Abstract
This narrative review seeks to summarize chemotherapeutic regimens commonly used for patients with newly diagnosed Philadelphia (Ph) chromosome–negative B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in the frontline setting and to describe the latest clinical research using the bispecific T-cell–engaging immunotherapy blinatumomab in the first-line treatment setting. Current standard-of-care chemotherapeutic backbones for newly diagnosed Ph-negative BCP-ALL are based on the same overarching treatment principle: to reduce disease burden to undetectable levels and maintain lasting remission. The adult treatment landscape has progressively evolved following the adoption of pediatric-inspired regimens. However, these intense regimens are not tolerated by all, and high-risk patients still have inferior outcomes. Therefore, designing more effective and less toxic strategies remains key to further improving efficacy and safety outcomes. Overall, the treatment landscape is evolving in the frontline, and integration of blinatumomab into different standard frontline regimens may improve overall outcomes with a favorable safety profile.
Subject terms: Acute lymphocytic leukaemia, Chemotherapy
Introduction
While outcomes in the first-line treatment setting for patients with Philadelphia (Ph)-negative B-cell precursor acute lymphoblastic leukemia (BCP-ALL) have significantly improved in recent years, especially for young adults, the prognosis for relapsed or refractory disease remains poor [1, 2]. Pivotal for preventing disease relapse is the optimal selection of frontline therapy, with the goal of improving cure rate among newly diagnosed patients and limiting long-term toxicities.
Treatment regimens for BCP-ALL remain among the most complex and intensive in oncology due to variable and often aggressive disease biology. Therefore, historical treatment strategies have combined multiple cytotoxic agents to maximize response, minimize development of resistance, and reduce overlapping toxicities [1, 3–5]. Key treatment goals include achieving disease remission, gaining rapid and deep control of measurable residual disease (MRD), preventing disease relapse, and improving survival (including reducing early mortality) while limiting toxicities and maintaining long-term quality of life.
Although individual BCP-ALL chemotherapy regimens can vary among different countries, the fundamental treatment principles are similar [4, 6]. Specific drug selection, dosing schedules, and treatment duration are tailored according to patient age, acute lymphoblastic leukemia (ALL) subtype (e.g., Ph chromosome status and other genetically defined groups), central nervous system (CNS) involvement, and early treatment response [4]. Despite these variances, standard chemotherapy backbone regimens have yielded approximately similar outcomes among similar cohorts of patients [7, 8].
Pediatric studies have played an important role in the treatment evolution of the BCP-ALL therapy landscape [9]. Although outcomes in younger adults with newly diagnosed BCP-ALL have improved following adoption of pediatric-inspired chemotherapy regimens, older adults continue to have poorer outcomes [10–13]. The 5-year overall survival (OS) in the past decade ranges from ~93% in children, ~73% in younger adults, ~28% to 50% in older adults (aged 60–69 years), and 10% to 20% in those older than 70 years [4, 13–15].
Attempts to improve outcomes for patients with newly diagnosed BCP-ALL have historically focused on intensifying cytotoxic agents and implementing a risk-stratified allogeneic hematopoietic stem cell transplantation (HSCT) in first remission for high-risk patients [1]. Today, chemotherapy agents within conventional age-adjusted regimens are, for the most part, being administered at their maximum tolerated doses and, thus, are limited in terms of further efficacy improvement [1, 3]. The focus to improve efficacy and safety has benefited from a better understanding of disease biology and the use of novel targeted agents across all treatment settings.
Blinatumomab (BLINCYTO®; Amgen Inc., Thousand Oaks, CA, USA), a bispecific T-cell–engaging immunotherapy, has established efficacy in adult and pediatric patients with Ph-negative relapsed/refractory BCP-ALL and MRD-positive BCP-ALL [16, 17]. In the frontline setting, the positive results reported by the E1910 phase 3 study have led to the US approval of blinatumomab in the consolidation phase as part of a multiagent chemotherapy backbone [17–19]. Common chemotherapy backbones used globally to treat Ph-negative BCP-ALL share substantial similarities, and incorporation of blinatumomab into these backbones will most probably have effects similar to those observed in the E1910 trial. In addition, phase 2 trials have evaluated the addition of blinatumomab as a bridge to HSCT or the replacement of chemotherapy and showed promising results for both efficacy and safety [17–27].
The goal of this narrative review is to descriptively summarize chemotherapeutic regimens commonly used for patients with newly diagnosed Ph-negative BCP-ALL to show that they are largely similar, both in terms of agents used and outcomes, and to underline the clinical benefits achieved when blinatumomab is incorporated into these regimens.
Methodology
Clinical trial data were obtained via a search of PubMed, ClinicalTrials.gov, and specialty national and international congresses. The PubMed search was conducted on October 4, 2024, using the search terms (acute lymphoblastic leukemia) AND (B-cell) AND (treatment) with articles also filtered by “clinical trial” or “review.” Overall, 6226 articles were identified. Titles and abstracts were manually scanned and were deemed relevant if describing Ph-negative BCP-ALL studies in the frontline setting. Of 421 clinical trial articles identified, 8 pertained to the frontline setting, but 3 were specific for MRD and were thus excluded. Of the 5 published articles, 3 were specific for blinatumomab. In addition, studies were identified from ClinicalTrials.gov, congresses, reviews, and documented files, including 12 congress abstracts specific for blinatumomab. Of the 1022 identified review articles, 6 were relevant and served as additional sources to support clinical data, including 2 articles specific for blinatumomab [1, 7, 10, 28–30].
Results
Commonalities across the Ph-negative BCP-ALL chemotherapy backbone landscape
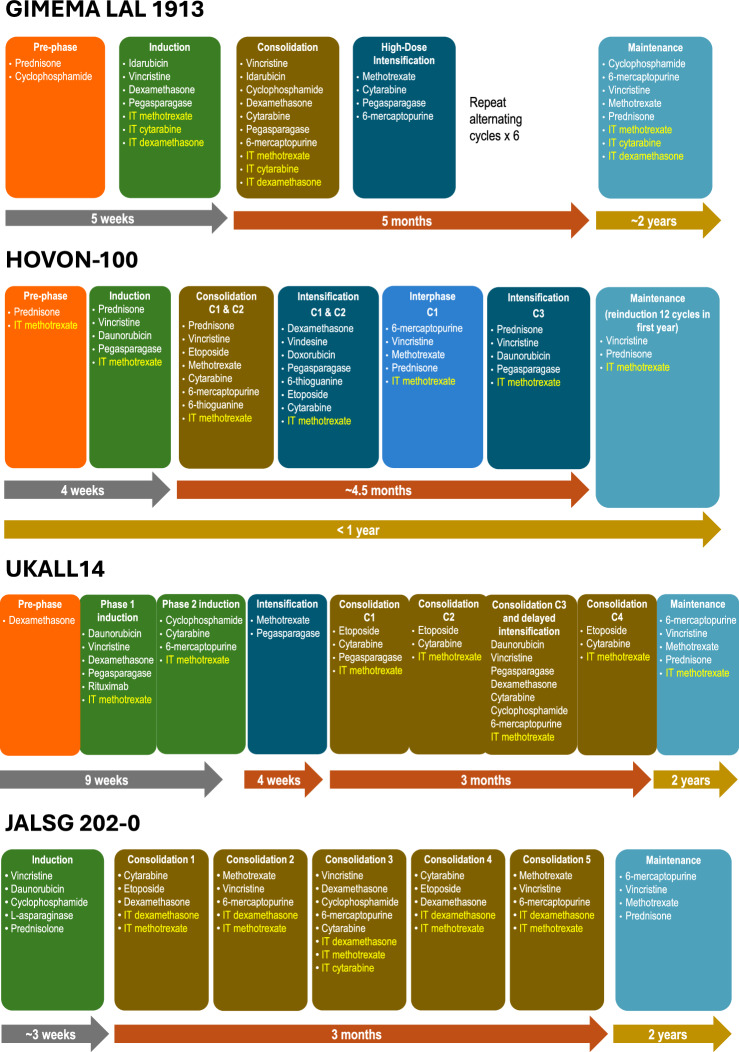
Many different chemotherapy regimens are commonly used to treat newly diagnosed Ph-negative BCP-ALL worldwide, varying from country to country (Table 1; Fig. 1) [4, 6, 31–36]. While selection of individual agents, dosing schedules, risk stratification, indications for HSCT, and therapy durations differ between treatment regimens, total treatment typically lasts approximately 2 to 3 years (Table 1; Table 2) [4, 37]. Treatment regimens for BCP-ALL are typically divided into three phases: induction, consolidation, and maintenance. In addition, CNS prophylaxis is also a critical component of the treatment regimens. In some treatment regimens, these phases are further subdivided into additional blocks of therapy, such as intensification or reinduction.
Table 1.
Individual Drugs Used in Common SOC Chemotherapy in Newly Diagnosed Adult Patients With Ph-Negative BCP-ALL.
| Chemotherapeutic Agent | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Common Regimen | Country | Vinca Alkaloid | Anthracycline | Corticosteroid | Asparaginase | Alkylating Agent | Antimetabolite | Topoisomerase Inhibitor | Rituximab* |
| CALGB 10403† [69] | US | Vincristine |
Daunorubicin Doxorubicin |
Prednisone Dexamethasone |
Pegaspargase | Cyclophosphamide |
Methotrexate (systemic and IT) Cytarabine (systemic and IT) 6-mercaptopurine 6-thioguanine |
||
| DFCI ALL† [70] | US | Vincristine | Doxorubicin |
Prednisone Dexamethasone Hydrocortisone (IT) |
Asparaginase |
Methotrexate (systemic and IT) Cytarabine (IT) 6-mercaptopurine |
|||
| PETHEMA ALL-96† [71] | Spain | Vincristine | Daunorubicin |
Prednisone Dexamethasone Hydrocortisone (IT) |
Asparaginase | Cyclophosphamide |
Methotrexate (systemic and IT) Cytarabine (systemic and IT) 6-mercaptopurine |
Teniposide | |
| Hyper-CVAD [72]† | US/Australia | Vincristine | Doxorubicin |
Prednisone Dexamethasone Methylprednisolone |
Cyclophosphamide |
Methotrexate (systemic and IT) Cytarabine (systemic and IT) 6-mercaptopurine |
|||
| USC/MSKCC ALL† [73] | US | Vincristine | Daunorubicin |
Prednisone Dexamethasone |
Pegaspargase | Cyclophosphamide |
Methotrexate (systemic and IT) Cytarabine 6-mercaptopurine 6-thioguanine |
Teniposide | |
| Linker 4-drug regimen [74] | US | Vincristine | Daunorubicin | Prednisone | Asparaginase |
Methotrexate (systemic and IT) Cytarabine 6-mercaptopurine |
Etoposide | ||
| CALGB 8811 Larson [75] | US | Vincristine |
Daunorubicin Doxorubicin |
Prednisone Dexamethasone |
L-asparaginase | Cyclophosphamide |
Methotrexate (systemic and IT) Cytarabine 6-mercaptopurine 6-thioguanine |
||
| MRC UKALLXII/ ECOG2993 [76] | US/UK | Vincristine | Daunorubicin |
Prednisone Dexamethasone |
L-asparaginase | Cyclophosphamide |
Methotrexate (systemic and IT) Cytarabine 6-mercaptopurine 6-thioguanine |
Etoposide | |
| Modified DFCI-01 [40] | Canada | Vincristine | Doxorubicin | Dexamethasone | Asparaginase |
Methotrexate (systemic and IT) Cytarabine 6-mercaptopurine |
|||
| GRAALL-2005† [77] | France | Vincristine | Daunorubicin |
Prednisone Dexamethasone Methylprednisolone (IT) |
L-asparaginase | Cyclophosphamide |
Methotrexate (systemic and IT) Cytarabine (systemic and IT) 6-mercaptopurine |
Etoposide | Rituximab |
| GRAALL-2014 [77, 78] | France | Vincristine | Daunorubicin |
Prednisone Dexamethasone Methylprednisolone (IT) |
L-asparaginase | Cyclophosphamide |
Methotrexate (systemic and IT) Cytarabine (systemic and IT) 6-mercaptopurine |
Etoposide | |
| GMALL [12, 79] | Germany | Vincristine | Idarubicin | Dexamethasone | Pegaspargase | Cyclophosphamide |
Methotrexate (systemic and IT) Cytarabine 6-mercaptopurine |
Rituximab | |
| GIMEMA LAL 1913 [60] | Italy | Vincristine | Idarubicin |
Prednisone Dexamethasone (systemic and IT) |
Pegaspargase | Cyclophosphamide |
Methotrexate (systemic and IT) Cytarabine (systemic and IT) 6-mercaptopurine Dexamethasone (IT) |
||
| HOVON-100 [61] | Netherlands, Belgium, France |
Vincristine Vindesine |
Daunorubicin Doxorubicin |
Prednisone Dexamethasone |
Pegaspargase |
Methotrexate (systemic and IT) Cytarabine 6-mercaptopurine 6-thioguanine |
Etoposide | ||
| UKALL14 [80] | UK | Vincristine | Daunorubicin |
Prednisolone Dexamethasone |
Pegaspargase | Cyclophosphamide |
Methotrexate (systemic and IT) Cytarabine 6-mercaptopurine |
Etoposide | Rituximab |
| JALSG 202-0 [81] | Japan | Vincristine |
Daunorubicin Doxorubicin |
Prednisolone Dexamethasone (systemic and IT) |
L-asparaginase | Cyclophosphamide |
Methotrexate (systemic and IT) Cytarabine (systemic and IT) 6-mercaptopurine |
Etoposide | |
Doses of individual agents used in these chemotherapy regimens are dependent on patient age, risk group, and other characteristics; therefore, individual adaptations to dose are common.
*With rituximab for CD20-positive disease.
†For AYA population and for adults < 65 years without substantial comorbidities.
ALL acute lymphoblastic leukemia, AYA adolescents and young adults, BCP-ALL B-cell precursor acute lymphoblastic leukemia, DFCI Dana-Farber Cancer Institute, GIMEMA Gruppo Italiano Malattie Ematologico dell’Adulto, GMALL German Multicenter Study Group in Adult ALL, GRAALL Group for Research on Adult Acute Lymphoblastic Leukemia, HOVON Haemato Oncology Foundation for Adults in the Netherlands, IT intrathecal, PH Philadelphia chromosome, SOC standard of care.
Fig. 1. Comparing Phases of Therapy in Ph-Negative BCP-ALL Treatment Regimens: CALGB 10403 (US), hyper-CVAD (US, Australia), UKALLXII/ECOG E2993 (US), GRAALL-2014 (France), PETHEMA ALL-96 (Spain), GIMEMA LAL 1913 (Italy), HOVON-100 (Netherlands, Belgium), UKALL14 (UK), JALSG 202-0 (Japan).
Gray arrow represents induction phase, orange arrow represents consolidation phase, and gold arrow represents maintenance phase. Yellow text represents CNS prophylaxis. ALL acute lymphoblastic leukemia, BCP-ALL B-cell precursor acute lymphoblastic leukemia, CNS central nervous system, HD high dose, IT intrathecal, Ph Philadelphia chromosome.
Table 2.
Summary of Efficacy Outcomes With Common SOC Chemotherapy Regimens in Newly Diagnosed Adult Patients With Ph-Negative BCP-ALL*.
| Common Regimen | N | Median Age (Range), y | Outcome Summary |
|---|---|---|---|
| PETHEMA ALL-96 [71] | 81 | 20 (15–30) |
• Median follow-up: 4.2 y • 6-year EFS: 61% (95% CI: 51–72) • 6-year OS: 69% (95% CI: 59–81) |
| MRC UKALLXII/ECOG2993 [82] | 1229 | 30 (14–65) |
• Median follow-up: 8.9 y • 5-year OS: 42% (95%CI: 39-44) |
| DFCI ALL [70] | 92 | 28 (18–50) |
• Median follow-up: 4.5 y • 4-year DFS: 66% (95% CI: 50–78) • 4-year OS: 68% (95% CI: 53–79) |
| Linker 4-drug regimen [74] | 84 | 27 (16–59) |
• Median follow-up: 5.6 y • 5-year EFS: 52% for 78 patients who achieved remission • 5-year DFS: 54% for patients who achieved remission • 5-year OS: 47% (overall population) |
| CALGB 10403 [69] | 295 | 24 (17.0–39.0) |
• Median follow-up: 64.2 mo (0.4–110) • 3-year EFS: 59% (95% CI: 54–65) • 3-year DFS: 81.7% (95% CI: 58.4–NE) • 3-year OS: 73% (68–78) |
| USC/MSKCC ALL [73] | 51 | 32 (18–57) |
• Median follow-up: NR • 7-year DFS: 58% • 7-year OS: 58% |
| CALGB 8811 Larson [75] | 197 | 32 (16–80) |
• Median follow-up: 43 mo (24–64) • Estimated median survival: 36 mo |
| GRAALL-2014 [22] | 104 | 36 (18–59) |
• Median follow-up: 4.3 y • 2.5-year DFS (Ph-negative BCP-ALL patients): 54% (95% CI: 43–63) • 2.5-year OS (Ph-negative BCP-ALL patients): 76% (95% CI: 66–83) |
| Hyper-CVAD [53] | 69† | 41 (32–50) |
• Median follow-up: 44 mo (26–53) • 4-year EFS: 59% (95%CI: 48-73) • 4-year OS: 68% (95% CI: 58–81) |
| GIMEMA LAL 1913 [60] | 139‡ | 41 (18–65) |
• Median follow-up: 38.7 mo (2.2–64.2) • 3-year EFS: 53.4% (95% CI: 45.5–62.8) • 3-year DFS: 61.4% (95% CI: 52.9–71.1) • 3-year OS: 64.9% (95% CI: 57–73.9) |
| HOVON-100[61] | 79§ | 42 (18–70) |
• Median follow-up: not reported • 5-year EFS (Ph-negative BCP-ALL patients): 48% (95% CI: 36–58) • 5-year DFS (Ph-negative BCP-ALL patients): 52% (95%CI: 40–63) • 5-year OS (Ph-negative BCP-ALL patients): 61% (95% CI: 49–71) |
| JALSG 202-0 [81] | 229|| | 43 |
• Median follow-up: 7.5–7.8 y • 5-year DFS (Hd-MTX): 58% (95% CI: 45–68); 56% (43–68) for BCP-ALL subgroup • 5-year DFS (Id-MTX): 32% (95% CI: 22–43); 35% (23–46) for BCP-ALL subgroup • 5-year OS (Hd-MTX): 64% (95% CI: 51–74) • 5-year OS (Id-MTX): 48% (95% CI: 37–59) |
| UKALL14 [80] | 288# | 45 (22–65) |
• Median follow-up: 53.7 mo (40.3–70.4) • Median EFS: 23.2 mo (95% CI: 19.2–34.0) • 3-year EFS: 43.7% (95% CI: 37.8–49.5); 43.1% (95% CI: 35.9–50.0) for Ph-negative BCP-ALL • Median OS: 40.1 mo (95% CI: 33.2–75.0); 48.8% (95% CI: 41.5–55.7) for Ph-negative BCP-ALL |
| ECOG1910 Control** [17, 19] | 112 | 50 (30–70) |
• Median follow-up: 3.6 y • 3-year RFS: 64.0% 3-year OS: 68.0% |
| Modified DFCI-01 [40] | 51†† | 65 (60–79) |
• Median follow-up: 21.6 mo (0.7–95) • 5-year DFS (Ph-negative–negative pts): 57.4% (95% CI: 32.8–75.8) • 5-year OS (Ph–negative pts): 40.5% (95% CI: 20.0–60.2) |
*Most of these studies include a mixed population of patients with ALL; however, most patients had BCP-ALL. Findings for the mixed population reported unless otherwise noted.
†Based on 67 patients with BCP-ALL and 2 patients with B-cell lymphoblastic lymphoma.
‡Includes 1 patient with lymphoblastic lymphoma.
§Ph-negative BCP-ALL population; HOVON-70 approach was used in patients aged ≤ 40 years.
||Includes 196 with BCP-ALL.
#Includes 202 with Ph-negative BCP-ALL.
**Time from randomization with patients already receiving induction therapy and MRD-negative.
††Includes 48 patients with BCP-ALL, of which 32 had Ph-negative BCP-ALL.
ALL acute lymphoblastic leukemia, BCP-ALL B-cell precursor acute lymphoblastic leukemia, DFCI Dana-Farber Cancer Institute, DFS disease-free survival, EFS event-free survival; GIMEMA Gruppo Italiano Malattie Ematologico dell’Adulto, GRAALL Group for Research on Adult Acute Lymphoblastic Leukemia, Hd high dose, HOVON Haemato Oncology Foundation for Adults in the Netherlands, Id intermediate dose, IQR interquartile range, MTX methotrexate, NE not estimable, NR not reported, OS overall survival, Ph Philadelphia chromosome, RFS relapse-free survival.
In general, induction therapy typically includes a combination of vincristine, anthracyclines, and corticosteroids, with or without asparaginase, and with or without cyclophosphamide [4]. During this phase, patients are generally admitted to the hospital for their initial workup and treatment and then may be discharged with close outpatient follow-up until remission is achieved, pending any complications necessitating inpatient care. The consolidation phase, commonly containing a combination of vincristine, anthracyclines, corticosteroids, asparaginase, cyclophosphamide, and various antimetabolite agents, varies in length and intensity across different protocols and is generally administered in the outpatient setting, although some intensive regimens require inpatient care [4, 38, 39]. Some regimens include an intensification phase before entering maintenance, which essentially is an abbreviated induction regimen. This is followed by a maintenance phase, consisting of daily antimetabolite therapy, usually 6-mercaptopurine (6-MP), weekly methotrexate, and in some studies monthly pulses of vincristine and corticosteroids known as the POMP regimen. Maintenance generally lasts around 2 years and is given in the outpatient setting [4]. Another crucial component of BCP-ALL therapy is CNS prophylaxis with intrathecal chemotherapy, CNS-directed systemic chemotherapy (high-dose methotrexate, high-dose cytarabine, asparaginase, dexamethasone) delivered throughout therapy, with or without cranial irradiation, and partly risk-adapted based on CNS status at diagnosis [4, 39].
In Table 1, many commonalities of conventional BCP-ALL treatment regimens are shown; most regimens include vincristine, daunorubicin or doxorubicin, dexamethasone (except for the Linker 4-drug regimen), and PEGylated asparaginase or historically native L-asparaginase (except the hyper-CVAD regimen). Cyclophosphamide is administered as the alkylating agent except in the Dana-Farber Cancer Institute (DFCI) ALL, modified DFCI-01 regimens, and pediatric regimens [4, 40]. Methotrexate and cytarabine are included in all chemotherapy backbones over a wide range of cumulative dosing levels, both systemically and intrathecally, with 6-MP and 6-thioguanine (6-TG) added to some regimens. Topoisomerase inhibitors, such as teniposide or etoposide, are also included in just over half of the treatment regimens.
The cytotoxic effects of chemotherapy inherently lead to acute and potentially long-term treatment-related adverse events (AEs). The incidence of selected AEs and treatment-related mortality for these regimens are shown in Table 3. High doses of myelosuppressive agents (e.g., anthracyclines, cytarabine, 6-MP, methotrexate, cyclophosphamide) and immunosuppressive corticosteroids within regimens can lead to complications such as neutropenic fever and infection, which require hospitalization and may be life-threatening [41]. Asparaginase-associated toxicities include hepatic dysfunction, venous sinus thrombosis, pancreatitis, and hypersensitivity/silent activation, while treatment with anthracyclines can induce cardiac toxicity [41–43]. Vinca alkaloids can induce short-term and even permanent neurotoxicity [44]. 6-TG is associated with an increased incidence of hepatic sinusoidal obstruction syndrome (also known as veno-occlusive disease) in some patients [45]. Other toxicities associated with ALL chemotherapy reported in clinical practice include myelopathy symptoms and encephalopathy associated with methotrexate, osteopenia/osteoporosis and metabolic syndrome with corticosteroids, acute complications with radiation therapy, and a broad spectrum of late effects [44, 46–49].
Table 3.
Incidence of Select Grade 3–5 AEs and Treatment-Related Mortality for Common SOC Chemotherapy Backbone Regimens.
| Common Regimen; Age Range | Treatment-Related AEs,* n (%) | ||||||
|---|---|---|---|---|---|---|---|
| Hepatic Dysfunction† | Pancreatic Dysfunction | Thrombosis‡ | Peripheral Nervous System Toxicity | Cardiovascular Toxicity | Most Commonly Reported Grade 3–4 AEs > 10% | Treatment-Related Deaths | |
| CALGB 10403 (n = 295); 17–39 y [69] |
Grade 3–4: 3 (1) Grade 5: 2 (1) |
Grade 3–4: 14 (5) Grade 5: 0 |
Grade 3–4: 33 (11) Grade 5: 0 |
CNS hemorrhage Grade 3–4: 4 (1) Grade 5: 0 |
Ventricular arrhythmia Grade 3–4: 1 (1) Grade 5: 1 ( < 0) |
ALT: 158 (54) Febrile neutropenia: 152 (52) Hyperglycemia: 103 (35) AST: 98 (33) Hyperbilirubinemia: 77 (26) Infection: 74 (26) Fatigue: 40 (14) Sensory neuropathy: 45 (15) Thrombosis: 33 (11) Hypertriglyceridemia: 33 (11) |
Total: 8 (3) During induction; 6 (2) |
| DFCI ALL (n = 92); 18–50 y [70] |
Grade 3–4: 56 (61) Grade 5: 1 (1) |
Grade 3–4: 9 (10) Grade 5: 1 (1) |
Grade 3–4:16 (17) Grade 5: 0 |
CNS hemorrhage Grade 3–4: 0 Grade 5: 1 (1) |
NR |
Neutrophils: 86 (93) Platelets: 75 (82) Febrile neutropenia: 30 (33) Infection (with grade 3–4 neutropenia): 49 (53) Hyperglycemia: 41 (45) Stomatitis: 10 (11) |
Total: NR During induction: 1 (1) |
| PETHEMA ALL-96 (n = 81); 15–30 y [71] |
Grade 3–4 during induction: 6 (8) Grade 3–4 during consolidation 1: 1 (1) |
NR | NR | NR | NR |
Severe infection During induction: 8 (10) During consolidation 1: 1 (1) Neutropenia During induction: 55 (68) During consolidation 1: 42 (52) Thrombocytopenia During induction: 36 (44) During consolidation 1: 13 (16) Febrile neutropenia During induction: 43 (54) During consolidation 1: 27 (39) |
Total: 2 (3) During induction: 1 (1) During consolidation: 1 (1) |
| Hyper-CVAD (n = 204); 16–79 y [83] |
Hepatic toxicity Any grade: 4 (2) |
NR | NR | NR | NR |
AE requiring hospitalization: 86 (42) Fever (of unknown origin): 47 (23) Sepsis: 22 (11) |
Total: 23 (11) During induction: 15 (7) |
| USC/MSKCC ALL (n = 51); 18–57 y [73] | NR | Grade 3–4: 7 (14) |
DVT Grade 3–4: 8 (16) |
NR | NR |
Transaminitis: 32 (63) Hyperbilirubinemia: 16 (31) Hyperglycemia: 17 (33) Hypertriglyceridemia: 9 (18) DVT: 8 (16) |
Total: 3 (6) In complete remission from neutropenic sepsis: 3 (6) |
| Linker 4-drug regimen (n = 29); 20–54 y [84] | NR | Grade 3–5: 1 (3) | Grade 3–5: 6 (19) | NR | NR |
Transaminitis: 17 (58) Hyperbilirubinemia: 12 (42) Hyperglycemia: 8 (29) Hypertriglyceridemia: 7 (26) Elevated GGT/alkaline phosphatase: 6 (19) Thrombosis: 6 (19) Hypoalbuminemia: 3 (10) |
Total: 8 (28) During induction: 1 (3) |
| CALGB 8811 Larson (n = 197); 16–80 y [75] | Severe AE§: 49 (25) | NR | NR | Severe AE‡: 3 (7) (5) | Severe AE‡: 5 (1) |
Leukopenia§: 193 (94) Thrombocytopenia‡: 185 (94) Anemia§: 128 (65) Infection§: 106 (54) |
Total: 28 (14) During induction: 17 (9) |
| MRC UKALLXII/ECOG2993 (n = 1153); 15–59 y [76] | NR | NR | Death due to thromboses: 2 ( < 1) | Death due to cerebral hemorrhage: 2 ( < 1) | NR | NR |
Total: not stated During induction: 54 (5) |
| ECOG1910 Control (n = 112); 30–70 y [17, 19] | NR | NR | NR | NR | NR |
Neutropenia: 97 (87) Thrombocytopenia: 77 (69) Leukopenia: 60 (54) Anemia: 41 (37) Febrile neutropenia : 26 (23) Lymphopenia: 26 (23) |
Total: 40 |
| Modified DFCI-01 (n = 51); 60–79 y [40] | Liver toxicity: 5 (10) | NR | NR | NR | Heart failure: 1 (2) |
Bacteremia: 21 (42) Febrile neutropenia: 12 (20) Hyperglycemia: 7 (14) Septic shock with ICU admission: 6 (12) |
Total: 26 (51) During induction: 10 (20) |
| GRAALL-2014 (n = 104); 18-59 y [22] | NR | NR | NR | NR | NR | NR | NR |
| GIMEMA LAL 1913 (n = 203); 18–65 y [60] |
Hepatobiliary disorder Grade 3–4: 23 (11) Grade 5: 1 (1) |
Grade 3–4: 2 (1) Grade 5: 0 |
Grade 3–4: 3 (2) Grade 5: 0 |
NR | NR | Hepatobiliary disorder: 23 (11) |
Cumulative treatment-related mortality 3-year rate: 15.6 (95%CI: 8.9–22.3) During induction: 2 (1) |
| HOVON-100 (n = 166); 18–70 y [61] | NR | NR | Grade ≥2: 50 (30) | NR | NR |
Infection: 74 (45) Gastrointestinal: 54 (33) Neurologic event: 22 (13) |
Fatal SAE: 13 (8) |
| UKALL14 (n = 286); 22–65 y [80] |
Hepatobiliary disorder Grade 3–4: 2 (1) Grade 5: 0 |
Pancreatitis Grade 3–4: 3 (1) Grade 5: 0 |
Grade 3–4: 8 (3) Grade 5: 0 |
Intracranial hemorrhage Grade 3–4: 0 Grade 5: 1( < 1) |
Grade 3–4: 4 (1) Grade 5: 0 |
Anemia: 138 (48) Neutrophil count decreased: 222 (78) White blood cell decreased: 169 (60) Febrile neutropenia: 41 (14) |
Total: 14 (5) 3-year non-relapse mortality: 23.7% (95% CI: 19.0–29.4) |
| JALSG 202-0 [81] (n = 343, induction; 229, combined treatment arms); NR (median 43 y) [81] | NR | Death due to pancreatitis: 1 ( < 1) | NR | NR | NR |
Grade 4 neutropenia: 53 (23) Grade 3–4 ALT elevation: 22 (10) |
Total: 19 (6) During induction: 17 (5) |
*AEs reported across all treatment phases.
†Hepatic dysfunction defined differently among studies. Use caution when interpreting these findings.
‡Includes thrombus and embolism.
§During induction phase.
AE adverse event, ALL acute lymphoblastic leukemia, ALT alanine aminotransferase, AST aspartate aminotransferase, CNS central nervous system, DFCI Dana-Farber Cancer Institute, DVT deep vein thrombosis, GGT gamma-glutamyl transferase, GIMEMA Gruppo Italiano Malattie Ematologico dell’Adulto, GMALL German multicenter study group in adult ALL, GRAALL Group for Research on Adult Acute Lymphoblastic Leukemia, HOVON Haemato Oncology Foundation for Adults in the Netherlands, ICU intensive care unit, NR not reported, SAE serious adverse event, SOC standard of care.
Chemotherapy backbone adaptation strategies
Selecting appropriate treatment intensity for a patient requires careful evaluation of comorbid conditions, performance status, and abilities to attend to activities of daily living [4]. The treatment intensity of standard multiagent chemotherapy regimens is modified by risk factor and across age-based subpopulations to maximize efficacy while maintaining an acceptable toxicity profile. Pediatric regimens are considered the most complex and intense. Common regimens used in young adults (aged 18–40 years) are either derived directly from the pediatric regimen or modified to increase tolerability (i.e., pediatric-inspired regimens (Table 1; Fig. 1) [1, 4, 38, 39, 50, 51]. For older patients (aged 55 or 60 and older), chemotherapy regimens are further modified with dose reduction/omission of anthracycline and other myelosuppressive agents (e.g., mini hyper-CVD or the age-adapted German Multicenter Study Group for Adult ALL [GMALL] backbone) [4, 25]. Consistently, similar age-based adaptations have been made to all other treatment regimens as well. The integration of targeted agents to existing standard-of-care (SOC) chemotherapy treatment backbones has also been used to improve efficacy in specific Ph-negative patient populations. Examples include the inclusion of inotuzumab ozogamicin for CD22-positive disease, rituximab or ofatumumab for patients with CD20-positive disease, or the use of blinatumomab in CD19-positive disease [4, 52–54].
Blinatumomab integration into SOC chemotherapy backbones
Studies have evaluated the role of adding blinatumomab to standard chemotherapeutic backbones for patients with newly diagnosed Ph-negative BCP-ALL (Table 4) [17–27, 55–59]. In a phase 2 study in patients aged 18 to 65 years, adding blinatumomab (2 cycles) after the first early consolidation cycle 3 and after late consolidation cycle 6 of the Gruppo Italiano Malattie Ematologico dell’Adulto (GIMEMA) LAL 1913 backbone resulted in 3-year disease-free survival (DFS) and OS of 66% and 71%, respectively, after a median follow-up of 37.5 months [23]. Whereas the GIMEMA LAL 1913 regimen alone historically reports a 3-year DFS and OS of 61% and 65%, respectively, after a median follow-up of 38.7 months [60].
Table 4.
Outcomes From Clinical Trials Evaluating Blinatumomab in Combination With Chemotherapy for Treatment of Newly Diagnosed Ph-Negative BCP-ALL.
| Regimen (NCT) | Treatment Schema | Study Phase | N | Age Range | Outcome Summary |
|---|---|---|---|---|---|
| Blinatumomab as a replacement for select chemotherapy cycles | |||||
| ALLG ALL08: Low-intensity hyper-CVAD + blinatumomab (ACTRN12617000084381) [24] | Four cycles of low-intensity chemotherapy, alternating with 4 cycles of blinatumomab consolidation and then 2 y of POMP maintenance | 2 | 30 | 40–67 y |
• Median follow-up: NR • Estimated 2-y median EFS: 61% (median EFS 36 mo) • Estimated 2-y median OS: 79% (median OS NR) • Safety: grade 3 CRS (n = 1); no treatment-related deaths |
| SWOG 1318; Blinatumomab induction and prednisone + vincristine + MTX + 6-MRP (NCT02143414) [56] | Two cycles of blinatumomab induction and 3 cycles of blinatumomab consolidation followed by 18 months of POMP | 2 | 29 | 66–84 y |
• MRD: 13 of 19 responders had available MRD data, with 12/13 (92%) MRD-negativity • Median follow-up: 3.14 y • Estimated 3-y DFS: 37% (95% CI: 17–57) • Estimated 3-y OS: 37% (lower 1-sided 90% CI: 26) • Binary 3-y OS: 34% (95% CI: 18–54) • Safety: most common grade 3-4 AEs, hyperglycemia (14%), dyspnea (10%), febrile neutropenia (10%), hypertension (10%), and lung infection (7%) |
| Alliance A041703, blinatumomab + inotuzumab ozogamicin (NCT03739814) [57, 58] | Two cycles of inotuzumab ozogamicin induction followed by blinatumomab consolidation for 2 cycles and up to another 3 cycles | 2 | 33 | 60–84 y |
• MRD: NR • Median follow-up: 22 mo • 1-y EFS: 75% (95% CI: 61–92) • 1-y OS: 84% (95% CI: 72–98) • 2 deaths in remission (n = 1 after HSCT), 1 death without remission from respiratory failure with sinusoidal occlusion syndrome of liver (n = 1) • Safety: Common grade 3-5 AEs, neutropenia (88%), thrombocytopenia (73%), anemia (42%), leukopenia (39%), lymphopenia (27%), febrile neutropenia (21%), and encephalopathy (12%) |
| Mini-hyper-CVD inotuzumab ozogamicin ± blinatumomab (NCT01371630) [55] | Four (4) cycles of mini-hyper-CVAD followed by 4 cycles of blinatumomab consolidation then maintenance with alternating blocks of 3 cycles of POMP and one cycle of blinatumomab for 12 cycles. A total of 4 cycles of inotuzumab ozogamicin were given during induction | 2 | 80 | 63–72 y |
• MRD: 94% negativity • Median follow-up: 92.8 mo • Median PFS (with blinatumomab): 56.4 mo (95% CI 11.3–69.7) • Median OS (with blinatumomab): 56.4 mo (95% CI 16.3–70.0) • 2-y PFS (with blinatumomab): 60.9% (95% CI: 24.0–76.5) • 5-y PFS (with blinatumomab): 41.8% (95% CI: 28.5–65.0) • 2-y OS (with blinatumomab): 59.6% (95% CI: 38.6–75.5) • 5-y OS (with blinatumomab): 40.9% (95% CI: 17.0–63.9) • Safety: most common grade 3–5 AEs, thrombocytopenia (78%), hyperglycemia (36%), febrile neutropenia (33%); no treatment-related deaths |
| GMALL BOLD (NCT 03480438) [25] | One cycle dose-reduced chemotherapy + 1 cycle blinatumomab as induction, followed by 3 cycles of blinatumomab alternating with age-adapted chemotherapy as consolidation | 2 | 47 | 56–76 y |
• MRD: NR • Median follow-up: 2.1 y • 3-y EFS: 60% • 1-y OS: 80% • 3-y OS: 67% • Safety: no unexpected events |
| Addition of blinatumomab to chemotherapy regimens | |||||
| GIMEMA LAL 1913 + sequential blinatumomab (NCT03367299) [20, 23] | Six cycles of chemotherapy with 2 cycles of sequential blinatumomab consolidation (cycles 2 and 6) | 2 | 149 | 18–65 y |
• MRD: 93% negativity rate • Median follow-up: 37.5 mo • 3-y DFS: 66% • 3-y OS: 71% • Safety: not stated |
| HOVON-146: Blinatumomab added to prephase and consolidation chemotherapy (based on HOVON-70)* (NCT03541083) [27, 59] | Chemotherapy with 1 cycle of prephase blinatumomab (for 2 weeks), followed by 2 cycles of blinatumomab consolidation (each 4 wk) | 2 | 71 (overall population) | 18–70 y |
• MRD: 91% negativity rate (n = 56) • Median follow-up: 43 mo • 4-y EFS: 49% (Ph-negative BCP-ALL patients) • 4-y OS: 70% (Ph-negative BCP-ALL patients) • Safety: grade 3 CRS (21%) |
| GRAALL-2014 + blinatumomab (NCT03709719) [21, 22] | Chemotherapy with 5 cycles of blinatumomab consolidation and maintenance | 2 | 94 | 18–59 y |
• MRD: 72% negativity rate • Median follow-up: 2.3 y • 2.5-y DFS: 72% (95% CI: 60–80) • 2.5-y OS: 79% (95% CI: 67–88) • Safety: not stated |
| Hyper-CVAD + blinatumomab (NCT02877303) [26] | Four cycles of chemotherapy, followed by 4 cycles of blinatumomab consolidation, then maintenance with alternating blocks of 3 cycles of POMP and one cycle of blinatumomab for 15 cycles | 2 | 38 |
18–39 y (n = 22) ≥40 y (n = 16) |
• Median follow-up: 37 mo • 3-y RFS: 73% (95% CI: 56–85) • 3-y OS: 81% (95% CI: 65–91) vs 4-y OS hyper-CVAD • Safety: most common grade 3–5 AEs, infection or febrile neutropenia (82%); no treatment-related deaths |
| E1910 Study (NCT02003222) [17–19] | Two cycles of blinatumomab, followed by 3 cycles of consolidation chemotherapy then cycle 3 of blinatumomab followed by cycle 4 of chemotherapy and then a fourth cycle of blinatumomab | 3 | 224 (blinatumo-mab plus chemo-therapy, n = 112; chemo-therapy alone, n = 112) | 30–70 y |
• MRD: only MRD-negative patients included • Median follow-up: 3.6 y • 3-y OS: 85% • 3-y RFS: 80% • Safety: higher incidence of grade ≥3 neuropsychiatric AEs with blinatumomab 23% versus chemotherapy alone (5%; P < 0.001) |
*The HOVON-70 regimen (developed for patients < 40 years old) was adapted for ages ≥ 40 years with reduced doses of anthracyclines, methotrexate, etoposide, and pegaspargase.
6-MP 6-mercaptopurine, AE adverse event, BCP-ALL B-cell precursor acute lymphoblastic leukemia, CRS cytokine release syndrome, DFS disease-free survival, EFS event-free survival, GIMEMA Gruppo Italiano Malattie Ematologico dell’Adulto, GRAALL Group for Research on Adult Acute Lymphoblastic Leukemia, HOVON Haemato Oncology Foundation for Adults in the Netherlands, MRD measurable residual disease, MTX Methotrexate, NR Not reported, OS overall survival, Ph Philadelphia chromosome, RFS relapse-free survival, SAE serious adverse event.
In the Haemato Oncology Foundation for Adults in the Netherlands (HOVON)–146 study, the HOVON group evaluated the addition of blinatumomab to both prephase (1 cycle, for 2 weeks only) and consolidation (2 cycles) within HOVON-70–based pediatric-inspired chemotherapy regimen in patients aged 18 to 70 years with Ph-positive and Ph-negative BCP-ALL [27, 59]. The 4-year event-free survival (EFS) and OS for patients with Ph-negative BCP-ALL were 49% and 70%, respectively, after a median follow-up of 43 months [59]. Findings from the historical phase 3 HOVON-100 backbone (for patients aged < 40 years, the HOVON-70 backbone, as mentioned above, was used), showed 5-year EFS and OS of 48% and 61%, respectively, for patients with Ph-negative BCP-ALL; median follow-up was 70 months for the overall population [61]. The GMALL Trial 08/2013 for newly diagnosed patients aged 18 to 55 years used upfront blinatumomab in all patients with MRD persistence > 0.01% after consolidation I followed by stem cell transplantation. The 3-year overall survival in this subgroup was 77% [62].
A phase 2 trial in high-risk adults aged 18 to 59 years (with complete remission after induction and first consolidation) evaluated frontline blinatumomab added to both consolidation (5 cycles) and maintenance therapy of the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL)–2014 regimen [22]. After a median follow-up of 2.3 years, 2.5-year DFS and OS were 72% and 79%, respectively. In contrast, after a median follow-up of 4.3 years with GRAALL-2014 alone, 2.5-year DFS and OS were 54% and 76%, respectively [22].
Results from a phase 2 single-arm study in patients aged ≥14 years (median [range], 37 [18–45] years) using the full-intensity hyper-CVAD backbone with 4 cycles of blinatumomab in the consolidation phase and 3 cycles in the maintenance phase (total of 7 blinatumomab cycles) reported a 3-year OS of 81%, with a median follow-up of 37 months [26]. In comparison, results from the historical hyper-CVAD regimen showed a 4-year OS of 68% [53].
Finally, the benefit of adding blinatumomab to a standard chemotherapy backbone to improve survival outcomes was conclusively demonstrated by the Eastern Cooperative Oncology Group (ECOG)–American College of Radiology Imaging Network (ACRIN) E1910 study [19]. The E1910 study was a phase 3, randomized controlled trial that compared modified UKALLXII/ECOG E2993 chemotherapy alone to the same chemotherapy plus four cycles of blinatumomab in adults aged 30 to 70 years with MRD-negative disease ( < 10-4) after induction and intensification therapy [17–19]. Among MRD-negative patients, 3-year OS was 85% for the blinatumomab arm versus 68% for chemotherapy alone (hazard ratio [HR], 0.41 [95% CI, 0.23–0.73]; P = 0.002) after a median follow-up of 3.6 years [19]. Additionally, 3-year relapse-free survival was 80% for the blinatumomab arm versus 64% for chemotherapy alone (HR, 0.53 [95% CI, 0.32–0.87]; P = 0.013) [19].
Blinatumomab with lower-intensity chemotherapy regimens
Results from studies evaluating chemo-minimizing strategies that use blinatumomab to replace some chemotherapy treatment in vulnerable patients (e.g., elderly) with newly diagnosed Ph-negative BCP-ALL have been reported. Final findings from the Australasian Leukemia and Lymphoma Group (ALLG) ALL08 phase 2 study in patients aged 40 to 67 years assessing the addition of blinatumomab consolidation to a low-intensity hyper-CVAD regimen estimated a 2-year median OS of 79% [24].
In the phase 2 SWOG 1318 study, blinatumomab was administered to elderly patients (median [range] age 75 [63–81] years; n = 29) as induction therapy for two cycles until reaching complete remission (CR) or CR with incomplete count recovery (CRi); this was followed by 3 cycles of blinatumomab post remission followed by POMP maintenance [56]. The estimated 3-year OS was 37%. The binary 3-year OS was 34%, which is significantly higher than historical rates of 10%.
The phase 2 Alliance A041703 study in older adults (n = 33; median [range] age, 71 [60–84] years) evaluated outcomes following treatment with inotuzumab ozogamicin during induction (3 cycles) and blinatumomab as consolidation therapy (2 cycles). After a median follow-up of 22 months, 1-year OS was 84%; 2 deaths occurred in remission [57]. With longer follow-up, this may improve on the rates reported for inotuzumab ozogamicin in combination with low intensity chemotherapy (mini-hyper-CVD) in older patients from a phase 2 study, 2-year OS of 66% [63].
An open-label phase 2 study has evaluated the combination of mini-hyper-CVD and inotuzumab ozogamicin with or without blinatumomab in patients aged 60 years or older [55]. Blinatumomab was added to both the consolidation phase (4 cycles) and during the dose-reduced POMP maintenance phase. Median PFS with blinatumomab (n = 31) was 56.4 months with a 2- and 5-year PFS of 61% and 42%, respectively, versus 34.7 months and 57% and 42%, respectively, without blinatumomab (n = 49). Median OS was 56.4 months with a 2- and 5-year OS of 60% and 41%, respectively, with blinatumomab versus 40.9 months and 65% and 47%, respectively, without blinatumomab.
In the GMALL BOLD study, eligible patients aged 56 to 76 years received one cycle of age-adapted induction chemotherapy and blinatumomab (induction phase), followed by three further cycles of blinatumomab alternating with age-adapted consolidation cycles [25]. With a median follow-up of approximately 2 years, the 3-year OS was 67%. Results from the historical GMALL standard regimen for older patients showed a 3-year OS of 49% [25].
Safety with blinatumomab-containing regimens
Limited toxicity data studies evaluating the addition or replacement of blinatumomab to SOC chemotherapy regimens have shown the combinations to be generally well tolerated (Table 4) [18, 24, 26, 27, 58]. Treatment with hyper-CVAD backbone plus 4 cycles of blinatumomab in both the consolidation and maintenance phases was safe, with the most common grade 3 to 5 events being infection or febrile neutropenia (82%), alanine transaminase or aspartate transaminase elevation (26%), or hyperglycemia (21%) [26]. Grade 3 cytokine release syndrome (CRS) was reported in only one patient (3%) and resolved within 24 h. Overall, 47% of patients developed a blinatumomab-related neurologic event of any grade, with only one patient (3%) discontinuing therapy due to treatment-related neurotoxicity. No treatment-related deaths were reported. In another study, the combination of blinatumomab added to consolidation of a low-intensity hyper-CVAD regimen was tolerable, and one case of grade 3 CRS was reported with no treatment-related deaths [24]. Treatment with HOVON-70–based chemotherapy plus 1 cycle of prephase blinatumomab followed by 2 cycles of blinatumomab consolidation reported grade ≥ 3 CRS in 21% of patients [27]. In the E1910 study with 4 cycles of consolidation blinatumomab, the safety results were consistent with the established safety profile of blinatumomab. Grade ≥ 3 treatment-related neuropsychiatric AEs were reported in 23% of 111 patients who started treatment with blinatumomab versus 5% of 112 patients receiving chemotherapy alone (P < 0.001) [18, 19]. In a phase 2 study in patients > 60 years old that used a combination of mini-hyper-CVD and inotuzumab with or without blinatumomab, the most common grade 3 and 4 AEs were thrombocytopenia (78%), hyperglycemia (36%), and febrile neutropenia (33%) and there were no early deaths on the trial. Seven deaths on study included five from neutropenic infections [55]. AEs related to cytopenia and immunosuppression are not unexpected and show that, despite low intensity chemotherapy with or without blinatumomab, these AEs still can occur, especially in older ( ≥ 70 years) patients. In the phase 2 SWOG 1318 study evaluating blinatumomab induction and consolidation followed by POMP maintenance in older patients, the most common grade 3 and 4 AEs were hyperglycemia (14%), dyspnea (10%), febrile neutropenia (10%), hypertension (10%), and lung infection (7%); 1 patient each developed a grade 3 CRS and a neurologic AE [56]. In the Alliance A041703 phase 2 study evaluating inotuzumab ozogamicin induction and blinatumomab consolidation in older patients, common grade ≥ 3 AEs were neutropenia (88%), thrombocytopenia (73%), anemia (42%), leukopenia (39%), lymphopenia (27%), febrile neutropenia (21%), and encephalopathy (12%) [58]. In the GMALL BOLD study, no unexpected safety events have been reported, and the overall mortality in induction and CR was low [25].
Discussion
Ph-negative BCP-ALL SOC treatment strategies aim to reduce the risk of relapse in frontline therapy, with the goal of improving cure rates while minimizing the risk of short- and long-term toxicities. The choice of an established Ph-negative BCP-ALL chemotherapy regimen in the frontline setting, including intensity, not only depends on clinical factors, such as age (e.g., younger adults vs older adults), comorbidities, and prognostic factors, but can vary from country to country, per health center, and according to physician choice. In many European countries treatment approaches are defined on a national level through multicenter study groups and are imbedded in long-term organizational infrastructures, such as reference laboratories, biobanks, or clinical registries [64, 65]. The efficacy and safety outcomes of these different chemotherapy regimens are largely similar, with no single regimen demonstrating clear superiority. The addition of blinatumomab to a standard chemotherapy backbone in the E1910 study has resulted in significantly improved overall survival and relapse-free survival outcomes. Promising results were also reported from single-arm phase 2 trials, adding blinatumomab into different standard chemotherapeutic backbone regimens and for different age groups [17–27]. The addition of blinatumomab has also supported the evaluation of dose-reduced chemotherapy regimens, allowing vulnerable (e.g., older) patients to be treated more effectively.
These data have led the current NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) to recommend blinatumomab as part of frontline multiagent consolidative therapy across all groups of adult patients with newly diagnosed Ph-negative BCP-ALL [4]. The approach was also supported by the current European Society for Medical Oncology and European LeukemiaNet recommendations [64, 66]. The E1910 study results [17–19], which span across both the younger and older adult subpopulations, have played a pivotal role in shaping the current guidelines [4]. The E1910 chemotherapy backbone was designed to accommodate patients aged 30 to 70 years by modifying the UKALLXII/ECOG E2993 regimen to be more “pediatric inspired” for younger adults ( < 55 years) and to reduce the intensity for older patients, defined as ≥ 55 years of age [18, 19]. This approach also aligns with current clinical practice, in which younger adult regimens are used up to the age of 55 years [66]. The lower age cut-off of 30 years was reportedly selected only to avoid a parallel recruiting clinical trial in the United States and was not based on any concerns regarding toxicity or efficacy in a younger adult population (18–29 years) [19].
Studies have also shown that the incorporation of blinatumomab into reduced-intensity chemotherapy regimens can improve both outcomes and safety profile in older populations, who historically have had poorer outcomes compared with their younger counterparts [24, 25, 55]. To conclusively demonstrate the efficacy of blinatumomab while reducing toxic chemotherapy in older patients, the ongoing phase 3 Golden Gate study (NCT04994717) is evaluating outcomes of blinatumomab alternating with low-intensity chemotherapy in induction, consolidation, and maintenance compared with age-adapted GMALL or hyper-CVAD regimens in patients aged ≥ 55 years with Ph-negative BCP-ALL [67]. Findings from the safety run-in provide support that this regimen is well tolerated with an acceptable safety profile [67].
Duration of therapy also represents a significant burden for patients with Ph-negative BCP-ALL. The use of blinatumomab in the frontline setting to reduce the overall duration of therapy has been addressed in a phase 2 study and is being investigated in an ongoing phase 3 study (Golden Gate, NCT04994717) [26, 67]. In addition, moving blinatumomab administration to an earlier timepoint in the treatment regimen may help induce deeper responses earlier on, helping further improve outcomes [25–27, 67]. This is also being explored in ongoing studies, such as the Golden Gate study.
The addition of blinatumomab into SOC frontline therapy in BCP-ALL, together with its favorable safety profile, has demonstrated a significant improvement in overall outcome in a randomized trial, and we believe these results can be applied to different standard backbones and different age groups [19]. However, data also show that blinatumomab is not effective for CNS disease due to poor penetration in the cerebrospinal fluid [68]. Therefore, particular attention should be paid to CNS prophylaxis. CNS-directed treatment should be continued and reinforced, especially if chemotherapy with potential CNS penetration is being omitted in the chemotherapy minimization approach per SOC in blinatumomab-containing regimens. Other essential considerations include relapse risk involving other sanctuary sites and the occurrence of CD19-negative relapses. It is also important to highlight that, although efficacy outcomes are generally similar across different chemotherapy regimens, studies differ in design and patient populations. For example, the E1910 study results are for patients who were in MRD-negative CR prior to start of consolidation [19]. Further, the transplantation indications, conditioning regimens, donor type, and rate of transplantation vary significantly among studies. Therefore, interpretation should be made with caution. Further studies will continue to generate evidence to highlight the extent to which and in which subgroups chemotherapy can be replaced by immunotherapy and whether the combination of different targeted agents may further improve results.
The future of BCP-ALL therapy belongs to optimal and combined use of targeted therapies. Therefore, it is reasonable to expect that further significant reductions in chemotherapy in the frontline setting will become SOC by using antibody-drug conjugates to de-bulk, with bispecific antibodies (including the newer generation of subcutaneous blinatumomab, which is under development) being used to drive a deeper response and chimeric antigen receptor T-cell therapy being used as further consolidation in select patients (eg, high-risk patients or patients who are MRD-positive after bispecific treatment).
Conclusions
Despite differences across the current SOC chemotherapeutic backbones for newly diagnosed Ph-negative BCP-ALL, they are all based on the same overarching treatment principles. The treatment landscape continues to evolve in the frontline setting, and data suggest that the integration of blinatumomab will add benefit regardless of the chemotherapy backbone.
Acknowledgements
The study was sponsored and funded by Amgen Inc. The authors thank Clare E. Lee, PhD, and Maryann T. Travaglini, PharmD (ICON plc, Blue Bell, PA, USA), whose work was funded by Amgen, for medical writing assistance in the preparation of this manuscript.
Author contributions
ACL was responsible for designing the review protocol. FZ and KV were responsible for extracting and analyzing the data and drafting the manuscript. EJJ, HMK, NG, BDS, SC, JHP, AWR, LG, SF, ACL, JMR, TFM, KM, FZ, KV, and NB were responsible for interpreting the results and providing feedback on the manuscript. AWR and KV were responsible for updating the reference list.
Competing interests
EJJ has received research grants from Pfizer, Takeda, Amgen, AbbVie, Novartis, Astex, Adaptive Biotechnologies, and Ascentage and served as a consultant and on advisory boards for Pfizer, Takeda, Amgen, AbbVie, BMS, Novartis, Genentech, Adaptive Biotechnologies, and Ascentage. HMK has received/served as honoraria/advisory board/consulting for AbbVie, Amgen, Amphista, Ascentage, Astellas, Biologix, Curis, Ipsen Biopharmaceuticals, KAHR Medical, Labcorp, Novartis, Pfizer, Shenzhen Target Rx, Stemline, and Takeda and has received research grants from AbbVie, Amgen, Ascentage, BMS, Daiichi-Sankyo, Immunogen, Jazz, and Novartis. NG has received institutional research funding from Amgen, Clinigen, Incyte, Jazz, Novartis, Pfizer, and Servier and received speaker honoraria or fees for advisory board participation from Amgen, AstraZeneca, Autolus, Celgene, Clinigen, Gilead, Incyte Jazz, Novartis, Pfizer, and Servier. BDS has served as a consultant and an educational advisor for Deciphera, Takeda, Kite, Novartis, Beigene, Pfizer, Jazz, BMS, Amgen, Adaptive, Lilly, Autolus, Syndax, Precision Biosciences, and Astra Zeneca; has received grants and served as an investigator for initiated trials for Kite, Jazz, and Servier; and has served on a data safety monitoring committee for Pepromene Bio. SC has served as a consultant and on advisory boards for Amgen, Incyte, Gilead, and AbbVie. JHP has received consulting fees from Affyimmune Therapeutics, Amgen, Autolus, Be Biopharma, Beigene, Bright Pharmaceutical Services, Caribou Biosciences, Curocell, Galapagos, In8Bio, Kite, Medpace, Minerva Biotechnologies, Pfizer, Servier, Sobi, and Takeda; received honoraria from OncLive, Physician Education Resource, and MJH Life Sciences; served on scientific advisory boards of Allogene Therapeutics, Artiva Biotherapeutics and Green Cross Biopharma; and received institutional research funding from Autolus, Genentech, Fate Therapeutics, InCyte, Servier, and Takeda. AWR has no competing interest. LG is an unpaid advisor to Amgen on the development of oncology products in pediatric patients. SF has served as an advisory board member for Amgen, Astellas, Gilead/Kite, Jazz, Pfizer, and Jazz; received speakers’ honoraria from Amgen, BMS, Gilead/Kite, Jazz, Pfizer, and Novartis; and received research funding from Amgen. ACL has received consulting honoraria from Amgen, AbbVie, Actinium, Sanofi, and Takeda, and research funding from Amgen, Autolus, Incyte, Kadmon/Sanofi, Kite/Gilead, Pharmacyclics, and Talaris. JMR has served as a speaker for and received advisory board honoraria from Pfizer, Amgen, Shire, and Ariad; has received research support from and participated in clinical trials with Amgen; and has participated in clinical trials with Pfizer and Ariad. TFM has served on the advisory board for Amgen, Incyte, Gilead, Novartis, and Pfizer. KM, FZ, and KV are employees of and shareholders in Amgen. NB has received personal/consulting fees from Amgen, Gilead Sciences, Novartis, Servier, and Pfizer.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jabbour E, Short NJ, Jain N, Haddad FG, Welch MA, Ravandi F, et al. The evolution of acute lymphoblastic leukemia research and therapy at MD Anderson over four decades. J Hematol Oncol. 2023;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafage-Pochitaloff M, Baranger L, Hunault M, Cuccuini W, Lefebvre C, Bidet A, et al. Impact of cytogenetic abnormalities in adults with Ph-negative B-cell precursor acute lymphoblastic leukemia. Blood. 2017;130:1832–44. [DOI] [PubMed] [Google Scholar]
- 3.Cooper SL, Brown PA. Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Lymphoblastic Leukemia Version 2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed August 21, 2024. To view the most recent and complete version of the guidelines, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- 5.Smirnova SY, Sidorova YV, Ryzhikova NV, Sychevskaya KA, Parovichnikova EN, Sudarikov AB. Evolution of tumor clones in adult acute lymphoblastic leukemia. Acta Nat. 2016;8:100–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Hoelzer D, Bassan R, Dombret H, Fielding A, Ribera JM, Buske C. Acute lymphoblastic leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v69–v82. [DOI] [PubMed] [Google Scholar]
- 7.Short NJ, Kantarjian H. Using immunotherapy and novel trial designs to optimise front-line therapy in adult acute lymphoblastic leukaemia: breaking with the traditions of the past. Lancet Haematol. 2023;10:e382–e388. [DOI] [PubMed] [Google Scholar]
- 8.Short NJ, Kantarjian H, Jabbour E. Optimizing the treatment of acute lymphoblastic leukemia in younger and older adults: new drugs and evolving paradigms. Leukemia. 2021;35:3044–58. [DOI] [PubMed] [Google Scholar]
- 9.Pieters R, Mullighan CG, Hunger SP. Advancing diagnostics and therapy to reach universal cure in childhood ALL. J Clin Oncol. 2023;41:5579–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pourhassan H, Agrawal V, Pullarkat V, Aldoss I. Positioning blinatumomab in the frontline of adult B-cell acute lymphoblastic leukemia treatment. Front Oncol. 2023;13:1237031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyer MB, Hsu M, Devlin SM, Tallman MS, Douer D, Park JH. Overall survival among older US adults with ALL remains low despite modest improvement since 1980: SEER analysis. Blood. 2017;129:1878–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goekbuget N, Stoltefuß A, Topp M, Schwartz S, Renzelmann A, Faul C, et al. Dose reduced chemotherapy in sequence with blinatumomab for newly diagnosed older patients with B-precursor adult lymphoblastic leukemia (ALL): results of the ongoing GMALL Bold trial. Blood. 2021;138:3399–401. [Google Scholar]
- 13.Sasaki K, Jabbour E, Short NJ, Jain N, Ravandi F, Pui CH, et al. Acute lymphoblastic leukemia: a population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980-2017. Am J Hematol. 2021;96:650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma H, Sun H, Sun X. Survival improvement by decade of patients aged 0-14 years with acute lymphoblastic leukemia: a SEER analysis. Sci Rep. 2014;4:4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulte D, Jansen L, Gondos A, Katalinic A, Barnes B, Ressing M, et al. Survival of adults with acute lymphoblastic leukemia in Germany and the United States. PloS One. 2014;9:e85554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl J Med. 2017;376:836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.BLINCYTO® (blinatumomab). Full Prescribing Information, Amgen Inc., Thousand Oaks, CA, 2024.
- 18.Litzow MR, Zhuoxin S, Paietta E, Mattison RJ, Hillard ML, Rowe JM. Consolidation therapy with blinatumomab improves overall survival in newly diagnosed adult patients with B-lineage acute lymphoblastic leukemia in measurable residual disease negative remission: results from the ECOG-ACRIN E1910 randomized phase III National Cooperative Clinical Trials Network trial. Blood. 2022;140:LBA–1. [Google Scholar]
- 19.Litzow MR, Sun Z, Mattison RJ, Paietta EM, Roberts KG, Zhang Y, et al. Blinatumomab for MRD-negative acute lymphoblastic leukemia in adults. N. Engl J Med. 2024;391:320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassan R, Chiaretti S, Della Starza I, Spinelli O, Santoro A, Elia L, et al. Preliminary results of the GIMEMA LAL2317 sequential chemotherapy-blinatumomab front-line trial for newly diagnosed adult Ph-negative B-lineage ALL patients. Eur Hematol Assoc Open Access Libr. 2021;324522:S114. [Google Scholar]
- 21.Boissel N, Huguet F, Graux C, Hicheri Y, Chevallier P, Kim R, et al. Frontline consolidation with blinatumomab for high-risk Philadelphia-negative acute lymphoblastic adult patients. Early results from the GRAALL-2014-QUEST phase 2. Blood. 2021;138:1232. [Google Scholar]
- 22.Boissel N, Huguet F, Leguay T, Hunault M, Kim R, Hicheri Y, et al. Blinatumomab during consolidation in high-risk Philadelphia chromosome (Ph)-negative B-cell precursor (BCP) acute lymphoblastic leukemia (ALL) adult patients: a two-cohort comparison within the GRAALL-2014/B study. Blood. 2022;140:507–9. [Google Scholar]
- 23.Chiaretti S, Della Starza I, Santoro A, Spinelli O, Elia L, De Propris MS, et al. Sequential chemotherapy and blinatumomab to improve minimal residual disease in adult Ph- B-lineage acute lymphoblastic leukemia. Final results of the phase II GIMEMA LAL2317 trial. Blood. 2023;142:826. [Google Scholar]
- 24.Fleming S, Reynolds J, Bajel A, Venn N, Kwan J, Moore J, et al. P365: Sequential blinatumomab with reduced intensity chemotherapy for older adults with newly diagnosed Ph-B-precursor acute lymphoblastic leukemia – final results of the ALLG ALL08 study. HemaSphere. 2023;7:e811479d. [Google Scholar]
- 25.Goekbuget N, Schwartz S, Faul C, Topp MS, Subklewe M, Renzelmann A, et al. Dose reduced chemotherapy in sequence with blinatumomab for newly diagnosed older patients with Ph/BCR::ABL negative B-precursor adult lymphoblastic leukemia (ALL): preliminary results of the GMALL Bold trial. Blood. 2023;142:964–6. [Google Scholar]
- 26.Jabbour E, Short NJ, Jain N, Thompson PA, Kadia TM, Ferrajoli A, et al. Hyper-CVAD and sequential blinatumomab for newly diagnosed Philadelphia chromosome-negative B-cell acute lymphocytic leukaemia: a single-arm, single-centre, phase 2 trial. Lancet Haematol. 2022;9:e878–e885. [DOI] [PubMed] [Google Scholar]
- 27.Rijneveld A, Gradowska P, Bellido M, de Weerdt O, Gadisseur A, Deeren D, et al. P366: Blinatumomab added to prephase and consolidation therapy in newly diagnosed precursor B-ALL in adults. A phase II HOVON trial. HemaSphere. 2022;6:266–7. [Google Scholar]
- 28.Lussana F, Cavallaro G, De Simone P, Rambaldi A. Optimal use of novel immunotherapeutics in B-cell precursor ALL. Cancers (Basel). 2023;15:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aureli A, Marziani B, Venditti A, Sconocchia T, Sconocchia G. Acute lymphoblastic leukemia immunotherapy treatment: now, next, and beyond. Cancers (Basel). 2023;15:3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirfakhraie R, Dehaghi BK, Ghorbi MD, Ghaffari-Nazari H, Mohammadian M, Salimi M, et al. All about blinatumomab: the bispecific T cell engager immunotherapy for B cell acute lymphoblastic leukemia. Hematol Transfus Cell Ther. 2024;46:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberta Health Services. Acute Lymphoblastic Leukemia in Adults. Available at: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-lyhe005-all.pdf. Accessed April 6, 2024.
- 32.Government of South Australia. Leukaemia - haematology - SA Health Approved Cancer Chemotherapy Protocol Register. Available at: https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/clinical+resources/clinical+programs+and+practice+guidelines/medical+conditions/cancer+haematology+and+oncology/cancer+therapy+protocols/leukaemia+-+haematology. Accessed April 6, 2024.
- 33.Hatta Y, Hayakawa F, Yamazaki E. JSH practical guidelines for hematological malignancies, 2018: I. leukemia-3. acute lymphoblastic leukemia/lymphoblastic lymphoma (ALL/LBL). Int J Hematol. 2020;112:439–58. [DOI] [PubMed] [Google Scholar]
- 34.Leukaemia Foundation of Australia, Australian Government Department of Health. Optimal care pathway for children, adolescents and young adults with acute leukaemia. Available at: https://www.cancer.org.au/assets/pdf/acute-leukaemia-in-children-adolescents-young-adults-1st-edition#_ga=2.169934627.2055769879.1678939651-1923260474.1675133706. Accessed April 6, 2024.
- 35.Princess Margaret Cancer Centre. Princess Margaret Cancer Centre Clinical Practice Guidelines: Leukemia Site Group, Acute Lymphoblastic Leukemia. Available at: https://www.uhn.ca/PrincessMargaret/Health_Professionals/Programs_Departments/Documents/CPG_Leukemia_AcuteLymphoblasticLeukemia.pdf. Accessed April 6, 2024.
- 36.Zhi Z. Chinese guidelines for diagnosis and treatment of adult acute lymphoblastic leukemia. Chin J Hematol. 2021;42:705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Cancer Society. Typical Treatment of Acute Lymphocytic Leukemia (ALL). Available at: https://www.cancer.org/cancer/types/acute-lymphocytic-leukemia/treating/typical-treatment.html. Accessed April 6, 2024.
- 38.Jabbour E, Kantarjian H. Hyper-CVAD in 2021: lessons learned and new approaches. Clin Lymphoma Myeloma Leuk. 2021;21:S82–S84. [DOI] [PubMed] [Google Scholar]
- 39.Rausch CR, Jabbour EJ, Kantarjian HM, Kadia TM. Optimizing the use of the hyperCVAD regimen: clinical vignettes and practical management. Cancer. 2020;126:1152–60. [DOI] [PubMed] [Google Scholar]
- 40.Martell MP, Atenafu EG, Minden MD, Schuh AC, Yee KW, Schimmer AD, et al. Treatment of elderly patients with acute lymphoblastic leukaemia using a paediatric-based protocol. Br J Haematol. 2013;163:458–64. [DOI] [PubMed] [Google Scholar]
- 41.Siegel S, Advani A, Seibel N, Muffly L, Stock W, Luger S, et al. Treatment of young adults with Philadelphia-negative acute lymphoblastic leukemia and lymphoblastic lymphoma: Hyper-CVAD vs. pediatric-inspired regimens. Am J Hematol. 2018;93:1254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burke PW, Hoelzer D, Park JH, Schmiegelow K, Douer D. Managing toxicities with asparaginase-based therapies in adult ALL: summary of an ESMO Open-Cancer Horizons roundtable discussion. ESMO Open. 2020;5:e000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zadeh C, AlArab N, Muwakkit S, Atweh LA, Tamim H, Makki M, et al. Stroke in Middle Eastern children with cancer: prevalence and risk factors. BMC Neurol. 2022;22:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Śliwa-Tytko P, Kaczmarska A, Lejman M, Zawitkowska J. Neurotoxicity associated with treatment of acute lymphoblastic leukemia chemotherapy and immunotherapy. Int J Mol Sci. 2022;23:5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanulla M, Schaeffeler E, Möricke A, Buchmann S, Zimmermann M, Igel S, et al. Hepatic sinusoidal obstruction syndrome and short-term application of 6-thioguanine in pediatric acute lymphoblastic leukemia. Leukemia. 2021;35:2650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levavi H, Khazak A, Pail O, Farina K, Kremyanskaya M, Mascarenhas J, et al. P389: Methotrexate neurotoxicity in adult ALL. HemaSphere. 2023;7:e599617c. [Google Scholar]
- 47.Majeed H, Gupta V. Adverse Effects of Radiation Therapy. StatPearls Publishing: Treasure Island (FL), 2023. [PubMed]
- 48.Kobza AO, Herman D, Papaioannou A, Lau AN, Adachi JD. Understanding and managing corticosteroid-Induced osteoporosis. Open Access Rheumatol. 2021;13:177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kulkarni S, Durham H, Glover L, Ather O, Phillips V, Nemes S, et al. Metabolic adverse events associated with systemic corticosteroid therapy-a systematic review and meta-analysis. BMJ Open. 2022;12:e061476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutchison RE, Murphy SB, Fairclough DL, Shuster JJ, Sullivan MP, Link MP, et al. Diffuse small noncleaved cell lymphoma in children, Burkitt’s versus non-Burkitt’s types. Results from the Pediatric Oncology Group and St. Jude Children’s Research Hospital. Cancer. 1989;64:23–8. [DOI] [PubMed] [Google Scholar]
- 51.Murphy SB, Bowman WP, Abromowitch M, Mirro J, Ochs J, Rivera G, et al. Results of treatment of advanced-stage Burkitt’s lymphoma and B cell (SIg+) acute lymphoblastic leukemia with high-dose fractionated cyclophosphamide and coordinated high-dose methotrexate and cytarabine. J Clin Oncol. 1986;4:1732–9. [DOI] [PubMed] [Google Scholar]
- 52.Maury S, Chevret S, Thomas X, Heim D, Leguay T, Huguet F, et al. Rituximab in B-lineage adult acute lymphoblastic leukemia. N. Engl J Med. 2016;375:1044–53. [DOI] [PubMed] [Google Scholar]
- 53.Jabbour E, Richard-Carpentier G, Sasaki Y, Konopleva M, Patel K, Roberts K, et al. Hyper-CVAD regimen in combination with ofatumumab as frontline therapy for adults with Philadelphia chromosome-negative B-cell acute lymphoblastic leukaemia: a single-arm, phase 2 trial. Lancet Haematol. 2020;7:e523–e533. [DOI] [PubMed] [Google Scholar]
- 54.Thomas DA, O’Brien S, Faderl S, Garcia-Manero G, Ferrajoli A, Wierda W, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28:3880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jabbour E, Short NJ, Senapati J, Jain N, Huang X, Daver N, et al. Mini-hyper-CVD plus inotuzumab ozogamicin, with or without blinatumomab, in the subgroup of older patients with newly diagnosed Philadelphia chromosome-negative B-cell acute lymphocytic leukaemia: long-term results of an open-label phase 2 trial. Lancet Haematol. 2023;10:e433–e444. [DOI] [PubMed] [Google Scholar]
- 56.Advani AS, Moseley A, O’Dwyer KM, Wood BL, Fang M, Wieduwilt MJ, et al. SWOG 1318: a phase II trial of blinatumomab followed by POMP maintenance in older patients with newly diagnosed Philadelphia chromosome–negative B-cell acute lymphoblastic leukemia. J Clin Oncol. 2022;40:1574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wieduwilt MJ, Yin J, Kour O, Teske R, Stock W, Byrd K, et al. Chemotherapy-free treatment with inotuzumab ozogamicin and blinatumomab for older adults with newly diagnosed, Ph-negative, CD22-positive, B-cell acute lymphoblastic leukemia: Alliance A041703. J Clin Oncol. 2023;41:7006. [Google Scholar]
- 58.Wieduwilt M, Yin J, Kour O, Teske R, Stock W, Byrd K, et al. Chemotherapy-free treatment with inotuzumab ozogamicin and blinatumomab for older adults with newly-diagnosed, Ph-negative, CD22-positive, B-cell acute lymphoblastic leukemia: ALLIANCE A041703. Presented at: European Hematology Association, June 8-11, 2023; Frankfurt, Germany.
- 59.Baalen EV, Gradowska P, Bellido M, de Weerdt O, Gadisseur A, Deeren D, et al. Blinatumomab added to prephase and consolidation therapy in adult B-ALL: a comparison of the consecutive HOVON-100 and H-146 studies. Presented at: European Hematology Association, June 13-16, 2024; Madrid, Spain.
- 60.Bassan R, Chiaretti S, Della Starza I, Spinelli O, Santoro A, Paoloni F, et al. Pegaspargase-modified risk-oriented program for adult acute lymphoblastic leukemia: results of the GIMEMA LAL1913 trial. Blood Adv. 2023;7:4448–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rijneveld AW, van der Holt B, de Weerdt O, Biemond BJ, van de Loosdrecht AA, van der Wagen LE, et al. Clofarabine added to intensive treatment in adult patients with newly diagnosed ALL: the HOVON-100 trial. Blood Adv. 2022;6:1115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goekbuget N, Stelljes M, Viardot A, Nachtkamp K, Steffen B, Schneller F, et al. First results of the risk-adapted, MRD-stratified GMALL trial 08/2013 in 705 adults with newly diagnosed acute lymphoblastic leukemia/lymphoma (ALL/LBL). Blood. 2021;138:362. [Google Scholar]
- 63.Kantarjian H, Ravandi F, Short NJ, Huang X, Jain N, Sasaki K, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2018;19:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gökbuget N, Boissel N, Chiaretti S, Dombret H, Doubek M, Fielding A, et al. Management of ALL in adults: 2024 ELN recommendations from a European expert panel. Blood. 2024;143:1903–30. [DOI] [PubMed] [Google Scholar]
- 65.Gokbuget N, Boissel N, Chiaretti S, Dombret H, Doubek M, Fielding A, et al. Diagnosis, prognostic factors, and assessment of ALL in adults: 2024 ELN recommendations from a European expert panel. Blood. 2024;143:1891–902. [DOI] [PubMed] [Google Scholar]
- 66.Hoelzer D, Bassan R, Boissel N, Roddie C, Ribera JM, Jerkeman M. ESMO Clinical Practice Guideline interim update on the use of targeted therapy in acute lymphoblastic leukaemia. Ann Oncol. 2024;35:15–28. [DOI] [PubMed] [Google Scholar]
- 67.Jabbour E, Aldoss I, Fleming S, Bajel A, Cannell P, Brüggemann M, et al. Blinatumomab alternating with low-intensity chemotherapy (CT) treatment for older adults with newly diagnosed Philadelphia (Ph)-negative B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is well tolerated and efficacious: safety run-in results for the phase 3 randomized controlled Golden Gate study. Blood. 2022;140:6134–6. [Google Scholar]
- 68.Lenk L, Alsadeq A, Schewe DM. Involvement of the central nervous system in acute lymphoblastic leukemia: opinions on molecular mechanisms and clinical implications based on recent data. Cancer Metastasis Rev. 2020;39:173–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stock W, Luger SM, Advani AS, Yin J, Harvey RC, Mullighan CG, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133:1548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeAngelo DJ, Stevenson KE, Dahlberg SE, Silverman LB, Couban S, Supko JG, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29:526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ribera J-M, Oriol A, Sanz M-A, Tormo M, Fernández-Abellán P, del Potro E, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the programa Español de Tratamiento en Hematología Pediatric-Based Protocol ALL-96. J Clin Oncol. 2008;26:1843–9. [DOI] [PubMed] [Google Scholar]
- 72.Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–61. [DOI] [PubMed] [Google Scholar]
- 73.Douer D, Aldoss I, Lunning MA, Burke PW, Ramezani L, Mark L, et al. Pharmacokinetics-based integration of multiple doses of intravenous pegaspargase in a pediatric regimen for adults with newly diagnosed acute lymphoblastic leukemia. J Clin Oncol. 2014;32:905–11. [DOI] [PubMed] [Google Scholar]
- 74.Linker C, Damon L, Ries C, Navarro W. Intensified and shortened cyclical chemotherapy for adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20:2464–71. [DOI] [PubMed] [Google Scholar]
- 75.Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood. 1995;85:2025–37. [PubMed] [Google Scholar]
- 76.Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–7. [DOI] [PubMed] [Google Scholar]
- 77.Huguet F, Chevret S, Leguay T, Thomas X, Boissel N, Escoffre-Barbe M, et al. Intensified therapy of acute lymphoblastic leukemia in adults: report of the randomized GRAALL-2005 clinical trial. J Clin Oncol. 2018;36:2514–23. [DOI] [PubMed] [Google Scholar]
- 78.Boissel N, Huguet F, Leguay T, Mathilde H-B, Graux C, Chalandon Y, et al. In adults with Ph-negative acute lymphoblastic leukemia (ALL), age-adapted chemotherapy intensity and MRD-driven transplant indication significantly reduces treatment-related mortality (TRM) and improves overall survival - results from the GRAALL-2014 trial. Blood. 2022;140:112–4.35427411 [Google Scholar]
- 79.Gökbuget N, Stoltefuß A, Schwartz S, Viardot A, Baumann L, Brüggemann M, et al. Dose reduced chemotherapy in combination with blinatumomab for newly diagnosed older patients with Ph-negative B-precursor all: first results of the bold trial. Presented at: European Hematology Association, June 11-21, 2020; Virtual Meeting.
- 80.Marks DI, Kirkwood AA, Rowntree CJ, Aguiar M, Bailey KE, Beaton B, et al. Addition of four doses of rituximab to standard induction chemotherapy in adult patients with precursor B-cell acute lymphoblastic leukaemia (UKALL14): a phase 3, multicentre, randomised controlled trial. Lancet Haematol. 2022;9:e262–e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sakura T, Hayakawa F, Sugiura I, Murayama T, Imai K, Usui N, et al. High-dose methotrexate therapy significantly improved survival of adult acute lymphoblastic leukemia: a phase III study by JALSG. Leukemia. 2018;32:626–32. [DOI] [PubMed] [Google Scholar]
- 82.Paietta E, Roberts KG, Wang V, Gu Z, Buck GAN, Pei D, et al. Molecular classification improves risk assessment in adult BCR-ABL1-negative B-ALL. Blood. 2021;138:948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garcia-Manero G, Kantarjian HM. The hyper-CVAD regimen in adult acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2000;14:1381–96. [DOI] [PubMed] [Google Scholar]
- 84.Wieduwilt MJ, Jonas BA, Schiller GJ, Liu L, Mulroney C, Mannis GN, et al. A phase II study of pegylated asparaginase, cyclophosphamide, rituximab, and dasatinib added to the UCSF 8707 (Linker 4-drug) regimen with liposomal cytarabine CNS prophylaxis for adults with newly diagnosed acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LBL): University of California Hematologic Malignancies Consortium study (UCHMC) 1401. Blood. 2018;132:4018. [Google Scholar]