Abstract
Nutritionists found beans still rarely consumed in Western diet, despite its high nutritional value was proven and recommended by authorities. The study aimed to propose a simple and effective way of manufacturing a new common bean-based product targeted to diabetics. The key components, including important protein (common beans) and antidiabetic (white mulberry) sources, were carefully selected due to their proven properties. The formulated product underwent extensive analysis: composition, sensory and structure attributes, antioxidant and anti-enzymatic activity showing its antidiabetic potential. Additionally, the proposed label information was presented. The results demonstrate that the proposed blend of ingredients yields a product of exceptional nutritional value, with significant levels of both soluble and insoluble dietary fibers (36.66–48.49%) and proteins (21.22–18.25%). Furthermore, the product exhibits notable antiradical and anti-enzymatic properties. Through precise control of ingredient proportions, conventional raw materials can be fortified with a diverse array of plant-based sources renowned for their health benefits, while maintaining a palatable taste for consumers. The designed product, a savory snack with added bioactive compounds, represents an interesting option for consumers, holding promise as a potential dietary option with low glycemic index (40) for diabetics (anti-glucosidase activity ranged 66.72–70.66%). This study emphasizes the potential of plant-protein-rich foods in offering health-promoting benefits and further supports the use of common beans and natural antidiabetic agents in developing innovative food products.
Keywords: Common bean, Mulberry, Diabetes mellitus, Food quality, Antioxidants, Herbal raw materials.
Subject terms: Nutrition, Metabolic disorders, Nutrition disorders, Lifestyle modification, Nutritional supplements, Nutrition therapy, Weight management
Introduction
Non-communicable diseases (NCDs) present a major global health challenge, with diabetes being a prominent concern. According to the World Health Organization, in 2021, an estimated 422 million individuals were living with diabetes worldwide. International Diabetes Federation projected that number to rise to 653 million by 20301. This chronic condition is characterized by elevated blood glucose levels and can lead to various complications, including cardiovascular disease, kidney disease, and nerve damage2. In the pursuit of effective strategies for managing and preventing diabetes, functional foods have emerged as a promising approach3. These foods extend beyond basic nutrition, offering additional health benefits, including improved blood glucose control.
Numerous studies have investigated the potential of functional foods in the prevention and management of diabetes. A notable randomized controlled trial published in 2018 revealed that the regular consumption of a polyphenol-rich beverage resulted in significant reductions in blood glucose levels and insulin resistance among individuals with type 2 diabetes mellitus (T2DM). Similarly, daily consumption of a functional food bar containing resistant starch and inulin improved glycemic control and insulin sensitivity in individuals with T2DM4. Promising evidence also surrounds other functional foods such as whole grains, legumes, nuts, and seeds in the context of diabetes management - a diet rich in these components was associated with a reduced risk of T2DM5, and glycemic control and lipid levels in individuals with diabetes6.
Incorporating polyphenol-rich beverages and snacks, containing ingredients with high and proven anti-diabetic value may offer a safe and effective approach to managing blood glucose levels and reducing the risk of diabetes-related complications and other lifestyle diseases7–11. From the group of legumes, bean seeds are rich in protein contributing to their antidiabetic activity, starch, B vitamins, with polyphenolic compounds contributing to their antioxidant and anticancer properties12,13. These compounds, including anthocyanins and proanthocyanidins, are particularly present in the bean seed coats14. From the group of herbs, white mulberry leaves (Morus alba L.) have been used in traditional Chinese medicine for treating diabetes, and its bioactive compounds – 1-deoxynojirimycin (DNJ) and morin, have antidiabetic effects by inhibiting enzymes and acting as antioxidants15. Many studies showed that white mulberry leaf significantly reduced blood glucose levels and enhances insulin secretion, supporting its role in diabetes management, both animals and human16,17. Thanks to its anti-glucosidase, anti-amylase and anti-hiperlipidemic activity Morus alba is worth considering as a valuable food component7.
People with diabetes mellitus need to carefully manage their diet to maintain appropriate blood glucose levels. However, most of available food products proposed for diabetes patients, are based on sugar constituents (sweeteners used to reduce the calorie content or the glycaemic index) or high-fat products with low-carb products. In contrast, functional foods produced by enrichment or recipe refinement, using valuable natural bioactive ingredients, instead of synthetic vitamins or minerals, are almost non-existent. Moreover, due to the low consumption of legume seeds in the Western diet, in this study Phaseolus vulgaris L. seeds as a base for designed snacks were used. Therefore, the main aim of this study was to compose such rare natural plant materials into one recipe, using the best technology to obtain a new tasty and nutritional snacks followed by analyzing their composition and prohealthy activity.
Results
Overall organoleptic and sensoric characteristic of the dessigned bean-snacks
As a result of various different initial combinations of selected ingredients, four different recipes (variants) of a designed new bean-based products were choosen by investigators. From the sensoric point of view, a form of crispy and nutritious bean-snacks were purposed as the most desirable. The selected ingredients were choosen by investigators according to their proven antidiabetic activity or prohealthy properties together with appropriate taste.
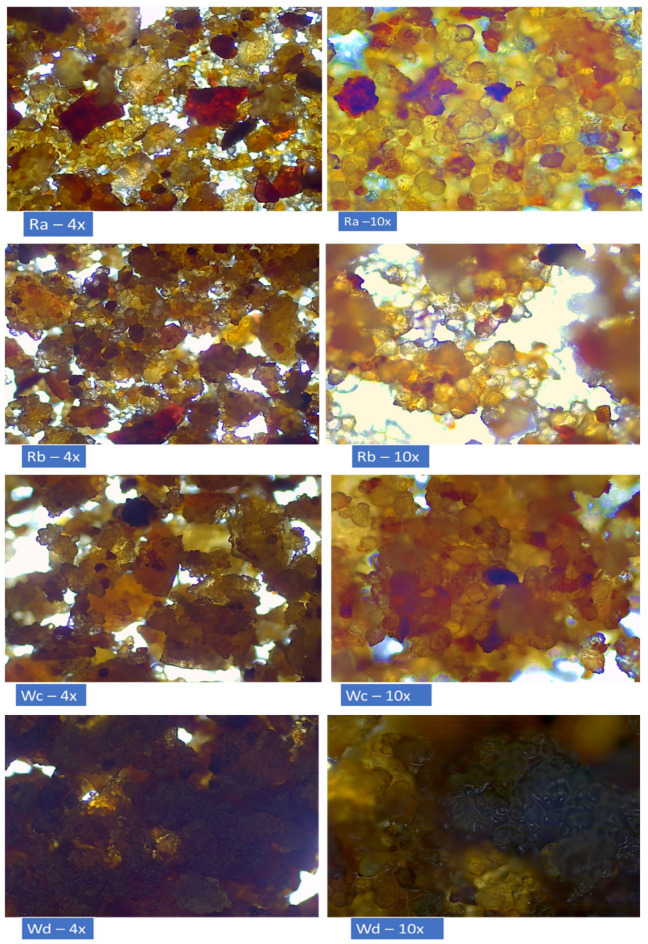
The observation of the surface morphology of food particles under an inverted system microscope using different magnification lenses can provide valuable insights into the physical and chemical properties of the particles18. The surface morphology (at x4 and x10 magnifications) of the analyzed bean-snacks suggests that it has undergone significant changes during the roasting and baking processes. The dehydration of the beans and mixture of additives resulted in a rough, uneven surface, while the coating of olive oil has created a glossy appearance. Moreover, the presence of paprika, basil, and rosemary (darker spots) can all be observed in detail. However, there were found any important changes in the structure of the samples (Fig. 1). This information can be useful in understanding the effects of the roasting and baking process on the product’s physical properties and in evaluating its overall quality.
Fig. 1.
Surface morphology observation under inverted system microscope (magnifications 4x and 10x).
From the sensorical point of view, in the paired comparison test, panelists scores resulted in statistically significant differences (α = 0.05) only in case of overall appearance between red-bean (Ra vs. Rb) samples. It was determined that Ra samples had received higher scores than Rb samples in visually appealing appearance (p < 0.05, Table 1). In paired comparison of white-bean snacks, Wc and Wd, no significant statistical differences were found in their overall appearance. On the other hand, results revealed significant differences in taste and texture between Wc and Wd snacks – Wd samples received higher scores in both taste and texture, indicating that this white-bean snack may be more flavourful and have a more desirable texture for consumers. It was shown by the panelists scores that while Wd had a superior taste and texture compared to Wc, the aroma of both products was very similar in panelists opinion.
Table 1.
Paired comparison test (sensory analysis) results in group of 26 panelists.
| Variant of bean-snacks | Ra | Rb | Wc | Wd |
|---|---|---|---|---|
| Overall Appearance | 115b | 67a | 102a | 80a |
| Taste | 95a | 87a | 67a | 115b |
| Aroma | 94a | 88a | 81a | 101a |
| Texture | 81a | 101a | 65a | 117b |
| Overall acceptability | 385a | 343a | 315a | 413a |
a, b – different letters show statistical differences between variants (in group of white bean-snacks, and red bean-snacks) (α = 0,05).
Basic composition of the bean-snacks
The basic composition of the four snacks was shown in Table 2.
Table 2.
Basic composition of the bean-snacks.
| Components [%] | Ra | Rb | Wc | Wd | ||||
|---|---|---|---|---|---|---|---|---|
| Dry matter | 96.99a ± 0.02 | 97.10a ± 0.01 | 98.12a ± 0.05 | 99.51b ± 0.02 | ||||
| Ash | 4.32b ± 0.07 | 4.38b ± 0.06 | 4.14a ± 0.07 | 4.30b ± 0.02 | ||||
| Fat | 24.27a ± 1.04 | 25.12a ± 0.18 | 25.73a ± 1.51 | 27.27b ± 1.21 | ||||
| Protein | 21.22b ± 2.23 | 20.56b ± 0.51 | 18.34a ± 0.13 | 18.25a ± 0.43 | ||||
| Carbohydrates | 47.19a ± 3.17 | 47.03a ± 0.40 | 49.90b ± 1.51 | 49.69b ± 1.50 | ||||
| SDF [%][% TDF] | 5.38 ± 2.84 | 13.4 | 7.75 ± 1.14 | 18.8 | 8.83 ± 0.46 | 24.1 | 10.40 ± 1.19 | 21.5 |
| IDF [%][% TDF] | 34.74 ± 6.88 | 86.6 | 33.51 ± 7.55 | 81.2 | 27.83 ± 6.22 | 75.9 | 38.09 ± 6.27 | 78.6 |
| TDF | 40.13a ± 5.87 | 41.26 a ± 8.32 | 36.66 a ± 6.27 | 48.49 b ± 7.42 | ||||
Ra, Rb – red-bean snacks, Wc, Wd – white-bean snacks; SDF – soluble dietary fiber, IDF – insoluble dietary fiber, TDF – total dietary fiber; a, b – different letters show statistical differences between variants (α = 0.05).
The variation in dry matter content observed between the four samples was attributed to differences in the composition of each sample and the time of the baking process. The statistically higher dry matter content was found for Wd snacks only, since the longest baking time was used (21 min). The significantly lower ash content in Wc (4.14%) variant was measured probably due to the lower addition of dried mulberry leaves. In case of fat content, in variant Wd slightly more (27.27%) fat was conducted, probably due to the highest addition of olive oil (17 mL).
Significant differences were found between white-bean and red-bean samples in protein content. The analyzed variants Ra and Rb had higher protein content (respectively: 21.22%, 20.56%) than snacks based on white beans (Wc, Wd).
Based on the conducted studies for the basic composition, the content of carbohydrates for each variant was calculated to obtain the sum of macroelements in product. Tha carbohydrates amounts ranged from 47.03% (Rb) to 49.90% (Wd), and for white-bean snacks were significantly higher than for red-bean snacks. It was also found that all variants were good sources of complex carbohydrates, such as fiber and starch. As the results were expressed as per 100 g of samples, red-bean snacks had a fiber content from 40.13% (Ra) – 41.26% (Rb), and white-bean snacks – 36.66% (Wc) – 48.49% (Wd). In the bean-snacks IDF fraction dominated (75.91–86.57% of TDF), while soluble dietary fiber (SDF) was found at level of 13.43–24.09% of TDF.
Antioxidant activity of the bean-snacks
The antioxidant and antiradical activity values of the four variants of the new bean-snack product were analyzed using several methods, including total phenolic content (TPC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), chelating activity, and ferric reducing antioxidant power (FRAP) (Table 3).
Table 3.
Antioxidant activity of common bean-snacks – different extraction processes.
| Test | Extraction type | Variant | |||
|---|---|---|---|---|---|
| Ra | Rb | Wc | Wd | ||
|
TPC [mM GAE] |
Me/For A | 9.06a ± 1.53 | 9.56b ± 1.72 | 8.71a ± 1.63 | 8.87a ± 1.19 |
| ACN/W/For A | 3.26a ± 0.34 | 4.67b ± 0.55 | 2.31a ± 0.80 | 0.68b ± 0.42 | |
|
DPPH· test [mM TE] |
Me/For A | 0.54a ± 0.04 | 0.78b ± 0.01 | 0.79a ± 0.01 | 0.81a ± 0.02 |
| ACN/W/For A | 0.78b ± 0.01 | 0.93a ± 0.01 | 0.95a ± 0.01 | 0.90a ± 0.01 | |
|
ABTS+· test [mM TE] |
Me/For A | 0.24a ± 0.01 | 0.33a ± 0.01 | 0.28a ± 0.02 | 0.37b ± 0.01 |
| ACN/W/For A | 0.13b ± 0.01 | 0.22a ± 0.01 | 0.29a ± 0.01 | 0.36b ± 0.01 | |
| Chelating activity [µmol EDTA] | Me/For A | 49.61a ± 0.05 | 49.56 a ±0.01 | 49.55a ± 0.05 | 49.72a ± 0.09 |
| ACN/W/For A | 50.61a ± 0.04 | 50.32a ± 0.16 | 50.21a ± 0.21 | 50.63a ± 0.07 | |
| FRAP test [mM FeSO4] | Me/For A | 3.42a ± 0.05 | 3.39a ± 0.06 | 3.43a ± 0.05 | 3.46a ± 0.04 |
| ACN/W/For A | 3.75a ± 0.01 | 3.66b ± 0.02 | 3.75a ± 0.01 | 3.70a ± 0.01 | |
Values in the table refer to the means of four replications ± standard deviation (SD). TE – Trolox equivalent; GAE – gallic acid equivalent; a, b – different letters show statistical differences between variants (α = 0,05).
All four variants of the designed snacks had comparable amounts of phenolics measured with Folin reagent, ranging from 8.71 mM GAE to 9.56 mM GAE in Me/ForA extracts, and 0.68 mM GAE to 4.67 mM GAE in ACN/W/ForA extracts.
The results from the DPPH· assay showed that the Ra variant of bean-snacks had the highest antiradical activity in this test, with a concentration of 0.535 mM TE (Me/ForA extracts), and 0.775 mM TE (ACN/W/ForA extracts) required to scavenge DPPH·. The other three variants (Rb, Wc, Wd) also showed good antiradical activity, with concentrations ranging from 0.776 to 0.811 mM TE for the Me/ForA, and 0.904–0.950 mM TE for the ACN/W/ForA extracts. Differences between samples were statistically significant.
In the ABTS+· assay tresults showed the Ra variant as being the highest scavenger, with a concentration of 0.241 mM TE for the Me/ForA, and 0.126 mM TE for the ACN/W/ForA extracts (statistically significant differences). It was in relation with results found in DPPH· test for Ra variant. The variants Rb, Wc, Wd showed such activity ranging 0.281–0.367 mM TE for the Me/ForA, and 0.216–0.355 mM TE for the ACN/W/ForA extracts.
The results from the chelating activity assay showed that all four variants of the product had comparable and high chelating activity with any statisticall differences, ranging from 49.55 to 49.72 µM EDTA for the Me/ForA, and 50.21–50.63 µM EDTA for the ACN/ForA extracts.
The ACN/W/ForA extraction method had higher FRAP values than the Me/ForA extraction for all designed snacks. For the Me/ForA extracts it ranged 3.39–3.46 mM FeSO4, while for the ACN/W/ForA extracts was 3.66–3.75 mM FeSO4 but no statisticall differences found between every variants. Overall, both extraction methods produced samples with relatively high FRAP values, proving that also in this area of activity bean-snacks had some antioxidant potential.
Anti-enzymatic activity of the bean-snacks
The results presented in Table 4 show the α-glucosidase inhibition activity percentage of different variants of bean-snacks. In anti-enzymatic tests the Me/ForA extracts of Ra snacks caused the lowest α-glucosidase inhibition activity at 66.72%, while the Wc variant - the highest (70.66%). In contrast, for the ACN/W/ForA extraction method, the Rb variant was found as the best inhibitor (63.84%), while the Wd variant as the worst one − 57.06%. When comparing the average inhibition activity for all variants in this test, inhibiton was at level of 67.90% in case of Me/ForA extracts, and ~ 19% lower in ACN/W/ForA (55.02%).
Table 4.
Anti-enzymatic activity of bean-snacks.
| Inhibition test | Extraction | Bean-snacks variant | |||
|---|---|---|---|---|---|
| Ra | Rb | Wc | Wd | ||
| α-glucosidase [%] * | Me/ForA | 66.72 ± 1.27 | 67.30 ± 2.24 | 70.66 ± 1.13 | 66.91 ± 0.78 |
| ACN/W/ForA | 49.19 ± 1.20 | 63.84 ± 0.93 | 50.00 ± 0.64 | 57.06 ± 0.58 | |
*acarbose was used as reference with 100% inhibition activity.
Label information proposal
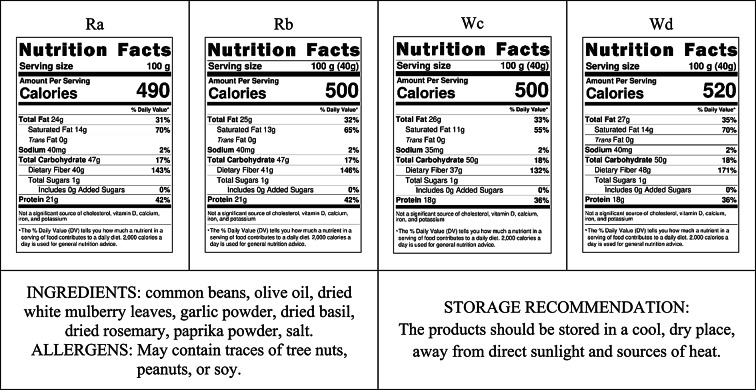
In Table 6 calculated nutritional values of each bean-snacks are shown, together with reference daily intake. Information about ingredients is obligatory to be included on the label, together with allergens and storage recommendation (Table 5.).
Table 5.
Nutritional value tables and recommendation for bean-snacks.
For designed food products above claims were proposed to be introduced on label, if product would be in interest of any food producers (Table 6).
Table 6.
Nutrition claims possible to be introduced to bean-snacks packaging.
| High protein | The product could be labelled as “high protein” because it contains 36.5–42.44% protein, which exceeds the reference daily intake (RDI) of 20%. |
|---|---|
| High fiber | The product could be labelled as “high fiber” because it contains 36.66–48.49% total dietary fiber, which exceeds the RDI of 15%. |
| Source of lipids | The product could be labelled as a “source of lipids” because it contains 24.27–27.27% lipids, which is above the threshold of 3%. |
| High energy | The product could be labelled as “high energy” because it contains 492.07–517 kcal/100 g, which exceeds the threshold of 400 kcal/100 g for “high energy” claims. |
Estimated GI and GL (for 40 g suggested portion) were calculated for designed variants of bean-snacks (Table 7). The glycaemic index of each food product was the same at 40. This means that all of the products have a similar effect on blood sugar levels compared to the reference food (glucose). However, the glycaemic load values of the products differed. Wc had the highest GL value of 5.30 per portion, indicating that it will have the greatest impact on blood sugar levels. This is likely due to its high carbohydrate content. In contrast, Wd had the lowest GL value of 0.48, indicating that it will have the least impact on blood sugar levels. Ra and Rb had similar GL values, with Ra having a GL of 2.80 and Rb having a GL of 2.31. Although Ra had a higher carbohydrate content, the difference in GL values was not significant.
Table 7.
Estimated glycemic index (GI) and glycemic load (GL) values of designed bean-snacks.
| Variant | Glycemic index [GI] | Glycemic load [GL] |
|---|---|---|
| Ra | 40a | 2.80a |
| Rb | 40a | 2.31a |
| Wc | 40a | 5.30a |
| Wd | 40a | 0.48b |
The estimated glycemic index (GI) was determined by using glucose as the standard food and the results were presented as a percentage of the glycemic response to the reference food; the stimated glycemic load (GL) was determined for 40 g portion of product; a, b – different letters show statistical differences between variants (α = 0,05).
Discussion
It is assumed that there are thousands of functional food products on the market, worldwide. The manufacturing technology of most of them is associated with the use of sugar and/or fat substitutes or low-calorie raw materials. On the other hand, products and raw materials with documented anti-diabetic value, including unpopular herbal raw materials, are only available as dietary supplements (according to law). In view of the increasing number of diabetics in the society, any new nutritional product should primarily fill gaps in the food range. It is worth noting that it is in the group of young consumers that pre-diabetic conditions are currently developing most rapidly, and the developed snack will be an excellent addition to their diet. It can replace highly processed snacks.
In this study, the main aim was to show possible prohealthy status of composed new common bean-based snacks designed by using rarely used natural anti-diabetic materials. By using the appropriate and easy technics tasty and nutritional snacks were produced.
In the paired comparison test panelists found such variants interesting. Such test is a valuable statistical analysis method that is commonly used in sensory evaluation and other fields to compare the performance or preferences of two items. The results of paired comparison tests allow for meaningful interpretation of the results and help to determine whether there is a significant difference between the items being compared and were analyzed according to Baryłko-Pikielna & Matuszewska19. In the literature sensory quality signals were found to be an important aspect for consumers when purchasing certain types of products20. From the technological point of view, sensory analysis is important to give a quantitative measure to the appearance, taste, aroma, texture of the product21. Multiple sensory attributes are shown to be linked with palatability of food product22. On the other hand, nowadays consumer behaviour in food choosing is more heterogeneic, and origin of the product was indicated as having the major influence on consumers in the market23.
When conducting chemical analyzes, the basic composition was first step to describe such new products. Ash content is an important parameter used in the analysis of food products as it provides valuable information about the mineral content of the food and its potential nutritional value. When comparing designed common bean snacks, similar amount of ash (4.00 g/100 g) was found for cv. IAC carioca Ete beans by De Almeida Costa et al.24. From a consumer’s point of view, it can be found as important, since the source of fat (olive oil) in final product were unsaturated fatty acids. The variations in fat content across the different variants provide a wide array of choices that can be tailored to the specific dietary preferences and health objectives of consumers. Proposed snacks alternatives directly cater to the growing demand for snacks that align with healthier eating patterns and embody the concept of ‘healthier’ options. This resonates particularly with health-conscious consumers who prioritize making dietary selections, as diabetes patients seems to be. Snacks were rich in protein due to common beans content. De Almeida Costa et al.24found in cooked common beans cv. IAC carioca Ete similar amount of protein (22.1 g/100 g). The differences at level 10–12% between analyzed here snacks could be in interest to consumers improving ther diet into plant protein components, which are essential in building and repairing processes in tissues, more ecological and valuable in anti-obesity diet than animal protein25. Protein also plays a crucial role in maintaining muscle mass, supporting immune function, and regulating hormones, therefore such snacks could be proposed as nutritious. However, it is worth to note that white-bean variants can be also found as a appropriate for vegan. Moreover, research showed that for individuals with diabetes, plant proteins are a better option than any other foods, especially in group of snacks typically based on mono- or dicarbohydates. Wang et al.26 indicated that soy isolate protein improved blood glucose level in diabetic rats, together with another diabetes parameters i.a. insulin level, HbA1c, hepatic glycogen, serum C-peptide, adiponectin level, cholesterol level. In diabetic older women, Ferreira et al.25 observed association between higher dietary protein/ energy ratio and lower obesity appearance. On the other hand, Tabaeifard et al.27noted plant protein intake can be linked to decreased risk of gestational diabetes mellitus. Since the common beans among legumes are known as essential amino acids sources – lysine, tyrosine, phenylalanine, it can be interested also from technological view. Scientists found that lysine plays a critical role in the process of post-translational modifications facilitated with enzymes lysyl hydroxylase and lysyl oxidase – enzymes directly involved in the synthesis and maturation of collagens28. That is another purpose to interest of such bean-snacks in vegan diabetes groups. Moreover, snacks were rich source of many types of carbohydrates, especially fiber. Fiber is important for providing sustained energy and promoting digestive health – improving digestal microorganism growth. It was already proven that an increase in the soluble dietary fiber intake by diabetic patients improve their blood glucose control, decrease hyperinsulinemia, decrease plasma lipid concentrations29. The highest percentage of addiditons in snacks (WML, garlic, rosemary, basil) in Wd variant resulted in the significantly highest amount of fiber (TDF) in analyzed samples. This suggests that it is a good source of dietary fiber, which can help promote digestive health and satiety. Other investigators found quite similar ratio SDF : IDF (4.7 g/100 g DM : 17.5 g/100 g DM) however with 2x less amount of TDF (22.2 g/100 g DM) in cooked soaked Pinto beans30. De Almeida Costa et al.24 in freeze-dried cooked common beans cv. IAC carioca Ete found crude fiber in much less amount of 6.26 g/100 g than in analyzed bean-snacks. The observed fiber content results carry substantial implications for digestive health and feelings of fullness in consumers. All variants, but especially Wd variant, characterized by their relatively high fiber content, contribute to supporting regular digestion, managing blood sugar levels, and offering sustained satiety. Bielefeld et al.31 in their review concluded that medium-to-long-term (over 6 weeks) legume consumption resulted in blood parameters in diabetes individuals, with lack of siginifact data for prediabetics and gestational diabetes mellitus.
To prove the prohealthy status of designed snacks antioxidant activity analyzes were conducted. The assessment of antioxidant activity is a critical aspect of evaluating the potential health benefits of food products32. The TPC assay measures the amount of flavonoids in a sample, which are antioxidants found in many plants. Phenolics are known for their ability to scavenge free radicals and reduce oxidative stress in the organism. Obtained results suggest that each variant was a good source of phenolics and may promote its benefits for consumers. The TPC in case of Me/ForA extracts was significantly higher when compared with results obtained by Fonseca Hernadez et al.33who found phenolics in amount of 0.44–0.45 mM GAE in the black bean extracts. It is worth noting that TPC level in other studies34 separately for dried white mulberry leaves was found as 3.376 mg GAE/g so it can be assumed that particularly the such mulberry leaves added in the formulation of these snacks gave a significant input to final effect. In the antiradical test (DPPH·assay) the ability of a sample to scavenge DPPH radicals was measured. DPPH radicals simulate some free radicals that be created in the organism in effect of oxidative damage to the body’s cells. The lower the concentration sample required to neutralize DPPH·, the higher the antiradical activity of the such sample is observed35. These values suggest that all four variants of the new product had significant DPPH·scavenging activity however, various proportions of ingredients resulted in test results. This is in relation with the results obtained by other authors who analyzed common bean cultivars and found their antiradical activity lower – ranged from 0.5 to 3.7 µmol TE/g DM36. On the other hand, in Spanish common beans 18.7 µmol TE/g (red beans) and 1.2 µmol TE/g (white beans) DPPH scavenging activity were found37. The second antiradical assay used in bean-snacks samples was the test with ABTS+· in which measurement of the ability of a sample to scavenge ABTS cation radicals is conducted. The lower the concentration of the sample required to neutralize the ABTS+·, the higher antiradical activity of the sample is noted. The ABTS+·results for the bean-snacks were significantly higher when compared with other common bean cultivars36 which ranged from 1.2 to 6.9 µmol TE/g DM. On the other hand, the antiradical activity for processes mulberry leaves was found at range of 17.235 µM TE/g34. The Ra variant emerged as the one with the highest antiradical activity in both DPPH and ABTS assays, closely followed by the other three variants. Statistically significant differences were observed between samples. These results denote the substantial antiradical potential of all four variants, partly indicating their ability to combat oxidative damage. The DPPH· assay in chemical way measures a sample’s capability to neutralize DPPH·, while the ABTS+· assay assesses the sample’s ability to scavenge ABTS+·.
The results of determining the antioxidant capacity of an extract depend largely on what technology and what free radical generator or oxidant is used for measurement. In some cases, individual antioxidants may act through multiple mechanisms in one system or through another single mechanism depending on the reaction system. mechanism depending on the reaction system. Furthermore, antioxidants may react differently to different sources of radicals or oxidants. No single test will accurately reflect all radical sources or all antioxidants in a mixed or complex system because many reaction characteristics and mechanisms, as well as different phase locations, are usually involved35. Matching the radical source and the characteristics of the antioxidant mechanisms of action are crucial in selecting appropriate antioxidants, as is consideration of the end-use of the results. It should be emphasized that there is no simple, universal method that can be used to accurately and quantitatively measure antioxidant capacity. Therefore, it is very useful to compare different analytical methods differing in terms of oxidation initiators and targets in order to understand the biological activity of an antioxidant38. In describing antioxidant status of snacks, the chelating activity and FRAP assays were conducted. The chelating activity assay measures the ability of a sample to chelate or bind some metal ions such as iron, which can promote the formation of free radicals in the cells. The higher the chelating activity of a sample, the higher its antioxidant activity. Any significant differences were found between proposed snacks. Comparing FRAP test values of Ra, Rb, Wc, Wd snacks and other common beans36 (1.4–8.5 µmol TE/g), it had been shown that additives implemented into recepture of bean-snacks resulted also in FRAP increasing. For comparison, Spanish common beans showed 34.1 µmol Fe2+/g (red beans) and 6.1 µmol Fe2+/g (white beans)37, while Chilean red-brown beans were from 804.26 µmol TE/g (var. Magnum) to 1352.96 µmol TE/g (var. Peumo) and white beans were from 238.75 µmol TE/g (var. Cisne) to 274.58 µmol TE/g (var. Coscorrón)39. When comparing two methods of extraction, the usage of Me/ForA mixture resulted in higher levels of total phenolic compounds together with antioxidant/antiradical activities (as measured by using DPPH, ABTS, and FRAP assays), than after usage ACN/W/ForA mixture.
Phenolic compounds are able to suppress formation of radical species by chelating metal ions (Fe, Cu) involved in initiation of free radical production process together with the inhibition of enzymes40. In analyzed samples the chelating activity however, did not show any significant difference between proposed extraction methods. There could be several reasons for the observed differences between the extraction methods. The choice of solvent and its polarity, pH, and other extraction conditions can all influence the extraction efficiency and selectivity of different compounds. Methanol is known to be a better solvent for phenolic compounds compared to acetonitrile, which could explain the higher levels of total phenolic compounds in the Me/ForA extracts in this study. Additionally, the formic acid (For A) in both extraction mixtures could help in the phenolics extraction by breaking down cell walls and interfering with intermolecular bonding.
In the area of possible anti-diabetic activity of designed snacks enzyme inhibition was measured. Overall, obtained results suggest that of the two extraction methods the Me/ForA mixture allowed more compounds with inhibition activity to be extracted from the bean-snacks samples compared to the ACN/W/ForA mixture. However, it is important to note that the standard deviation was also slightly higher for the Me/ForA extracts, indicating greater variability among the variants. Notably, all variants exhibited α-glucosidase inhibition activity, and variant Ra consistently displayed the highest activity, regardless of the extraction method used.
From the future consumers’ point of view labels are the first insight into product they are about to buy. For producers, labels are essential elements of every food product. Mandatory information on the packaging of the product, in accordance with the Regulation 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers41, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC, and Commission Regulation (EC) No 608/2004 Text with EEA relevance is the indication of the nutritional value of the product. Nutritional value on food labels provides information about the nutrient content of a food product and are obligatory. It allows consumers to make informed decisions about the foods they consume and helps individuals maintain a healthy and balanced diet. Moreover, nutritional claims provide information about the composition and potential health benefits. These claims can help consumers make informed decisions about the foods they eat and can assist in managing health conditions and achieving dietary goals.
Then considering such new snacks as targeted to diabetics, some anti-diabetic parameters can be included into product label. The glycaemic index (GI) is a measure of how quickly blood glucose levels rise after consuming 50 g of digestible carbohydrates from a specific food product, compared to the rate of glucose level rise after consuming the same amount of carbohydrates from glucose or white bread. The GI of glucose is considered to be 100, and other products are rated in comparison to this value. It is important to note that the GI only applies to products that contain carbohydrates, such as bread, grains, pasta, rice, vegetables, or fruits. Products that are solely sources of protein and fats, like meat, fish, and oils, do not fall under this category. In the case of diabetes, it is recommended to consume foods with a low GI (< 55) and optionally foods with a moderate GI (55–70). These foods result in a gradual increase in blood glucose levels and reduce fluctuations throughout the day. Conversely, products with a high GI (> 70) should be avoided since they cause a rapid increase in blood glucose levels. Therefore, it is essential to avoid including such foods in one’s daily diet42. Estimated glycemic index parameter can be proposed as easy (because without human) and quick way to estimate the glycemic index of designed food product, especially at the stage of designing process of new products. Designed snacks had GI at level of 40. Recently, diabetic patients have been paying more attention to not only the GI but also the glycemic load (GL). The GL considers not only the type of product consumed but also its portion size. The glycemic load is determined by multiplying the GI by the carbohydrate content of a given portion of the product. While the glycemic index predicts the extent to which a food’s carbohydrates increase blood glucose, the total increase in blood glucose after eating a particular meal depends on both the quality (represented by the GI) and the quantity of carbohydrates consumed. Foods that have a high glycemic index may have a low glycemic load, depending on the size of the consumed portion of the product. GL can be low (< 10), medium (10–20), or high (> 20)42. The results showed significant differences in GL values among the bean-snacks because of its composition, indicating significant differences in their potential decreasing impact on blood sugar levels. In pairs, variants Ra and Wc showed higher GL values while Rb and Wd – lower. Observed differences may be due to the fact that both products had the same GI value, suggesting that Rb could have a lower carbohydrate content but a higher GI value than Ra.
Conclusions
Of equal importance is the food product’s potential as an antidiabetic aid. As the prevalence of type 2 diabetes mellitus (T2DM) rises globally, the pursuit of natural means to manage and prevent this condition becomes increasingly relevant. The blend of ingredients in this newly developed food product displays promising antidiabetic properties, particularly in terms of their anti-enzymatic activity, which could aid in balancing blood sugar levels and enhancing insulin sensitivity. Notably, the active components within white mulberry leaves exhibit inhibitory effects on carbohydrate breakdown, thus contributing to the maintenance of healthy blood sugar levels.
This study highlights the potential of this dry-savory snack based on legumes, recommended by nutritionists, showcasing its remarkable antioxidant content and possible antidiabetic properties, positioning it as a favorable dietary option for those aiming to enhance their well-being, particularly individuals with diabetes, and those seeking diabetes prevention.
Noteworthy, the possibility of using white mulberry leaves as a food ingredient is still unregulated within the European Union food law, which is probably a constraint on the possible implementation of the product. However, it is already worthwhile to develop technological solutions for the industry for the smooth market introduction of this nutritious product in the future.
Concluding, future research should probably explore the mechanisms behind these observed differences, investigating the specific compounds responsible for the antioxidant (its optimization) and enzyme inhibition activities. Additionally, the potential health implications of consuming bean-snacks, especially for individuals with specific dietary needs, could be further explored through clinical trials and longitudinal studies.
Materials and methods
Material
Plant material
Two types of canned beans from the Polish market were used: red common beans Phaseolus vulgaris L. (Dobra Nasza, Poland; origin Poland; bought in local market) and white beans (Pudliszki, Poland; origin Poland; bought in local market). Moreover, dried white mulberry leaves Morus alba L. (WML) var. Żółwińska wielkolistna (bought in Institute of Natural Fibres and Medicinal Plants, Poland), extra virgin olive Olea europaea L. oil (La Espanola, Spain; bought in local market), dried garlic Allium sativum L. powder (Kamis, Poland; origin Poland; bought in local market), dried rosemary Salvia rosmarinus L. (Kamis, Poland; origin Poland; bought in local market), dried red paprika Capsicum annuum L. powder (Kamis, Poland; origin India; bought in local market), dried basil Ocimum basilicumL. (Prymat, Poland; origin Poland; bought in local market), table salt (o’Sole, Poland; origin Poland; bought in local market) were used as components in final product. In case of WML (leaves conditioned for 2 h) preparations previously described34 were used as a component.
Preparation of the Four variants of the product
In the primary stage, 20 variants of snacks with different quantitative compositions were proposed and prepared. According to sensory characteristic made by investigators 4 variants were chosen – 2 variants made of white beans, 2 variants made of red beans (Fig. 2.). Before using, canned beans were gently washed with tap water for 3 min and dried with towel paper. All of the ingredients were weighed according to recipes shown in Table 8.
Fig. 2.
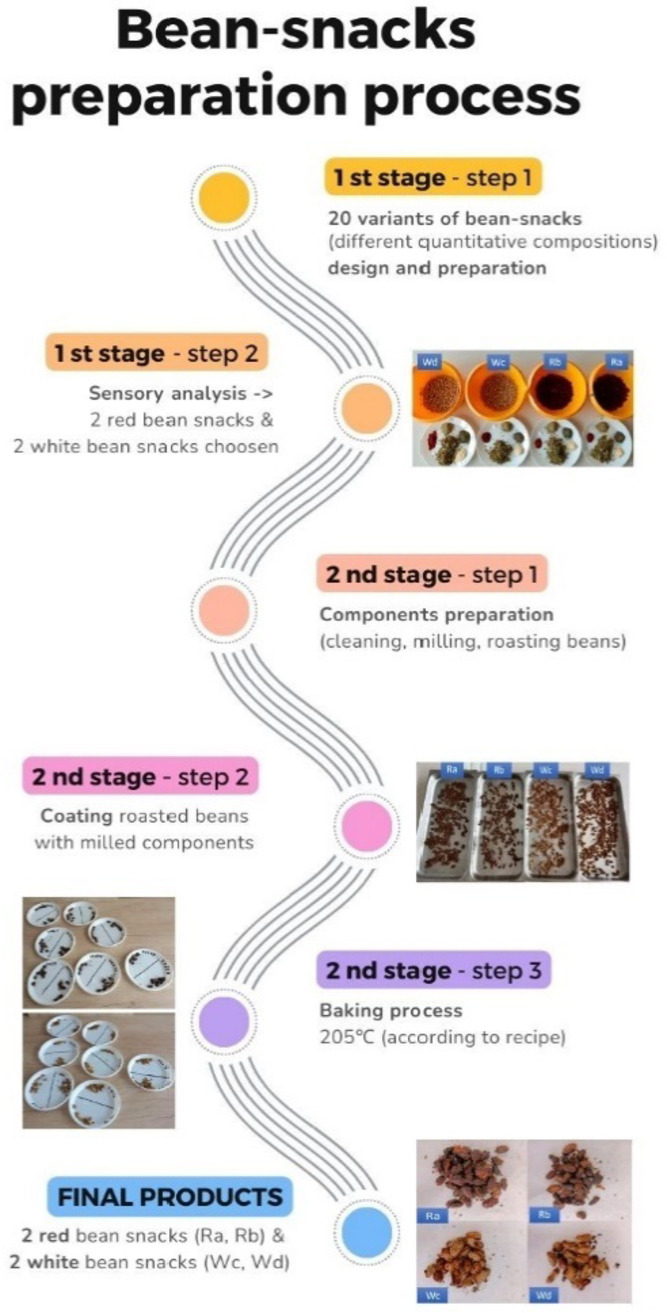
Scheme of processing of common beans into bean-snacks.
Table 8.
The composition of four variants of new bean-based products.
| Ingredients | Ra | Rb | Wc | Wd |
|---|---|---|---|---|
| Canned beans [g] | 100.00 | 100.00 | 100.00 | 100.00 |
| Olive oil [mL] | 14.50 | 16.00 | 13.50 | 17.00 |
| Dried WML [g] | 1.90 | 2.20 | 1.80 | 2.20 |
| Garlic powder [g] | 2.20 | 1.90 | 2.40 | 2.70 |
| Basil [g] | 0.13 | 0.27 | 0.29 | 0.43 |
| Rosemary [g] | 0.68 | 0.96 | 0.87 | 1.16 |
| Red paprika [g] | 0.41 | 0.27 | 0.65 | 0.58 |
| Salt [g] | 0.68 | 0.82 | 0.43 | 0.58 |
| Baking temperature [℃] | 205 | 205 | 205 | 205 |
| Baking time [min] | 19 | 20 | 18 | 21 |
R – red common bean, W – white common bean.
The other ingredients (WML, garlic, basil, rosemary, paprika) were milled to obtain a powder form, and mixed well with the beans, ½ of olive oil and salt. Beans were roasted using a frying pan (½ olive oil) for 3 min. Roasted beans were mixed well with milled fraction, and baked at a temperature of 205℃ for 18, 19, 20, or 21 min. To obtain the best sensory results, specific baking time was used for each variant of bean-snacks (convection oven Combidampfer, CCC101, Rational, Germany).
Reagents
All of the chemicals were of analytical grade, purchased from POCH, Gliwice, Poland, or Merck, Darmstadt, Germany.
Methods
Microstructure surface
The surface microstructure of bean-snacks was studied using a scanning electron microscope (SEM) Zeiss EVO 40 with the electron accelerating voltage of 17 kV. The 4x magnification lens provided a broad overview of the granules, while the 10x magnification lens allowed for a more detailed observation of the granule surface43.
Sensory analysis – paired comparison test
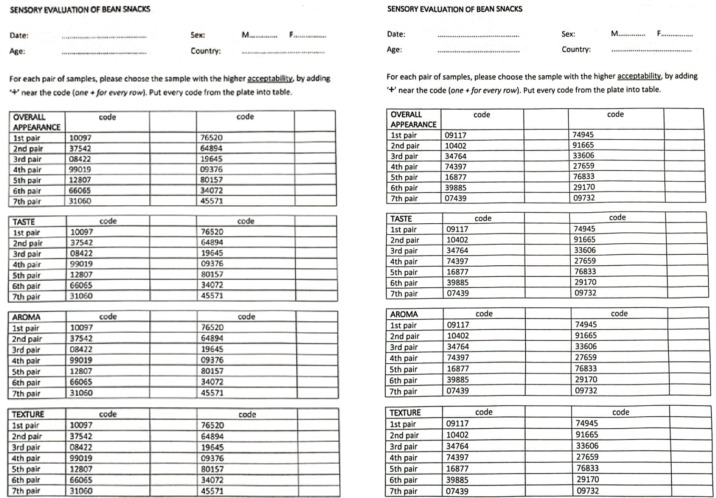
The sensory analysis of the 4 variants has been performed by 26 panelists from different cultures and countries, including Asian, African, and Polish, in the sensoric laboratory at the Department of Gastronomy Science and Functional Foods, Poznań University of Life Sciences, under equal and ambient conditions according to Polish safety requirements PN-EN ISO 8589:201044. No ethical approval was required for this study. Participants were informed about the study’s aim and that their participation was entirely voluntary. Volunteers could stop the analysis at any point, the responses were anonymous. The authors did not ask for sensitive data or personal information. Paired comparison semi-consument test was used according to Baryłko-Pikielna&Matuszewska19. During the evaluation, the panelists were presented with 4 variants of bean-snacks in pairs (one pair of white bean-snacks, one pair of red bean-snacks, and filled the sensory characteristic evaluation card (Fig. 3) to rate the best product from each pair on various attributes such as flavor, texture, overall appearance, aroma.
Fig. 3.
Sensory evaluation cards.
Extraction process
For extraction two methods were used (Fig. 4). The first method of extraction in Met/ForA45: the powdered samples (0.5 g) from each variant (Ra, Rb, Wc, Wd) were extracted with 10 mL of 70% methanol containing 0.5% formic acid, in a water bath shaker at room temperature for 24 h and then centrifuged (4000 rpm, 25 °C, 15 min). The supernatants were filtrated (via Büchner funnel) and stored in the fridge.
Fig. 4.
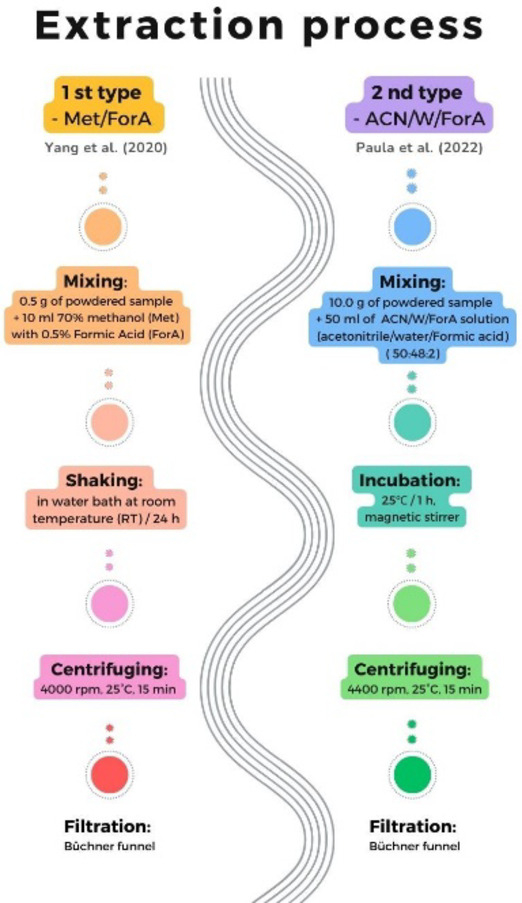
Extraction process scheme.
The second method of extraction (ACN/W/ForA)46: a mixture of acetonitrile, water, and formic acid in the proportion of 50:48:2 was used to prepare the extraction solution. The extraction was carried out by mixing 50 mL of the ACN/W/ForA solution and 10 g of samples followed by incubation at 25℃ for 1 h using magnetic stirrer. Once the extraction process was complete, the liquid extracts were centrifuged (4400 rpm, 25 °C, 15 min), and supernatants were collected.
Basic composition
Dry matter analysis involved collecting three repetitions (5.00 g ± 0.01) of each variantand the results referred to the percentage47. For ash content each variant (2.00 g ± 0.01) in three repetitions was analyzed47. Fat content analysis involved weighing 4.00 g ± 0.01 of each sample (three repetitions)47. Protein content analysis has been conducted using the Kjeldahl method (Kjeltec 2200, Foss Tecator)47. The content of total dietary fiber (TDF), soluble dietary fiber (SDF), and insoluble fractions (IDF) was determined using the method described by Asp et al., and the results were converted to dry matter (DM) and expressed as % of DM48.
Antioxidant activity: total phenolic content (TPC) with Folin reagent, DPPH· scavenging activity, ABTS+· scavenging activity, Fe2+ chelating activity, ferric reducing antioxidant power (FRAP)
The total content of phenolic compounds (TPC) in extracts was determined spectrophotometrically (λ = 765 nm) in four repetitions. Results were expressed as mg per g of dry matter for gallic acid equivalent (GAE)49.
The DPPH·(2,2-diphenyl-1-picrylhydrazyl) scavenging activity method was based on spectrophotometric measurement (λ = 517 nm). A standard curve was generated for Trolox (TE). The scavenging activity was expressed as the inhibitory level [%] of the hydrogen-donating activity of the sample in four replicates50.
The ABTS+· (2,2’-azino-bis(3-ethylbenzothiazoline)−6-sulfonic acid) scavenging activity analysis was based on the spectrophotometric measurement of the alteration in absorbance at λ = 734 nm). The ABTS+· scavenging activity was made in four replicates51.
Determination of iron (II) chelating ability was based on spectrophotometric (λ = 562 nm) and the results obtained for four repetitions were expressed as equivalent of EDTA (ethylenediaminetetraacetic acid) standard52.
The Ferric Reducing Antioxidant Power (FRAP) assay is based on the ability of antioxidants to reduce ferric ions (Fe3+) to ferrous ions (Fe2+) in the presence of a reducing agent. Using a spectrophotometer, the absorbance of the solutions (three repetitions) was measured at λ = 593 nm53.
Anti-enzymatic activity: α-glucosidase inhibition activity
In order to detect the inhibition ability of α-glucosidase in extracts, the reference sample set was made up of solutions of aqueous acarbose (Merck, Germany) in concentrations ranging from 1 to 5 mg/mL. Three separate trials were conducted for each concentration tested. Spectrophotometric method developed by Szczepaniak et al.54 was used to determine the inhibition of α-glucosidase in the aqueous extracts. The absorbance of the released p-nitrophenol was measured at λ = 405 nm. Samples were made in triplicates.
Label information proposal - nutritional value, nutritional claims, estimated Glycemic Index, estimated glycemic load
Nutritional information was prepared according to European Union rules and Regulation (EU) No 1169/201141. Claims were selected in accordance with to European Union rules on nutrition and health claims and Regulation (EC) No 1924/200655. Estimated Glycemic Index was calculated according to Dodd et al.56. The calculation method used the amount of available carbohydrates in each component in relation to whole product carbohydrates, and the glycemic index of each component57. Estimated glycemic load parameter was calculated with method by Olendzki et al.58 for suggested portion of product (40 g) according to formula: GL = (GI x the amount of carbohydrates in portion)/ 100. This parameter was considered together with the composition, the components’ sizes.
Statistical analysis
The data, which represents the average results of the experiments, was subjected to one-way ANOVA analysis on Statistica software version 13.3 (Tibco, United States). The differences between the statistical data were determined using Tukey’s test with a significance level of p < 0.05.
Acknowledgements
Authors would thank Maciej Jarzębski, PhD (PULS, Poland) and Professor Krystyna Szymandera-Buszka (PULS, Poland) for support given during research.
Author contributions
M.P.: resources, data curation, investigation, conceptualization, methodology, validation, writing, review, editing, supervision, funding acquisition; N.A.: visualization, investigation, writing. All authors contributed to reviewing and editing the final manuscript.
Funding
This research was funded by the Department of Gastronomy Science and Functional Foods, Faculty of Food Science and Nutrition, Poznań University of Life Sciences, Poland (statutory fundings). The publication was financed by the Polish Minister of Science and Higher Education as part of the Strategy of the Poznan University of Life Sciences for 2024–2026 in the field of improving scientific research and development work in priority research areas.
Data availability
The datasets of the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Federation, I. D., IDF, Facts & Fig. (2022). https://idf.org/about-diabetes/diabetes-facts-figures/
- 2.WHO & Diabetes (2021). https://www.who.int/health-topics/diabetes#tab=tab_1. (Accessed: 15th March 2024).
- 3.Alkhatib, A. et al. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 9, 1310. (2017). https://www.mdpi.com/2072-6643/9/12/1310 [DOI] [PMC free article] [PubMed]
- 4.Pérez-Jiménez, J., Neveu, V., Vos, F. & Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: an application of the phenol-explorer database. Eur. J. Clin. Nutr.64, S112–S120 (2010). https://www.nature.com/articles/ejcn2010221 [DOI] [PubMed] [Google Scholar]
- 5.Kaya, N., Kurtoğlu, S. & Gökmen Özel, H. Does meal-time insulin dosing based on fat‐protein counting give positive results in postprandial glycaemic profile after a high protein‐fat meal in adolescents with type 1 diabetes: a randomised controlled trial. J. Hum. Nutr. Diet.33, 396–403. 10.1111/jhn.12711 (2020). https://onlinelibrary.wiley.com/doi/full/ [DOI] [PubMed] [Google Scholar]
- 6.Wang, P., Sheng, Y. & Samadi, M. Effects of almond consumption on lipid profile in patients with type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Arch. Physiol. Biochem.130, 128–135 (2024). https://pubmed.ncbi.nlm.nih.gov/34624199/ [DOI] [PubMed] [Google Scholar]
- 7.Przeor, M. Some common Medicinal plants with antidiabetic activity, known and available in Europe (a Mini-review). Pharmaceuticals15, 3390 (2022). https://www.mdpi.com/1424-8247/15/1/65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mo, J., Rashwan, A. K., Osman, A. I., Eletmany, M. R. & Chen, W. Potential of Chinese Bayberry (Myrica rubra Sieb. Et zucc.) Fruit, Kernel, and Pomace as Promising Functional ingredients for the development of Food products: a Comprehensive Review. Food Bioprocess. Technol. 1–19. 10.1007/S11947-023-03313-9 (2024).
- 9.Kochadai, N., Khasherao, B. Y. & Sinija, V. R. N. Effect of Radiofrequency Pre-treatment on the Extraction of Bioactives from Clitoria ternatea and Hibiscus rosa sinensis and Insights to Enzyme Inhibitory Activities. Food Bioprocess Technol. 15, 571–589. (2022). https://link.springer.com/article/10.1007/s11947-022-02770-y
- 10.Wei, H. et al. A systematic review of the medicinal potential of mulberry in treating diabetes mellitus. Am. J. Chin. Med. 46, 1743–1770. (2018). https://www.worldscientific.com/doi/abs/10.1142/S0192415X1850088X [DOI] [PubMed]
- 11.Gramza-Michalowska, A. & Regula, J. Use of tea extracts (Camelia Sinensis) in jelly candies as polyphenols sources in human diet. Asia Pac. J. Clin. Nutr.16, 43–46 (2007). [PubMed] [Google Scholar]
- 12.Kaur, B., Ranawana, V. & Henry, J. The Glycemic Index of Rice and Rice products: a review, and table of GI values. Crit. Rev. Food Sci. Nutr.56, 215–236. 10.1080/10408398.2012.717976 (2016). https://www.tandfonline.com/doi/abs/ [DOI] [PubMed] [Google Scholar]
- 13.Villegas, R. et al. Prospective study of Dietary Carbohydrates, Glycemic Index, Glycemic load, and incidence of type 2 diabetes Mellitus in Middle-aged Chinese women. Arch. Intern. Med.167, 2310–2316 (2007). https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/413531 [DOI] [PubMed] [Google Scholar]
- 14.Sidorova, Y. et al. Hypoglycemic and hypolipidemic effect of Vaccinium myrtillus L. leaf and Phaseolus vulgaris L. seed coat extracts in diabetic rats. Nutrition41, 107–112 (2017). https://linkinghub.elsevier.com/retrieve/pii/S0899900717300874 [DOI] [PubMed] [Google Scholar]
- 15.Zhao, X. et al. Mulberry (Morus alba L.) leaf polysaccharide ameliorates insulin resistance- and adipose deposition-associated gut microbiota and lipid metabolites in high-fat diet-induced obese mice. Food Sci. Nutr.10, 617–630. 10.1002/fsn3.2689 (2022). https://onlinelibrary.wiley.com/doi/full/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammadi, J. & Naik, P. R. Evaluation of hypoglycemic effect of Morus alba in an animal model. Indian J. Pharmacol.40, 15–18. 10.4103/0253-7613.40483 (2008). https://journals.lww.com/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi, M. et al. Effects of the timing of acute mulberry leaf extract intake on postprandial glucose metabolism in healthy adults: a randomised, placebo-controlled, double-blind study. Eur. J. Clin. Nutr.77, 468–473 (2023). https://www.nature.com/articles/s41430-023-01259-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarzębski, M. et al. Heme iron as potential iron fortifier for food application – characterization by material techniques. Rev. Adv. Mater. Sci. 62, 1. (2023). https://www.degruyter.com/document/doi/10.1515/rams--0128/html (2023).
- 19.Baryłko-Pikielna, N. & Matuszewska, I. Sensoryczne badania żywności: podstawy, metody, zastosowania. (2009).
- 20.Jürkenbeck, K. & Spiller, A. Importance of sensory quality signals in consumers’ food choice. Food Qual. Prefer. 90, 104155 (2021). https://linkinghub.elsevier.com/retrieve/pii/S0950329320304249 [Google Scholar]
- 21.Iannario, M., Manisera, M., Piccolo, D. & Zuccolotto, P. Sensory analysis in the food industry as a tool for marketing decisions. Adv. Data Anal. Classif. 6, 303–321. (2012). https://link.springer.com/article/10.1007/s11634-012-0120-4
- 22.Santosh, O., Bajwa, H. K., Bisht, M. S. & Chongtham, N. Antioxidant activity and sensory evaluation of crispy salted snacks fortified with bamboo shoot rich in bioactive compounds. Appl. Food Res.1, 100018. 10.1016/j.afres.2021.100018 (2021). [Google Scholar]
- 23.Van Loo, E. J., Grebitus, C. & Roosen, J. Explaining attention and choice for origin labeled cheese by means of consumer ethnocentrism. Food Qual. Prefer. 78, 103716. 10.1016/j.foodqual.2019.05.016 (2019). [Google Scholar]
- 24.De Almeida Costa, G. E., Silva Queiroz-Monici, D., Pissini Machado Reis, K., De Oliveira, A. C. & S. M. & Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem.94, 327–330 (2006). https://linkinghub.elsevier.com/retrieve/pii/S0308814604008519 [Google Scholar]
- 25.d. Á. Ferreira, E. et al. Higher dietary protein/energy ratio is associated with a lower risk for obesity in older women with type 2 diabetes: cross-sectional analysis of Japanese patients with type 2 diabetes mellitus (JDDM75). Hum. Nutr. Metab.36 https://linkinghub.elsevier.com/retrieve/pii/S2666149724000197 (2024). [Google Scholar]
- 26.Wang, S. et al. Protective effect of soy isolate protein against streptozotocin induced gestational diabetes mellitus via TLR4/MyD88/NF-κB signaling pathway. Biomed. Pharmacother. 168, 115688 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Tabaeifard, R., Moradi, M., Arzhang, P. & Azadbakht, L. Association between protein intake and risk of gestational diabetes mellitus: a systematic review and dose–response meta-analysis of cohort studies. Clin. Nutr.43, 719–728. 10.1016/j.clnu.2024.01.027 (2024). [DOI] [PubMed] [Google Scholar]
- 28.Añazco, C., Ojeda, P. G. & Guerrero-Wyss, M. Common beans as a source of amino acids and cofactors for collagen biosynthesis. Nutrients15, 21, 4561 (2023). https://www.mdpi.com/2072-6643/15/21/4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandalia, M. et al. Beneficial effects of High Dietary Fiber Intake in patients with type 2 diabetes Mellitus. N Engl. J. Med.342, 1392–1398. 10.1056/NEJM200005113421903 (2000). http://www.nejm.org/doi/abs/ [DOI] [PubMed] [Google Scholar]
- 30.Kutoš, T., Golob, T., Kač, M. & Plestenjak, A. Dietary fibre content of dry and processed beans. Food Chem.80, 231–235 (2003). https://linkinghub.elsevier.com/retrieve/pii/S0308814602002583 [Google Scholar]
- 31.Bielefeld, D., Grafenauer, S. & Rangan, A. The effects of legume consumption on markers of glycaemic control in individuals with and without diabetes mellitus: a systematic literature review of randomised controlled trials. Nutrients12, 1–18 (2020). https://www.mdpi.com/2072-6643/12/7/2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locatelli, D. A., Nazareno, M. A., Fusari, C. M. & Camargo, A. B. Cooked garlic and antioxidant activity: correlation with organosulfur compound composition. Food Chem.220, 219–224. 10.1016/j.foodchem.2016.10.001 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Fonseca Hernadez, D., Orozco-Avila, I., Lugo-Cervantes, E. & Mojica, L. Black Bean (Phaseolus vulgaris L.) Phenolic Extract exhibits antioxidant and anti-aging potential. Curr. Dev. Nutr.4, 24 (2020). https://linkinghub.elsevier.com/retrieve/pii/S247529912307542X [Google Scholar]
- 34.Przeor, M. et al. Functional properties and antioxidant activity of Morus alba L. leaves var. Zolwinska Wielkolistna (WML-P)— the effect of controlled conditioning process. Antioxidants9, 668. https://www.mdpi.com/2076-3921/9/8/668 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haider, K., Haider, M. R., Neha, K. & Yar, M. S. Free radical scavengers: an overview on heterocyclic advances and medicinal prospects. Eur. J. Med. Chem.204, 112607 (2020). https://linkinghub.elsevier.com/retrieve/pii/S0223523420305791 [DOI] [PubMed] [Google Scholar]
- 36.Carbas, B. et al. Antinutrients, Phenolic Composition, and antioxidant activity of Common Bean cultivars and their potential for Food Applications. Antioxidants9, 186 (2020). https://www.mdpi.com/2076-3921/9/2/186/htmNutrients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madrera, R. R., Negrillo, A. C., Valles, B. S. & Fernández, J. J. F. Phenolic content and antioxidant activity in seeds of common bean (Phaseolus vulgaris L). Foods10 (4), 864–864 (2021). https://www.mdpi.com/2304-8158/10/4/864/htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prior, R. L., Wu, X. & Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in Foods and Dietary supplements. J. Agric. Food Chem.53, 4290–4302. 10.1021/jf0502698 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Nina, N. et al. Chemical profile and bioactivity of Chilean bean landraces (Phaseolus vulgaris L). J. Funct. Foods. 104, 105513 (2023). https://linkinghub.elsevier.com/retrieve/pii/S1756464623001135 [Google Scholar]
- 40.Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr.16, 33–50 (1996). [DOI] [PubMed] [Google Scholar]
- 41.EUR-Lex. Regulation – 1169/2011 - EN - Food Information to Consumers Regulation - EUR-Lex. (2011). https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R1169. (Accessed: 15th March 2024).
- 42.Lu, Z. Q. & Yan, J. Dietary Carbohydrate and Age-Related Cataract. in Handbook of Nutrition, Diet and the Eye (ed. Preedy, V. R.) 271–277Academic Press, doi: (2014). 10.1016/B978-0-12-401717-7.00027-7
- 43.Wiszumirska, K. et al. Characterization of biodegradable food contact materials under Gamma-Radiation treatment. Mater. (Basel)16, 2. (2023). https://www.mdpi.com/1996-1944/16/2/859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polski Komitet Normalizacyjny. PN-EN ISO 8589:2010. (2010). https://sklep.pkn.pl/pn-en-iso-8589-2010e.html
- 45.Yang, Q. Q. et al. Phenolic content and in vitro antioxidant activity in common beans (Phaseolus vulgaris L.) are not directly related to anti-proliferative activity. Food Biosci.36, 100662 (2020). https://linkinghub.elsevier.com/retrieve/pii/S2212429219310855 [Google Scholar]
- 46.Paula, L. C. et al. Effect of extrusion and autoclaving on the biological potential of proteins and naturally-occurring peptides from common beans: antioxidant and vasorelaxant properties. Food Chem. X. 13, 100259. 10.1016/j.fochx.2022.100259 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.AOAC. Official methods of analysis. (2000).
- 48.Asp, N., Johansson, C., Hallmer, H. & Siljestroem, M. Rapid enzymatic assay of insoluble and soluble dietary fiber. J. Agric. Food Chem.31, 476–482. 10.1021/jf00117a003 (1983). [DOI] [PubMed] [Google Scholar]
- 49.Przeor, M. & Flaczyk, E. Antioxidant properties of paratha type flat bread enriched with white mulberry leaf extract. Indian J. Tradit Knowl.15, 237–244 (2016). [Google Scholar]
- 50.Amarowicz, R., Pegg, R. & Bautista, D. Antibacterial activity of green tea polyhenols against Escherichia coli K12. Nahrung44, 60–62. 10.1002/(sici)1521-3803(20000101)44:1%3C60::aid-food60%3E3.0.co;2-l (2000). [DOI] [PubMed]
- 51.Re, R. et al. Antioxidant activity an improved ABTS radical cation decolorization assay. Free Radic Biol. Med.26, 1231–1237. 10.1016/S0891-5849(98)00315-3 (1999). [DOI] [PubMed] [Google Scholar]
- 52.Tang, S., Joe, K., Sheehan, D. & Buckley, D. Antioxidative mechanisms of tea catechins in chicken meat system. Food Chem.76, 45–51. 10.1016/S0308-8146(01)00248-5 (2002). [Google Scholar]
- 53.Benzie, I. & Strain, J. Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. in Methods in Enzymology (eds. Abelson, J. & Simon, M.) 15–27Academic Press, (1999). 10.1016/s0076-6879(99)99005-5 [DOI] [PubMed]
- 54.Szczepaniak, O., Cielecka-Piontek, J., Kobus-Cisowska, J. & Hypoglycaemic Antioxidative and phytochemical evaluation of Cornus mas varieties. Eur. Food Res. Technol.247, 183–191 (2021). https://linkinghub.elsevier.com/retrieve/pii/S0002916523024711 [Google Scholar]
- 55.EUR-Lex. Regulation – 1924/2006 - EN - EUR-Lex. (2006). https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32006R1924. (Accessed: 15th March 2024).
- 56.Dodd, H., Williams, S., Brown, R. & Venn, B. Calculating meal glycemic index by using measured and published food values compared with directly measured meal glycemic index. Am. J. Clin. Nutr.94, 992–996 (2011). https://linkinghub.elsevier.com/retrieve/pii/S0002916523024711 [DOI] [PubMed] [Google Scholar]
- 57.Atkinson, F., Brand-Miller, J., Foster-Powell, K., Buyken, A. & Goletzke, J. International tables of glycemic index and glycemic load values 2021: a systematic review. Am. J. Clin. Nutr.114, 1625–1632 (2021). https://www.sciencedirect.com/science/article/pii/S0002916522004944#ec1 [DOI] [PubMed] [Google Scholar]
- 58.Olendzki, B. C. et al. Methodology for adding glycemic index and glycemic load values to 24-hour dietary recall database. Nutrition22, 1087–1095 (2006). https://linkinghub.elsevier.com/retrieve/pii/S0899900706002747 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets of the current study are available from the corresponding author on reasonable request.