Abstract
Microvascular obstruction (MVO) is linked with adverse clinical outcome in acute coronary syndrome (ACS) patients, therefore, early prediction of MVO with non-invasive peripheral microcirculation is crucial in facilitating optimal treatment. Current study aims to analyze the significance of opisthenar microvessel area (OMA, measured using optical coherence tomography, OCT) in predicting coronary stenosis (Gensini score, GS) and short-term cardiac recovery of ACS patients and the results were compared to those of arterial stiffness parameters (Pulse Wave Velocity, PWV; Ankle-Brachial Index; ABI). Results showed that cardiac functional parameters (e.g. ejection fraction, EF; fractional shortening, FS) and OMA were higher in normal/low risk (GS 0–≤ 20, n = 69) compared to medium/high risk patients (GS > 20, n = 44, P < 0.0001). Furthermore, OMA, EF or FS was negatively associated with the severity of coronary stenosis (P < 0.0001). In addition, OMA was negatively correlated with heart and liver damage parameters (e.g. creatine kinase, CK; creatine kinase muscle brain, CKmB; lactate dehydrogenase, LDH; hydroxybutyrate dehydrogenase, HDBH; aspartate aminotransferase, AST; alanine aminotransferase, ALT) or with inflammatory markers (neutrophil, neutrophil/lymphocyte ratio, NEU, NEU/LYM (NLR); systemic immune inflammation index, SII and system inflammation response index, SIRI), indicating clinical significance of OMA. Conversely, PWV and ABI were not associated with coronary stenosis or organ damage markers. Receiver Operator Characteristic (ROC) analysis showed high specificity and sensitivity of OMA for coronary stenosis (specificity 0.864 or sensitivity 0.87). In conclusion, OMA shows negative associations with the severity of coronary stenosis and adverse clinical parameters indicate that microcirculation measurement from opisthenar possesses prognostic value for severe ACS patients.
Keywords: Coronary stenosis, Opisthenar microcirculation, Pulse wave velocity, Optical coherence tomography angiography, Cardiac function
Subject terms: Interventional cardiology, Biomarkers, Medical research
Introduction
Acute coronary syndrome (ACS) is one of the most severe types of heart diseases which leads to sudden cardiac death and heart failure worldwide. Recent prevalence of percutaneous coronary intervention (PCI) and pharmacological treatment restore coronary perfusion and the pumping function of the heart, but myocardial damage during coronary occlusion and post-reperfusion remains a major obstacle for optimal clinical outcome of ACS patients1,2. Microvascular dysfunction is one of the severe comorbidities post-hemodynamic disorders in ACS patients and is the target for intensive research.
Investigations of microvascular pathophysiology using non-invasive or invasive image techniques (e.g. myocardial contrast echocardiography or pressure sensor/thermistor-tipped guidewire3,4) indicated 30–40% incidence of microvascular obstruction (MVO) and delayed microvascular perfusion (dMVP), incidences peak within 24–72 h with or without sufficient recovery4,5. Importantly, ACS patients with MVO and dMVP showed reduced EF and increased mortality rate during 6, 12 to 65 months’ follow up6,7, suggesting that insufficient microvascular perfusion post-PCI or antithrombotic therapy define adverse outcome including heart failure or recurrence of coronary occlusion. Early detection and preventive measure of microvascular dysregulation is crucial to guide the interventions for optimal treatment and the improvement of clinical outcome. However, direct assessment of microvascular circulation in the heart is technically demanding, noninvasive procedures become a necessity.
Peripheral microcirculation provides optimal alternative to analyze the pathological progression of ACS patients8. For example, recent reports presented optical coherence tomography (OCT) measurement of retinal fundus vascular density showed significant reduction in myocardial infarction (MI) patients compared to those of healthy controls9. In addition, peripheral arterial tonometry to measure flow-mediated dilatation in the finger tips has been shown to be associated with adverse clinical outcome in ACS patients10,11. The downregulation of microcirculation was associated with increased risk of coronary artery disease (CVD). Capillary density from the dorsum of the middle phalanx was reduced in patients with premature CVD, and impaired peripheral microvascular blood flow predicts the incidence of cardiovascular events12. Recently, our lab has investigated the opisthenar microcirculation area (OMA, i.e. the back of the hand microvessel area) which showed significant reduction in hypertensive patients13. Furthermore, OMA and heart rate normalized OMA (OMA-HR) were shown to be associated with cardiac functional parameters in ACS patients treated with PCI14. Until recently, the implications of OMA on the extent of coronary stenosis has not been studied, which may provide prognostic values for better management of ACS patients.
In this study, we grouped the patients according to the Gensini score since such an angiographic and comprehensive scoring system is well established to assess the extent of atherosclerotic plaque, the severity of stenosis and clinical outcome15. By analyzing OMA in low and high Gensini score patients, the correlations with the extent of coronary stenosis, organ damage and inflammatory markers, we aimed to provide direct information of peripheral microcirculation index in the significance of pathogenesis in ACS patients.
Methods
Study design and participants
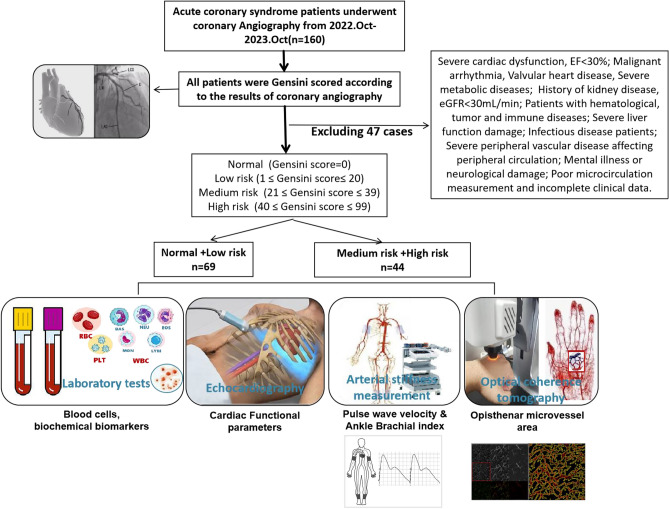
Database of 160 in hospital ACS patients admitted to the Cardiology Department of Yanbian University Affiliated Hospital in China from October 2022 to October 2023 were recruited to the analysis. As shown in Fig. 1, both OMA and arterial stiffness parameters were measured in the Central Laboratory of the Science and Technology Building. 47 cases were excluded from the analysis according to the exclusion criteria: Severe cardiac dysfunction (patients EF < 30% in intensive care units); Malignant arrhythmia, Valvular heart disease, Severe metabolic diseases; History of kidney disease, eGFR < 30 mL/min; Patients with hematological, tumor and immune diseases; Severe liver function damage; Infectious disease patients; Severe peripheral vascular disease affecting peripheral circulation; Mental illness or neurological damage; Poor microcirculation measurement and incomplete clinical data. Therefore, 113 patients were included in the study. Base on electrocardiographic diagnosis and myocardial damage markers, ACS patients could be categorized to acute myocardial infarction (AMI) and unstable angina with ST-segment elevation myocardial infarction (STEMI) or non-ST-segment elevation myocardial infarction (NSTEMI) patients. Those with no significant changes in electrocardiogram and myocardial injury markers were unstable angina. Among 113 patients, n = 32 are AMI, n = 21 are STEMI and n = 11 are NSTEMI). 67 were angina (unstable angina 47, stable angina 20) patients. 14 did not show angiography abnormalities.
Fig. 1.
Design of the study including ACS patients’ exclusion criteria and examination methods used in the study.
Gensini score (GS) is the sum of the degree of coronary arterial disease × corresponding coefficients of each branch. The degree of coronary arterial disease is defined as follow: 0 points for no vascular obstruction, 1 point for 1% to 24% vascular obstruction, 2 points for 25% to 50% vascular obstruction, 4 points for 51% to 75% vascular obstruction. Those with 76% to 90% blood vessel blockage were scored 8 points, those with 91% to 99% blood vessel blockage were scored 16 points, and those with complete blood vessel occlusion were scored 32 points. Corresponding coefficients was defined as follow: the common left main trunk coefficient is 5; the proximal, middle and distal coefficients of the left anterior descending branch are 2.5, 1.5 and 1.0, the first and second diagonal branches are 1.0 and 0.5 respectively; the proximal, middle and distal coefficients of the left circumflex branch are the same as the left anterior descending branch, the remaining posterior descending branch is 1.0, and the posterior collateral branch is 0.5; the right coronary coefficient is 1.0, and the collateral coefficients of other small blood vessels are all recorded as 0.5. As such, the subjects were divided into 4 groups according to the calculated GS (Table 1): normal (GS = 0), low-risk group (1 ≤ GS ≤ 20), medium risk group (21 ≤ GS ≤ 39), and high-risk group (40 ≤ GS ≤ 99). After calculating the scoring results, 113 patients were divided into 2 groups: normal + low risk group, GS ≤ 20 points, n = 69; medium + high risk group, GS > 20, n = 44.
Table 1.
Gensini score evaluation criteria.
| Lesion site | Coefficient | Stenosis degree | Score |
|---|---|---|---|
| Left trunk | 5.0 | 0% | 0 |
| Proximal left anterior descending branch or circumflex branch | 2.5 | 1–24% | 1 |
| Middle left anterior descending branch or circumflex branch | 1.5 | 25–50% | 2 |
| Distal left anterior descending branch or circumflex branch | 1.0 | 51–75% | 4 |
| Right coronary artery | 1.0 | 76–90% | 8 |
| First diagonal branch or posterior descending branch | 1.0 | 91–99% | 16 |
| Second diagonal branch or the posterior branch or blunt edge branch | 0.5 | 100% | 32 |
Gensini score is the sum of coronary stenosis degree score and lesion site score. The lesion score is the product of a single lesion score and coefficient: the coefficient indicates the importance of stenosis in different positions of the coronary artery system. Gensini score with ≤ 20 points was regarded as low risk; > 20 as medium to high risk.
Patients of normal + low risk group (0–20 points, n = 69) and medium + high risk group (> 21 points, n = 44) were compared with hemodynamic parameters including OMA, OMA-HR, cardiac functional parameters and arterial stiffness parameters (pulse wave velocity, PWV; ankle brachial index, ABI). Laboratory parameters including the blood cell counts, biochemical indexes for heart, liver and kidney functions and metabolic substrates (glucose, total glycerol, triglyceride, low-density lipoprotein, LDL and high-density lipoprotein, HDL) were measured from the venous blood on hospital admission and before the medical interventions. Color Doppler ultrasound examination was performed for the heart function analysis in the ultrasound medical department 2–3 days after the coronary arterial interventions.
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Affiliated Hospital of Yanbian University, and the written informed consent and medical research questionnaire were signed before the test population is examined. The personal information of the test population is completely confidential.
Optical coherence tomography (OCT) angiography measurement of OMA area
All participants used a non-invasive imaging system (OCT model, Micro VCC, UK, Fig. 1) to obtain opisthenar microcirculation image from the back of their hands (1 cm from the middle bone, Fig. 1). The testing personnel adjust the relevant parameters of the instrument, adjust the lens of the device to closely fit the back of the patient’s hand as much as possible, and prevent the pressure applied by the lens from affecting the microcirculation blood flow at the back of the hand.
Briefly, a high-speed super luminescent diode (central wavelength: l060 nm, repetition rate: 100 kHz, spectral bandwidth: 100 nm) scanned the field of view (4 mm × 4 mm) with 16 μm/pixel resolution. Laser reflectance of the surface of flowing red blood cells depict microvessels under the skin. Longitudinal (x-axis) and cross-sectional area (y axis) were scanned and blood vessels in the upper layer of epidermal structure (small arteries, venules and capillaries generated blood vessel diagram) were processed for image acquisition. The total microvascular area measured in the field and the vessel density, total number of connections, total blood vessel length, average blood vessel length, total number of end points of the measurements were quantified and calculated automatically in the software (Angio Tool). Each patient measure opisthenar microcirculation twice at room temperature (23–25 °C) 2–3 days after coronary interventions.
Arterial stiffness measurement
Brachial ankle pulse wave velocity (baPWV) and ankle brachial index (ABI) were measured using an automated device (HBP-8000, Omron, Japan) on the same day when OMA was measured. The patient is in a supine position for 5–10 min in a quiet state. The cuff is placed at the site of brachial and ankle artery pulsation, ensuring that the device is in normal use and the machine is connected. By inputting gender, date of birth, height information and measuring, blood pressure, pulse wave velocity (PWV) and ankle brachial index (ABI) was obtained. Record the average value of two consecutive measurements.
Statistical analysis
SPSS 23.0 (IBM) statistical software was used for data analysis. Data were represented as mean ± standard deviation (mean ± SD) or in quartiles (P25, P75). Non-parametric statistical analysis and multivariate analysis were used for comparison between groups. Pearson correlation analysis was used to analyze the association of each test parameter, The Receiver operating characteristic (ROC) curve was used to explore the relationship between OMA, OMA-HR, and coronary arterial stenosis. P < 0.05 was considered statistically significant.
Results
General information and clinical characteristics of two study groups
Table 2 showed that among general information, HR, DBP, MAP, and SaO2 were statistically significant between normal + low risk and medium + high risk groups (p < 0.05). Biochemical examination results showed significant differences in biomarkers of heart, liver and kidney functions (in particular, CK, CK-MB, LDH, HBDH, ALT, AST and Crea, p < 0.05). In blood routine tests, WBC, NEU and LYM, as well as RD-CV were significantly different between groups (p < 0.05).
Table 2.
Comparison of general information, biochemical indicators, blood routine between two group patients.
| Variable | Normal + Low risk n = 69 | Medium risk + High risk n = 44 | P | |
|---|---|---|---|---|
| General information | AGE(y) | 59.43 ± 7.65 | 59.88 ± 8.09 | 0.894 |
| HTN history | 37 | 19 | 0.281 | |
| Smoking history | 30 | 23 | 0.363 | |
| Sex | M = 40 F = 29 | M = 32 F = 12 | 0.113 | |
| BMI (kg/m2) | 25.75 ± 2.94 | 24.80 ± 3.87 | 0.133 | |
| HR (60–100)/min | 71.26 ± 9.25 | 74.86 ± 11.53 | 0.046* | |
| SBP (90–120) mmHg | 135 (117.5, 152.5) | 125.5 (114, 137) | 0.097 | |
| DBP (60–90) mmHg | 78 (72.5, 89) | 75 (70, 79.7) | 0.014* | |
| MAP (70–105) mmHg | 98.51 ± 14.47 | 92.26 ± 10.99 | 0.031* | |
| Heart | CK (0–200) U/L | 77 (55, 118.5) | 149.5 (75.5, 1363.5) | 0.000** |
| CK-MB (0–25) U/L | 14 (9, 18) | 20.5 (11.5, 113.7) | 0.001** | |
| LDH (0–220) U/L | 179 (157, 220) | 245.5 (183.5, 578.5) | 0.000** | |
| HBDH (0–295) U/L | 145 (128.5, 175) | 236 (142.5, 492.5) | 0.000** | |
| Kidney | BUN (2.5–7.0) mmol/L | 5.2 (4.3, 5.95) | 5.3 (4.5, 6.5) | 0.360 |
| CREA (75–115) mmol/L | 65 (53.5, 72) | 68 (60, 79.5) | 0.026* | |
| Liver | AST (0–40) U/L | 19 (16, 24.5) | 45.5 (22, 181.7) | 0.000** |
| ALT (0–40) U/L | 18 (14, 27) | 39.5 (21, 55.75) | 0.000** | |
| TB (5.1–25.6) umol/L | 11.1 (8.35, 14.7) | 11.45 (7.77, 14.17) | 0.793 | |
| TP (60–80 g)/L | 64 (61, 70.5) | 63 (60, 68.5) | 0.178 | |
| Metabolism | GLU (3.9–6.1) mmol/L | 5.11 (4.68, 5.58) | 5.28 (4.68, 6.13) | 0.197 |
| TG (0.48–1.88) mmol/L | 1.44 (1.05, 1.97) | 1.38 (0.88, 1.72) | 0.406 | |
| TC < 5.18 mmol/L | 3.83 (3.12, 5.06) | 4.21 (3.21, 5.39) | 0.300 | |
| LDL (0.00–3.12) mmol/L | 2.18 (1.83, 2.78) | 2.53 (1.85, 3.45) | 0.084 | |
| HDL > 0.9 mmol/L | 1.05 (0.9, 1.24) | 1.03 (0.96, 1.21) | 0.588 | |
| SaO2 (95–100%) | 0.97 (0.95, 0.98) | 0.975 (0.97, 0.98) | 0.004** | |
| Blood routine | WBC (4–10) × 10^9/L | 6.37 (5.43, 8.08) | 7.28 (6.0, 9.67) | 0.039* |
| RBC (3.5–5.5) × 10^12/L | 4.51 ± 0.57 | 4.41 ± 0.51 | 0.189 | |
| HGB (110–160) g/L | 141.84 ± 17.75 | 139.31 ± 15.93 | 0.511 | |
| PLT (100–300) × 10^9/L | 207 (181, 242) | 199.5 (183.5, 232) | 0.768 | |
| RDW-SV (37-50fL) | 43 (40.5, 44.5) | 44 (41, 45) | 0.056 | |
| RDW-CV (11.5–14.5%) | 13 (12, 13) | 13 (12.25, 14) | 0.033* | |
| NEU (1.8–6.3) × 10^9/L | 4.04 (2.92, 5.44) | 5.2 (3.58, 7.69) | 0.005** | |
| LYM(1.1–3.2) × 10^9/L | 1.71 (1.45, 2.47) | 1.54 (1.1, 2.0) | 0.024* | |
| MON (0.1–0.6) × 10^9/L | 0.46 ± 0.18 | 0.49 ± 0.20 | 0.235 | |
| EOS (0.02–0.5) × 10^9/L | 0.1 (0.05, 0.18) | 0.075 (0.02, 0.15) | 0.240 | |
| BAS (0–0.06) × 10^9/L | 0.03 (0.02, 0.04) | 0.025 (0.02, 0.04) | 0.181 |
Normal + Low risk: Gensini score ≤ 20, Medium + High risk: Gensini points > 20; P . Normal + Low risk vs. Medium + High risk *P < 0.05, **P < 0.01.
Comparison of parameters of cardiac function, arterial stiffness and microvessels
Table 3 showed that EF, FS, and CO levels were lower and LVDS, EDV, ESV, E and E/e ' were greater in medium + high risk group (p < 0.05). PWV or ABI were not different between two groups (p > 0.05). Notably, OMA and OMA-HR were significantly reduced in medium + high risk group (p < 0.001).
Table 3.
Comparison of cardiac function, arterial stiffness and OMA in two groups patients.
| Variable | Normal + Low risk n = 69 | Medium risk + High risk n = 44 | P | |
|---|---|---|---|---|
| Echocardiography | EF (0.57–0.75) | 0.62 (0.60, 0.63) | 0.59 (0.57, 0.61) | 0.000** |
| FS (0.20–0.40) | 0.31 (0.30, 0.32) | 0.30 (0.27, 0.31) | 0.000** | |
| SV (70–90) ml | 73 (70, 74) | 70 (68, 74) | 0.087 | |
| CO (3–6) l/min | 5.2 (4.9, 5.3) | 5.0 (4.5, 5.1) | 0.000** | |
| DT (180–240) ms | 210 (188, 239) | 200 (188, 227) | 0.268 | |
| LVDD | 46 (43, 48.5) | 47 (45, 49.7) | 0.107 | |
| LVDS | 28 (26, 30) | 30 (28, 32) | 0.007** | |
| EDV | 117.74 (113.33, 120.97) | 121.67 (114.87, 129.69) | 0.005** | |
| ESV | 45.33 (43.18, 48) | 49.66 (45.5, 55) | 0.000** | |
| E | 0.6 (0.5, 0.7) | 0.7 (0.6, 0.8) | 0.013** | |
| A | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.212 | |
| e′ | 0.10 (0.09, 0.13) | 0.10 (0.08, 0.12) | 0.074 | |
| E/A | 0.83 (0.67, 0.97) | 0.89 (0.75, 1.14) | 0.171 | |
| E/e′ | 5.69 (5.0, 7.5) | 7.5 (6.0, 9.17) | 0.000** | |
| Arterial stiffness | BaPWV R (M/S) | 1563 (1387, 1819) | 1601 (1419, 1877) | 0.398 |
| BaPWV L (M/S) | 1578 (1394, 1758) | 1593 (1480, 1838) | 0.244 | |
| ABI.R | 1.11 ± 0.10 | 1.09 ± 0.09 | 0.477 | |
| ABI.L | 1.09 ± 0.09 | 1.08 ± 0.09 | 0.540 | |
| Microvessels | OMA (pixel) | 27,549.05 ± 2301.48 | 25,995.11 ± 1745.01 | 0.000** |
| OMA-HR (pixel/m) | 392.31 ± 57.18 | 355.81 ± 62.45 | 0.001** |
*P < 0.05, **P < 0.01.
Correlation with the severity of coronary stenosis
Correlation analysis in Table 4 showed that severity of coronary stenosis was negatively correlated with: (1) EF (r = − 0.45, p < 0.01), FS (r = − 0.41, p < 0.01), SV (r = − 0.068, p < 0.05), and CO. (r = − 0.325, p < 0.01); (2) OMA (r = − 0.373, p < 0.01) and OMA-HR (r = − 0.221, p < 0.05). PWV or ABI were not correlated with the severity of coronary stenosis (p > 0.05).
Table 4.
Correlation with the severity of coronary stenosis.
| Variable | Degree of coronary stenosis | ||
|---|---|---|---|
| r | p | ||
| Echocardiography | EF (0.57–0.75) | − 0.450 | 0.000** |
| FS (0.20–0.40) | − 0.410 | 0.000** | |
| SV (70–90) ml | − 0.068 | 0.006** | |
| CO (3–6) l/min | − 0.325 | 0.001** | |
| DT (180–240) ms | − 0.130 | 0.180 | |
| Arterial stiffness | BaPWV R (M/S) | 0.134 | 0.158 |
| BaPWV L (M/S) | 0.156 | 0.098 | |
| ABI.R | − 0.062 | 0.514 | |
| ABI.L | − 0.010 | 0.919 | |
| Microvessel | OMA (pixel) | − 0.373 | 0.000** |
| OMA-HR (pixel/m) | − 0.221 | 0.019* | |
| Inflammation | NEU | 0.210 | 0.026* |
| LYM | − 0.202 | 0.032* | |
| MONO | 0.147 | 0.121 | |
| NLR | 0.220 | 0.019* | |
| MLR | 0.288 | 0.002** | |
| PLR | 0.120 | 0.204 | |
| SII | 0.140 | 0.138 | |
| SIRI | 0.302 | 0.001** | |
*P < 0.05, **P < 0.01.
Since inflammation is known to be correlated strongly with the disease progression, we compared the markers from blood routine results. As shown in Table 4, except for LYM, which showed a significant negative correlation, the severity of coronary stenosis was positively correlated with NEU, NLR, MLR and SIRI (p < 0.05).
Correlation between cardiac function, arterial stiffness and microvessels with biochemical indicators
Correlation analysis were conducted between biochemical parameters and cardiac function, arterial stiffness and microvessels in all patients. As shown in Table 5, OMA and OMA-HR showed significant and negative correlations with heart and liver function parameters (CK, CK-MB, LDH, HDBH, AST, ALT, p < 0.05); similar correlations were observed with heart functional parameters such as EF and FS with heart, kidney and liver functional indexes (CK-MB, CREA, AST, TP, p < 0.05). However, PWV or ABI showed no correlation with any of organ function parameters.
Table 5.
Correlation analysis between biochemical indicators with cardiac function, arterial stiffness and OMA in all patients.
| Variable N = 113 | CK | CK-MB | LDH | HDBH | BUN | CREA | AST | ALT | TB | TP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| EF (0.57–0.75) | − 0.183 | 0.053 | 0.146 | 0.122 | − 0.094 | 0.323 | 0.113 | 0.235 | 0.065 | 0.494 | − 0.209 | 0.026* | − 0.220 | 0.019* | − 0.089 | 0.349 | − 0.125 | 0.189 | − 0.231 | 0.014* |
| FS (0.20–0.40) | − 0.101 | 0.288 | 0.231 | 0.014* | − 0.049 | 0.607 | 0.117 | 0.217 | 0.099 | 0.297 | − 0.178 | 0.060 | − 0.255 | 0.006** | − 0.092 | 0.333 | − 0.077 | 0.418 | − 0.207 | 0.027* |
| BaPWV R (M/S) | − 0.028 | 0.776 | 0.012 | 0.903 | − 0.032 | 0.739 | − 0.073 | 0.442 | 0.146 | 0.123 | 0.007 | 0.942 | − 0.010 | 0.920 | − 0.041 | 0.688 | 0.006 | 0.952 | 0.050 | 0.596 |
| BaPWV L (M/S) | − 0.087 | 0.358 | − 0.057 | 0.549 | − 0.085 | 0.370 | − 0.105 | 0.268 | 0.170 | 0.072 | 0.130 | 0.168 | − 0.040 | 0.671 | − 0.069 | 0.470 | − 0.058 | 0.543 | 0.092 | 0.333 |
| ABI.R | − 0.157 | 0.096 | − 0.174 | 0.066 | − 0.108 | 0.255 | − 0.044 | 0.642 | 0.027 | 0.780 | 0.058 | 0.544 | − 0.160 | 0.090 | − 0.164 | 0.083 | − 0.048 | 0.615 | − 0.019 | 0.844 |
| ABI.L | − 0.159 | 0.093 | − 0.154 | 0.102 | − 0.097 | 0.306 | − 0.096 | 0.313 | 0.026 | 0.788 | − 0.015 | 0.873 | − 0.166 | 0.078 | − 0.071 | 0.458 | − 0.215 | 0.022* | − 0.058 | 0.545 |
| OMA (pixel) | − 0.121 | 0.202 | − 0.075 | 0.431 | − 0.204 | 0.030* | − 0.176 | 0.062 | − 0.103 | 0.277 | − 0.027 | 0.780 | − 0.223 | 0.018* | − 0.089 | 0.348 | − 0.177 | 0.060 | 0.152 | 0.109 |
| OMA-HR(pixel/m) | − 0.225 | 0.017* | − 0.234 | 0.012* | − 0.198 | 0.035* | − 0.227 | 0.016* | − 0.122 | 0.198 | − 0.084 | 0.375 | − 0.262 | 0.005** | − 0.218 | 0.020* | − 0.094 | 0.320 | 0.051 | 0.592 |
*P < 0.05, **P < 0.01.
Correlation analysis between inflammation and cardiac function, arterial stiffness and microvessels parameters
Table 6 showed results of inflammatory indicators and cardiac function in all patients. EF or FS showed negative correlations with NEU, NEU/LYM ratio (NLR), MON/LYM ratio (MLR) and SIRI (p < 0.05). Similar analysis between inflammatory indicators and OMA or OMA-HR were conducted (Table 6) and the results showed that OMA or OMA-HR were negatively correlated with NEU, NLR, SII and SIRI (p < 0.05).
Table 6.
Correlation analysis between inflammation and cardiac function, arterial stiffness and OMA in all patients.
| Variable N = 113 | NEU | LYM | MONO | NLR | MLR | SII | SIRI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| EF (0.57–0.75) | − 0.183 | 0.053* | 0.146 | 0.122 | − 0.094 | 0.323 | − 0.209 | 0.026* | − 0.220 | 0.019* | − 0.125 | 0.189 | − 0.231 | 0.014* |
| FS (0.20–0.40) | − 0.101 | 0.288 | 0.231 | 0.014* | − 0.049 | 0.607 | − 0.178 | 0.060 | − 0.255 | 0.006** | − 0.077 | 0.418 | − 0.207 | 0.027* |
| BaPWV R (M/S) | − 0.042 | 0.660 | − 0.134 | 0.158 | − 0.217 | 0.021* | 0.053 | 0.587 | − 0.163 | 0.084 | 0.054 | 0.568 | − 0.175 | 0.064 |
| BaPWV L (M/S) | 0.035 | 0.715 | − 0.113 | 0.234 | − 0.187 | 0.047* | 0.093 | 0.325 | − 0.154 | 0.105 | 0.058 | 0.541 | − 0.109 | 0.248 |
| ABI.R | − 0.259 | 0.006** | 0.021 | 0.825 | − 0.047 | 0.619 | − 0.189 | 0.045* | 0.016 | 0.863 | − 0.167 | 0.077 | − 0.164 | 0.083 |
| ABI.L | − 0.276 | 0.003** | 0.010 | 0.916 | 0.018 | 0.853 | − 0.226 | 0.016* | 0.058 | 0.542 | − 0.188 | 0.047* | − 0.161 | 0.088 |
| OMA (pixel) | − 0.151 | 0.110 | 0.136 | 0.152 | 0.014 | 0.885 | − 0.182 | 0.053 | − 0.136 | 0.151 | − 0.119 | 0.210 | − 0.198 | 0.035* |
| OMA-HR (pixel/m) | − 0.276 | 0.003** | 0.125 | 0.186 | − 0.005 | 0.958 | − 0.225 | 0.017* | − 0.145 | 0.126 | − 0.210 | 0.026* | − 0.277 | 0.003** |
*P < 0.05, **P < 0.01.
Intriguingly, PWV was shown to be negatively correlated with MON (p < 0.05) whereas ABI was negatively correlated with NEU, NLR and SII (p < 0.05).
AUC analysis of OMA and OMA-HR
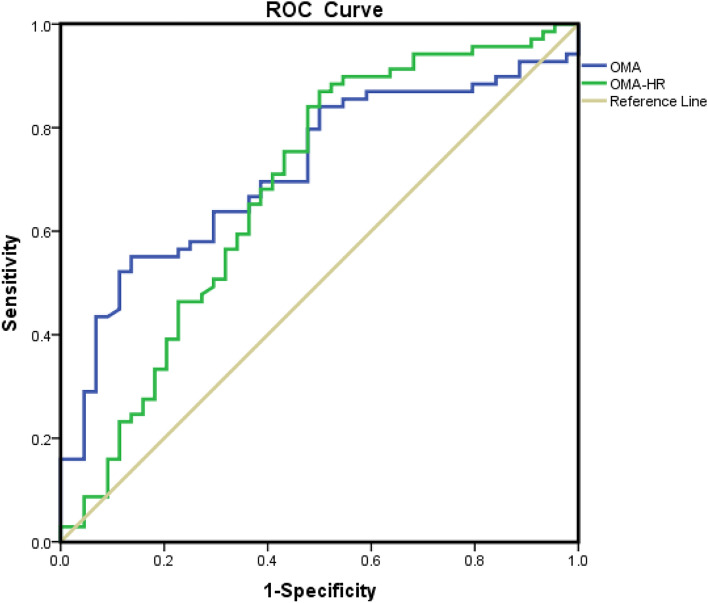
We went on and analyzed the sensitivity and specificity of OMA and OMA-HR for the degree of coronary stenosis (Fig. 2). AUC of OMA is 0.717 (specificity 0.864, sensitivity 0.551), with a cutoff value of 27,693 pixels. AUC of OMA-HR is 0.681 (sensitivity 0.87, specificity 0.5), with a cutoff value of 339.64 pixels/m.
Fig. 2.
Receiver Operator Characteristic (ROC) curve predicting coronary stenosis. Opisthenar microvessel area (OMA): area under the curve (AUC) 0.717, cut-off 27,693 pixel, sensitivity 0.551, specificity 0.864. Heart rate normalized OMA (OMA-HR): area under the curve 0.681, cut-off 339.64 pixel/m, sensitivity 0.87, specificity 0.5.
Discussion
The main findings of this study are the identification of OMA (measured using optical coherence tomography OCT from ACS patients 2–3 days after the medical interventions), which was significantly reduced in medium + high risk coronary stenosis patients, that is associated with the GS determination of the severity of coronary stenosis. The results showed that EF, FS, SV, CO as well as OMA were negatively correlated with the severity of coronary stenosis and NEU, NEU/LYM ratio, MON/LYM ratio and SIRI were positively correlated with the severity of coronary stenosis. OMA was also associated with heart and liver damage parameters in ACS patients. Arterial stiffness indexes, PWV or ABI were not affected and were not associated with the severity of coronary stenosis. AUC analysis indicated high sensitivity and specificity of OMA and OMA-HR with the coronary stenosis in ACS patients. Taken together, through comprehensive analysis of cardiac function, main arterial properties, peripheral microvessels, inflammatory indexes and biochemical organ damage indexes, we provide robust evidence to suggest that non-invasive OMA can be a valuable parameter to predict the severity of coronary arterial diseases.
Hemodynamic coherence following acute coronary syndrome is important and sufficient blood flow through microcirculation is essential in maintaining target organ functions. In general, cardiac function is assessed using echocardiography where EF and FS are regarded as golden standard to represent the functional recovery following the therapy of ACS patients. In fact, measured indexes are often within normal range in the groups examined, but EF, FS, CO and SV were significantly higher in normal + low risk group compared to those of medium + high risk group (Table 3), which was accompanied by lower end-diastolic and end-systolic volume or length indexes (e.g. EDV, ESV, LVDD). These results are consistent with greater heart, kidney and liver damage indexes in medium + high risk patients. Furthermore, NEU is greater and LYM is smaller, indicative of inflammatory profiling changes with the extent of the diseases. Importantly, OMA and OMA-HR were significantly reduced in medium + high risk patient group where cardiac function was reduced, suggesting impaired microcirculation in peripheral organs due to insufficient hemodynamic coherence. The analysis results suggest that OMA or OMA-HR could be additional functional indicators of the recovery of ACS patients. This is important because peripheral microcirculation such as retinal microvessels measured from coronary heart disease patients or systemic hypertensive patients showed similar reduction16–18. It should be noted that neither PWV nor ABI were shown to be different between normal + low risk and medium + high risk patient groups. As such, arterial stiffness indexes which represent arterial properties of the patients are not affected by the disease or the recovery of the disease, per se.
Changes in the microcirculation post- therapy in ACS patients are important in understanding the functional recovery and cardiovascular stratification. Accumulating evidences showed that in majority of STEMI patients post-PCI, microvascular perfusion of the heart was reduced when detected with cardiac magnetic resonance (CMR) imaging19. Endothelial dysfunction or microvascular thrombi may cause the disruption of coronary reperfusion and clinical consequence can be huge due to the progression of irreversible myocardial hemorrhage at the infarct core, which could be associated with mortality and re-hospitalization due to heart failure20.
Due to the impracticality of measuring microcirculation of the heart post-therapy, peripheral sources such as skin microcirculation can be optimal alternative. Indeed, skin microcirculation is considered to be a representative vascular bed for assessing microvascular reactivity and has been proposed as a prognostic marker of the restoration of normal microcirculation in ACS patients21,22. In the current study, the significance of OMA is further confirmed by showing clear associations between OMA/OMA-HR and the severity of coronary stenosis (Table 4). Similar associations were observed with EF, FS, SV and CO as well as inflammatory indexes such as NEU, LYM, NLR, MLR and SIRI (Table 6), supporting predictive values in ACS patients. It should be noted that OMA or OMA-HR were significantly associated with heart, liver functional parameters from blood test results and inflammatory indexes on admission to the hospital, further indicating the prognostic values in assessing the severity of ACS patients. PWV or ABI were not associated with the severity of coronary stenosis, discriminating macro- and microvessels in such a clinical significance.
Over the last two decades, techniques and consensus of measuring microcirculation in various diseases are overwhelmed, with non-invasive and robust optimal coherence tomography angiography (OCTA) being central to detect morphological and functional changes of the organ microcirculation (such as retina, sublingual, cerebrum, gut, and opisthenar). As the current analysis suggests, microcirculation results are convincing in assessing clinical implications or outcomes in patients including acute coronary syndrome7,8. E.g. impaired retinal or cerebral microcirculation is shown to be prevalent in high risk coronary arterial disease population23. The AUC analysis further supports the high specificity and sensitivity of OMA and OMA-HR on assessing the disease status.
Taken together, Gensini score classification of ACS patients revealed that peripheral microcirculation, OMA, associates with the severity of coronary stenosis. Larger population studies are needed to determine hemodynamic coherence for comprehensive understanding of the functional recovery of ACS patients. Microcirculation is important in better therapeutic management in severe ACS patients.
Author contributions
C.C., J.W., T.T.H., B.L.Z., Z.Y.G., X.J., Z.H.Z., are involved in the patients’ database analysis, measurement, data analysis and writing. M.H.L., J.X.L. are involved in statistics, L.C., W.H.X., X.N.H., Y.H.Z. are involved in project design, manuscript review and finalization.
Funding
National Natural Science Foundation of China, Grant/Award Number: NSFC31860288 and NSFC32260213; National Natural Science Foundation of China, Grant/Award Number: 82100307; Korean National Research Foundation of Korea (NRF), Grant/Award Number: 2023R1A2C2007021; Korean Society of Hypertension (Grant number KSH-R-2020); BK21 Four for Seoul National University College of Medicine.
Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dedovic, V., Stankovic, S., Asanin, M. & Vukcevic, V. Coronary microcirculation: The next frontier in the management of STEMI. J. Clin. Med.12(4), 1602 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scarsini, R. et al. Angiography-derived and sensor-wire methods to assess coronary microvascular dysfunction in patients with acute myocardial infarction. JACC Cardiovasc. Imaging16(7), 965–981 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Aarnoudse, W. et al. Myocardial resistance assessed by guidewire-based pressure-temperature measurement: in vitro validation. Catheter. Cardiovasc. Interv.62(1), 56–63 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Galiuto, L., Garramone, B., Scarà, A., Rebuzzi, A. G., Crea, F., La Torre, G., Funaro, S., Madonna, M., Fedele, F., Agati, L., AMICI Investigators. The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: results of the multicenter AMICI study. J. Am. Coll. Cardiol.51(5), 552–9 (2008). [DOI] [PubMed]
- 5.Aggarwal, S., Xie, F., High, R., Pavlides, G. & Porter, T. R. Prevalence and predictive value of microvascular flow abnormalities after successful contemporary percutaneous coronary intervention in acute ST-segment elevation myocardial infarction. J. Am. Soc. Echocardiogr.31(6), 674–682 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Xie, F., Qian, L., Goldsweig, A., Xu, D. & Porter, T. R. Event free survival following successful percutaneous intervention in acute myocardial infarction depends on microvascular perfusion. Circ. Cardiovasc. Imaging13, e010091 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Yoon, G. S. et al. The index of microcirculatory resistance after primary percutaneous coronary intervention predicts long-term clinical outcomes in patients with ST-segment elevation myocardial infarction. J. Clin. Med.10(20), 4752 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva, M., Paiva, L., Teixeira, R., Ferreira, M. J. & Gonçalves, L. Microcirculation function assessment in acute myocardial infarction: A systematic review of microcirculatory resistance indices. Front. Cardiovasc. Med.9, 1041444 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu, J. Y. et al. Ocular microvascular alteration in patients with myocardial infarction-a new OCTA study. Sci. Rep.14(1), 4552 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komura, N. et al. Impaired peripheral endothelial function assessed by digital reactive hyperemia peripheral arterial tonometry and risk of in-stent restenosis. J. Am. Heart Assoc.5(6), e003202 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montone, R. A. et al. Endothelial dysfunction as predictor of angina recurrence after successful percutaneous coronary intervention using second generation drug eluting stents. Eur. J. Prev. Cardiol.25(13), 1360–1370 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Tibirica, E., Souza, E. G., De Lorenzo, A. & Oliveira, G. M. Reduced systemic microvascular density and reactivity in individuals with early onset coronary artery disease. Microvasc. Res.97, 105–108 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Guo, Z. Y. et al. Opisthenar microvessel area as a sensitive predictive index of arterial stiffness in hypertensive patients. Sci. Rep.11(1), 23616 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, C. et al. Enlargement of opisthenar microcirculatory area predicts impaired heart function in severe acute coronary syndrome patients. Microcirculation30(4), e12803 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Charach, L. et al. Using the Gensini score to estimate severity of STEMI, NSTEMI, unstable angina, and anginal syndrome. Medicine (Baltimore)100(41), e27331 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun, C. et al. Systemic hypertension associated retinal microvascular changes can be detected with optical coherence tomography angiography. Sci. Rep.10(1), 9580 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, W. H. et al. Retinal microvascular change in hypertension as measured by optical coherence tomography angiography. Sci. Rep.9(1), 156 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, J. et al. Retinal and choroidal vascular changes in coronary heart disease: An optical coherence tomography angiography study. Biomed. Opt. Express10(4), 1532–1544 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lechner, I. et al. Clinical outcomes associated with various microvascular injury patterns identified by CMR after STEMI. J. Am. Coll. Cardiol.83(21), 2052–2062 (2024). [DOI] [PubMed] [Google Scholar]
- 20.Carrick, D. et al. Myocardial hemorrhage after acute reperfused ST-segment-elevation myocardial infarction: Relation to microvascular obstruction and prognostic significance. Circ. Cardiovasc. Imaging9(1), e004148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holowatz, L. A., Thompson-Torgerson, C. S. & Kenney, W. L. The human cutaneous circulation as a model of generalized microvascular function. J. Appl. Physiol.105(1), 370–372 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Mahé, G., Humeau-Heurtier, A., Durand, S., Leftheriotis, G. & Abraham, P. Assessment of skin microvascular function and dysfunction with laser speckle contrast imaging. Circ. Cardiovasc. Imaging5(1), 155–163 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Mejia-Renteria, H. et al. Coronary microvascular dysfunction is associated with impaired cognitive function: the Cerebral-Coronary Connection study (C3 study). Eur. Heart J.44(2), 113–125 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.