Abstract
People still hold the concept of using cell-based treatments to regenerate missing neurons in high esteem. CD117+ cells are considered favorable stem cells for regenerative medicine. The objective of this research was to examine the impact of Alginate-Gelatin (Alg-Gel) hydrogel on the process of neurogenic differentiation of CD117+ cells utilizing a cytokines secretion test conducted in a laboratory setting. To achieve this objective, bone marrow-CD117+ cells were isolated using the MACS technique and then transformed into neuron cells using a neurogenic differentiation medium. The characterization of enriched CD117+ cells has been done with flow cytometry as well as immunocytochemistry. Next, the cells underwent western blotting assay to evaluate the signaling pathways. Subsequently, the culture media was obtained from both groups in order to determine cytokine levels. The study revealed that the Alg-Gel hydrogel had a notable impact on enhancing the protein expression of neuron markers such as β-tubulin and Wnt/catenin signaling pathway components in CD117+ neurogenic differentiated cells. Furthermore, the cultured medium from the experimental group exhibited a notable abundance of IL-6 and IL-10 in comparison to the control group. The observed in vitro effects of Alg-Gel hydrogel on neurogenic differentiation of CD117+ cells are likely to be caused by the cytokines that are released.
Keywords: Alginate-gelatin hydrogel, CD117+ hematopoietic stem cells, Neurogenic differentiation, Regenerative medicine
1. Introduction
In general, stem cells possess distinctive characteristics that render them advantageous in the treatment of a variety of diseases and injuries and in the field of regenerative medicine [1,2]. Their capacity to secrete growth factors, migrate to damaged regions of the body, and differentiate into multiple cell types renders them a promising tool for the repair and regeneration of tissues and organs [3,4]. The expression of growth factors and chemokines is responsible for the migration and return of stem cells to the injured regions of the brain following transplantation [5]. Stem cells differentiate into host tissue cells at the site of injury, replacing necrotic and injured neuronal tissue [6,7]. Stem cells further mitigate injury and encourage endogenous cells to repair and restore the normal functionality of neurons through their paracrine mechanisms [8]. Furthermore, stem cells secrete a variety of neuroprotective growth factors that activate numerous signaling pathways, thereby improving differentiation, survival, and the preservation of neuronal functions [9]. Additionally, stem cells possess angiogenesis, immunomodulatory, and anti-inflammatory paracrine functions through the production of interleukin (IL) and growth factors [10,11]. Neurodegeneration is characterized by a gradual decline in brain functionality that is a result of the loss of neuronal and other cells in the central nervous system [12]. When employed as a therapeutic approach, stem cells have the potential to prevent the deterioration of neurons and facilitate the repair of the wounded brain's circuitry [13].
The bone marrow (BM) is home to hematopoietic stem cells (HSCs) that are capable of self-regeneration. Consequently, hematopoietic stem cell transplantation (HSCT) is a widely used treatment for a variety of hematologic malignancies and immune system disorders [14]. The niche, a specialized milieu in the BM, is the location of the cellular kit (C-Kit) or CD117+ cells. This niche is home to a variety of biochemical agents, including growth factors, adhesion molecules, and extracellular matrix (ECM) components [15]. Together, these factors contribute to the complex process of cell contact, proliferation, and differentiation.
For an extended period, HSCs have been the subject of research and application in the treatment of specific neurological disorders; however, there have been conflicting results. Autologous and allogeneic hematopoietic stem cell transplantation (AHSCT and Allo-HSCT, respectively) are effective treatments for specific neurological conditions [16]. Neuroinflammation in multiple sclerosis, neuromyelitis optica spectrum disorder, and myasthenia gravis can be halted by AHSCT. Nevertheless, the clinical benefits are primarily due to the peripheral immune reset that is induced by the conditioning regimen rather than the direct interaction between the nervous system and HSC [17]. Allo-HSCT has been rarely employed in auto-immune neurological disorders due to a high risk of treatment-related mortality, despite the prospective benefits of the graft-vs.-autoreactivity effect and self-tolerant immune reconstitution [18].
Numerous researchers have developed three-dimensional (3D) environments that serve as replicas of the natural environments of HSCs [19]. The medium enhances cellular potential by facilitating cell attachment and enhancing cell-cell and cell-matrix interactions [20]. A number of three-dimensional fibrous habitats that resemble cell compartments in living organisms have been identified; however, additional research is required [21]. This research introduces a novel method that employs a hydrogel-based technique with sodium alginate (Alg) to accelerate the proliferation of HSCs in 3D culture. The proposed porous hydrogel technology was employed to establish a novel 3D cell culture and processing approach, which was the unique feature of this work [15]. The hypothesis is that BM-CD117+ cells in the alginate-gelatin (Alg-Gel) hydrogel have neurogenic potential and can be considered for further assessment in future studies.
2. Materials and methods
2.1. Reagents
We acquired all plates and supplements for cell culture, unless otherwise indicated, from SPL Life Sciences Co., Ltd. (Gyeonggi-do, Korea) and GIBCO (UK), respectively. The monoclonal antibodies utilized in the Western blot were acquired from SantaCruz, Inc. (SantaCruz, CA). The CD117 microbeads and magnetic activated cell sorting (MACS) column were purchased from Miltenyi Biotechnology Company.
The cells were divided into two groups: group I, which comprised CD117+ HSCs without Alg-Gel coating and in the presence of neurogenic differentiation, and group II, which comprised CD117+ HSCs with Alg-Gel coating and in the presence of neurogenic differentiation.
2.2. Enrichment of CD117+ HSCs
To isolate CD117+ HSCs, four-to nine-week-old male Rattus Norvegicus were employed. Fathi et al. (2020) previously reported the isolation of BM-mononuclear cells (MNCs) and enrichment of CD117+ HSCs [22]. To put it briefly, the femur and tibia were removed and flushed with washing buffer (PBS supplemented with 5 % FBS) following the euthanasia of the rats. The MNCs were then gathered after the BM content was centrifuged in Ficoll-Paque (Lymphodex, Inno-TRAIN, REF: 002041600) [23]. Following an LS MACS column pass through, the isolated MNCs were resuspended and treated with CD117 microbeads. Eventually, the column was flushed in order to recover enhanced CD117+ cells. Following the enrichment of CD117+ HSCs, flow cytometry as well as immunocytochemistry (ICC) were used to evaluate the cell purity, as previously documented by Farahzadi et al. (2023) [24].
2.3. Neurogenic differentiation of CD117+ HSCs
The neurogenic differentiation medium stimulated CD117+ HSCs to undergo neurogenic differentiation. In order to do this, 14 days were spent cultivating 10 × 105 CD117+ HSCs/12-well plates in the neurogenic differentiation medium, both with and without the Alg-Gel layer. Twice a week, new medium was added to the old one. Following the completion of neurogenic differentiation, the induction of neurons was verified using western blotting of the expression of β-Tubulin III. Following the conclusion of the differentiation phase, cells were subjected to flow cytometry, western blotting, and ELISA analysis after the Alg-Gel was eliminated by washing them with PBS. After the differentiation period, the cells were washed with PBS to remove the Alg-Gel. Subsequently, they were subjected to flow cytometry, western blotting, and ELISA investigation.
2.4. Apoptosis and cell proliferation investigation by Annexin V/PI
Neurogenic differentiated CD117+ cells were subjected to the Annexin V/PI assay. In this regard, 20 × 104 of these cells/well were rinsed twice with PBS, resuspended in the binding buffer (ref no.: 00-0055-56, e-bioscience), and maintained at 4 °C in the dark for 20 min. Cells were incubated with FITC-conjugated Annexin V (ref no.: 11-8005-74, e-bioscience) for 15 min at 25 °C in the subsequent step. Following this, the cells were rinsed with binding buffer and then exposed to the PI solution. The data were analyzed using FlowJo software version. X.0.7. 49, and flow cytometry was conducted using FACSCalibur (BD Bioscience) [25].
2.5. Western blot analysis for protein expression assessment
The neurogenic differentiated CD117+ cells from both groups were extracted, and western blotting was performed in accordance with the methodology outlined previously [26]. The cell protein sample was electrophoresed on 12 % polyacrylamide slab gels and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were subsequently incubated with primary antibodies (1:1000) against β-Actin (sc-47778), β3 Tubulin (TU-20) (sc-51670), Wnt-3 (sc-74537), β-catenin (sc-7963), mTOR (sc-517464), p-mTOR (59. Ser 2448) (sc-293133), and Telomerase Reverse Transcriptase (TERT) (E-AB-33070) for 16 h at 4 °C. The membranes were subsequently incubated with secondary antibody for 60 min at 25 °C. Subsequently, the membranes were rinsed and protein bands were identified by employing ECL with X-ray film.
2.6. Cytokine measurement by ELISA
Culture media was obtained from both control and experimental groups. The ELISA was conducted in accordance with the manufacturer's instructions (R&D Systems, China). In summary, a 96-well plate was coated with detection Reagent A for 16 h at 4 °C. Next, the 96-well plate was coated with IL-6 and IL-10 antibodies, and cell culture media was added. The ELISA sandwich technique was used to detect the presence of the media [24].
2.7. Telomere Length (TL) measurement by real time PCR
The absolute Telomere length (aTL) measurement method was previously reported by O'Callaghan and Fenech (2011) [27]. The Genomic DNA Extraction Mini Kit was employed to isolate genomic DNA from the control and experimental groups in accordance with the manufacturer's instructions. Briefly, proteinase K was incubated with cells, ethanol was introduced, and the mixture was meticulously transferred from the sample mixture to the BG column. The flow through was discarded after the mixture was centrifuged and rinsed. The membrane center of the BG column was injected with 30–50 μl of elution buffer. The DNA fragment was stored at 4 °C until it was needed for aTL measurement, after the column was centrifuged for 2 min to elute the DNA. 20 ng/μl of DNA is necessary for aTL measurement. O'Callaghan and Fenech (2011) have previously comprehensively reported the telomere standard curve and single copy gene (SCG) standard curve, which are included in the necessary diagrams [27]. The primer's sequences previously have been used by O'Callaghan and French (2011) (Table 1) [27].
Table 1.
Primer sequences used in absolute telomere length assay.
| Oligomer name | Primer pair sequence (5′-3′) |
|---|---|
| Telomere standard | (TTAGGG)14 |
| 36B4 standard | 5′CCTTGTCTCCAGTCTTTATCAGCTGCACATCGCTCTGAGGA AGAGAAGAGCAGTTACCACCCAGACACACAGAAG 3′ |
| Telo | Fwd: CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGG TTTGGGTT Rev: GGCTTGCCTTACCCTTACCCTTACCC TTACCCTTACCCT |
| 36B4 | Fwd: CTTCTGTGTGTCTGGGTGGT Rev: CCTTGTCTCCAGTCTTTATCAG |
2.8. Statistical analysis
Sidak's multiple comparisons test following a two-way ANOVA was used to analyze the results from Fig. 2, Fig. 3, Fig. 4. Also, the results from Fig. 5 were analyzed using an unpaired t-test. The statistical significance was established at P < 0.05 using GraphPad Prism version 6.01.
Fig. 2.
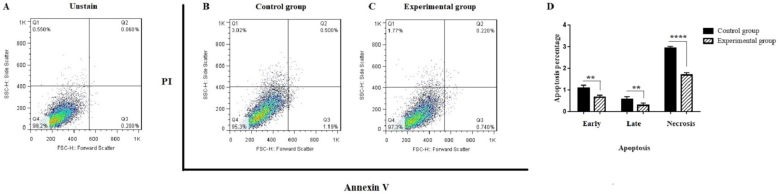
Assessment of apoptosis rate via the Annexin V/PI test. A transition from the bottom-right quadrant panel (early apoptosis) to the top-right quadrant panel (late apoptosis) and the top-left quadrant panel (necrosis) was noted. (A) Unstained cells; (B) Control group; (C) Experimental group. The quantification of apoptosis is presented in Part D. Values are expressed as mean ± SD from independent experiments (∗∗P < 0.01 and ∗∗∗∗P < 0.0001).
Fig. 3.
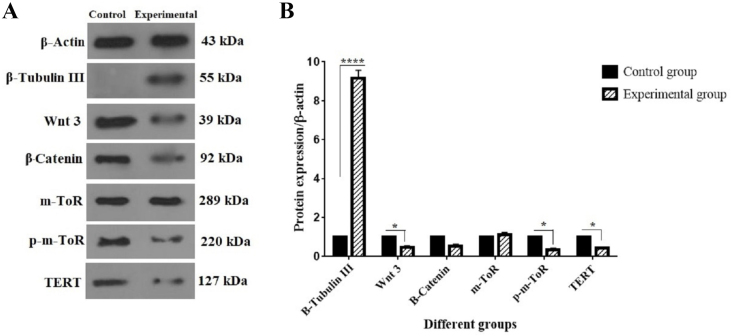
Effect of Alg-Gel hydrogel on protein expression of neurogenic differentiated CD117+ cells. (A) The protein bands of β-Actin, β-Tubulin III, Wnt3, β-Catenin, m-TOR, p-m-TOR, and TERT. (B) The protein expression levels of the mentioned proteins. 1 × 106 cells/well from the two groups of cells (control and experimental) were collected. Following the isolation of whole protein, western blotting was performed as described before. Values are mean ± SD from independent experiments (∗P < 0.05 and ∗∗∗∗p < 0.0001).
Fig. 4.
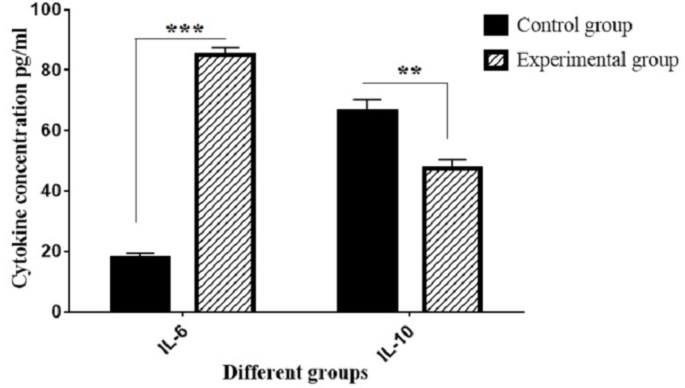
The secretion levels of cytokines IL-6 and IL-10 from two groups: control (CD117+ HSCs without Alg-Gel coating and in the presence of the neurogenic differentiation) and experimental (CD117+ HSCs with Alg-Gel coating and in the presence of the neurogenic differentiation) (∗∗∗P < 0.001 and ∗∗P < 0.01; compared with control group).
Fig. 5.
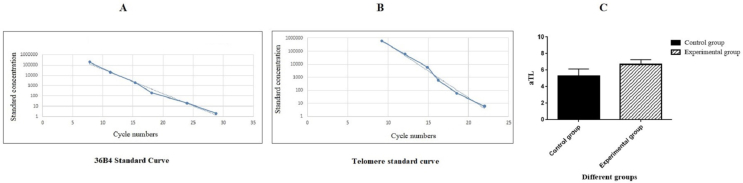
aTL measurement of CD117+ neurogenic differentiated cells in the presence of Alg-Gel hydrogel. A: The standard curve for calculating genome copies using the 36B4 copy number; B: The standard curve for calculating the length of telomere sequence per reaction tube; C: aTL of CD117+ neurogenic differentiated cells in the experimental group were compared to the control group.
3. Results
3.1. Identification of BM-CD117+ cells
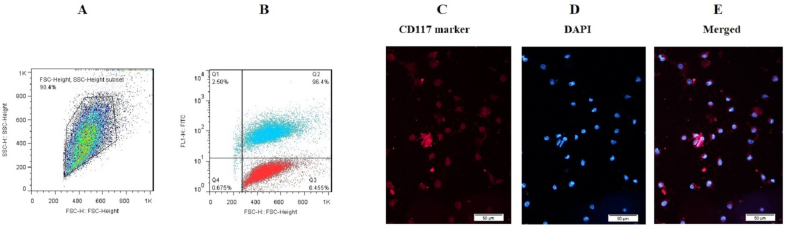
The MACs method was used to enrich BM-CD117+ HSCs, and flow cytometry analysis was employed to evaluate the purity of the cell enrichment. The flow cytometry results demonstrated that the CD117 expression of enriched BM-CD117+ cells were high (96.4 %) (Fig. 1A and B). The cell population of CD117+ cells following the MACs method is illustrated in Fig. 1A. The CD117+ cells that were enriched exhibited a purity of 96.4 %. In other words, the enriched cells exhibited elevated levels of CD117+ expression (Fig. 1B). The findings are consistent with those of the preceding reports. The protein expression of CD117-related markers in enriched CD117+ cells was assessed using the ICC method in addition to flow cytometry (Fig. 1C–E). The CD117 marker fluorescence is depicted in red, while the nuclei are stained with DAPI in blue.
Fig. 1.
Characterization of BM-CD117+ cells. (A) The total number of cells used for CD117 evaluation; (B) 96.4 % of cells were positive for the CD117 marker; (C–E) The enriched BM-CD117+ cells were characterized through ICC investigation. The presence of CD117 cells was confirmed by immunofluorescence imaging, as evidenced by the positive color reaction. Red cells exhibited PE-conjugated CD117, while blue portions exhibited DAPI nuclear staining (bar = 20 μm).
3.2. Apoptosis investigation by Annexin V/PI assay
To evaluate the impact of Alg-Gel hydrogel on apoptosis, cells from two control and experimental groups were harvested, and Annexin V/PI analysis was conducted via flow cytometry, as previously reported by Farahzadi et al. (2023) [28]. As illustrated in Fig. 2, the percentage of early apoptosis, late apoptosis, and necrosis have significantly reduced in the experimental group in the presence of Alg-Gel hydrogel versus control group 1.5, 1.98, and 1.71-folds, respectively (∗∗P < 0.01, ∗∗P < 0.01, and ∗∗∗∗P < 0.0001).
3.3. Alg-Gel hydrogel changed the protein expression of Wnt3, β-catenin, m-TOR, p-m-TOR, and TERT
The protein expression of signaling pathway components was evaluated to investigate the effect of Alg-Gel hydrogel on the neurogenic differentiation of CD117+ HSCs. This panel evaluated Wnt3, β-Catenin, m-TOR, and p-m-TOR as elements in the signaling pathways. Fig. 3 indicates that the protein levels of Wnt3 and p-m-TOR were significantly decreased by 0.45-fold and 0.34-fold in the experimental group relative to the control group, respectively (∗P < 0.05). The expression level of TERT protein, a crucial component of the telomerase enzyme and an aging marker, significantly decreased by 0.42-fold in the experimental group compared to the control group (∗P < 0.05). No substantial alterations were seen in the expression levels of β-Catenin and m-TOR proteins.
3.4. Confirmation of neurogenic differentiation of BM-CD117+ cells
After 14 days of culturing CD117+ cells in neurogenic differentiation media, the protein expression of β-tubulin III, a neuronal marker, is examined. In summary and according to Fig. 3, the neurogenic differentiated CD117+ cells residing in the BM express β-tubulin III as a neuron marker. With more details, the expression level of β-tubulin III, significantly increased by 9.16-fold in the experimental group compared to the control group (∗∗∗∗P < 0.0001).
3.5. Cytokine secretion measurement of neurogenic differentiated CD117+ cells by ELISA
Fig. 4 illustrates that, in the presence of Alg-Gel hydrogel, neurogenic differentiated CD117+ cells produce several growth factors that promote neurogenesis. The cytokines IL-6 and IL-10 obtained from the cultured media of two control and experimental groups were analyzed using the ELISA sandwich technique. To this end, culture media were collected from each group. The ELISA procedure was conducted according to the manufacturer's instructions. The findings indicated that the secretion of IL-6 was significantly increased and IL-10 was significantly decreased in the experimental group compared to the control group, respectively (∗∗∗P < 0.001 and ∗∗P < 0.01).
3.6. aTL measurment
The effect of Alg-Gel on the aTL of the CD117+ neurogenic differentiated cells was investigated by quantitative real-time PCR. In order to achieve this, genomic DNA from the experimental and control groups was extracted at the end of the treatment period, and real-time PCR was used. To calculate the telomere sequence content in kb for each sample, two standard curves were drawn. Graphs A and B in Fig. 5 show the standard curves related to the calculation of the genome copies using the 36B4 copy number and length of the telomere sequence per reaction tube and, respectively. As can be seen in Fig. 5C, there was no significant difference in aTL between the experimental group (9.65 Kbp) and the control group (6.25 Kbp).
4. Discussion
The utilization of biomaterials as scaffolds has been demonstrated to be advantageous for MSC-based treatment in numerous disease models [29,30]. The selection of scaffolds and seeding cells was crucial for attaining the desired therapeutic outcome, as it was contingent upon the type of illness [31,32]. For instance, MI necessitated a scaffold that could be utilized in an ischemic milieu and a seeding cell with a robust cardiomyogenic potential [33,34]. Stem cell transplantation has been proposed as a state-of-the-art treatment for disease for several years, with promising results [35]. Nevertheless, there was an ongoing debate regarding the origin of stem cells and the use of different forms of hydrogels. Scaffold application was generally considered a viable method for enhancing stem cell treatment [36,37]. Nevertheless, the relevant studies were significantly dissimilar due to the use of a variety of scaffolds in previous years. It is desirable for a scaffold to be bioactive and to offer a growth medium that is comparable to the ECM found in nature [38].
The biocompatibility, non-thrombogenic nature, delicate gelation process, and similarity to the ECM of the ionotropic Alg hydrogel biomaterial have made it a popular choice for drug administration in tissue engineering and regenerative medicine [39,40]. The utilization of biomaterials in conventional spinal care may be stimulated by the integration of Alg hydrogels into clinical spine research [41]. Alg is a promising candidate for investigation in spinal pathologies, including spinal cord injury (SCI), due to their potential as "antidegenerative" and "pro-regenerative" agents [42]. The utilization of Alg hydrogels as a matrix for neural stem cell growth has been demonstrated in numerous studies [43]. Ashton et al. (2007) have previously demonstrated a method for the synthesis of Alg hydrogel scaffolds that are incorporated with poly (lactide-co-glycolide) microspheres and have adjustable degradation rates in stem cell cultures. The authors emphasized a substantial increase in the rate of proliferation of neuronal progenitor cells that were cultured in hydrogel Alg that were degrading [44]. In another study, Banerjee et al. (2009) examined the impact of the modulus of Alg hydrogels on the proliferation and differentiation of neural stem cells [45]. Nerve outgrowth and astrocyte reactions were also observed at the stump of two transected spinal cords of juvenile rats that were implanted with Alg at the site of the lesion by Kataoka et al. (2004) [45]. In contrast to collagen polymers that served as controls, there was substantial growth. The authors demonstrated an increase in the expression of β-tubulin III within Alg hydrogel scaffolds by adjusting the concentration of Alg and calcium ions. It has been employed as a controlled release carrier of numerous bioactive cytokines to enhance self-healing and promote endogenous regeneration [46]. In vitro and adult rat models were employed by Prang et al. (2006) to demonstrate the feasibility of Alg hydrogel scaffolds for axonal regrowth following acute SCI [47]. In an entorhinal-hippocampal slice culture model, anisotropic capillary hydrogels facilitated directed central nervous system axonal growth and enabled longitudinally oriented reinnervation in vitro and integration into the spinal cord without significant inflammatory reactions in vivo. In 2015, Günther et al. conducted a study in which 2-mm long Alg hydrogels were implanted into the C5 hemisection lesion of a rat spinal cord [48]. The hydrogels were inoculated with BM stromal cells (BMSCs) that expressed brain-derived neurotrophic factor (BDNF) or green fluorescent protein as a control. Macrophages, blood vessels, Schwann cells, and numerous BMSCs were observed in the scaffold channels during the four-week assessment. Additionally, the axon numbers in the Alg group were 3–4 times greater than those in the control group. In contrast to axons in Alg-based scaffolds, which exhibited axons in linear orientation with respect to the hydrogel channel wall, lesions filled with BMSCs without Alg hydrogels exhibited random axon orientation. These findings demonstrate that Alg hydrogel scaffolds can be instrumental in the facilitation of axonal regeneration [48]. The effects of the same Alg-based grafts on subtotal cervical hemi sections were further investigated by Tobias et al. in a subsequent investigation conducted in 2005. The study evaluated the function of the forelimb and hindlimb, as well as the proliferation of axons, in the absence of immunosuppression. The results indicated that the Alg graft resulted in a partial recovery of forelimb and hindlimb function in comparison to the group that did not receive the Alg graft. An abundance of axonal promoters, such as neurofilament (RT-97), 5-HT, CGRP, and GAP-43, was observed in the Alg graft group during immunohistochemical examination [49]. A BDNF-releasing Alg graft induced axonal reorganization and behavioral recovery, indicating that Alg grafts are a viable approach for the therapeutic recovery of injured SCI. In 2019, Schackel et al. conducted a study in which they grafted poly-l-ornithine and laminin-coated Alg hydrogels into a cervical hemi section of adult female rats promptly following injury. The implants' potential for axonal regeneration was further bolstered by the authors' report that they remained firmly integrated and exhibited indications of host cell migration and neurite extension [50].
In addition to the function and importance of numerous hydrogel varieties, stem cell utilization has garnered attention for an extended period.
Many studies have been reported on various types of stem cells and neurodegenerative diseases, but the research on CD117+ cells has not been reported. The significance of MSCs, their derived factors, and the associated signaling pathways in ameliorating aging, age-related illnesses, and neurodegenerative diseases such as Alzheimer's disease (AD) has garnered increased attention from researchers. Farahzadi et al. (2020) [28] demonstrated the influence of MSCs on Aβ-treated brain cells via the mTOR, AMPK, GSK-3β, and Wnt/β-catenin signaling pathways, which are crucial in the context of AD [51]. This study aims to investigate the neurogenic differentiation potential of BM-resident CD117+ HSCs utilizing Alg-Gel hydrogel as a scaffold. One of the study's unforeseen findings was the observation that, unlike hydrogel's capacity to enhance the production of some cytokines like IL-6, the experimental group's TL and TERT protein expression were diminished, maybe influencing the differentiation of the cells.
Genomic instability resulting from TL reduction may be a significant contributor to age-related illnesses. TL is being widely investigated as a potential epigenetic marker linked to several neurodegenerative diseases, such as AD. Previous investigations on TL in AD yield inconsistent results [52]. Rolyan et al. (2016) indicated that knockout mice for the telomerase RNA component (TERC) (G3Terc−/−), characterized by shortened telomeres, exhibit a decreased quantity of amyloid plaques. Evidence indicates that shorter telomeres are causally linked to an increased risk of AD. The functional impact of telomere shortening on brain aging and AD remains unclear; thus, additional research is required to investigate the specifics [53]. Contrary to the increase in the neuronal differentiation of cells shown in our study in the presence of Alg-Gel, we did not observe a significant change in TL.
Biodegradable hydrogels that facilitate the three-dimensional (3D) differentiation of stem cells into neurons are extensively sought after in biomedical research for investigating drug neurotoxicity and for producing cell-laden biomaterials for neural tissue regeneration [54]. The findings of this work related to the role of Alg-Gel hydrogel on increasing neurogenic differentiation are consistent with those of a study conducted by Distler et al. (2021), who demonstrated that incorporation of laminin into oxidized Alg-Gel hydrogel matrices is essential for optimizing these hydrogels for 3D neuronal cell culture applications of human induced pluripotent stem cell (hiPSC) derived neutrospheres [54]. Considering each of these interpretations, there is contention over the potential role of the percentage of BM-derived CD117+ cells in neurogenic regeneration. Comprehending the fundamental reasons behind these controversial discoveries is essential to comprehending the practical importance of BM-derived CD117+ cells. Thus, scaffolds that replicate the conditions of in vivo cell development are frequently employed in ongoing studies.
In this study, the protein expressions of β-Tubulin as a marker of neurogenic differentiation, as well as the Wnt3, β-Catenin, m-TOR, and p-m-TOR protein expression, were examined to investigate the impact of the Alg-Gel hydrogel mechanism on the neurogenic differentiation of CD117+ HSCs at the cellular and molecular levels. Also, the aTL measurement, like TERT protein expression, was investigated to better evaluate the effect of Alg-Gel hydrogel on the aging of neurogenic differentiated cells. In the following, for evaluating the secreted cytokines in this direction, IL-6 and IL-10 were measured with Elisa.
In comparing the experimental group to the control group, our findings demonstrated that the Alg-Gel hydrogel significantly enhanced the protein expression of β-Tubulin III and significantly decreased the protein expression of Wnt3, p-m-TOR, and TERT. Also, contrary to our expectation, no significant change was observed in the TL, and the decrease in the TERT protein expression may be due to the fact that when the cells enter the differentiation phase, a decrease in the expression of the Tret gene is observed in them.
We also found that there is a correlation between the rising and decreasing levels of IL-6 and IL-10, respectively, and neurogenic marker expression. The cytokines that are produced have significant impacts on paracrine way cytokines, which are crucial for neurogenesis processes associated to stem cells. These effects include anti-remodeling, anti-apoptosis, and anti-inflammatory properties [55]. According to research by Perez-Asensio et al. (2013) [56], IL-10 reduces neuronal differentiation and ultimately impairs endogenous neurogenesis. The in vivo neuronal development of subventricular zone progenitors is consistently boosted and the integration of new neurons in the adult olfactory bulb is increased when IL-10 is absent [56].
Our results show that the IL-10 decreased in the experimental group, and this result is in line with the previous study by Perez-Asensio et al. (2013) [56].
Based on the previously stated findings, it's noteworthy that we are the first to show that Alg-Gel hydrogel may promote the expression of β-Tubulin III as a neurogenic marker, hence inducing the neurogenic differentiation of CD117+ HSCs. In this sense, the cytokines that are secreted IL-6, and IL-10 may be the cause of its actions.
5. Conclusion
In summary, our study suggests that Alg-Gel hydrogel may enhance the protein expressions of neurogenic-related markers in CD117+ stem/progenitor cells, which are associated with cytokines such as, IL-6, and IL-10. Tyrosine kinase activity is essential for the in vitro functional neurogenic differentiation of CD117+ stem/progenitor cells; hence, it is recommended that future research use this assay to examine the CD117 receptor expression.
Author contributions
J. P as the executives of the project, had the main contribution to conception and design, the performance of experiments, data analysis, and manuscript writing, and supervised the manuscript preparation.
Funding
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Fathi E., Farahzadi R. Mesenchymal stem cells as a cell-based therapeutic strategy targeting the telomerase activity of KG1 acute myeloid leukemia cells. Acta Med Iran. 2022;60(2):71–77. [Google Scholar]
- 2.Heidari H.R., Fathi E., Montazersaheb S., Mamandi A., Farahzadi R., Zalavi S., et al. Mesenchymal stem cells cause telomere length reduction of molt-4 cells via caspase-3, BAD and P53 apoptotic pathway. Int J Mol Cell Med. 2021;10(2):113–122. doi: 10.22088/IJMCM.BUMS.10.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazloumi Z., Farahzadi R., Rafat A., Dizaji Asl K., Karimipour M., Montazer M., et al. Effect of aberrant DNA methylation on cancer stem cell properties. Exp Mol Pathol. 2022;125 doi: 10.1016/j.yexmp.2022.104757. [DOI] [PubMed] [Google Scholar]
- 4.Yang W., Ding N., Luo R., Zhang Q., Li Z., Zhao F., et al. Exosomes from young healthy human plasma promote functional recovery from intracerebral hemorrhage via counteracting ferroptotic injury. Bioact Mater. 2023;27:1–14. doi: 10.1016/j.bioactmat.2023.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian S., Chen X., Wu W., Lin H., Qing X., Liu S., et al. Nucleus pulposus cells regulate macrophages in degenerated intervertebral discs via the integrated stress response-mediated CCL2/7-CCR2 signaling pathway. Exp Mol Med. 2024;56(2):408–421. doi: 10.1038/s12276-024-01168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma R.S. Breaking dogma for future therapy using stem cell - where we have reached? Indian J Med Res. 2016;143(2):129–131. doi: 10.4103/0971-5916.180195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng W., Yu L., Wu J., Wang F., Liu X., Ren S., et al. Clinical characteristics and long-term follow-up outcomes of myelin oligodendrocyte glycoprotein antibody-associated disease in Han Chinese participants. Medicine (Baltim) 2023;102(40) doi: 10.1097/MD.0000000000035391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baraniak P.R., McDevitt T.C. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5(1):121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu G., Ding J., Yang N., Ge L., Chen N., Zhang X., et al. Evaluating the pro-survival potential of apoptotic bodies derived from 2D- and 3D- cultured adipose stem cells in ischaemic flaps. J Nanobiotechnol. 2024;22(1):333. doi: 10.1186/s12951-024-02533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C., Ge H., Zhang S., Liu D., Jiang Z., Lan C., et al. Hematoma evacuation via image-guided para-corticospinal tract approach in patients with spontaneous intracerebral hemorrhage. Neurol Ther. 2021;10(2):1001–1013. doi: 10.1007/s40120-021-00279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y., Wang C., Zhou T., Xie F., Liu Z., Xu H., et al. Lumican promotes calcific aortic valve disease through H3 histone lactylation. Eur Heart J. 2024;45(37):3871–3885. doi: 10.1093/eurheartj/ehae407. [DOI] [PubMed] [Google Scholar]
- 12.Shen B., Xiao S., Yu C., Zhang C., Zhan J., Liu Y., et al. Cerebral hemodynamics underlying ankle force sense modulated by high-definition transcranial direct current stimulation. Cerebr Cortex. 2024;34(6) doi: 10.1093/cercor/bhae226. [DOI] [PubMed] [Google Scholar]
- 13.Thompson L.H., Björklund A. Reconstruction of brain circuitry by neural transplants generated from pluripotent stem cells. Neurobiol Dis. 2015;79:28–40. doi: 10.1016/j.nbd.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Kelly S., Sola C., De Lima M., Shpall E. Ex vivo expansion of cord blood. Bone Marrow Transplant. 2009;44(10):673–681. doi: 10.1038/bmt.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J.E., Lee E.J., Wu Y., Kang Y.G., Shin J.-W. The combined effects of hierarchical scaffolds and mechanical stimuli on ex vivo expansion of haematopoietic stem/progenitor cells. Artif Cell Nanomed Biotechnol. 2019;47(1):585–592. doi: 10.1080/21691401.2019.1573180. [DOI] [PubMed] [Google Scholar]
- 16.de Vasconcelos P., Lacerda J.F. Hematopoietic stem cell transplantation for neurological disorders: a focus on inborn errors of metabolism. Front Cell Neurosci. 2022;16 doi: 10.3389/fncel.2022.895511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharrack B., Saccardi R., Alexander T., Badoglio M., Burman J., Farge D., et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE) Bone Marrow Transplant. 2020;55(2):283–306. doi: 10.1038/s41409-019-0684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Wijmeersch B., Sprangers B., Rutgeerts O., Lenaerts C., Landuyt W., Waer M., et al. Allogeneic bone marrow transplantation in models of experimental autoimmune encephalomyelitis: evidence for a graft-versus-autoimmunity effect. Biol Blood Marrow Transplant. 2007;13(6):627–637. doi: 10.1016/j.bbmt.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Soffer-Tsur N., Peer D., Dvir T. ECM-based macroporous sponges release essential factors to support the growth of hematopoietic cells. J Contr Release. 2017;257:84–90. doi: 10.1016/j.jconrel.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Batnyam O., Shimizu H., Saito K., Ishida T., Suye S.-i., Fujita S. Biohybrid hematopoietic niche for expansion of hematopoietic stem/progenitor cells by using geometrically controlled fibrous layers. RSC Adv. 2015;5(98):80357–80364. [Google Scholar]
- 21.Zhao M., Kang M., Wang J., Yang R., Zhong X., Xie Q., et al. Stem cell-derived nanovesicles embedded in dual-layered hydrogel for programmed ROS regulation and comprehensive tissue regeneration in burn wound healing. Adv Mater. 2024;36(32) doi: 10.1002/adma.202401369. [DOI] [PubMed] [Google Scholar]
- 22.Fathi E., Azarbad S., Farahzadi R., Javanmardi S., Vietor I. Effect of rat bone marrow derived-mesenchymal stem cells on granulocyte differentiation of mononuclear cells as preclinical agent in cellbased therapy. Curr Gene Ther. 2022;22(2):152–161. doi: 10.2174/1566523221666210519111933. [DOI] [PubMed] [Google Scholar]
- 23.Fathi E., Pashutan M.K., Farahzadi R., Charoudeh H.N. L-carnitine in a certain concentration increases expression of cell surface marker CD34 and apoptosis in the rat bone marrow CD34+ hematopoietic stem cells. Iran J Vet Res. 2021;22(4):264. doi: 10.22099/ijvr.2021.39045.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farahzadi R., Fathi E., Mesbah-Namin S.A., Vietor I. Granulocyte differentiation of rat bone marrow resident C-kit(+) hematopoietic stem cells induced by mesenchymal stem cells could be considered as new option in cell-based therapy. Regen Ther. 2023;23:94–101. doi: 10.1016/j.reth.2023.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fathi E., Vietor I. Mesenchymal stem cells promote caspase expression in Molt-4 leukemia cells via GSK-3α/and ERK1/2 signaling pathways as a therapeutic strategy. Curr Gene Ther. 2021;21(1):81–88. doi: 10.2174/1566523220666201005111126. [DOI] [PubMed] [Google Scholar]
- 26.Farahzadi R., Valipour B., Anakok O.F., Fathi E., Montazersaheb S. The effects of encapsulation on NK cell differentiation potency of C-kit+ hematopoietic stem cells via identifying cytokine profiles. Transpl Immunol. 2023;77 doi: 10.1016/j.trim.2023.101797. [DOI] [PubMed] [Google Scholar]
- 27.O'Callaghan N.J., Fenech M. A quantitative PCR method for measuring absolute telomere length. Biol Proced Online. 2011;13:3. doi: 10.1186/1480-9222-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farahzadi R., Sanaat Z., Movassaghpour-Akbari A.A., Fathi E., Montazersaheb S. Investigation of L-carnitine effects on CD44+ cancer stem cells from MDA-MB-231 breast cancer cell line as anti-cancer therapy. Regenerative Therapy. 2023;24:219–226. doi: 10.1016/j.reth.2023.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang L., Yang Y., Li Y., Yang G., Luo T., Xue Y., et al. Knitted silk mesh-like scaffold incorporated with sponge-like regenerated silk fibroin/collagen I and seeded with mesenchymal stem cells for repairing Achilles tendon in rabbits. Acta Bioeng Biomech. 2018;20(3):77–87. [PubMed] [Google Scholar]
- 30.Subbarayan R., Girija D.M., Rao S.R. Human umbilical cord tissue stem cells and neuronal lineages in an injectable caffeic acid–bioconjugated gelatin hydrogel for transplantation. J Cell Physiol. 2019;234(3):1967–1977. doi: 10.1002/jcp.26834. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Zhai W., Cheng S., Li J., Zhang H. Surface-functionalized design of blood-contacting biomaterials for preventing coagulation and promoting hemostasis. Friction. 2023;11(8):1371–1394. [Google Scholar]
- 32.Zhao Y., Hu J., Sun X., Yang K., Yang L., Kong L., et al. Loss of m6A demethylase ALKBH5 promotes post-ischemic angiogenesis via post-transcriptional stabilization of WNT5A. Clin Transl Med. 2021;11(5):e402. doi: 10.1002/ctm2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Zhai W., Zhang H., Cheng S., Li J. Injectable polyzwitterionic lubricant for complete prevention of cardiac adhesion. Macromol Biosci. 2023;23(4) doi: 10.1002/mabi.202200554. [DOI] [PubMed] [Google Scholar]
- 34.Fu P.C., Wang J.Y., Su Y., Liao Y.Q., Li S.L., Xu G.L., et al. Intravascular ultrasonography assisted carotid artery stenting for treatment of carotid stenosis: two case reports. World J Clin Cases. 2023;11(29):7127–7135. doi: 10.12998/wjcc.v11.i29.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traverse J.H. Using biomaterials to improve the efficacy of cell therapy following acute myocardial infarction. Journal of cardiovascular translational research. 2012;5:67–72. doi: 10.1007/s12265-011-9330-y. [DOI] [PubMed] [Google Scholar]
- 36.Chen J., Zhan Y., Wang Y., Han D., Tao B., Luo Z., et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018;80:154–168. doi: 10.1016/j.actbio.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Bai R., Tian L., Li Y., Zhang J., Wei Y., Jin Z., et al. Combining ECM hydrogels of cardiac bioactivity with stem cells of high cardiomyogenic potential for myocardial repair. Stem Cell Int. 2019:2019. doi: 10.1155/2019/6708435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantore S., Crincoli V., Boccaccio A., Uva A.E., Fiorentino M., Monno G., et al. Recent advances in endocrine, metabolic and immune disorders: mesenchymal stem cells (MSCs) and engineered scaffolds. Endocr Metab Immune Disord - Drug Targets. 2018;18(5):466–469. doi: 10.2174/1871530318666180423102905. [DOI] [PubMed] [Google Scholar]
- 39.Ruvinov E., Cohen S. Alginate biomaterial for the treatment of myocardial infarction: progress, translational strategies, and clinical outlook: from ocean algae to patient bedside. Adv Drug Deliv Rev. 2016;96:54–76. doi: 10.1016/j.addr.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Lv K., Li Q., Zhang L., Wang Y., Zhong Z., Zhao J., et al. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics. 2019;9(24):7403. doi: 10.7150/thno.32637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S., Sandner B., Schackel T., Nicholson L., Chtarto A., Tenenbaum L., et al. Regulated viral BDNF delivery in combination with Schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury. Acta Biomater. 2017;60:167–180. doi: 10.1016/j.actbio.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Perale G., Rossi F., Sundstrom E., Bacchiega S., Masi M., Forloni G., et al. Hydrogels in spinal cord injury repair strategies. ACS Chem Neurosci. 2011;2(7):336–345. doi: 10.1021/cn200030w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H., Ji Q., Chen X., Sun Y., Xu Q., Deng P., et al. Accelerated bony defect healing based on chitosan thermosensitive hydrogel scaffolds embedded with chitosan nanoparticles for the delivery of BMP2 plasmid DNA. J Biomed Mater Res. 2017;105(1):265–273. doi: 10.1002/jbm.a.35900. [DOI] [PubMed] [Google Scholar]
- 44.Ashton R.S., Banerjee A., Punyani S., Schaffer D.V., Kane R.S. Scaffolds based on degradable alginate hydrogels and poly(lactide-co-glycolide) microspheres for stem cell culture. Biomaterials. 2007;28(36):5518–5525. doi: 10.1016/j.biomaterials.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 45.Banerjee A., Arha M., Choudhary S., Ashton R.S., Bhatia S.R., Schaffer D.V., et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30(27):4695–4699. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruvinov E., Leor J., Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials. 2011;32(2):565–578. doi: 10.1016/j.biomaterials.2010.08.097. [DOI] [PubMed] [Google Scholar]
- 47.Prang P., Müller R., Eljaouhari A., Heckmann K., Kunz W., Weber T., et al. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27(19):3560–3569. doi: 10.1016/j.biomaterials.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 48.Günther M.I., Weidner N., Müller R., Blesch A. Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater. 2015;27:140–150. doi: 10.1016/j.actbio.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Tobias C.A., Han S.S., Shumsky J.S., Kim D., Tumolo M., Dhoot N.O., et al. Alginate encapsulated BDNF-producing fibroblast grafts permit recovery of function after spinal cord injury in the absence of immune suppression. J Neurotrauma. 2005;22(1):138–156. doi: 10.1089/neu.2005.22.138. [DOI] [PubMed] [Google Scholar]
- 50.Schackel T., Kumar P., Günther M., Liu S., Brunner M., Sandner B., et al. Peptides and astroglia improve the regenerative capacity of alginate gels in the injured spinal cord. Tissue Eng. 2019;25(7–8):522–537. doi: 10.1089/ten.TEA.2018.0082. [DOI] [PubMed] [Google Scholar]
- 51.Farahzadi R., Fathi E., Vietor I. Mesenchymal stem cells could Be considered as a candidate for further studies in cell-based therapy of Alzheimer's disease via targeting the signaling pathways. ACS Chem Neurosci. 2020;11(10):1424–1435. doi: 10.1021/acschemneuro.0c00052. [DOI] [PubMed] [Google Scholar]
- 52.Kota L.N., Bharath S., Purushottam M., Moily N.S., Sivakumar P.T., Varghese M., et al. Reduced telomere length in neurodegenerative disorders may suggest shared biology. J Neuropsychiatry Clin Neurosci. 2015;27(2):e92–e96. doi: 10.1176/appi.neuropsych.13100240. [DOI] [PubMed] [Google Scholar]
- 53.Rolyan H., Scheffold A., Heinrich A., Begus-Nahrmann Y., Langkopf B.H., Hölter S.M., et al. Telomere shortening reduces Alzheimer's disease amyloid pathology in mice. Brain. 2011;134(Pt 7):2044–2056. doi: 10.1093/brain/awr133. [DOI] [PubMed] [Google Scholar]
- 54.Distler T., Lauria I., Detsch R., Sauter C.M., Bendt F., Kapr J., et al. Neuronal differentiation from induced pluripotent stem cell-derived neurospheres by the application of oxidized alginate-gelatin-laminin hydrogels. Biomedicines. 2021;9(3) doi: 10.3390/biomedicines9030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duran J.M., Makarewich C.A., Sharp T.E., Starosta T., Zhu F., Hoffman N.E., et al. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ Res. 2013;113(5):539–552. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez-Asensio F.J., Perpiñá U., Planas A.M., Pozas E. Interleukin-10 regulates progenitor differentiation and modulates neurogenesis in adult brain. J Cell Sci. 2013;126(Pt 18):4208–4219. doi: 10.1242/jcs.127803. [DOI] [PubMed] [Google Scholar]