Abstract
Tregs and M2-type macrophages are essential for immune surveillance. CD38 + NK cells are involved in immunoregulation by modulating cytokine secretion. This study investigated how CD38 + NKs affect Tregs and macrophages in colorectal cancer (CRC). Higher proportions of CD38 + NKs and Tregs were detected in bloods and tumor tissues of CRC patients than that in the samples from healthy controls (HCs). Compared with CD38 + NKs from HCs, the NK cells from CRC promoted the differentiation of Tregs from CD4 + T cells, and secreted increased levels of IL-10, TGF-β and TNF-α and decreased levels of IFN-γ. CD38 + NKs from CRC expressed higher levels of CD38, NF-κB and acetyl-NF-κB and lower levels of Sirt1. When CRC CD38 + NK cells were treated with anti-CD38 monoclonal antibody, the above trends were reversed. CRC CD38 + NKs with treatment of NF-κB inhibitor also showed opposite effects on cytokine secretion and CD4 + T-cell differentiation. After treatment with a Sirt1 activator, NF-κB signaling was inhibited in these CD38 + NKs, whereas treatment with a Sirt1 inhibitor activated NF-κB signaling. The supernatants of CRC CD38 + NK culture promoted M0 macrophage polarization to M2-type. We suggest that CD38 modulates cytokine secretion by NK cells through Sirt1/NF-κB signaling pathway, thereby suppressing immune surveillance in tumorigenesis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79008-8.
Subject terms: Colorectal cancer, Tumour immunology
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors worldwide1. The tumorigenesis and development of CRC involve multiple abnormalities in the immune response2. CD4 + CD25 + forkhead box protein 3+ (FOXP3+) regulatory T cells (Tregs) suppress immune responses and facilitate tumor cell escape3–5. Naive CD4 + T cells can differentiate into helper T (Th) cells and Tregs under the induction of cytokines6. IFN-γ is well known for stimulating the differentiation of helper T1 (Th1) cells, but it inhibits Treg differentiation7,8; furthermore, TGF-β can induce the differentiation of Tregs8. In addition, macrophages play important roles in tumor growth and immune surveillance. In brief, M1-type macrophages inhibit tumor growth, whereas M2 macrophages promote tumor growth; M2-type macrophages are thus termed tumor-associated macrophages (TAMs)9,10. The polarization of macrophages in vitro is also influenced by cytokines, such as IFN-γ and TNF-α, which promote M1 polarization, whereas IL-10 and TGF-β induce M2 polarization11,12.
Natural killer cells (NK cells) are part of the innate immune system13. They possess functions that range widely from immune responses against tumor cells and viral infection14,15 to immunoregulatory activities, which are performed through the secretion of chemokines and cytokines16,17. CD38 functions as an enzyme with NAD-depleting and intracellular signaling activity or as a receptor with adhesive functions. CD38 can be expressed on the cell surface, where it may face the extracellular milieu or the cytosol. Structurally, CD38 is a type II transmembrane glycoprotein that is expressed by NK, plasma and plasmacytoid dendritic cells18. Anti-CD38 monoclonal antibodies can bind to CD38 on the cell surface to suppress CD38 activity19. CD38 modulates features of immune cells and is involved in the immunosuppression of tumors such as esophageal cancer (EC)20 and CRC21. Ayelet et al. reported that, in glioma transplantation models, CD38 knockout mice presented attenuated glioma expansion and had longer lifespans than did wild-type mice22. CD38 + NK cells constitute a subtype of NK cells. These cells have been found in multiple myeloma23, systemic lupus erythematosus24 and gouty arthritis patients25. Our group reported that CD38 + NK cells from patients with rheumatoid arthritis (RA) modulated T-cell immune balance by regulating cytokine secretion26–28. We recently reported that the proportion of CD38 + NK cells was significantly greater in patients with positive lymph node metastasis. Furthermore, a high proportion of CD38 + NK cells is associated with poor prognosis in CRC patients29. However, the role of CD38 + NK cells from tumors in the differentiation of CD4 + T cells remains unclear. In addition, the mechanism by which CD38 regulates cytokine secretion by NK cells has not been well investigated.
In this study, we measured the proportions of CD38 + NK cells and Tregs in the blood of CRC patients and healthy controls (HCs). CD38 + NK cells were isolated from the peripheral blood of HCs and from those with CRC. These NK cells were separately cocultured with CD4 + T cells in transwell chambers to observe the effect of CD38 + NK cells on CD4 + T-cell differentiation. The proportions of Th1, Th2, Th17 and Treg cells were determined after coculture. The supernatants of CD38 + NK cells were collected to determine the levels of cytokines. We also investigated the effects of CD38 + NK cells on the polarization of macrophages. PCR, Western blotting and transcriptome analyses were conducted to explore the mechanism by which CD38 modulates cytokine secretion by NK cells.
CD38 can inhibit sirtuin 1 (Sirt1) by breaking down nicotinamide adenine dinucleotide (NAD+)30–32. The CD38-NAD-Sirt1 axis affects inflammatory responses in many diseases32–35. As a deacetylase, Sirt1 is involved in the deacetylation of various nonhistone targets, including NF-κB30. Sirt1 physically interacts with the RelA/p65 subunit of NF-κB and inhibits its transcription via the deacetylation of RelA/p6536, 37. Antagonistic crosstalk between NF-κB and Sirt1 regulates innate immunity and energy metabolism37,38. Thus, the present study also investigated the effect of CD38 on the Sirt1 and NF-κB axis. Furthermore, transcriptome analysis was conducted to explore the regulatory mechanism of CD38 + NK cells. This investigation may be useful for explaining the role of CD38 + NK cells in CD4 + T-cell differentiation, macrophage polarization and the causes of immune surveillance disruption in tumors.
Materials and methods
Sample collection
The study included patients with a confirmed diagnosis of lung cancer (LC), esophageal cancer (EC) or colorectal cancer (CRC) on the basis of pathological assessment of biopsied samples. The exclusion criteria included the presence of comorbidities such as tuberculosis, diabetes mellitus or chronic heart failure, as well as incomplete medical records. Additional exclusion criteria for patients with colorectal cancer and esophageal cancer included the following: (1) multiple primary colorectal cancers; (2) other malignant tumors within five years; and (3) emergency surgery indications such as digestive tract obstruction, perforation or bleeding. After applying the inclusion and exclusion criteria, blood samples were collected from patients with LC (n = 86), EC (n = 37) or CRC (n = 125). In addition, 142 blood samples were collected from HCs. (Supplementary Table 1).
This study was designed according to the Declaration of Helsinki of the World Medical Association. The experimental design was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFYWZLL27438). All patients signed informed consent forms.
Measurement of CD38 + NK cells in peripheral blood
Fluorescein isothiocyanate (FITC)-conjugated anti-human CD3 (BioLegend, USA), phycoerythrin (PE)-conjugated anti-human CD56 (BioLegend), and allophycocyanin (APC)-conjugated anti-human CD38 (BD Biosciences) were added to the blood samples. FITC IgG2a, PE IgG1 and APC IgG1 were used as controls. CD38 + NK cells (CD3- CD56 + CD38+) and CD38 + NK-like T cells (CD3 + CD56 + CD38+) were detected via flow cytometry (Agilent, USA).
Separation of CD38 + NK cells
Peripheral blood samples from HCs or CRC patients were collected and maintained in heparin sodium anticoagulant. Peripheral blood mononuclear cells (PBMCs) were obtained via density gradient centrifugation with a Ficoll lymphocyte isolation solution (TBD Science, China).
The NK cell biotin-antibody cocktail (Miltenyi, Germany) was added to the PBMCs. Then, an antibiotin microbead solution (Miltenyi, Germany) was added to the mixture. Negative selection of NK cells was performed by adding the cell suspension to an LS column (Miltenyi). Then, CD38 biotin (Miltenyi, Germany) was added to the NK cell suspension. After centrifugation and resuspension of the pellet, antibiotin microbeads were added to the mixture. Positive selection of CD38 + NK cells was performed by loading the aforementioned mixture into an MS column (Miltenyi, Germany).
Separation and stimulation of naive CD4 + T cells
PBMCs from HCs were mixed with a naive CD4 + T-cell and biotin-antibody cocktail (Miltenyi, Germany). Then, an antibiotin microbead cocktail (Miltenyi, Germany) was added. The naive CD4 + T cells were negatively selected by loading the mixture onto an LS column (Miltenyi, Germany). The CD4 + T cells were subsequently activated via incubation with anti-human CD3 (500 ng/ml, T&L Biological Technology, China) and anti-human CD28 (500 ng/ml, T&L Biological Technology) for 24 h. After the cells were washed with a 1× PBS solution, they were used for coculture.
Coculture experiments
CD38 + NK cells (approximately 1 × 106/ml) from HCs or CRC patients were incubated with an anti-CD38 monoclonal antibody at a concentration of 5 µg/ml (CD38 mAb, Johnson & Johnson, USA) or control IgG at a concentration of 5 µg/ml (C-IgG, Proteintech) for 24 h. After incubation, these NK cells were washed with 1× PBS solution. The cells were resuspended at a concentration of 1 × 106/ml in RPMI-1640 (Gibco, USA) containing 10% human heat-inactivated pooled AB serum (Gibco, USA) and then were seeded into the upper chamber of a transwell apparatus with 0.4-µm pores (Corning-Costar, USA). Activated naive CD4 + T cells (approximately 1 × 105/ml) from HCs were seeded in the lower chamber of the transwell apparatus. After coculturing at 37 °C in the presence of 5% CO2 for 48 h, the cells were harvested, and the proportions of Tregs and Th17, Th1 and Th2 cells in the CD4 + T-cell population were determined via flow cytometry.
CD38 + NK cells were also pretreated with resveratrol (RSV, MCE, USA), an activator of Sirt1, at a concentration of 20 µM; nicotinamide (NAM, MCE), an inhibitor of Sirt1, at a concentration of 100 µM; or pyrrolidinedithiocarbamate ammonium (PDTC, MCE), an inhibitor of NF-κB, at a concentration of 25 µM. Following washing, these pretreated NK cells were cocultured with activated naive CD4 + T cells as described above.
Measuring cytokine levels via flow cytometry
The pretreated CD38 + NK cells (1 × 106/ml) were cultured alone for 48 h, and the supernatant was collected for cytokine detection. A customized human essential immune response panel mix-and-match kit (BioLegend, USA) was used. Assay buffer and beads were added to the samples. After centrifugation, the detection antibodies from the kit were added to the pellets. Then, streptavidin (SA)-PE was added to the mixture. Flow cytometry (Agilent) was performed to determine the levels of cytokines, including IL-10, TNF-α, IFN-γ and TGF-β. Data analysis was conducted on the LEGENDplex chip platform.
Quantifying tregs via flow cytometry
A human regulatory T-cell staining kit (Multi Sciences, China) was used. Anti-human CD4-FITC and anti-human CD25-APC antibodies were added to blood samples or cocultured CD4 + T cells. Next, 1× fixation/permeabilization working solution was added to the mixture. After centrifugation, the pellets were resuspended in 1×permeabilization buffer, and then anti-human FOXP3-PE antibody was added to the suspension. The proportion of CD4 + CD25 + FOXP3 + Tregs was determined via flow cytometry (Agilent).
Quantifying Th1, Th2, and Th17 cells via flow cytometry
A human Th1/Th2/Th17 staining kit (Multi Sciences) was used. PMA/ionomycin and BFA/monensin mixtures were added to the CD4 + T-cell suspensions. The mixture was incubated at 37 °C for 4 h. After centrifugation and resuspension, anti-human APC-Cy7-CD4 was added. Following treatment with FIX & PERM, anti-human FITC-IFN-γ, anti-human APC-IL-4, and anti-human PE-IL-17 A antibodies were added to the mixture. Th1 cells (CD4 + IFN-γ+), Th2 cells (CD4 + IL-4+) and Th17 cells (CD4 + IL-17 A+) were detected via flow cytometry (Agilent).
Culturing macrophages with the culture supernatant of CD38 + NK cells
CD38 + NK cells from HCs or CRC patients were treated with a CD38 mAb (5 µg/ml, Proteintech) or C-IgG (5 µg/ml, Proteintech) for 24 h. After incubation, these NK cells were washed with 1× PBS solution. The cells were resuspended to a concentration of 1 × 106/ml and cultured for 48 h at 37 °C in the presence of 5% CO2. The culture supernatant was subsequently collected.
THP-1 cells were treated with phorbol 12-myristate 13-acetate (PMA, 320 nM, Sigma, USA) for 24 h to induce their differentiation into M0-type macrophages. The culture medium was subsequently replaced with the culture supernatant of the CD38 + NK cells. After being cultured at 37 °C in the presence of 5% CO2 for 48 h, the cells were collected. Human TruStain FcX (BioLegend, USA) was added to the cell suspensions, which were subsequently incubated for 10 min at room temperature. Anti-CD68-FITC (BioLegend), anti-CD86-APC (BioLegend) and anti-CD206-PE (BioLegend) antibodies were used to detect M1-type (CD68 + CD86+) and M2-type (CD68 + CD206+) macrophages via flow cytometry (Agilent).
Cell proliferation assay
CD38 + NK cells were incubated with a CD38 mAb (Proteintech) at final concentrations of 0, 1, 5 and 10 µg/ml at 37 °C in 5% CO2. After incubation for 2, 4, 6 and 24 h, CCK-8 reagent (MedChemExpress, USA) was added. After incubation with the reagent for 3 h, the absorbance at 450 nm was measured via a microplate reader.
Cell apoptosis assay
An Annexin V-APC/PI apoptosis kit (Elabscience, China) was used. CD38 + NK cells were treated with a CD38 mAb (Proteintech) at final concentrations of 0, 1, 5, and 10 µg/ml at 37 °C in 5% CO2 for 24 h. The cells were collected and suspended in 1× Annexin V binding buffer. Then, the Annexin-V-APC and PI staining solutions were added to the mixture. The stained cells were analyzed via flow cytometry (Agilent).
Real-time PCR
Total RNA was extracted from CD38 + NK cells and reverse transcribed to generate cDNA. Real-time quantitative PCR (Thermo Fisher Scientific, USA) was performed to measure the expression levels of Sirt1, NF-κB and CD38. GAPDH expression was used as the internal control. The PCR primer sequences are shown in Supplementary Table 2.
Western blot analysis
CD38 + NK cells were lysed in RIPA lysis buffer (Elabscience, China). The subsequent protein samples were separated via SDS–PAGE and transferred onto PVDF membranes (Merck Millipore, USA). Antibodies against Sirt1 (Cell Signaling Technology, USA), NF-κB (Cell Signaling Technology), acetyl-NF-κB (Cell Signaling Technology) and CD38 (Abcam, UK) were incubated with the membranes. GAPDH (Elabscience, China) expression was used as the internal control. The relative protein expression levels were quantified with ImageJ software.
Transcriptome analysis of CD38 + NK cells
CRC CD38 + NK cells were collected from 10 patients and pooled in equal volumes, while control CD38 + NK cells were collected from 10 healthy people and pooled. Total RNA from CD38 + NK cells was extracted and reverse transcribed to generate cDNA. Sequencing was performed on an Illumina platform. HISAT2 was used to align the sequences of the clean reads with the specified reference genome. The differentially expressed genes (DEGs) were defined as protein-coding genes that exhibited significant variable importance in the projection values, with a fold change (FC) > 1.5 and a p value < 0.05. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was performed to identify the pathways in which more than 2 DEGs were enriched.
Immunofluorescence (IF)
Tissue arrays with formalin-fixed paraffin-embedded CRC tissues were obtained from Outdo (Shanghai, China). Information regarding the CRC patients is provided in Supplementary Table 3. Formalin-fixed paraffin-embedded tissues were deparaffinized via regular processes.
-
Triple IF for CD38 + NK cells.
The first primary antibody (rabbit against CD3; Servicebio, China) was incubated with the samples at 4 °C overnight. After washing, the slides were incubated for 50 min with the secondary antibody (CY5-labeled goat anti-rabbit IgG, Servicebio). Following washing, the second and third primary antibodies (rabbit against CD38 and mouse against CD56; Servicebio) were incubated with the slides at 4 °C overnight. After washing, the slides were incubated for 50 min with secondary antibodies (Alexa Fluor 488-labeled goat anti-mouse IgG and CY3-labeled goat anti-rabbit IgG; Servicebio).
-
Double IF for Tregs.
The slides were incubated with primary antibodies (rabbit against FOXP3 and mouse against CD4; Servicebio) at 4 °C overnight. After washing, the slides were incubated for 50 min with secondary antibodies (Alexa Fluor 488-labeled goat anti-rabbit IgG and CY3-labeled goat anti-mouse IgG; Servicebio).
DAPI was then added to stain the nucleus. Images were acquired via Case Viewer Systems (Servicebio). The numbers of colabeled cells and single-labeled cells in each field were counted via the fluorescent cell counting function of ImageJ (Media Cybernetics, USA). The proportions of Tregs and CD38 + NK cells were calculated on the basis of fluorescent cell counts.
Statistical analysis
Graphpad Prism 8.3.0 (USA) was used for assessments of normality and variance homogeneity. One-way ANOVA was performed for multigroup significance assessments. The least significant difference (LSD) method was used for pairwise comparisons. A t test was used to analyze quantitative data with a normal distribution, whereas the Mann‒Whitney U test was used to analyze those without a normal distribution. Spearman correlation analysis was applied for relationship analysis. All reported P values were two-tailed, and a p value < 0.05 was considered statistically significant.
Results
The proportions of CD38 + NK cells and tregs were increased in CRC patients
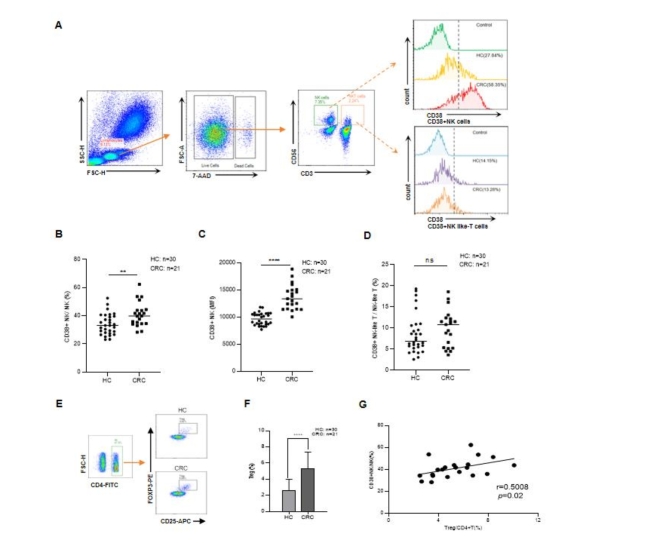
The proportions of CD38 + NK and CD38 + NK-like T cells in blood samples were detected via flow cytometry). Compared with those in HCs, the proportions of CD38 + NK cells among total NK cells were significantly greater in the blood of patients with LC (P < 0.0001, Fig. 1A and B), patients with EC (P = 0.0008, Fig. 1C) and patients with CRC (P < 0.0001, Fig. 1D). The difference was particularly notable for CRC patients. Notably, the mean fluorescence intensity (MFI) for CD38 on CD38 + NK cells from CRC patients was greater than that on CD38 + NK cells from HCs (Supplementary Table 1). Furthermore, compared with that in HCs, the proportion of CD38 + NK-like T cells in tumors was not significantly different (Supplementary Fig. 1A–C). We also detected the proportion of Tregs in the blood of CRC patients and HCs). The proportion of Tregs in CRC patients was greater than that in HCs (P = 0.0002, Fig. 1E and F). A positive correlation between the proportions of Tregs and CD38 + NK cells was found in the peripheral blood of CRC patients (P = 0.0034, r = 0.6086) (Fig. 1G). There was no significant correlation between CD38 + NK cells and Tregs in the peripheral blood (P > 0.05).
Fig. 1.
The proportions of CD38 + NK cells and Tregs in the peripheral blood of cancer patients. (A) Gating strategies for CD38 + NK and CD38 + NK-like T cells. CD38 + NK cells were gated as CD3- CD56 + CD38+, and CD38 + NK-like T cells were gated as CD3 + CD56 + CD38+. Proportions of CD38 + NK cells out of all NK cells in the peripheral blood of HCs (n = 144) and patients with LC (n = 93) (B), EC (n = 37) (C) and CRC (n = 126) were measured (D). E. Gating strategy for Tregs. The Tregs were gated as CD4 + CD25 + FOXP3+. (F) Proportions of Tregs in the peripheral blood of HCs and CRC patients. (G) Correlation between CD38 + NK cells and Tregs in CRC. HCs: healthy controls, LC: lung cancer, EC: esophageal cancer, CRC: colorectal cancer. *: P < 0.05, **: P < 0.01, ***: P < 0.001, and ****: P < 0.0001.
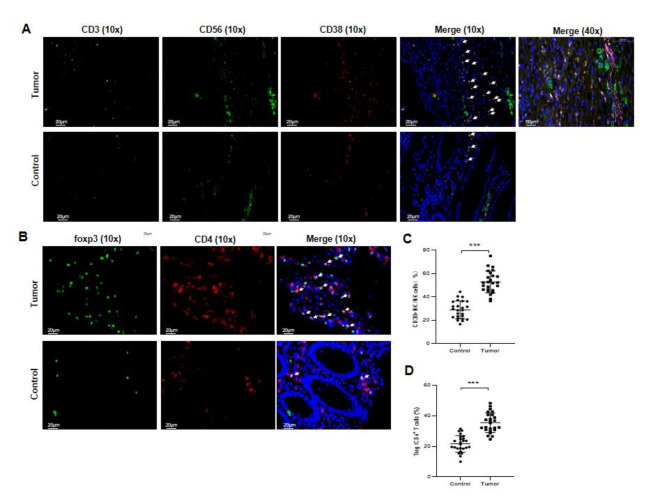
H&E staining was performed to examine tumor tissue and normal colorectal tissue (Supplementary Fig. 1D). IF was used to locate CD38 + NK cells and Tregs in tissues. CD3 (labeled with pink), CD38 (labeled with green) and CD56 (labeled with red) were expressed mainly on the cell membrane. CD56 and CD38, but not CD3, were coexpressed in some cells (shown in orange) in the tumor tissues. The proportion of CD38 + NK cells (CD3- CD56 + CD38+) in CRC tumor tissues was greater than that in normal colorectal tissues (Fig. 2A and C). CD4 (labeled with red) was expressed mainly on the cell membrane, while FOXP3 (labeled with green) was expressed in the nucleus. CD4 and Foxp3 were coexpressed in some cells in tumor tissues. The proportion of Tregs in CRC tumor tissues was greater than that in normal tissues (Fig. 2B and D).
Fig. 2.
Representative immunofluorescent images of CD38 + NK cells and Tregs in tumor tissues and normal colorectal tissues. Immunofluorescence of CD38 + NK cells (CD3- CD56 + CD38+) (A) and Tregs (CD4 + FOXP3+) (B) in tumor tissues and adjacent normal tissues from CRC patients. Magnification: 10x and 40x. The proportions of CD38 + NK cells (C) or Tregs (D) in tumor tissues and adjacent normal tissues were compared. Five fields were collected from each of the five samples. **: P < 0.01 and ***: P < 0.001.
CD38 + NK cells affect the differentiation of CD4 + T cells
CD38 + NK cells were separated from peripheral blood collected from HCs or CRC patients, while CD4 + T cells were isolated from HCs. The extracted CD38 + NK cells and CD4 + T cells were highly pure (Supplementary Fig. 2).
To observe the effect of CD38 + NK cells on the differentiation of CD4 + T cells, these two sets of cells were cocultured in transwells. CD38 + NK cells were pretreated with a CD38 mAb or C-IgG. Cell proliferation and apoptosis assays were performed to determine the best concentration of antibody that could affect CD38 + NK cells without harming cell viability. CD38 mAb at 1–5 µg/ml had no significant effect on the proliferation or apoptosis of CD38 + NK cells (Supplementary Fig. 3). Notably, 5 µg/ml of the antibody was the best dose for inhibiting CD38 activity to the maximum extent without affecting NK cell viability.
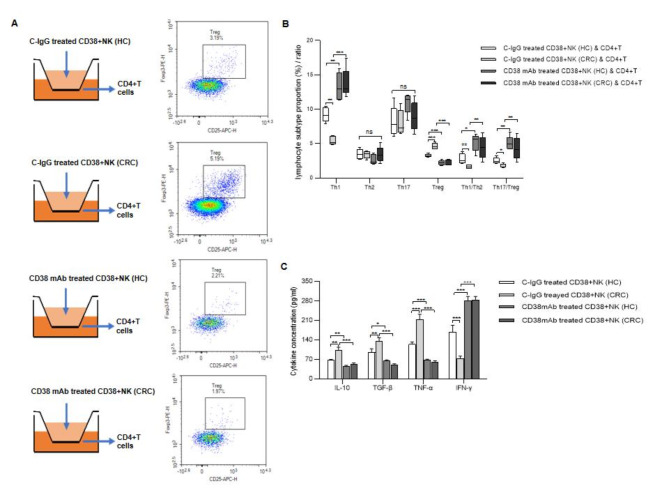
Compared with those from HCs, CD38 + NK cells from CRC patients more strongly promoted the differentiation of Tregs (P < 0.001) and suppressed the differentiation of Th1 cells (P = 0.003), meanwhile the NK cells didn’t affect the level of Th17 cells. When CD38 + NK cells from HCs or CRC patients were pretreated with a CD38 mAb before coculture, the proportion of Tregs in the population of CD4 + T cells was decreased, and the proportion of Th1 cells increased. The Th17/Treg and Th1/Th2 ratios were also elevated in CD4 + T cells following coculture with CD38 + NK cells that were pretreated with the CD38 mAb (Fig. 3A and B, Supplementary Fig. 4).
Fig. 3.
The effect of CD38 + NK cells on CD4 + T-cell differentiation. (A) CD38 + NK cells from HCs or CRC patients were pretreated with C-IgG or a CD38 mAb and cocultured with CD4 + T cells in transwell chambers. Representative images showing the effects of CD38 + NK cells from different groups on the differentiation of CD4 + T cells into Tregs. (B) Proportions of Tregs and Th1, Th2, and Th17 cells and the Th17/Treg and Th1/Th2 ratios in the CD4 + T-cell population after coculture. (C) Cytokine levels in the culture supernatants of CD38 + NK cells subjected to different treatments. CD38 + NK (HC): CD38 + NK cells isolated from the blood of healthy controls; CD38 + NK (CRC): CD38 + NK cells isolated from the blood of CRC patients; CRC: colorectal cancer. ns: not statistically significant; *: P < 0.05, **: P < 0.01 and ***: P < 0.001.
Cytokine levels in the culture supernatant of CD38 + NK cells were measured. IL-10, TGF-β and TNF-α were significantly elevated (P = 0.0015,P = 0.0055 and P < 0.001, respectively), and IFN-γ was decreased (P < 0.001) in the supernatants of CD38 + NK cells from CRC patients compared with those from HCs. Compared with those in the cells treated with C-IgG, the levels of IL-10, TGF-β and TNF-α were lower, and the level of IFN-γ was greater in the supernatant of the CD38 + NK cells treated with the CD38 mAb (Fig. 3C).
CD38 mediates Sirt1/NF-κB signaling to regulate cytokine secretion in NK cells, thereby affecting CD4 + T-cell differentiation
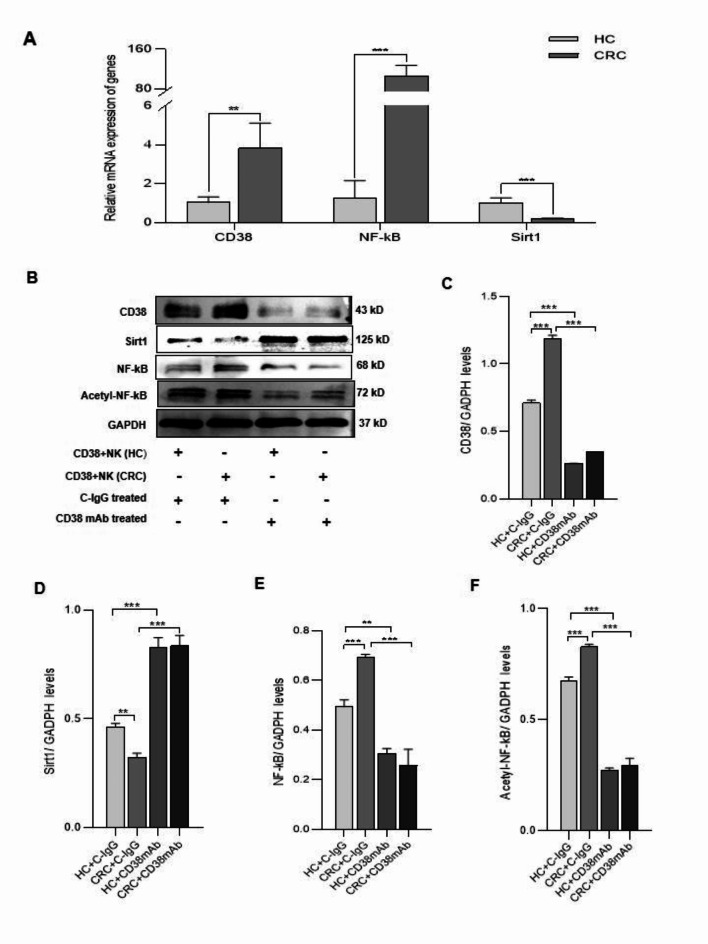
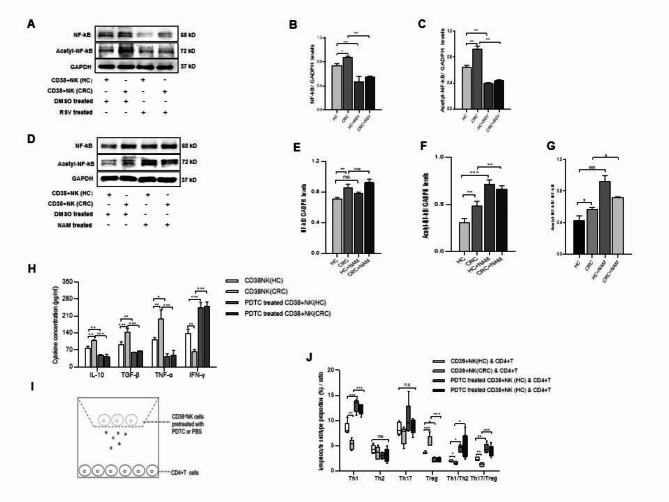
To explore the mechanism by which CD38 + NK cells affect CD4 + T-cell differentiation, the gene expression levels of CD38, Sirt1 and NF-κB in CD38 + NK cells were analyzed. Real-time PCR showed that the CD38 + NK cells from CRC patients had lower expression of Sirt1 (P < 0.001) and higher expression of CD38 and NF-κB (P = 0.0056, p < 0.001, respectively) than did the cells from HCs (Fig. 4A). Western blot analysis revealed that, compared with those in HCs, the expression of CD38 (P < 0.001), NF-κB (P < 0.001) and acetyl-NF-κB (P < 0.001) in CD38 + NK cells from CRC patients was increased and that of Sirt1 (P = 0.002) was decreased. When the CD38 + NK cells were treated with the CD38 mAb, the levels of the aforementioned proteins showed opposite trends (Fig. 4B–F). The results indicated that CD38 on NK cells regulates the levels of Sirt1, NF-κB and acetyl-NF-κB.
Fig. 4.
The effect of CD38 on the expression of Sirt1, NF-κB and acetyl-NF-κB in NK cells. (A) Gene expression levels of Sirt1, CD38, and NF-κB in CD38 + NK cells were determined via real-time PCR. (B) Protein expression levels of CD38, Sirt1, NF-κB, and acetyl-NF-κB were measured via Western blot analysis. Quantitation of the protein expression was performed for CD38 (C) Sirt1 (D), NF-κB (E), and acetyl-NF-κB (F) in CD38 + NK cells from different groups. GAPDH was used as an internal control. CD38 + NK (HC): CD38 + NK cells isolated from the blood of HCs; CD38 + NK (CRC): CD38 + NK cells isolated from the blood of CRC patients; HCs: healthy controls; CRC: colorectal cancer. CD38 mAb: anti-CD38 monoclonal antibody; C-IgG: control IgG. **: P < 0.01 and ***: P < 0.001.
RSV is an activator of Sirt1. To determine whether CD38 plays a role on NF-κB via Sirt1, CD38 + NK cells from HCs and CRC patients were treated with 20 µΜ RSV. Cells treated with an equal volume of DMSO were used as controls. Western blot analysis revealed that the expression levels of NF-κB and acetyl-NF-κB were significantly decreased in CD38 + NK cells treated with RSV (Fig. 5A-C). CD38 + NK cells from HCs or CRC patients were also treated with NAM, an inhibitor of Sirt1. After treatment with NAM (100 µM), the expression of acetyl-NF-κB was significantly increased in CD38 + NK cells from CRC patients and in CD38 + NK cells from HCs (Fig. 5D–G). To investigate the role of NF-κB in cytokine secretion from CD38 + NK cells, we compared cytokine levels in the supernatants of CD38 + NK cells treated with PDTC, an NF-κB inhibitor, or PBS. Both CD38 + NK cells from HCs and those from CRC patients that were pretreated with PDTC showed reduced secretion of IL-10 (p = 0.0049, p < 0.001, respectively), TGF-β (P = 0.0068, P < 0.001, respectively) and TNF-α (P = 0.0216, P < 0.001, respectively) and increased secretion of IFN-γ (P < 0.001, P < 0.001, respectively) (Fig. 5H). CD38 + NK cells treated with PDTC or PBS were also cocultured with CD4 + T cells (Fig. 5I). CD38 + NK cells treated with PDTC promoted the differentiation of Th1 cells, inhibited the differentiation of Tregs, and increased the ratios of Th1/Th2 and Th17/Treg (Fig. 5J).
Fig. 5.
The effect of CD38-mediated Sirt1/NF-κB signaling on cytokine secretion and the resultant modulation of CD4 + T-cell differentiation. Expression levels of NF-κB and acetyl-NF-κB proteins in CD38 + NK cells treated with RSV (Sirt1 activator) or DMSO (A) and quantitation for NF-κB (B) and acetyl-NF-κB (C). Expression levels of NF-κB and acetyl-NF-κB proteins in CD38 + NK cells treated with NAM (Sirt1 inhibitor) or DMSO (D) and quantitation for NF-κB (E) and acetyl-NF-κB (F). (G) Acetyl-NF-κB expression was compared with NF-κB expression. (H) Cytokine levels in the culture supernatant of CD38 + NK cells treated with PDTC (NF-κB inhibitor) or PBS. (I). CD4 + T cells were cocultured with CD38 + NK cells pretreated with PDTC or PBS. (J). Proportions of Tregs, Th1, Th2, and Th17 cells and the ratios of Th17/Treg and Th1/Th2 cells among CD4 + T cells cocultured with CD38 + NK cells that were pretreated with PDTC or PBS. CD38 + NK (HC): CD38 + NK cells isolated from the blood of healthy controls; CD38 + NK (CRC): CD38 + NK cells isolated from the blood of CRC patients; CRC cells: colorectal cancer; RSV: resveratrol; NAM: nicotinamide; DMSO: dimethyl sulfoxide. ns: not statistically significant; *: P < 0.05, **: P < 0.01 and ***: P < 0.001.
CD38 + NK cells from CRC promoted the polarization of M2 macrophages
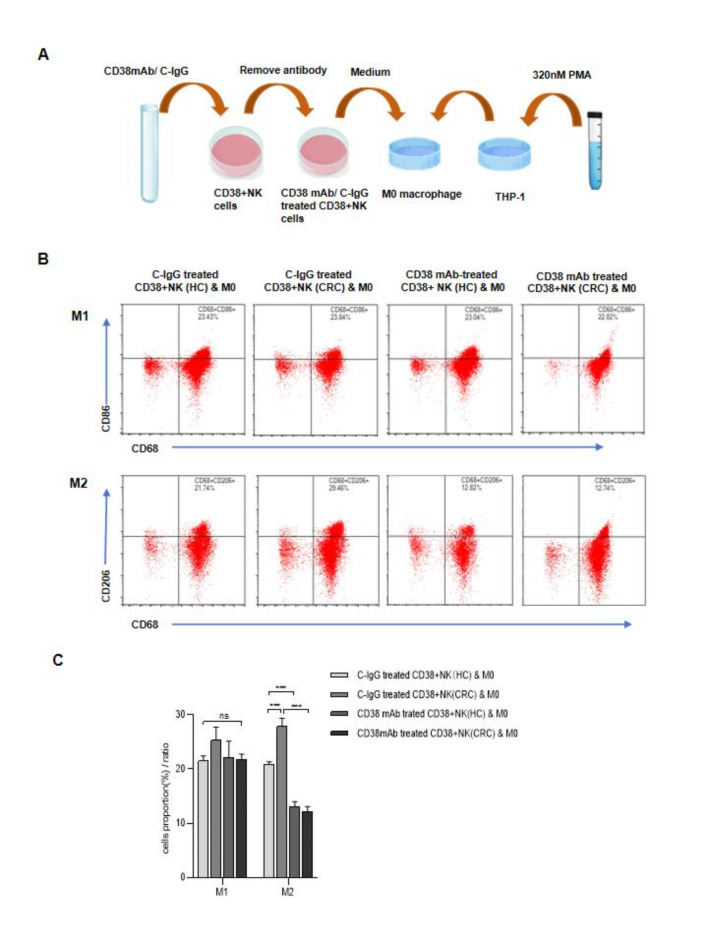
To determine whether CD38 on NK cells affects the polarization of macrophages, M0-type macrophages derived from THP-1 cells were incubated with the supernatant of CD38 + NK cells (Fig. 6A). Compared with those from HCs, the supernatants of CD38 + NK cells from CRC patients could more significantly promote the polarization of M2 macrophages (P < 0.001). The polarization of M1 macrophages was not significantly different between the two groups (P = 0.27). When M0 macrophages were incubated with the supernatant of CD38 + NK cells pretreated with the anti-CD38 mAb, the proportion of M1 macrophages did not significantly differ, whereas the proportion of M2 macrophages significantly decreased; the M1/M2 ratio increased (Fig. 6B and C).
Fig. 6.
Effects of CD38 + NK cells on macrophage polarization. (A) M0 macrophages derived from THP-1 cells were cultured with the culture supernatant of CD38 + NK cells from HCs or CRC patients and pretreated with a CD38 mAb or C-IgG. (B) Representative samples from different groups showing the effect of CD38 + NK cell culture supernatant on macrophage polarization. (C) Proportions of M1 and M2 macrophages after the treatment of M0 macrophages with the culture supernatant of CD38 + NK cells from different groups. CD38 + NK (HC): CD38 + NK cells isolated from the blood of healthy controls; CD38 + NK (CRC): CD38 + NK cells isolated from the blood of CRC patients; HCs: healthy controls; CRC: colorectal cancer; CD38 mAb: anti-CD38 monoclonal antibody; C-IgG: control IgG. ns: not statistically significant; **: P < 0.01 and ***: P < 0.001.
Transcriptome analysis of CD38 + NK cells
To fully understand the regulatory mechanism in CD38 + NK cells, we conducted transcriptome analysis. Compared with the results from the cells from HCs, there were 638 upregulated genes, including CD38, and 190 downregulated genes in the cells from CRC patients (Supplementary Fig. 5A, Supplementary File 1). KEGG functional analysis (based on the top 20 DEGs) revealed that the DEGs between the two groups were associated with a variety of signaling pathways, specifically for cytokine production and regulation, including cytokine–cytokine receptor interaction, cytokine and cytokine receptor, the chemokine signaling pathway, the IL-17 signaling pathway, the TNF signaling pathway, hematopoietic cell lineage, the Toll‒like receptor signaling pathway, the NF‒kappa B signaling pathway, the NOD‒like receptor signaling pathway and tryptophan metabolism. (Supplementary Fig. 5B, Supplementary File 2).
Discussion
In this study, we found that the proportions of CD38 + NK cells were significantly increased in the blood of LC, EC and CRC patients. The increase was particularly notable for CRC patients, and the MFI of CD38 on CD38 + NK cells from CRC patients was greater than that on CD38 + NK cells from HCs. The proportion of Tregs was positively correlated with the levels of CD38 + NK cells in CRC. Compared with what was observed in normal colorectal tissues, the proportions of CD38 + NK cells and Tregs were also elevated in tumor tissues, although we are not sure if there was a significant correlation between CD38 + NK cells and Tregs in tumor tissues. It is well known that Tregs facilitate cancer progression by modulating immune surveillance and inhibiting the antitumor immune response39. Increased levels of Tregs are associated with the progression and poor survival of patients with a variety of cancers. The above observations suggest a high abundance of CD38 + NK cells in tumors40,41, leading to high infiltration of Tregs in tumors and disruption of immune surveillance. NK cells are divided into two types: CD56lo/CD16 + NK cells and CD56hi/CD16- NK cells. CD56lo/CD16 + NK cells are highly cytotoxic, and CD56hi NK cells can produce cytokines and perform a regulatory function42. The present study did not assess the CD38 expression level in CD56hi or CD56dim NK cells.
To clarify the relationship between CD38 + NK cells and Tregs, CD38 + NK cells were cocultured with naive CD4 + T cells in transwell chambers. The population of Tregs in the CD4 + T cell pool cocultured with CRC CD38 + NK cells was greater than that among those cocultured with CD38 + NK cells from HCs, while the proportion of Th1 cells was significantly lower. When CD38 + NK cells were pretreated with a CD38 mAb, the proportions of Th1 cells, Th1/Th2 cells and Th17/Treg cells were elevated, and the proportion of Tregs was decreased. Tosolini et al. reported that CRC patients with high numbers of Th1 cells had prolonged disease-free survival43. The dominance of Th1 cells has antitumor effects, and a decrease in their numbers promotes tumor development44. The results of this study suggest that a high level of CD38 + NK cells from patients with CRC can disrupt the immune response by stimulating CD4 + T cells to differentiate into Tregs and by suppressing their differentiation into Th1 cells and that CD38 plays a key role in this process.
The differentiation of CD4 + T cells is regulated by cytokines8. TGF-β induces the differentiation of Tregs, and IFN-γ induces the differentiation of Th1 cells44. NK cells can secrete numerous cytokines, such as IFN-γ, TNF-α, IL-10, and TGF-β13,45,46. We analyzed the supernatant of CD38 + NK cells from CRC patients, and, relative to HC cells, we found that there were increased levels of secreted IL-10, TGF-β and TNF-α and decreased levels of IFN-γ. When the cells were treated with the anti-CD38 mAb, the above cytokines showed the opposite trends. These results suggest that CD38 on NK cells modulates CD4 + T-cell differentiation by regulating cytokine secretion.
As a proinflammatory signaling pathway, NF-κB has been recognized for its role in regulating cytokine secretion47,48. NF-κB can promote the expression of TNF-α in NK cells13; the activation of NF-κB can upregulate TGF-β secretion in endothelial cells49 and IL-10 secretion in microglia50. In the present study, real-time PCR and Western blotting revealed that the expression of CD38, NF-κB and acetyl-NF-κB was increased, whereas that of Sirt1 was decreased in CD38 + NK cells from CRC patients compared with those from HCs. Pretreatment of the cells with a CD38 mAb increased the level of Sirt1 and decreased the levels of NF-κB and acetyl-NF-κB. Furthermore, pretreatment of CD38 + NK cells with NAM upregulated the expression of acetyl-NF-κB, while RSV treatment downregulated NF-κB and acetyl-NF-κB; these data suggest that CD38 on NK cells activates NF-κB signaling by suppressing Sirt1. In addition, pretreatment of CD38 + NK cells with PDTC promoted the secretion of IFN-γ and inhibited the secretion of IL-10, TGF-β and TNF-α. Furthermore, pretreating with PDTC promoted the differentiation of Th1 cells and inhibited the differentiation of Tregs. These results indicate that CD38 on NK cells modulates cytokine secretion via Sirt1/NF-kB signaling, thereby affecting the differentiation of CD4 + T cells into Tregs or Th1 cells. Previous studies reported that CD38 deficiency in macrophages led to reductions in NF-κB and acetyl-NF-κB via Sirt136,37,51,52.
In our study, decreased CD38 expression was detected in CD38 + NK cells when they were treated with an anti-CD38 mAb. We previously used C3G and 78c, a natural product and chemical, respectively, to treat CD38 + NK cells and reported similar observations25,27,28,52,53. In the previous studies of ours, CD38 suppresses Sirt1 or Sirt6 expression, and Sirt1 or Sirt6 also suppresses CD38 expression. When CD38 activity is suppressed by treatment with an anti-CD38 antibody, Sirt1 or Sirt6 become active and inhibit CD38 expression.
The polarization of macrophages is also influenced by cytokines. IFN-γ and TNF-α stimulate the polarization of M1, whereas IL-10 and TGF-β promote the polarization of M211,12. The current study revealed that the culture supernatant of CD38 + NK cells from CRC patients stimulated the polarization of M2 phenotypes. The stimulatory effect was suppressed when the NK cells were treated with an anti-CD38 mAb. These results confirmed that CD38 on CD38 + NK cells could influence the secretion of cytokines, thereby facilitating the polarization of M2 macrophages.
In the present study, we conducted a transcriptome analysis of CD38 + NK cells. KEGG functional analysis revealed that DEGs between CD38 + NK cells from CRC patients and those from HCs were significantly associated with signaling pathways related to cytokine production and regulation, including cytokine‒cytokine receptor interactions, cytokine and cytokine receptor pathways, the chemokine signaling pathway, the IL-17 signaling pathway, the TNF signaling pathway and the NF‒kB signaling pathway. This analysis was consistent with significant changes that were observed in IL-10, TGF-b, and TNF-a production and NF-kB expression in CRC CD38 + NK cells, indicating the activation of cytokine production and regulation in the tumor CD38 + NK cells.
In this study, we found that CD38 + NK cells from the peripheral blood of CRC patients promoted the differentiation of Tregs. However, we previously reported that CD38 + NK cells from RA patients or HC suppress the differentiation of Tregs26–28.26,27,28 This may explain why there was no significant correlation between CD38 + NK cells and Tregs in the peripheral blood of HCs as we found in the current study. This finding might be attributed to the source of the NK cells, as the immune cells in RA exhibit an different status with that from tumor patients. Another possible explanation is the differences in coculture conditions. In a previous study, CD38 + NK cells were cocultured with MNCs, whereas in the present study, CD38 + NK cells were cocultured with CD4 + T cells. Macrophages and dendritic cells in MNCs are the main sources of IL-653,54, which inhibits the differentiation of Tregs54. In the present study, CD38 + NK cells from CRC patients produced a lower level of IFN-g IFN-gamma than did CD38 + NK cells from HCs. CRC CD38 + NK cells that were treated with an anti-CD38 antibody recovered the production of IFN-g to a high levels. We previously reported that CD38 + NK cells from individuals with RA produced more IFN-g than did NK cells from healthy individuals26,27. IFN-g IFN-gamma suppresses the transition of CD4 + T cells to Tregs7. The above observations suggest that CD38 + NK cells from tumors produce little IFN-gIFN-gamma, which leads to increased differentiation of Tregs from CD4 + T cells.
Conclusion
This study revealed that CD38 in CD38 + NK cells from CRC patients modulated cytokine secretion through Sirt1/NF-kB signaling, thereby affecting the differentiation of CD4 + T cells and the polarization of macrophages. The expression of CD38 in CD38 + NK cells from CRC patients was greater than it was in cells from HCs, which further promoted the differentiation of Treg cells and inhibited the differentiation of Th1 cells. Moreover, CD38 + NK cells from CRC patients promoted the polarization of M2 macrophages. This finding may be useful for understanding the mechanism underlying tumor surveillance. In the future, CD38 could function as a promising target for regulating immune surveillance in patients with tumors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; XW and XC took part in drafting, KF revising or critically reviewing the article; XS gave final approval of the version to be published; XW have agreed on the journal to which the article has been submitted; KF and XC agree to be accountable for all aspects of the work.
Funding
This study was supported by the Shandong Provincial Key R & D programs (2017GSF18174) and Science and Technology Projects in Qingdao West Coast New District (2021-3).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Ethics Committee of the Affiliated Hospital of Qingdao University approved all the experiments (QYFYWZLL27438). All the patients had signed informed consents. We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kehua Fang and Xiaotian Chang contributed equally to this work.
Contributor Information
Kehua Fang, Email: kehua.fang@163.com.
Xiaotian Chang, Email: changxt@126.com.
References
- 1.Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71 (3), 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Ding, P. et al. C5aR1 is a master regulator in colorectal tumorigenesis via Immune modulation. Theranostics. 10 (19), 8619–8632 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahrami, A. et al. Modulation of regulatory T cells by natural products in cancer. Cancer Lett.459, 72–85 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Itahashi, K., Irie, T. & Nishikawa, H. Regulatory T-cell development in the tumor microenvironment. Eur. J. Immunol.52 (8), 1216–1227 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Sarkar, T., Dhar, S. & Sa, G. Tumor-infiltrating T-regulatory cells adapt to altered metabolism to promote tumor-immune escape. Curr. Res. Immunol.2, 132–141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Najafi, S. & Mirshafiey, A. The role of T helper 17 and regulatory T cells in tumor microenvironment. Immunopharmacol. Immunotoxicol. 41 (1), 16–24 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Piconese, S. & Barnaba, V. Stability of regulatory T cells undermined or endorsed by different Type-1 cytokines. Adv. Exp. Med. Biol.850, 17–30 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Dong, C. Cytokine regulation and function in T cells. Annu. Rev. Immunol.39, 51–76 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Owen, J. L. & Mohamadzadeh, M. Macrophages and chemokines as mediators of angiogenesis. Front. Physiol.4, 159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bardi, G. T., Smith, M. A. & Hood, J. L. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine. 105, 63–72 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duque, G. A. & Descoteaux, A. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol.5, 491 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hesketh, M. et al. Macrophage phenotypes regulate scar formation and chronic wound healing. Int. J. Mol. Sci.18 (7), 1545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaszubowska, L. et al. NK cells of the oldest seniors represent constant and resistant to stimulation high expression of cellular protective proteins SIRT1 and HSP70. Immun. Ageing. 15, 12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature. 517 (7534), 293–301 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Cording, S. et al. Innate lymphoid cells in defense, immunopathology and immunotherapy. Nat. Immunol.17 (7), 755–757 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Bryceson, Y. T. et al. Molecular mechanisms of natural killer cell activation. J. Innate Immun.3 (3), 216–226 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Hazeldine, J. & Lord, J. M. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res. Rev.12 (4), 1069–1078 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Weers, M. et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol.186 (3), 1840–1848 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Piedra-Quintero, Z. L. et al. CD38: An immunomodulatory molecule in inflammation and autoimmunity. Front. Immunol.11, 597959 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karakasheva, T. A. et al. CD38-Expressing myeloid-derived suppressor cells promote tumor growth in a murine model of esophageal cancer. Cancer Res.75 (19), 4074–4085 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karakasheva, T. A. et al. CD38 + M-MDSC expansion characterizes a subset of advanced colorectal cancer patients. JCI Insight. 3 (6), e97022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy, A. et al. CD38 deficiency in the tumor microenvironment attenuates glioma progression and modulates features of tumor-associated microglia/macrophages. Neuro Oncol.14 (8), 1037–1049 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu, H. T. & Zhao, X. Y. Regulation of CD38 on multiple myeloma and NK cells by monoclonal antibodies. Int. J. Biol. Sci.18 (5), 1974–1988 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humbel, M. et al. Restoration of NK Cell cytotoxic function with Elotuzumab and Daratumumab promotes elimination of circulating plasma cells in patients with SLE. Front. Immunol.12, 645478 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, M. et al. Single-cell analysis in blood reveals distinct immune cell profiles in gouty arthritis. J. Immunol.210 (6), 745–752 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Wang, H. et al. T-cell immune imbalance in rheumatoid arthritis is associated with alterations in NK cells and NK-Like T cells expressing CD38. J. Innate Immun.14 (2), 148–166 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, H. et al. Potential therapeutic effects of cyanidin-3-O-glucoside on rheumatoid arthritis by relieving inhibition of CD38 + NK cells on Treg cell differentiation. Arthritis Res. Ther.21 (1), 220 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang, X. et al. Compound 78c exerts a therapeutic effect on collagen-induced arthritis and rheumatoid arthritis. Clin. Exp. Rheumatol.41 (7), 1384–1395 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Wang, X. et al. Overexpression of circulating CD38 + NK cells in colorectal cancer was associated with lymph node metastasis and poor prognosis. Front. Oncol.14, 1309785 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber, K. & Superti-Furga, G. After the grape rush: Sirtuins as epigenetic drug targets in neurodegenerative disorders. Bioorg. Med. Chem.19 (12), 3616–3624 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Zapata-Pérez, R. et al. NAD(+) homeostasis in human health and disease. EMBO Mol. Med.13 (7), e13943 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aksoy, P. et al. Regulation of SIRT 1 mediated NAD dependent deacetylation: A novel role for the multifunctional enzyme CD38. Biochem. Biophys. Res. Commun.349 (1), 353–359 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Liang, H. et al. MEGF6 prevents sepsis-induced acute lung injury in mice. Int. Immunopharmacol.123, 110727 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Ding, Q. et al. CD38 deficiency promotes skeletal muscle and brown adipose tissue energy expenditure through activating NAD(+)-Sirt1-PGC1alpha signaling pathway. Can. J. Physiol. Pharmacol.101 (7), 369–381 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Dong, M., Wang, S. & Pei, Z. Mechanism of CD38 via NAD(+) in the development of non- alcoholic fatty liver disease. Int. J. Med. Sci.20 (2), 262–266 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian, Y. et al. CD38 Deficiency promotes inflammatory response through activating Sirt1/NF-κB-Mediated inhibition of TLR2 expression in macrophages. Mediators Inflamm.8736949 2018 (2018). [DOI] [PMC free article] [PubMed]
- 37.Kauppinen, A. et al. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal.25 (10), 1939–1948 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Yeung, F. et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J.23 (12), 2369–2380 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Togashi, Y., Shitara, K. & Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol.16 (6), 356–371 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Núñez, N. G. et al. Tumor invasion in draining lymph nodes is associated with treg accumulation in breast cancer patients. Nat. Commun.11 (1), 3272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka, A. & Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell. Res.27 (1), 109–118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghaemdoust, F., Keshavarz-Fathi, M. & Rezaei, N. Natural killer cells and cancer therapy, what we know and where we are going. Immunotherapy. 11 (14), 1231–1251 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Tosolini, M. et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res.71 (4), 1263–1271 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Powell, M. D. et al. Ikaros Zinc Finger transcription factors: Regulators of cytokine signaling pathways and CD4(+) T helper cell differentiation. Front. Immunol.10, 1299 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark, S. E. et al. IL-10-producing NK cells exacerbate sublethal streptococcus pneumoniae infection in the lung. Transl Res.226, 70–82 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krammer, S. et al. Rhinovirus suppresses TGF-β-GARP presentation by Peripheral NK cells. Cells. 12 (1), 129 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vulpis, E. et al. Genotoxic stress modulates the release of exosomes from multiple myeloma cells capable of activating NK cell cytokine production: Role of HSP70/TLR2/NF-kB axis. Oncoimmunology. 6 (3), e1279372 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li, C. et al. TREM2 inhibits inflammatory responses in mouse microglia by suppressing the PI3K/NF-κB signaling. Cell. Biol. Int.43 (4), 360–372 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao, Y. et al. Endothelial cell metabolic memory causes cardiovascular dysfunction in diabetes. Cardiovasc. Res.118 (1), 196–211 (2022). [DOI] [PubMed] [Google Scholar]
- 50.Prichard, A. et al. Brain rhythms control microglial response and cytokine expression via NF-κB signaling. Sci. Adv.9 (32), eadf5672 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zulaziz, N. et al. Photodynamic therapy mediates innate immune responses via fibroblast-macrophage interactions. Hum. Cell.28 (4), 159–166 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Zhou, H. et al. Downregulation of Sirt6 by CD38 promotes cell senescence and aging. Aging (Albany NY). 14 (23), 9730–9757 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang, S. et al. Historical overview of the interleukin-6 family cytokine. J. Exp. Med.217 (5), e20190347 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hunter, C. A. & Jones, S. A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol.16 (5), 448–457 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.