panel) and quantification of the number of tumor metastatic nodules (right panel). ***p < 0.001, ****p < 0.0001 as compared to the shCon or Vector group. Data are presented as mean ± SD
ZNF384 promotes transcriptional activity of LIN28B in serous ovarian cancer
In our previous bioinformatics analysis, Lin-28 homolog B (LIN28B) was screened as a potential target of ZNF384. We hypothesized that the oncogenic effect of ZNF384 might be exerted by promoting the function of the RNA-binding protein LIN28B. Both western blot and qPCR assays showed a positive correlation between ZNF384 treatment and LIN28B expression in SOC cells, suggesting that LIN28B is indeed regulated by ZNF384 (Fig. 6a-b). Next, we examined whether ZNF384 promoted LIN28B expression via increasing its promoter activity. We downloaded the -2000/ + 100 bp DNA sequence of LIN28B from the UCSC website, and then uploaded this sequence to the JASPER database to predict the sequence that may be recognized by ZNF384. The binding sites were shown in Fig. 6c. We constructed luciferase reporters containing different lengths of the LIN28B promoter and co-transfected them with ZNF384 overexpression plasmid into OVCAR-8 cells. As shown in Fig. 6c, the luciferase activity was enhanced in the ZNF384 overexpression group compared with the vector group. Furthermore, the luciferase activity of the LIN28B promoter sequence (-1364 ~ + 31) was significantly lower than that of the sequence (-1969 ~ + 31) under ZNF384 overexpression conditions, therefore, we speculated that the main regulatory region of ZNF384 on LIN28B promoter was the -1969 ~ -1364 region, which contained three binding sites: site7, site8, and site9. Next, we proposed to design primers for each of these three sites to further verify whether ZNF384 directly binds to these sites using ChIP assay, but due to the close distance between site 7 and site 8, only a pair of primers can be used for detection. As demonstrated in Fig. 6d, the left panel corresponds to sites 7 and 8, and the right panel corresponds to sites 9. The positive products were detected in both the Input group and the anti-ZNF384 group, indicating that ZNF384 could directly bind to these sites, while negative products were detected in the anti-IgG group, suggesting that the binding of ZNF384 to the above sites is specific. Next, we further assessed whether the regulation of LIN28B by ZNF384 affects the malignant phenotype of SOC cells by constructing the LIN28B overexpression plasmid and transfecting it into ZNF384-knockdown stably transfected cells. The transfection efficiency was verified by western blot and qPCR (Fig. 6e). The viability of cells transfected with LIN28B was enhanced compared to the vector group, indicating that LIN28B partially restored the knockdown effect of ZNF384 (Fig. 6f). Similarly, both wound healing and transwell experiments demonstrated that LIN28B mediated the regulation of ZNF384 on the migration and invasion capacity of SOC cells (Fig. 6g-h). Taken together, ZNF384 mediated the malignant behavior of SOC cells through transcriptional activation of LIN28B expression.
Fig. 6.
ZNF384 promotes transcriptional activity of LIN28B in serous ovarian cancer. a-b Western blot and qPCR analysis of LIN28B expression in OVCAR-8, OVCAR-3, and SKOV3 cells with ZNF384 knockdown or overexpression. ***p < 0.001, ****p < 0.0001 as compared to the shCon or Vector group. c Predicted binding site and sequence names of LIN28B promoter (left panel). Dual-luciferase vectors containing LIN28B promoter sequences with different binding sites were co-transfected with ZNF384 overexpression plasmid into OVCAR-8 cells, and luciferase reporter gene assay was performed after 48 h (right panel). d The binding of ZNF384 to the LIN28B promoter was verified by ChIP experiments. e OVCAR-8 cells were transfected with LIN28B overexpression plasmid, and the transfection efficiency was verified by western blot and qPCR. f Cell proliferation of OVCAR-8 cells with LIN28B overexpression was detected by CCK8 assay. g-h The migration (g) and invasion (h) capacity of the OVCAR-8 cells transfected with LIN28B by wound healing and transwell assays. *p < 0.05, **p < 0.01. Data are presented as mean ± SD. qPCR, quantitative real-time PCR; ChIP, chromatin immunoprecipitation
LIN28B promotes the malignant behavior of serous ovarian cancer cells by regulating UBD
Based on previous experiments, we have clarified the role of LIN28B in the development of SOC, but its molecular mechanism is still unclear. In this part, we mainly carried out relevant studies on the downstream molecular targets of LIN28B as well as the regulatory mechanism. Firstly, we predicted that UBAP2L, UBE2T, ubiquitin D (UBD), UBE3A, UBE3C, and USP1 might be the downstream factors of LIN28B through literature research and ENCORI website, and the binding relationship between the above factors and LIN28B has not been reported. Next, we used RIP and qPCR assays to detect the enrichment of the above predictors in anti-IgG and anti-LIN28B antibodies, using SOX2 as a negative control. As shown in Fig. 7a, UBAP2L, UBE2T, UBD, UBE3A, UBE3C, and USP1 were notably enriched in the anti-LIN28B RIP but not anti-IgG RIP. Notably, the role of UBE2T and USP1 in ovarian cancer has been previously reported, while the GEPIA database showed no significant changes in the expression of UBAP2L, UBE3A, and UBE3C in ovarian cancer (Cui et al. 2022; Song et al. 2022). In addition, we found that UBD expression was higher in ovarian cancer samples than in normal tissues through GEPIA database analysis, so UBD was selected as the downstream factor of LIN28B for follow-up studies. The information on the predicted binding motif of LIN28B was shown in Fig. 7b. To demonstrate the physical interaction between LIN28B and UBD mRNA, we performed RNA pull-down experiments, which showed that LIN28B could bind to the wild-type UBD mRNA (lane 3). We further generated a UBD mutant Probe (UBDdel probe), which the predicted LIN28B binding site (AUGAGAA) was deleted. The results showed that LIN28B could bind to wild-type UBD mRNA (UBDWT probe, lane 3). In addition, the binding of UBD to LIN28B was attenuated after the deletion of the predicted UBD binding site (UBDdel probe; lane 4), suggesting that LIN28B may bind UBD by specifically recognizing this site. We further explored the relationship between UBD mRNA and LIN28B. Western blot and qPCR assays confirmed that knockdown of LIN28B resulted in decreased UBDL mRNA and protein levels (Fig. 7c). On this basis, we used actinomycin D for mRNA degradation experiments to inhibit mRNA transcription. As shown in Fig. 7d, the results indicated that LIN28B knockdown enhanced the degradation of UBD mRNA. In summary, the above observations suggested that LIN28B can bind to UBD and enhance the stability of UBD mRNA.
Fig. 7.
LIN28B promotes the malignant behavior of serous ovarian cancer cells by regulating UBD. a Downstream factors that LIN28B may regulate were verified by RIP assay. SOX2 was used as a negative control. b Schematic diagram of predicted LIN28B motif and UBD binding sites. The binding of LIN28B and UBD was verified by RNA pull-down assay, and the expression of LIN28B in OVCAR-8 cells was detected by western blot. c Western blot and qPCR analysis of UBD expression in OVCAR-8 cells with LIN28B knockdown. d The half-life of UBD mRNA was detected in cells depicted in (c). e OVCAR-8 cells were transfected with UBD overexpression plasmid, and the transfection efficiency was verified by western blot and qPCR. Cell proliferation (f), migration (g), and invasion (h) ability of OVCAR-8 cells after transfection with UBD were detected by CCK-8, wound healing, and transwell assays, respectively. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are presented as mean ± SD. RIP, RNA-binding protein immunoprecipitation; qPCR, quantitative real-time PCR
Next, we further verified whether LIN28B could promote SOC by targeting UBD. UBD overexpression plasmid was co-transfected with shLIN28B into OVCAR-8 cells to further assess the cell proliferation, migration, and invasion abilities. The UBD protein expression in OVCAR-8 cells transfected with shLIN28B or co-transfected with shLIN28B + UBD was detected by western blot. the UBD expression decreased with the knockdown of LIN28B, and qPCR also showed similar experimental results, suggesting that LIN28B may regulate the expression of UBD in SOC cells (Fig. 7e). The proliferation of OVCAR-8 cells transfected with shLIN28B was slower than that of the shNC group, while UBD partially reversed the inhibitory effect of shLIN28B (Fig. 7f). In addition, transfection with shLIN28B inhibited the migration and invasion of SOC cells compared to the shNC group, whereas UBD significantly restored this trend (Fig. 7g-h). Collectively, these data demonstrated that LIN28B promoted SOC progression by regulating UBD expression in SOC cells.
Discussion
SOC is the most lethal malignancy of the female reproductive system (Prat 2015). Research conducted over the past 20 years has led to a profound understanding of its cellular and molecular basis. For example, the origin of HGSOC has been elucidated, and serous tubal epithelial carcinoma is the precursor lesion of HGSOC which helps guide the development of prevention programs for patients with genetic susceptibility (Yamamoto et al. 2016). Although the diagnosis and treatment technology of SOC has made great progress, its clinical outcome is still not optimistic due to the late clinical stage of most patients at the time of diagnosis and the high recurrence rate after treatment. Therefore, the discovery of new potentially effective targeted genes and biomarkers is the key to early tumor prevention and effective treatment.
ZNF384 belongs to a family of zinc finger proteins and acts as transcription factors that can shuttle between nucleoplasm and respond to changes in cell shape and cytoskeletal structure by altering DNA structure (Janssen and Marynen 2006). In addition, ZNF384 is consistently highly expressed in a variety of malignant tumors, such as lung cancer, hepatocellular cancer, and has a significant clinical correlation with worsening prognosis (He et al. 2019; Yan et al. 2022; Young et al. 2016). Recent studies have identified ZNF384 as a potential biomarker for psoriasis and linked it to excessive inflammation and metabolic disorders, further demonstrating the critical role of ZNF384 in the disease process (Liu et al. 2022). In our research, we found that ZNF384 has a gene variant rate of 7% in SOC, was overexpressed in SOC, and ZNF384 expression was strongly related to the prognosis of patients. It is suggested that ZNF384 may be involved in the progression of SOC as an oncogene. On this basis, we revealed that ZNF384 knockdown inhibited the proliferation, migration, and invasion of SOC cells, while overexpression of ZNF384 promoted the malignant behavior of SOC cells. Meanwhile, by constructing a xenograft mice model, we found that ZNF384 knockdown in vivo significantly inhibited tumor growth as well as the invasive ability of tumor cells. Combined with the above findings, we reasonably hypothesized that SOC cells express high levels of ZNF84 to enhance their malignant behaviors through some unknown mechanisms.
The pathogenic mechanism of ZNF384 has been extensively studied in a variety of cancers. In hepatocellular carcinoma, ZNF384 can proliferate tumor cells by increasing cell cycle protein expression (He et al. 2019; Xiao et al. 2022). In addition, ZNF384 has been shown to bind to the APOBEC3B promoter and regulate A3B expression in cervical cancer, thereby accelerating cancer progression (Mori et al. 2015). To further explore the molecular mechanisms by which ZNF384 regulates SOC cell progression, we validated the target genes of ZNF384. The analysis results of the JASPER website suggested that the LIN28B promoter could be recognized by ZNF384, indicating that ZNF384 might transcriptionally activate LIN28B.
LIN28B is a highly conserved RNA-binding protein discovered in 1997, and in recent years the expression and active participation of LIN28B has been found in embryonic stem cell differentiation (Moss et al. 1997; Zhou et al. 2020). LIN28B has previously been reported to be important in the transformation of cancer stem-like cells, promotes tumor invasiveness and metastasis, and is overexpressed in multiple tumor types associated with advanced disease (Viswanathan et al. 2009; Xu et al. 2023; Yuan and Tian 2018). LIN28B is associated with poor prognosis of ovarian cancer by a molecular mechanism related to the negative regulation of let-7 family microRNAs, altering the expression of downstream genes and tumor development (Busch et al. 2016). Our study showed that ZNF384 and LIN28B expression are positively correlated in SOC cells, and ZNF384 could recognize specific sequences on the LIN28B promoter, thereby promoting the transcriptional activity of LIN28B. Moreover, LIN28B could partially restore the tumor suppressor effect of ZNF384 knockdown. Therefore, we suggested that the ZNF384/LIN28B regulatory axis has a very important role in SOC. As an RNA-binding protein, LIN28B may have multiple target RNAs. We found that LIN28B could bind UBD mRNA by RBPsuite and ENCORI website analysis, and the analysis of the GEPIA database showed that UBD expression was elevated in ovarian cancer tissues. UBD is also a marker factor for precancerous lesions, and past studies revealed that UBD imparts malignant characteristics to non-tumorigenic cells and enhances malignancy-associated features in cancer cells (Gao et al. 2014; Zhang et al. 2020). In addition, we noted an interesting phenomenon that UBD expression in tumors was somewhat tissue-specific, and transcriptional upregulation was observed in the ovary (Lee et al. 2003). Our study verified that LIN28B could bind to UBD and thus enhance the stability of UBD mRNA. In tumor cells, the expression of UBD was positively correlated with that of LIN28B. Furthermore, the rescue experiment results showed that UBD partially reversed the inhibitory effect of LIN28B knockdown on the malignant behaviors of SOC cells. Overall, these results suggested that ZNF384 is deeply involved in SOC carcinogenesis and progression by upregulating the expression of UBD via promoting the transcriptional activity of LIN28B and promoting the malignant behavior of tumor cells.
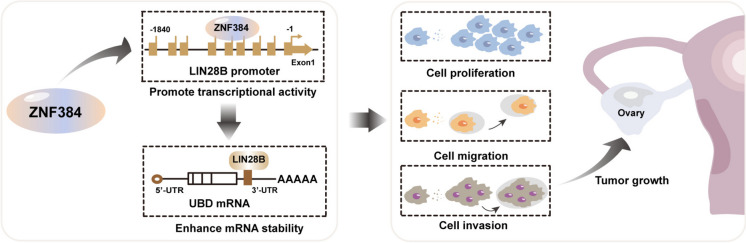
In summary, we systematically demonstrated the biological role of ZNF384 in SOC occurrence and development, confirmed the oncogene role of ZNF384 in SOC, and then revealed the molecular mechanisms by which ZNF384 regulates cell proliferation (Fig. 8). Our results suggested that ZNF384 regulates the malignant development of SOC and is a potential prognostic marker for SOC.
Fig. 8.
Mechanism of ZNF384 promoting serous ovarian cancer progression. ZNF384 is involved in the malignant progression of serous ovarian cancer by promoting the proliferation, migration, and invasion of serous ovarian cancer cells. Its mechanism of action is related to the binding of the LIN28B promoter to promote its transcriptional activity, which further binds UBD mRNA, affects mRNA stability, and enhances its expression
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Min Wang conceives and designs experiments. Ye Yang, Runze He, Dongxiao Li, Tianli Mu and Ziteng Kuang conduct the experiments. Runze He collects clinical samples. Ye Yang and Runze He perform statistical analysis. Ye Yang writes initial draft of manuscript. Min Wang revises manuscript. All authors have critically revised manuscript and approved its final version.
Funding
This study was supported by the Outstanding Scientific Fund of Shengjing Hospital (under Grant 201705).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
All procedures involving human subjects were approved by Shengjing Hospital of China Medical University (Approval No. 2023PS426K), and animal experiments were performed with consent from the ethics committee of China Medical University (Approval No. CMU2023049).
Consent for publication
All authors have read the paper and agree that it can be published.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Boehm KM, Aherne EA, Ellenson L, Nikolovski I, Alghamdi M, Vázquez-García I, Zamarin D, Long Roche K, Liu Y, Patel D, et al. Multimodal data integration using machine learning improves risk stratification of high-grade serous ovarian cancer. Nat Cancer. 2022;3:723–33. 10.1038/s43018-022-00388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch B, Bley N, Müller S, Glaß M, Misiak D, Lederer M, Vetter M, Strauß HG, Thomssen C, Hüttelmaier S. The oncogenic triangle of HMGA2, LIN28B and IGF2BP1 antagonizes tumor-suppressive actions of the let-7 family. Nucleic Acids Res. 2016;44:3845–64. 10.1093/nar/gkw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CL, Chen TJ, Li WS, Lee SW, Yang CC, Tian YF, Lin CY, He HL, Wu HC, Shiue YL, et al. Upregulated ubiquitin D is a favorable prognostic indicator for rectal cancer patients undergoing preoperative concurrent chemoradiotherapy. Onco Targets Ther. 2022;15:1171–81. 10.2147/ott.S378666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui P, Li H, Wang C, Liu Y, Zhang M, Yin Y, Sun Z, Wang Y, Chen X. UBE2T regulates epithelial-mesenchymal transition through the PI3K-AKT pathway and plays a carcinogenic role in ovarian cancer. J Ovarian Res. 2022;15:103. 10.1186/s13048-022-01034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dochez V, Caillon H, Vaucel E, Dimet J, Winer N, Ducarme G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12:28. 10.1186/s13048-019-0503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Theng SS, Zhuo J, Teo WB, Ren J, Lee CG. FAT10, an ubiquitin-like protein, confers malignant properties in non-tumorigenic and tumorigenic cells. Carcinogenesis. 2014;35:923–34. 10.1093/carcin/bgt407. [DOI] [PubMed] [Google Scholar]
- Grieco A, Rzeczkowska P, Alm C, Palmert MR. Investigation of peripubertal expression of Lin28a and Lin28b in C57BL/6 female mice. Mol Cell Endocrinol. 2013;365:241–8. 10.1016/j.mce.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Fan X, Li Y, Chen M, Cui B, Chen G, Dai Y, Zhou D, Hu X, Lin H. Overexpression of zinc finger protein 384 (ZNF 384), a poor prognostic predictor, promotes cell growth by upregulating the expression of Cyclin D1 in hepatocellular carcinoma. Cell Death Dis. 2019;10:444. 10.1038/s41419-019-1681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsmoortel HH, Bresolin S, Lammens T, Cavé H, Noellke P, Caye A, Ghazavi F, de Vries A, Hasle H, Labarque V, et al. LIN28B overexpression defines a novel fetal-like subgroup of juvenile myelomonocytic leukemia. Blood. 2016;127:1163–72. 10.1182/blood-2015-09-667808. [DOI] [PubMed] [Google Scholar]
- Izar B, Tirosh I, Stover EH, Wakiro I, Cuoco MS, Alter I, Rodman C, Leeson R, Su MJ, Shah P, et al. A single-cell landscape of high-grade serous ovarian cancer. Nat Med. 2020;26:1271–9. 10.1038/s41591-020-0926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen H, Marynen P. Interaction partners for human ZNF384/CIZ/NMP4–zyxin as a mediator for p130CAS signaling? Exp Cell Res. 2006;312:1194–204. 10.1016/j.yexcr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol. 2013;24(Suppl 10):x16-21. 10.1093/annonc/mdt463. [DOI] [PubMed] [Google Scholar]
- Lee CG, Ren J, Cheong IS, Ban KH, Ooi LL, Yong Tan S, Kan A, Nuchprayoon I, Jin R, Lee KH, et al. Expression of the FAT10 gene is highly upregulated in hepatocellular carcinoma and other gastrointestinal and gynecological cancers. Oncogene. 2003;22:2592–603. 10.1038/sj.onc.1206337. [DOI] [PubMed] [Google Scholar]
- Liu S, Yuan X, Su H, Liu F, Zhuang Z, Chen Y. ZNF384: A potential therapeutic target for psoriasis and alzheimer’s disease through inflammation and metabolism. Front Immunol. 2022;13: 892368. 10.3389/fimmu.2022.892368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QX, Wang KN, Li JH, Zhang H, Chen ZH, Zhou XJ, Cao XC, Wang P, Yu Y. ZNF384-ZEB1 feedback loop regulates breast cancer metastasis. Mol Med. 2022;28:111. 10.1186/s10020-022-00541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Takeuchi T, Ishii Y, Kukimoto I. Identification of APOBEC3B promoter elements responsible for activation by human papillomavirus type 16 E6. Biochem Biophys Res Commun. 2015;460:555–60. 10.1016/j.bbrc.2015.03.068. [DOI] [PubMed] [Google Scholar]
- Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–46. 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- Nakamoto T, Yamagata T, Sakai R, Ogawa S, Honda H, Ueno H, Hirano N, Yazaki Y, Hirai H. CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases. Mol Cell Biol. 2000;20:1649–58. 10.1128/mcb.20.5.1649-1658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres LC, Cushing-Haugen KL, Köbel M, Harris HR, Berchuck A, Rossing MA, Schildkraut JM, Doherty JA. Invasive epithelial ovarian cancer survival by histotype and disease stage. J Natl Cancer Inst. 2019;111:60–8. 10.1093/jnci/djy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat J. Pathology of cancers of the female genital tract. Int J Gynaecol Obstet. 2015;131(Suppl 2):S132-145. 10.1016/j.ijgo.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Psilopatis I, Schaefer JI, Arsenakis D, Bolovis D, Levidou G (2023) SOX11 and epithelial-mesenchymal transition in metastatic serous ovarian cancer. Biomedicines 11. 10.3390/biomedicines11092540. [DOI] [PMC free article] [PubMed]
- Ren J, Fu J, Ma T, Yan B, Gao R, An Z, Wang D. LncRNA H19-elevated LIN28B promotes lung cancer progression through sequestering miR-196b. Cell Cycle. 2018;17:1372–80. 10.1080/15384101.2018.1482137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shago M, Abla O, Hitzler J, Weitzman S, Abdelhaleem M. Frequency and outcome of pediatric acute lymphoblastic leukemia with ZNF384 gene rearrangements including a novel translocation resulting in an ARID1B/ZNF384 gene fusion. Pediatr Blood Cancer. 2016;63:1915–21. 10.1002/pbc.26116. [DOI] [PubMed] [Google Scholar]
- Song A, Wang Y, Jiang F, Yan E, Zhou J, Ye J, Zhang H, Ding X, Li G, Wu Y, et al. Ubiquitin D promotes progression of oral squamous cell carcinoma via NF-Kappa B signaling. Mol Cells. 2021;44:468–80. 10.14348/molcells.2021.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Jiang Y, Jiang Y, Lin Y, Liu J. ML323 suppresses the progression of ovarian cancer via regulating USP1-mediated cell cycle. Front Genet. 2022;13: 917481. 10.3389/fgene.2022.917481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov KM, Pearson DS, Wu Z, Han A, Triboulet R, Seligson MT, Powers JT, Osborne JK, Kane S, Gygi SP, et al. LIN28 phosphorylation by MAPK/ERK couples signalling to the post-transcriptional control of pluripotency. Nat Cell Biol. 2017;19:60–7. 10.1038/ncb3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, O’Sullivan M, Lu J, Phillips LA, Lockhart VL, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41:843–8. 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos MC, van der Wurff AA, Bulten J, Kruitwagen R, Feijen H, van Kuppevelt TH, Hendriks T, Massuger LF. Limited independent prognostic value of MMP-14 and MMP-2 expression in ovarian cancer. Diagn Pathol. 2016;11:34. 10.1186/s13000-016-0485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Yang K, Liu P, Ma D, Lei P, Liu Q. Deoxyribonuclease 1-like 3 inhibits hepatocellular carcinoma progression by inducing apoptosis and reprogramming glucose metabolism. Int J Biol Sci. 2022;18:82–95. 10.7150/ijbs.57919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhou Y, Yang J, Gu Y, Zhang E, Yuan W, Wang C, Jin G, Ma H, Hu Z. Hypomethylation-activated cancer-testis gene LIN28B promotes cell proliferation and metastasis in gastric cancer. Gene. 2022;813: 146115. 10.1016/j.gene.2021.146115. [DOI] [PubMed] [Google Scholar]
- Xu TP, Yu T, Xie MY, Fang Y, Xu TT, Pan YT, Ma P, Shu YQ. LOC101929709 promotes gastric cancer progression by aiding LIN28B to stabilize c-MYC mRNA. Gastric Cancer. 2023;26:169–86. 10.1007/s10120-022-01348-z. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Ning G, Howitt BE, Mehra K, Wu L, Wang X, Hong Y, Kern F, Wei TS, Zhang T, et al. In vitro and in vivo correlates of physiological and neoplastic human Fallopian tube stem cells. J Pathol. 2016;238:519–30. 10.1002/path.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Hayakawa F, Yasuda T, Odaira K, Minamikawa Y, Tange N, Hirano D, Kojima Y, Morishita T, Tsuzuki S, et al. ZNF384-fusion proteins have high affinity for the transcriptional coactivator EP300 and aberrant transcriptional activities. FEBS Lett. 2019;593:2151–61. 10.1002/1873-3468.13506. [DOI] [PubMed] [Google Scholar]
- Yan DW, Li DW, Yang YX, Xia J, Wang XL, Zhou CZ, Fan JW, Wen YG, Sun HC, Wang Q, et al. Ubiquitin D is correlated with colon cancer progression and predicts recurrence for stage II-III disease after curative surgery. Br J Cancer. 2010;103:961–9. 10.1038/sj.bjc.6605870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhou Y, Yang Y, Zeng C, Li P, Tian H, Tang X, Zhang G (2022) Zinc finger protein 384 enhances colorectal cancer metastasis by upregulating MMP2. Oncol Rep 47. 10.3892/or.2022.8260 [DOI] [PMC free article] [PubMed]
- Yong W, Yu D, Jun Z, Yachen D, Weiwei W, Midie X, Xingzhu J, Xiaohua W. Long noncoding RNA NEAT1, regulated by LIN28B, promotes cell proliferation and migration through sponging miR-506 in high-grade serous ovarian cancer. Cell Death Dis. 2018;9:861. 10.1038/s41419-018-0908-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SK, Shao Y, Bidwell JP, Wek RC. Nuclear matrix protein 4 is a novel regulator of ribosome biogenesis and ccontrols the unfolded protein response via repression of Gadd34 expression. J Biol Chem. 2016;291:13780–8. 10.1074/jbc.M116.729830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Tian J. LIN28B promotes the progression of colon cancer by increasing B-cell lymphoma 2 expression. Biomed Pharmacother. 2018;103:355–61. 10.1016/j.biopha.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Yuan R, Wang K, Hu J, Yan C, Li M, Yu X, Liu X, Lei J, Guo W, Wu L, et al. Ubiquitin-like protein FAT10 promotes the invasion and metastasis of hepatocellular carcinoma by modifying β-catenin degradation. Cancer Res. 2014;74:5287–300. 10.1158/0008-5472.Can-14-0284. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhao Y, Wu J, Liangpunsakul S, Niu J, Wang L. MicroRNA-26-5p functions as a new inhibitor of hepatoblastoma by repressing lin-28 homolog B and aurora kinase a expression. Hepatol Commun. 2018;2:861–71. 10.1002/hep4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Chen L, Zhang Z, Cao J, He L, Li L. Ubiquitin-like protein FAT10: A potential cardioprotective factor and novel therapeutic target in cancer. Clin Chim Acta. 2020;510:802–11. 10.1016/j.cca.2020.09.016. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nair GG, Russ HA, Belair CD, Li ML, Shveygert M, Hebrok M, Blelloch R. LIN28B impairs the transition of hESC-derived β cells from the juvenile to adult state. Stem Cell Rep. 2020;14:9–20. 10.1016/j.stemcr.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Bai W, Cheng Q, Fang J. ZNF384-Related Fusion Genes in Acute Lymphoblastic Leukemia. Cancer Control. 2023;30:10732748231182788. 10.1177/10732748231182787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.