Abstract
The regulation of long non-coding RNAs (lncRNAs) has been implicated in the pathogenesis of sepsis-induced acute kidney injury (SI-AKI). Nevertheless, the specific roles of individual lncRNAs in this process remain unclear. This study investigated the expression of lncRNA AP001007 in lipopolysaccharide (LPS)-induced HK-2 cells and in the peripheral blood of sepsis patients. The result shows that LPS treatment downregulated the expression of AP001007 in HK-2 cells and that circulating levels of AP001007 were lower in sepsis patients. Furthermore, overexpressing AP001007 in HK-2 cells improved cell viability, mitochondrial activity, and survival when exposed to LPS. Additionally, LPS-treated HK-2 cells secreted fewer pro-inflammatory cytokines when AP001007 was overexpressed. Similar protective effects were observed in human kidney organoids (HKOs) subjected to LPS. These findings suggest that AP001007 confers protection against LPS-induced damage in HK-2 cells and HKOs, highlighting its potential as a regulator of SI-AKI.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79367-2.
Keywords: Long non-coding RNA, AP001007, Sepsis, Kidney, Lipopolysaccharide
Subject terms: Cell biology, Chemical biology, Immunology
Introduction
Sepsis, a life-threatening condition, affects approximately 19.4 million adults globally and contributes to over 5.3 million deaths annually1. This disorder develops when the body’s reaction to an infection is dysregulated, which can cause extensive inflammation and harm to several organs, including the kidneys2. In individuals with sepsis, sepsis-induced acute kidney damage (SI-AKI) is a frequent consequence3, marked by a significant reduction in renal function. The severity of SI-AKI can vary widely, ranging from mild impairment to complete kidney failure4. Prompt recognition and treatment of SI-AKI are crucial to prevent further complications and improve patient prognosis5.
The etiology of SI-AKI remains incompletely understood. Various theories have been proposed to explain its occurrence, including dysregulated systemic and renal hemodynamics, dysfunction of microvascular and endothelial cells, renal tubular epithelial cells being harmed, and the cytokine storm triggered by infection6,7. However, each of these theories has its limitations in fully explaining all aspects of AKI in the context of sepsis5. Consequently, further thorough research is needed to clarify the exact mechanisms behind the beginning and development of SI-AKI.
Long non-coding RNAs (lncRNAs) are RNA molecules exceeding 200 base pairs that do not code for proteins. Originally, they were thought to be “junk” transcripts, meaning they had no purpose8. However, accumulating evidence has revealed their essential roles in various mechanisms that are both physiological and pathological9. Recent research has further emphasized the involvement of long non-coding RNAs (lncRNAs) in the pathogenesis of ischemic acute kidney injury (SI-AKI)10. For example, NEAT1 enhances the LPS-induced damage in HK2 cells by sequestering miR-22-3p, a suppressor of the NF-κB signaling pathway11. NEAT1 enhances the LPS-induced damage in HK2 cells by sequestering miR-22-3p, a suppressor of the NF-κB signaling pathway12,13. Given the abundance of lncRNAs, more characterization is required to clarify the roles of additional lncRNAs in SI-AKI regulation.
In a prior investigation, multiple lncRNAs exhibiting divergent expression patterns in untreated HK-2 cells compared to those exposed to LPS were discovered, which serves as a widely validated in vitro model for SI-AKI14. Specifically, this study demonstrated how the lncRNA CDK6-AS1 significantly contributes to LPS-induced HK-2 cell injury14. Building upon these findings, our current study delves into the expression profile and functional implications of another differentially expressed lncRNA, AP001007. The first thing that was done was to look at how much AP001007 was expressed in peripheral blood samples taken from sepsis patients. Additionally, the goal was to clarify the function of AP001007 in human kidney organoids (HKOs) derived from human embryonic stem cells (hESCs) and in HK-2 cells treated with LPS. We aim to obtain an additional understanding of the possible role of AP001007 in the pathophysiology of SI-AKI.
Materials and methods
Cell culture
Human renal tubular epithelial HK-2 cells were grown in DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) and 5 ng/mL EGF (Invitrogen, USA) at 37 °C in an incubator with 5% CO2. The cells were received from Xiamen Immocell Biotechnology, China. The cells were incubated with 5 µg/mL LPS for a whole day in order to treat them with LPS.
Plasmid construction and transfection
The AP001007 overexpression plasmid, named AP001007 OE, was constructed using the pLV-EF1a-mcs-puro vector and sequenced by GeneChem (Shanghai, China). The primers utilized in the construction of plasmids are enumerated below: AP001007 OE-F: 5′-CTAGAGCTAGCGAATTCAGGGACGCAGCAGTGAGG-3′, AP001007 OE-R: 5′-CAGCGGCCGCGGATCCACATTTTTGTTGAAAGAGTG-3′. The transfection of cells was carried out utilizing Lipofectamine 2000 (Invitrogen, USA), adhering strictly to the manufacturer’s prescribed protocol. The transfection of HKO was accomplished through lentivirus infection. In summary, a total of 9 micrograms of AP001007 OE and the packaging plasmids (3 µg of pMD2G and 6 µg of pspax2) were co-transfected into 293T cells using Lipofectamine 2000. Following a 48-hour incubation period, the culture medium was gathered and processed through centrifugation at 30,000 rpm for a duration of 2 h. In conclusion, the supernatant was delicately eliminated, and the virus titer was precisely measured utilizing an ELISA-based kit (Catalog number: BF06203, Biodragon, China). The HKOs were infected for 12 h with the AP001007 OE virus (MOI 10) while 8 µg/mL polybrene was present.
Induction of HKO development
The method to develop HKO was adapted from a published protocol15. The hESC line H1 was cultured in Matrigel-coated 6-well plates using mTeSR1 medium (STEMCELL, USA) within a controlled environment of a humidified incubator maintained at 5% CO2 levels. The medium underwent daily refreshment, and the cells were cultivated over a period of four days until achieving a confluence of 70%. The cells were then separated using Tryple Express (Gibco, USA), suspended in mTeSR1 media with 10 µM Y27632, and seeded at a density of 5.0 × 105 cells/mL (0.1 mL) into Ultra-Low Attachment 96-well plates (Corning, USA). Afterward, the plates were centrifuged at 820 rpm to pellet the cells and cultured overnight. On the following day, the cells were cultured in Y27632-free mTeSR1 medium to induce embryoid body (EB) development. Next, the EBs were induced to form the posterior primitive streak (PPS) using APEL2 medium containing 8 μm CHIR99021 and 5% PFHM2, and this day was designated as differentiation day 0 (D0). Throughout the four-day process, the culture medium was replaced every alternate day. Subsequently, from D4 to D7, the PPSs were induced to develop intermediate mesoderm (IM) by utilizing APEL2 culture medium enriched with 10% KnockOut Serum Replacement (KSR, Gibco, USA), 200 ng/mL FGF9, and 1 μg/mL heparin. This enriched medium was also refreshed every alternate day to maintain optimal conditions for development. From D7 to D12, the IMs were initially treated with APEL2 medium containing 10% KSR and 5 µM CHIR99021 for 1 h, followed by culture with APEL2 medium supplemented with 10% KSR, 200 ng/mL FGF9, and 1 µg/mL heparin. Throughout the five-day period, the culture medium underwent alternation every second day. Subsequently, on the twelfth day, the HKO cells commenced their maturation process and were subjected to further cultivation in APEL2 medium enriched with 10% KSR and 1 µg/mL heparin for a duration ranging from six to twelve days. During this period, the medium was replaced every alternate day. The morphology of the cell culture and organoid was observed at the indicated time points and imaged using a Jiangnan inverse bright-field microscopy equipped with a digital camera (China).
Blood collection
Five milliliters of peripheral venous blood were collected from fasting volunteers to assess the concentrations of AP001007. The clinical features of patients are shown in supplementary Table S1. The patient inclusion standard refers to the international Pediatric Sepsis Consensus16. Patients with the following conditions were excluded from the cohort: Immunodeficiency or currently receiving immunosuppressive treatment; Coexisting chronic heart, liver, or kidney diseases; Coexisting blood disorders or malignant tumors; Severe malnutrition; Coexisting chronic inflammatory diseases; History of severe drug allergies; Recent use of medications that could affect the outcome of this treatment. This study was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital (#2022KD0133), and written informed consent was obtained from all participants.
Quantitative PCR (qPCR)
HK-2 cells, blood samples, or HKOs were used to extract total RNA using a TIANGEN kit (catalog number: 4992858, China), and cDNA was synthesized using a kit from TIANGEN (catalog number: KR118, China). SYBR Green (Invitrogen, USA) was used for qPCR on an ABI 7500 machine. Using the 2 − ΔΔCt technique, the relative expression of target genes was determined. Table 1 contains a list of qPCR primers.
Table 1.
qPCR primers.
| Name | Sequence (5′–3′) |
|---|---|
| 18 S rRNA forward primer | ACCCGTTGAACCCCATTCGTGA |
| 18 S rRNA reverse primer | GCCTCACTAAACCATCCAATCGG |
| IL-1β forward primer | CCACAGACCTTCCAGGAGAATG |
| IL-1β reverse primer | GTGCAGTTCAGTGATCGTACAGG |
| IL-6 forward primer | AGACAGCCACTCACCTCTTCAG |
| IL-6 reverse primer | TTCTGCCAGTGCCTCTTTGCTG |
| IL-18 forward primer | GAGAGTGATTGAGAGTGGACCAC |
| IL-18 reverse primer | CACAACCCTCTGCACCCAGTTT |
| TNF-α forward primer | CTCTTCTGCCTGCTGCACTTTG |
| TNF-α reverse primer | ATGGGCTACAGGCTTGTCACTC |
| Bcl-2 forward primer | ATCGCCCTGTGGATGACTGAGT |
| Bcl-2 reverse primer | GCCAGGAGAAATCAAACAGAGGC |
| Bax forward primer | TCAGGATGCGTCCACCAAGAAG |
| Bax reverse primer | TGTGTCCACGGCGGCAATCATC |
| AP001007 forward primer | CAGCCCATCTCCGCTCCACT |
| AP001007 reverse primer | TCTCCGCAGCCTCGTCTT |
| NPHS1 forward primer | GTCTGCACTGTCGATGCCAATC |
| NPHS1 reverse primer | CCAGTTTGGCATGGTGAATCCG |
| PODXL forward primer | CCTGAACCTCACAGGAAACACC |
| PODXL reverse primer | TGGAACAGATGCCAGCCGTATG |
| ECAD forward primer | GCCTCCTGAAAAGAGAGTGGAAG |
| ECAD reverse primer | TGGCAGTGTCTCTCCAAATCCG |
| GATA3 forward primer | ACCACAACCACACTCTGGAGGA |
| GATA3 reverse primer | TCGGTTTCTGGTCTGGATGCCT |
| AQP1 forward primer | TATGCGTGCTGGCTACTACCGA |
| AQP1 reverse primer | GGTTAATCCCACAGCCAGTGTAG |
MTT assay
To measure cell viability, the cells were seeded onto 96-well plates, achieving a density of 5 × 103 cells per well. Following a 6-hour incubation, the cells underwent transfection with AP001007 OE plasmids utilizing Lipofectamine 2000. The transfected cells were then cultured for an additional 8 h. Subsequently, the cells were cultured in DMEM medium containing 5 µg/mL LPS. After a 24-hour incubation, MTT (5 mg/mL) was introduced to the cells and incubated at 37 °C for 4 h. Finally, the culture medium was aspirated, and 150 µL of DMSO was added to each well to dissolve the crystallized MTT. The absorbance at 490 nm (OD490) was then measured using a microplate reader.
Annexin V/PI and JC-1 staining
Using the Annexin V/PI kit (catalog number: A211-01, Vazyme, China) and the JC-1 staining kit (catalog number: C2006, Beyotime, China), respectively, the apoptosis and mitochondrial membrane potential (MMP) of the LPS-treated control and AP001007-overexpressing cells were measured, adhering to the manufacturer’s instructions. A flow cytometer (Beckman, USA) was then used to analyze the resuspended cells using flow cytometry.
Enzyme-linked immunosorbent assay (ELISA)
LPS-treated control and AP001007-overexpressing cells were incubated for 24 h, following which the culture media were harvested and centrifuged. Subsequently, the supernatants were assayed using ELISA kits provided by Beyotime (China), specifically: IL-1β (catalog number: PI305), IL-18 (catalog number: PI640), IL-6 (catalog number: PI330), and TNF-α (catalog number: PT518).
Western blotting
After lysing the cells or HKOs for 30 min on ice in RIPA buffer with protease inhibitors (catalog number: P0013C, Beyotime, China), the supernatants were collected by centrifugation at 4 °C. A BCA kit was used to measure the protein concentration (catalog number: P0012S, Beyotime, China). Following denatured samples, 10% SDS-PAGE gels were loaded, electrophoresis was used to separate the samples, and PVDF membranes were transferred. The membranes were then blocked with 5% skim milk for an hour at room temperature (RT), incubated with the primary antibody for an additional night at 4 °C, and then given three Tris Buffered Saline with Tween 20 (TBST) washes (catalog number: ST671, Beyotime, China). Subsequently, the membranes were exposed to signal development using an ECL kit (catalog number: P0018S, Beyotime, China) after being incubated with secondary antibody solutions at room temperature for one hour and cleaned with TBST. X-ray film was used to display the results and protein levels were analyzed using Image J (NIH, USA). Table 2 contains information about antibodies.
Table 2.
Antibody information for Western blotting.
| Classification | Name of antibody | Manufacturer | Catalog number | Dilution |
|---|---|---|---|---|
| Primary antibody | β-actin | Proteintech | 66009-1-Ig | 1:2000 |
| p65 | 10745-1-AP | 1:3000 | ||
| P-p65 | Cell Signaling Technology | 3033 | 1:1000 | |
| Bcl-2 | 15,071 | 1:1000 | ||
| Bax | 89,477 | 1:1000 | ||
| Cleaved caspase 3 | 9664 | 1:1000 | ||
| Cleaved caspase 9 | 20,750 | 1:1000 | ||
| Secondary antibody | HRP Goat Anti-Mouse IgG(H + L) | 7076 | 1:1000 | |
| HRP Goat Anti-Rabbit IgG(H + L) | 7074 | 1:1000 |
Immunohistochemistry (IHC) and immunofluorescence (IF)
The HKOs were incubated with LPS for a duration of 24 h. Subsequently, they were collected and prepared for fixation using 4% paraformaldehyde for 2 h at RT. This was followed by paraffin embedding and sectioning. For IHC, the sections underwent rehydration, treatment with 3% H2O2, antigen retrieval, blocking with 5% goat serum, and overnight incubation with primary antibody solutions at 4 °C. Subsequently, the sections were washed with PBST (PBS + 0.1% Tween 20) and incubated with secondary antibody solutions for 1 h at RT, followed by another wash with PBST. For IHC, the sections were stained with DAB, counterstained with hematoxylin, dehydrated, cleared, and mounted. The resulting specimens were observed and imaged using a Jiangnan XD202 microscope (China). Additionally, for immunofluorescence (IF), the sections were counterstained with DAPI, mounted, and then observed and imaged using an LSM 900 confocal microscope (Zeiss, Germany). A detailed list of the antibodies utilized for both IHC and IF is presented in Table 3.
Table 3.
Antibody information for IHC and IF.
| Classification | Name of antibody | Manufacturer | Catalog number | Dilution |
|---|---|---|---|---|
| Primary antibody | CK19 | Proteintech | 10712-1-AP | 1:200 |
| ECAD | 20874-1-AP | 1:200 | ||
| PODXL | Abclonal | A10200 | 1:200 | |
| Second antibody | CoraLite488-Goat Anti-Rabbit IgG(H + L) | Proteintech | SA00013-2 | 1:500 |
| HRP Goat Anti-Rabbit IgG(H + L) | PR30011 | 1:500 |
Statistical analysis
The statistical program SPSS 22.0 was used for the analyses. The information is displayed as mean ± SD. One-way ANOVA followed by post-hoc Tukey’s HSD was utilized to analyze the differences among multiple data sets, and unpaired two-tailed Student’s t-tests were employed to evaluate the significance between two data sets. Less than 0.05 was the threshold for statistical significance.
Results
AP001007 expression was downregulated in LPS-treated HK-2 cells and the peripheral blood of sepsis patients
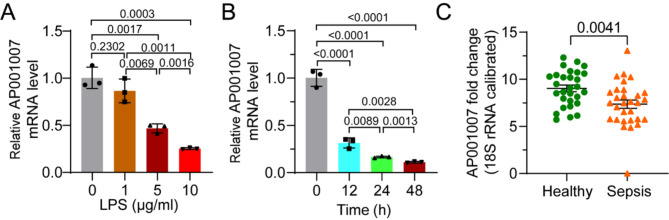
In order to learn more about how LPS treatment affects AP001007 expression in HK-2 cells, the cells were subjected to different LPS doses and performed qPCR analysis. The findings showed that LPS inhibits AP001007 expression in a dose-dependent manner (Fig. 1A). Furthermore, a reduction was observed in AP001007 expression with increasing duration of LPS treatment, indicating a time-dependent effect (Fig. 1B). Additionally, the analysis of circulating AP001007 mRNA levels in sepsis patients compared to healthy volunteers revealed a significant decrease (Fig. 1C), as determined by qPCR. These findings suggest that sepsis may trigger the downregulation of AP001007 expression.
Fig. 1.
AP001007 expression is downregulated by LPS and in sepsis patients’ peripheral blood. (A) qPCR results showing AP001007 levels in HK-2 cells treated with different concentrations of LPS. (B) qPCR data depicting the expression of AP001007 in HK-2 cells treated with 5 µg/mL LPS for different durations. (C) qPCR outcome illustrating the circulating AP001007 in control volunteers and sepsis patients.
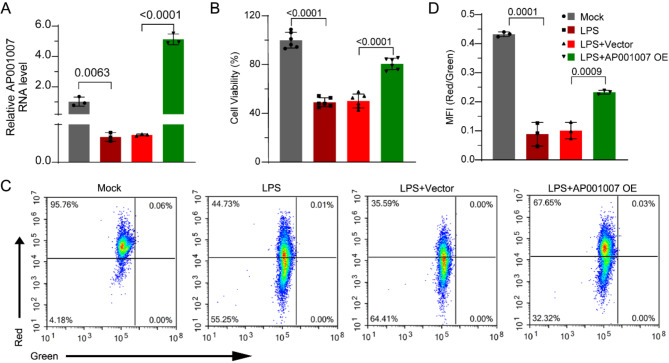
Overexpression of AP001007 improved the viability, mitochondrial function, and survival of LPS-treated HK-2 cells
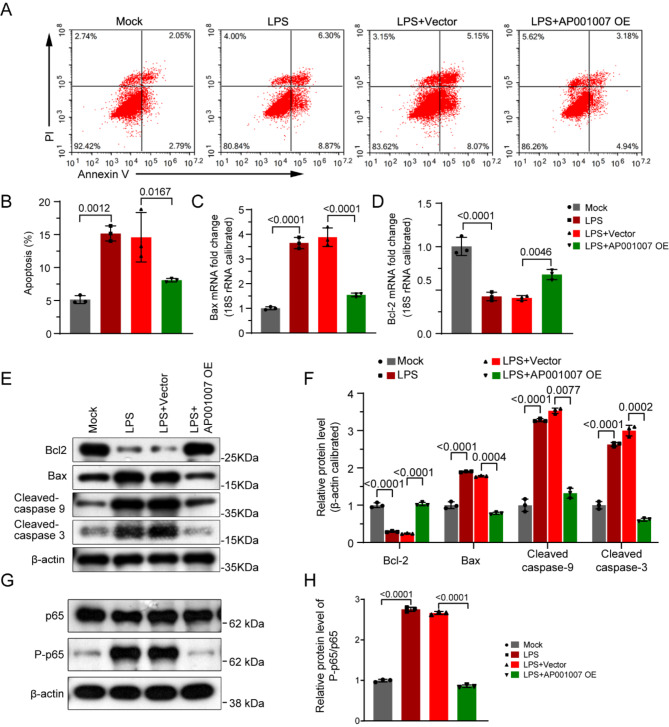
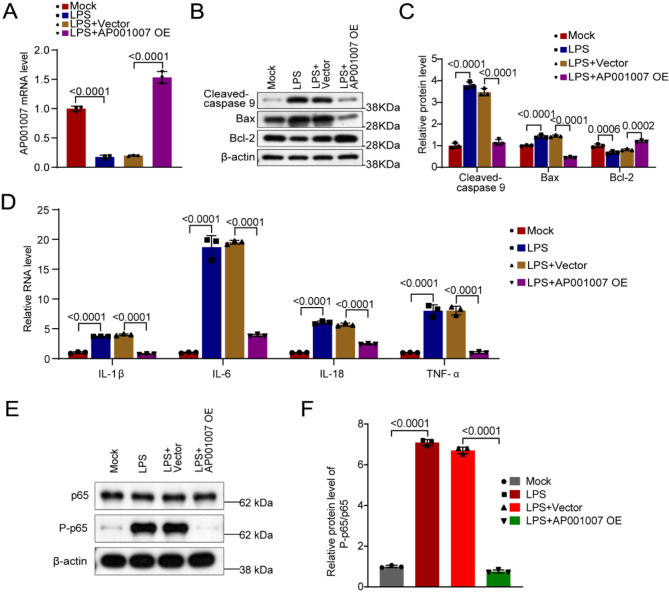
To explore the influence of AP001007 on HK-2 cells treated with LPS, those experiments were conducted to overexpress AP001007 (Fig. 2A). Our MTT assay results showed that AP001007 overexpression significantly improved the viability of LPS-treated HK-2 cells (Fig. 2B). Additionally, excessive expression of AP001007 notably reversed the reduction in mitochondrial potential in LPS-treated HK-2 cells, indicating improved mitochondrial function (Fig. 2C,D). Furthermore, AP001007 overexpression attenuated the LPS-induced increase in apoptosis in HK-2 cells, as demonstrated by Annexin V/PI staining (Fig. 3A,B). This finding was supported by qPCR and Western blotting analyses, which revealed elevated levels of the anti-apoptotic factor Bcl-2 and reduced levels of the pro-apoptotic factors Bax, Cleaved-caspase 9, and Cleaved-caspase 3 in LPS-treated HK-2 cells overexpressing AP001007 (Fig. 3C–F). Additionally, the overactivated NF-κB pathway by LPS was attenuated by excessive AP001007, as evidenced by the P-p65 levels (Fig. 3G,H). These results collectively suggest that AP001007 effectively enhances the viability and survival of LPS-treated HK-2 cells, possibly by repressing the NF-κB pathway.
Fig. 2.
Overexpression of AP001007 improved the viability and mitochondrial function in LPS-treated HK-2 cells. (A) qPCR data showing the expression of AP001007 in HK-2 cells with the indicated treatments. (B) MTT assay results depicting the viability of HK-2 cells with the indicated treatments. (C) JC-1 staining outcome illustrating the mitochondrial membrane potential in HK-2 cells with the indicated treatments. (D) The quantification results of data shown in (C). Mock defines PBS treatment and vector defines transfection with empty backbone plasmid for AP001007 overexpression (OE).
Fig. 3.
Excessive AP001007 alleviated LPS-triggered extra apoptosis in HK-2 cells. (A) Annexin V/PI staining results showing the apoptosis of HK-2 cells with the indicated treatments. (B) Quantification of the apoptosis results. (C,D) qPCR data depicting the expression of Bax (C) and Bcl-2 mRNA levels (D) in HK-2 cells with the indicated treatments. (E) Western blotting outcome illustrating the expression of Bcl-2, Bax, Cleaved-caspase 9, and Cleaved-caspase 3 in HK-2 cells with the indicated treatments. GAPDH was used as the internal control. (F) Quantification of the Western blotting outcome in (E). (G) Western blotting data indicating the levels of p65 and P-p65 in HK-2 cells with the indicated treatments. (H) Quantification of the Western blotting data in (G).
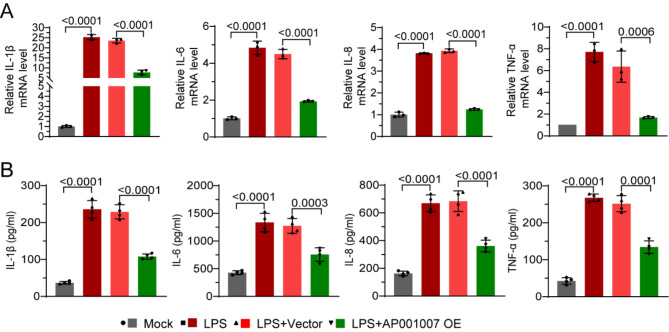
AP001007 inhibited the secretion of pro-inflammatory cytokines by LPS-induced HK-2 cells
The effect of AP001007 overexpression on LPS-treated HK-2 cells’ production of pro-inflammatory cytokines was then evaluated. Our qPCR study showed that when AP001007 was overexpressed in HK-2 cells, the mRNA levels of IL-1β, IL-6, IL-18, and TNF-α, which were considerably elevated by LPS treatment, were dramatically downregulated (Fig. 4A). ELISA findings consistently verified that AP001007 overexpression led to a substantial reduction in the production of IL-1β, IL-6, IL-18, and TNF-α by LPS-treated HK-2 cells (Fig. 4B). These results imply that AP001007 may be a useful treatment for kidney cell inflammation brought on by sepsis.
Fig. 4.
Overexpression of AP001007 inhibited pro-inflammatory cytokine secretion by LPS-treated HK-2 cells. (A) qPCR data showing the expression of IL-1β, IL-6, IL-18, and TNF-α by HK-2 cells with the indicated treatments. (B) ELISA results illustrating the levels of IL-1β, IL-6, IL-18, and TNF-α in the culture medium of HK-2 cells with the indicated treatments.
Overexpression of AP001007 protected HKO from LPS-triggered damage
To further elucidate whether AP001007 confers protection against SI-AKI, the HKOs were established (Fig. S1A,B), which serve as a model for SI-AKI when exposed to LPS17. qPCR analysis revealed that the HKOs exhibited elevated expression of the podocyte markers NPHS1 and PODXL, and collecting duct markers GATA3 and ECAD (Fig. S1C). Similarly, IF staining demonstrated the expression of collecting duct markers CK19 and ECAD, as well as the podocyte marker PODXL, in the HKOs (Fig. S1D). Additionally, they expressed proximal tubule marker AQP1, as indicated by qPCR data (Fig. S1E). These results confirm the successful establishment of the HKOs.
The initial evaluation focused on the impact of various LPS concentrations on HKO proliferation to investigate the effect of AP001007 on LPS-treated HKOs. Additionally, qPCR analysis showed that HKOs treated with 1 µg/mL LPS exhibited the highest expression levels of pro-inflammatory cytokines (Fig. S2). Therefore, 1 µg/mL LPS was selected for these subsequent experiments.
Subsequently, AP001007 was overexpressed in LPS-treated HKOs and confirmed successful overexpression by qPCR (Fig. 5A). Moreover, the upregulation of Bcl2 expression and downregulation of Bax and Cleaved caspase 9 levels in LPS-treated HKOs upon AP001007 overexpression suggested a reduction in apoptosis (Fig. 5B,C). Furthermore, in HKOs, the overexpression of AP001007 significantly reduced the production of pro-inflammatory cytokines triggered by LPS treatment (Fig. 5D). Additionally, excessive AP001007 markedly decreased the LPS-induced P-p65 levels in HKOs (Fig. 5E,F). These findings collectively indicate that AP001007 protects HKOs from LPS-induced damage.
Fig. 5.
Excessive AP001007 protects HKOs from LPS-triggered damage. (A) qPCR data showing the levels of AP001007 in HKOs with the indicated treatments. (B) Western blotting data illustrating the levels of Cleaved-caspase 9, Bax, and Bcl-2 in HKOs with the indicated treatments. β-actin was employed as the internal control. (C) Quantification of the Western blotting data is shown in (B). (D) qPCR results indicating the expression of IL-1β, IL-6, IL-18, and TNF-α in HKOs with the indicated treatments. (E) Western blotting data indicating the levels of p65 and P-p65 in HKOs with the indicated treatments. (F) Quantification of the Western blotting data in (E).
Discussion
The high mortality rate among patients with SI-AKI underscores the urgent need for effective treatments. Despite ongoing research efforts, the complex etiology of SI-AKI poses challenges in developing successful therapies. However, the emerging role of lncRNAs in disease pathogenesis has sparked interest in exploring these molecules as potential therapeutic targets18. Yet, given the vast number of lncRNAs in the human genome, there is still much to learn about their functions and potential therapeutic applications across different disease contexts.
Upon screening for differentially expressed lncRNAs in LPS-exposed HK-2 cells, we discovered AP001007 as a putative modulator of SI-AKI. This inference was bolstered by the observed significant downregulation of AP001007 following LPS treatment. Our observation of decreased AP001007 expression in sepsis patient peripheral blood further corroborates this finding. Additionally, our functional analyses demonstrated that increased AP001007 expression attenuated LPS-induced damage in HK-2 cells, leading to improved cell viability, mitochondrial function preservation, cell survival promotion, and suppression of pro-inflammatory cytokine secretion. Notably, comparable protective effects were evident in LPS-treated HKOs upon AP001007 overexpression. Collectively, these results underscore the potential of AP001007 as a protective agent against SI-AKI, indicating that its downregulation may play a role in the pathogenesis of this condition.
The limited understanding of AP001007’s function underscores the novelty of our findings. While it has been suggested that AP001007 could be utilized for prognostic prediction in glioma19, its biological role remains largely unexplored. Notably, AP001007, also known as PKNOX2-AS1 due to its genomic location near PKNOX2, presents an intriguing contrast to the known functions of PKNOX2. Tumor suppressor PKNOX2 has been linked to stomach and lung malignancies. It works by inhibiting the PI3K/AKT/mTOR cascade, which promotes cell survival and proliferation, and by activating the production of P53 and IGFBP5 20,21. However, our findings reveal a divergent role for AP001007 in LPS-treated HK-2 cells and HKOs, where it appears to promote cell proliferation and survival. This suggests a complex interplay between AP001007 and its neighboring gene PKNOX2, warranting further investigation into their respective functions and potential regulatory mechanisms.
LncRNAs have been identified to regulate the expression of adjacent genes via various mechanisms, including transcription interference, chromatin remodeling, and RNA processing modulation22,23. Whether AP001007 achieves its goal of regulating PKNOX2 expression is still unknown, though. It would be intriguing to assess the PKNOX2 level in HK-2 cells and HKOs treated with LPS since this might offer insights into the possible function of PKNOX2 in SI-AKI.
Given that PKNOX2 has never been reported to regulate inflammation, it is likely that AP001007 inhibits the secretion of pro-inflammatory cytokines by regulating other inflammation regulators, such as miRNAs that control the NF-κB pathway, as lncRNAs can function as sponges for miRNAs, thereby affecting their expression and downstream targets24. Indeed, our analysis revealed that the level of NF-κB pathway indicator, P-p65 25, was downregulated by AP001007 overexpression. Alternatively, AP001007 can modulate the expression of cytokine genes by altering the chromatin structure, which is another common mechanism by which lncRNAs exert their function26. Further investigations would be essential to test these possibilities.
Notably, our conclusions are based on in vitro experiments and have not yet been validated by in vivo studies. Additionally, these findings, particularly the reduced levels of circulating AP001007 in young sepsis patients, need to be confirmed in a larger cohort with a broader age range.
Conclusion
In summary, our research has provided evidence that AP001007 possesses the ability to alleviate the pain from LPS-induced in both HK-2 cells and HKOs, thereby emphasizing its possible protective function in SI-AKI. Thus, targeted overexpression of AP001007 could potentially yield therapeutic benefits for sepsis patients with AKI.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Sheng Lin, Zuxiu Ren, Lili Li, and Hong Ye designed and directed the experiments. Sheng Lin, Zuxiu Ren, and Lili Li performed the experiments and wrote the manuscript. Suqin Xia and Rongrong Yang collected and analyzed the data and performed the experiments. All authors read and approved the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Funding
This research was funded by Joint Funds for the Innovation of Science and Technology, Fujian province (Grant number: 2021Y9161).
Data availability
All the data supporting the conclusions of this article have been included in the manuscript. The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Policy and ethics
The described research complies with all relevant ethical regulations. The study was conducted in accordance with the declaration of Helsinki and approved by the Ethics Committee of Fujian Maternity and Child Health Hospital (#2022KD0133), and written informed consent was obtained from all participants. All authors confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sheng Lin, Zuxiu Ren and Lili Li.
References
- 1.Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet395, 200–211. 10.1016/S0140-6736(19)32989-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lelubre, C. & Vincent, J. L. Mechanisms and treatment of organ failure in sepsis. Nat. Rev. Nephrol.14, 417–427. 10.1038/s41581-018-0005-7 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Peerapornratana, S., Manrique-Caballero, C. L., Gomez, H. & Kellum, J. A. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int.96, 1083–1099. 10.1016/j.kint.2019.05.026 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White, K. C. et al. Sepsis-associated acute kidney injury in the intensive care unit: incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes. A multicenter, observational study. Intensive Care Med.49, 1079–1089. 10.1007/s00134-023-07138-0 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarbock, A. et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative Workgroup. Nat. Rev. Nephrol.19, 401–417. 10.1038/s41581-023-00683-3 (2023). [DOI] [PubMed] [Google Scholar]
- 6.He, F. F. et al. Sepsis-induced AKI: from pathogenesis to therapeutic approaches. Front. Pharmacol.13, 981578. 10.3389/fphar.2022.981578 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fani, F. et al. Recent advances in the pathogenetic mechanisms of sepsis-associated acute kidney injury. J. Nephrol.31, 351–359. 10.1007/s40620-017-0452-4 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Mattick, J. S. et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol.24, 430–447. 10.1038/s41580-022-00566-8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Statello, L., Guo, C. J., Chen, L. L. & Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol.22, 96–118. 10.1038/s41580-020-00315-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y. et al. Non-coding RNAs in sepsis-associated acute kidney Injury. Front. Physiol.13, 830924. 10.3389/fphys.2022.830924 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou, Y. et al. Downregulation of lncRNA NEAT1 alleviates sepsis-induced acute kidney injury. Cent. Eur. J. Immunol.47, 8–19. 10.5114/ceji.2022.115628 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, M., Wei, J., Shang, F., Zang, K. & Zhang, P. Down-regulation of lncRNA SNHG5 relieves sepsis-induced acute kidney injury by regulating the miR-374a-3p/TLR4/NF-kappaB pathway. J. Biochem.169, 575–583. 10.1093/jb/mvab008 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Wang, M., Wei, J., Shang, F., Zang, K. & Ji, T. Long non–coding RNA CASC2 ameliorates sepsis–induced acute kidney injury by regulating the miR–155 and NF–kappaB pathway. Int. J. Mol. Med.45, 1554–1562. 10.3892/ijmm.2020.4518 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Wu, L., Zhang, R., Lin, S., Lin, M. & Wang, J. Silencing CDK6-AS1 inhibits LPS-induced inflammatory damage in HK-2 cells. Open. Med. (Wars). 16, 1256–1264. 10.1515/med-2021-0314 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takasato, M., Er, P. X., Chiu, H. S. & Little, M. H. Generation of kidney organoids from human pluripotent stem cells. Nat. Protoc.11, 1681–1692. 10.1038/nprot.2016.098 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein, B., Giroir, B. & Randolph, A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med.6, 2–8. 10.1097/01.Pcc.0000149131.72248.E6 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Zhang, W. et al. Kidney organoids as a novel platform to evaluate lipopolysaccharide-induced oxidative stress and apoptosis in acute kidney injury. Front. Med. (Lausanne)8, 766073. 10.3389/fmed.2021.766073 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkle, M., El-Daly, S. M., Fabbri, M. & Calin, G. A. Noncoding RNA therapeutics—challenges and potential solutions. Nat. Rev. Drug Discov.20, 629–651. 10.1038/s41573-021-00219-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, X. et al. An immune gene-related five-lncRNA signature for to predict glioma prognosis. Front. Genet.11, 612037. 10.3389/fgene.2020.612037 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song, M., Zhang, N., Cao, F. & Liu, J. PKNOX2 suppresses lung cancer cell proliferation by inhibiting the PI3K/AKT/mTOR axis. Exp. Ther. Med.25, 217. 10.3892/etm.2023.11917 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang, L. et al. PKNOX2 suppresses gastric cancer through the transcriptional activation of IGFBP5 and p53. Oncogene38, 4590–4604. 10.1038/s41388-019-0743-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wight, M. & Werner, A. The functions of natural antisense transcripts. Essays Biochem.54, 91–101. 10.1042/bse0540091 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faghihi, M. A. & Wahlestedt, C. Regulatory roles of natural antisense transcripts. Nat. Rev. Mol. Cell Biol.10, 637–643. 10.1038/nrm2738 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paraskevopoulou, M. D. & Hatzigeorgiou, A. G. Analyzing MiRNA-LncRNA interactions. Methods Mol. Biol.1402, 271–286. 10.1007/978-1-4939-3378-5_21 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Christian, F., Smith, E. L. & Carmody, R. J. The regulation of NF-κB subunits by phosphorylation. Cells5. 10.3390/cells5010012 (2016). [DOI] [PMC free article] [PubMed]
- 26.Bohmdorfer, G. & Wierzbicki, A. T. Control of chromatin structure by long noncoding RNA. Trends Cell Biol.25, 623–632. 10.1016/j.tcb.2015.07.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the conclusions of this article have been included in the manuscript. The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.