Abstract
The rice weevil Sitophilus oryzae is one of the primary insects that infest stored grains, causing both quantitative and qualitative losses. The use of synthetic pesticides to control it has led to the emergence of several problems related to human health and the environment, which has prompted the search for safer alternatives for their control. In this study, the effectiveness of two essential oils, cumin (Cuminum cyminum) and basil (Ocimum basilicum), was evaluated as insecticides for controlling the rice weevil at three different times intervals and various concentrations. (GC-MS) analysis revealed that both oils contain several key compounds, such as procanal (26.07%), ˠ- terpinene (15.78%), for C. cyminum and linalool (56.7%), cadinol, epi-α (11.4%) for O. basilicum, in addition to some secondary components. The results showed that, the lethal concentration (LC50) of cumin was 49%, 45%, and 33% lower than that of basil at the 24, 48, and 72-h assessment periods, respectively demonstrating the superiority of cumin over basil. Regarding the effects on the levels of certain enzymes activity, the results indicated that both oils had a significant impact on the activity of both enzymes ALP and GOT, while there was no significant effect on (GOT), α-amylase, and acetylcholinesterase (AChE) compared to control. Based on the obtained results, it can be concluded that tested essential oils could be developed as a safe and effective alternatives for controlling rice weevil.
Keywords: Cuminum cyminum, Enzymatic activity, Essential oils, Ocimum basilicum, Sitophilus oryzae, Toxicity effect
Subject terms: Ecology, Plant sciences, Environmental sciences
Introduction
The Food and Agriculture Organization (FAO) reported that the world could face significant food shortages unless global food production increases by 70% by 20501,2. This situation necessitates urgent efforts to maximize food production. However, during extended storage periods, stored products are vulnerable to various deterioration factors that lead to substantial economic losses and pose health risks3. Stored-product insects cause post-harvest losses due to encouraging bacterial and fungal infections, ranging from 10 to 20% of the total cereal grain and legume production in developed and developing countries, respectively4–6. One of the most destructive pests of stored cereal grains globally is the rice weevil, Sitophilus oryzae L. (Coleoptera: Curculionidae), which is a cosmopolitan insect7. The different stages (eggs, larvae, and pupae) feed and develop concealed within the seed kernels, causing huge losses. Chemical pesticides are extensively used in pest management8–11. The convenience of managing stored product pests depends mainly on the synthetic pesticides, such as organophosphorus, carbamates, and pyrethroid insecticides/fumigants, as a rapid method to control stored-product insect pests worldwide12,13. Using of chemical pesticides causes many harms to mammals and the environment. Also after continuous using of chemical pesticides insects gain resistance against these chemical pesticides and lead these pesticides to be not efficient4,5,8,12,13. Therefore, there is an urgent need to develop new methods for pest control using natural products or new safety materials. Alternative methods can be used without environmental side effects on environment14,15. Additionally, increasing consumer awareness of chemical or pesticide-free food products led to the exploration of potential natural compounds for pest control as mentioned by Perumal16 who reported that Acacia nilotica seed has excellent insecticidal action against major agricultural insect pests. Nowadays, researchers are focusing on natural products, such as essential oils, which can offer protection to stored products against pests, are safe for humans, and are eco-friendly17,18. Botanical insecticides (secondary defence metabolites) have been used as plant protectants with a profound historical tradition of more than 3000 years19. Essential oils (EOs) are natural aromatic components rich in terpenoids (monoterpenes and sesquiterpenes), phenylpropanoids, and ketones20,21. The U.S. Food and Drug Administration (FDA) has classified certain essential oils (EOs) and their bioactive compounds as generally recognized as safe (GRAS). As a result, extensive research has been conducted on essential oils and their bioactive compounds in relation to stored product insects, due to their natural biological properties, including toxicity22, as well as their antifeedant and ovicidal activity. Essential oils (EOs) extracted from different plant species have been the target of new research aimed at their application as natural agricultural pesticides against various stored product insects, aromatic plants can protect itself especially during the production and mature stage and that refers to its essential oils which has repellent effect against insects, studies curried out to evaluate the insecticidal activities of several essential oils and found that all tested essential oils had repellent, contact and fumigant effect against stored grain insects4,8,23–25. Study also carried out to evaluate the effect of the essential oils components separately and found that the component have the same effect as the oil against stored grain insects4. Many studies have discussed the potential of essential oils in the activity of some enzymes. Oils extracted from Acacia nilotica seed cotyledons showed decrease in acetylcholinesterase enzymes and an increase in glutathione S-transferase activity against insect pests26. Also, cajeput oil (Melaleuca cajuputi) exhibit larvicidal activity against larvae of Anopheles stephensi resulting indecrease in detoxifying enzymes(acetylcholinesterase (AchE), α β-carboxylesterase)27. Kiran and Prakash28 reported that acetylcholinesterase (AChE) activity in S. oryzae was inhibited by the essential oil of eastern teaberry Gaultheria procumben, as well as changes in the antioxidative defense system, such as superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), and oxidized glutathione (GSSG). Numerous components of essential oils can exhibit insecticidal activity by affecting the neurotransmitters in the synapses, such as octopamine and GABA, and by inhibiting acetylcholinesterase (AChE) which induces mortality in most insects19,29. Bhavya et al.30 reported that holy basil (Ocimum tenuiflorum) essential oil and its component eugenol affect acetylcholinesterase activity in vivo. In addition, Ahmed et al.31 concluded that both plant powders of Rubus fruticosus and Valeriana jatamansi are effective against granary weevil by altering alpha-amylase enzyme activity, indicating that both can be used as bio-pesticides against stored grain pests. Omar et al.29 reported that essential oils (EOs) of cumin (Cuminum cyminum L.) seeds and basil (Ocimum basilicum L.) have a mortality effect on Tribolium castaneum insects in addition to their impact on enzymatic activity (total protein, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, and α-amylase). Also, Derbalah et al.17 reported that some plant-derived monoterpenes and yucca extract could be used as alternatives to chemical insecticides against the red flour beetle, T. castaneum in addition to their effect on some enzymes (Acetylcholinesterase, ά-amylase, and alkaline phosphatase). In this context, current study was conducted under laboratory conditions to evaluate the insecticidal activity of cumin (C. cyminum) and basil (O. basilicum) (Herb) essential oils against S. oryzae. Additionally, study the mode of action of the two tested essential oils on S. oryzae regarding to its effects on various biochemical parameters of the tested insect.
Materials and methods
Tested essential oils
Essential oils of cumin (Cuminum cyminum) and basil (Ocimum basilicum) available in Egypt were sourced from Hashem Brothers Company for Essential Oils and Aromatic Products, located at 69 Abd Elone’m Riad St., Dokki, Giza, Egypt.
Rearing of stored product insect
Rice weevils, Sitophilus oryzae were obtained from stock colonies maintained at the laboratory of Stored Product Pests, Sakha Agricultural Research Station, Agriculture Research Center (ARC), Egypt. It was cultured using conditioned whole wheat and maintained at 28 ± 2 °C, 70 ± 5% RH, and a 16:8 light: dark photoperiod. Adult insects (1–2 weeks old) were used in all experiments. The insect cultures were maintained in the laboratory without insecticide exposure. All the experiments were conducted under similar environmental conditions8.
Enzymatic activity assay chemicals
Phosphate buffer, chemical reagents for the estimation of protein, alpha-amylase, aspartate aminotransferase (AST), alkaline phosphatase (ALP), and alanine aminotransferase (ALT). All chemical reagents used for this estimation were purchased from the Egyptian Company for Biotechnology (Spectrum Diagnostics, Egypt).
Chemical analysis and identification of essential oils
The constituents of essential oils for the two tested plants were analyzed by gas chromatography-mass spectrometry (GC/MS) using the model (HP5890) made in the USA system with an HP column (60 m × 0.25 mm, 0.25 μm film thickness) (HP, 5 ms). The initial temperature was 60 °C and the maximum temperature was 250 °C for 65.3 min. The injector temperature was 240 °C. Relative percentage amounts were calculated from the peak’s total area using apparatus software. The compounds were identified by matching the mass spectral data with those obtained from a computer library (Wiley 275. L)32,33. All steps of sample preparation, extraction, and analysis were performed at the Analytical Laboratory of Hashem Brothers Company for Essential Oils and Aromatic Products.
Toxicity bioassay of cumin and basil essential oil against adults of Sitophilus oryzae using mixing with media method
Batches of whole-wheat grains weighting 20 g were introduced into 50 ml jars. Five dilutions of cumin oil (12, 14, 16, 18, and 20 µg/ml) and (10, 15, 20, 25, and 30 µg/ml) for basil oil were diluted in acetone and subsequently used to treat wheat as 1 ml solution for each replicate. The treated wheat grains were left to dry for 20 min and received 10 unsexed adult rice weevils per jar, which were covered with muslin cloth and kept under the same laboratory conditions as for rearing the insects. Wheat treated with acetone served as the control. Cumulative mortality counts were recorded after 1, 2, and 3 days of treatment and corrected using Abbott’s formula34. Each concentration was replicated thrice.
Effect of Lethal exposure of cumin and Basil essential oil on biochemical parameters in Sitophilus oryzae in vivo
Preparation of enzyme extract
One hundred adult insects were subjected to a derived lethal concentration (LC50) of the essential oil for three days at room temperature (27 ± 2 °C). After the exposure period, live insects were removed and homogenized in phosphate buffer (pH 7.4, 100 mmoL/L) in a glass-Teflon homogenizer at different intervals. Three replicates for each treatment were used in the biochemical experiments. Subsequently, the homogenate was centrifuged at 10,000 rpm and cooled (4 °C) for 10 min, and the supernatant was stored on ice for further enzyme assessment28.
Biochemical determination
Glutamic oxaloacetic transaminase (GOT) activity in vivo
GOT was determined calorimetrically following the method of Reitman and Frankel35. Initially, 0.5 ml of the GOT buffer substrate was incubated at 37 °C for 5 min. Next, 0.1 ml of the sample supernatant was added and incubated for an additional 50 min at 37 °C. Afterward, 0.5 ml of color reagent (2,4-dinitrophenylhydrazine) was added, and the mixture was allowed to sit at room temperature for 20 min. Following this, 5 ml of 0.4 N NaOH was added, and the mixture was thoroughly mixed. Absorbance was measured at 505 nm against distilled water, and the enzyme activity (in units per ml) was calculated using a standard curve.
Assay of alkaline phosphatase enzyme (ALP)
Alkaline phosphatase (ALP) activity was determined spectrophotometrically in the supernatant at 505 nm according to the method of Belfield and Goldberg36. In the procedure, 0.025 ml of the supernatant was mixed with 0.5 ml of buffer substrate (phenyl phosphate, pH 10) and incubated for 20 min at 37 °C. Then, 0.25 ml of the enzyme inhibitor EDTA and 4-amino-phenazone were added, followed by 0.25 ml of potassium ferricyanide. The mixture was incubated in the dark at room temperature for 5 min. Absorbance readings were taken at 510 nm for both the sample (A_sample) and the standard (A_standard) against a reagent blank.
The quantity of Alkaline Phosphatase was determined according to the following equation:

Determination of α-amylase
Alpha-amylase in the supernatant was determined spectrophotometrically at 660 nm against a blank, according to Caraway method37. Initially, 0.5 ml of buffered substrate was incubated at 37 °C for 3 min. Then, 0.01 ml of the supernatant was added, thoroughly mixed, and incubated at 37 °C for 7.5 min. After incubation, 0.5 ml of working reagent and 4 ml of distilled water were added and mixed well. The absorbance of both the sample and the blank was measured at 660 nm against distilled water.
Alpha-amylase activity was calculated using the following formula:

The quantity of alpha-amylase was determined according to the following equation:
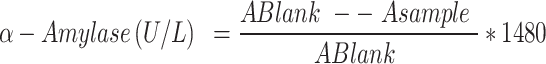
Total protein contents assay
Total protein content was estimated using the method described by Gornall et al.38. In this procedure, 1 ml of biuret reagent was mixed with 0.025 ml of insect supernatant in a test tube and incubated at 37 °C for 10 min. After incubation, the absorbance of both the sample and the standard was measured at 550 nm against a blank reagent.
Total protein concentration was determined according to the following equation: Total protein concentration (g/dL) = Sample/Standard × 5.
Acetylcholinesterase assay
AChE activity was assayed photometrically using U.V. spectrophotometer at 405 nm according to Kendel and Bottger38. The enzyme catalyzes the hydrolysis of butyrylthiocholine (BTC), producing thiocholine, which reduces ferricyanide (III) to ferrocyanide (II), resulting in a decrease in absorbance.
Reagents were prepared by mixing R1 buffer with R2 (DTNB) and R3 (BTC), followed by the combination of R3 and reconstituted R2 to form the MONOREAGENT. One ml of MONOREAGENT was mixed with 20 µl of serum, and absorbance was measured at 405 nm at 30, 60, and 90 s using the following equation:

Statistical analysis
The experiments were performed in triplicate, and the data are presented as the mean ± SE. The results were analyzed by one-way analysis of variance (ANOVA), followed by the Least Significant Difference test for mean separation. P values of ≤ 0. 05 were considered significant. LC50 (the lethal concentration for 50% mortality) was determined by log-probit analysis, and the data were analyzed by determining chi-square values and degrees of freedom. Data analysis was performed using SPSS program version 24.0 for Windows (SPSS Inc., IBM Corp.)
Results
GC–MS analysis of essential oils
The results of the present study showed an insecticidal effect of two botanical insecticides represented in the essential oil of cumin (C. cyminum) and basil O. basilicum (Herb). Both tested essential oils showed insecticidal activity against one of the stored products of the insect rice weevil, S. oryzae with significant differences between them.
Chemical analysis of both tested essential oils showed varied chemical components, as shown in Tables 1 and 2. The main components of cumin oil are propanal (26.07%) and ˠ- terpenene (15.78%). While for basil, O. basilicum (Herb) mainly composed of linalool (56.7%) and α- bergamoten (9.2%) These components are classified as fatty acids, flavonoids, and alkaloids, which are anti-insecticidal compounds.
Table 1.
The main component of cumin essential oil, Cuminum cyminum seeds analyzed by gas chromatography-mass spectrometry (GC–MS).
| Compounds | % Percent composition | RT (retention time min) |
Molecular formula |
|---|---|---|---|
| β-pinene | 10.37 | 6.8 | C10H16 |
| p-cymene | 7.45 | 8.24 | C10H14 |
| ˠ-terpinene | 15.78 | 9.6 | C10H16 |
| Ethanone | 1.99 | 14.57 | C8H12 O |
| Propanal | 26.07 | 16.9 | C3H6 O |
| Isopropylidene | 10.48 | 18.54 | C11H18 |
| Isopropylidene | 1.63 | 18.58 | C11H18 |
| Benzenemethanol | 6.62 | 18.72 | C10H14O |
| Benzenemethanol | 11.32 | 19.02 | C10H14O |
Table 2.
The main component of basil essential oil, analyzed by gas chromatography-mass spectrometry (GC–MS).
| Compounds | % Percent composition |
R.T (retention time min) |
Molecular formula |
|---|---|---|---|
| Linalool | 56.7 | 12.6 | C10H18O |
| α-bergamoten | 9.2 | 15.54 | C15H24 |
| Germacrene D | 3.3 | 25.053 | C15H24 |
| γ-cadinene | 5.4 | 25.89 | C15H24 |
| Viridiflorol | 1.7 | 26.033 | C15H26O |
| Cadinol, epi-α | 11.4 | 28.58 | C15H26O |
Toxicity bioassay
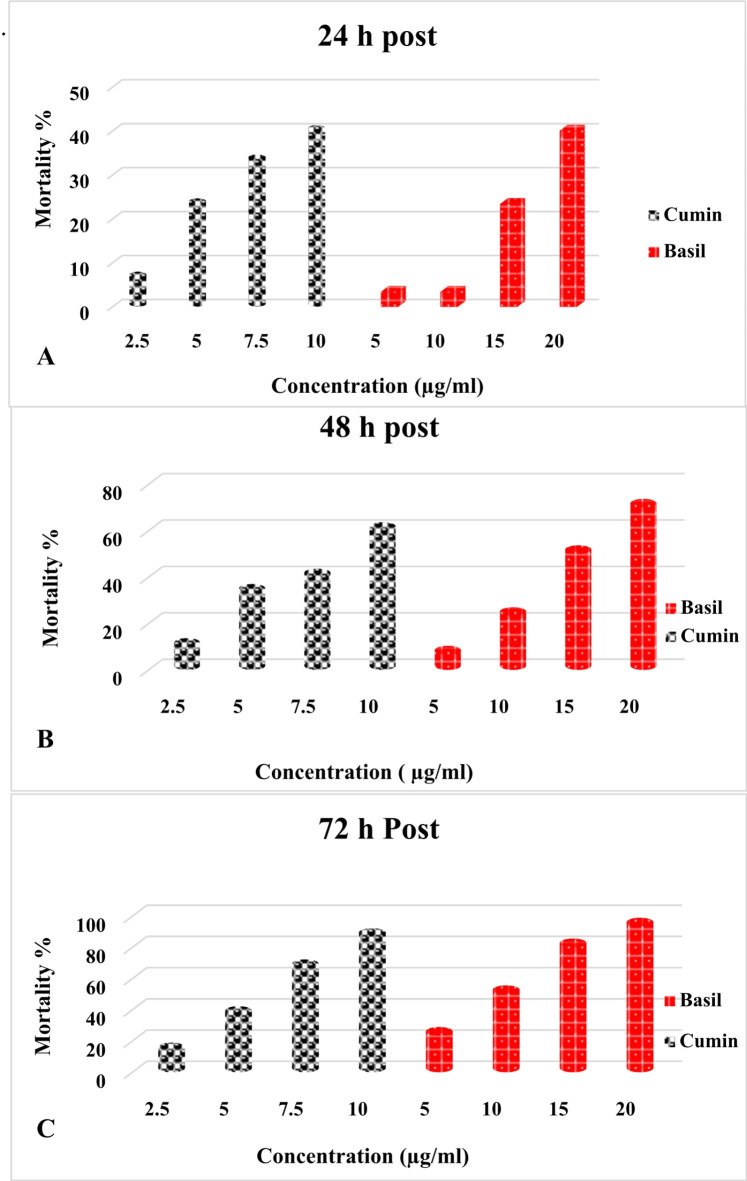
The results presented in Fig. 1A, B, and C demonstrate that the tested essential oil exhibited significant toxicity against S. oryzae insect after different exposure periods, where higher concentrations of tested oils for short periods were more effective than lower concentrations for longer periods. Cumin oil had the highest toxicity rate compared to basil oil toward the tested insect. Results indicated that increasing exposure time up to (72 h) achieved 86.66% mortality for cumin oil and 93.33% for bail oil with the superiority of cumin over basil oil according to LC50.
Fig. 1.
Efficacy of cumin and basil oil against Sitophilus arise after different period of exposure (A), (B), and (C). Bars represent mortality means of three replicates. The lowercase superscript letters above each bar represents the results from post hoc Duncan comparisons between treatments. Bars with the same letter were not statistically different from one another.
The calculated (LC50) for both the tested oils on S. oryzae insect are summarized in Table 3. Where Cumin had the lowest LC50 value all over the test period, recording (11.04, 8.51 and 6.16 μg/ml ) for 1, 2 and 3 days post-exposure, respectively, while the LC50 of basil oil was (21.91, 15.59 and 9.94 μg/ml ) respectively, for the same exposure period.
Table 3.
Lethal concentrations of cumin (Cuminum cyminum) and basil, Ocimum basilicum (herb) essential oil against Sitophilus Oryzae.
| Tested oil | LC50 Value (µg/ml) | Confidence limits 95% | Slope value | Chi-square (X2) | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Cumin | 24 h | 11.04 | 8.89 | 18.95 | 1.63 | 1.22 |
| 48 h | 8.51 | 7.17 | 11.04 | 1.56 | 1.25 | |
| 72 h | 6.16 | 5.25 | 7.05 | 1.84 | 0.04 | |
| Basil | 24 h | 21.91 | 18.94 | 29.20 | 2.68 | 1.22 |
| 48 h | 15.59 | 13.71 | 18.08 | 2.09 | 0.26 | |
| 72 h | 9.94 | 7.68 | 11.75 | 1.39 | 0.45 | |
Enzymatic bioassay
Data in Table 4 revealed the change in the activity of total protein, alkaline phosphatase, glutamic oxaloacetic transaminase (GOT), alpha-amylase, and acetylcholinesterase in S. oryzae insects exposed to LC50 of cumin and basil essential oil after 3 days of exposure.
Table 4.
Changes in some biochemical parameter activity in Sitophilus Oryzae after 72 h of treatment with the LC50 of cumin (Cuminum cyminum) and basil, (Ocimum basilicum) (herb) essential oil under laboratory conditions.
| Mean of enzyme | Control | Cumin (Cuminum cyminum) | Change% | Basil (Ocimum basilicum) | Change% |
|---|---|---|---|---|---|
| Total protien (g/dl) | 8.29 a | 8.74 a | + 5.43% | 8.46 a | + 2.05% |
| Alkalin phosphatese (IU/L) | 105.17 b | 104.02 b | −1% | 145.55 a | + 38% |
| (AST/GOT) (U/L) | 220 a | 150 ab | −68% | 38 b | −83% |
| α-amylas (U/L) | 142.307 a | 213.46 a | + 50% | 552.63 a | + 288% |
|
AChE (U/L) |
7976.88 a | 10425.78 a | + 30.70% | 8186.95 a | + 0.026% |
Values in the row followed by the same letters are not significantly different (p > 0.05) according to ANOVA and Duncan multiple comparison tests.
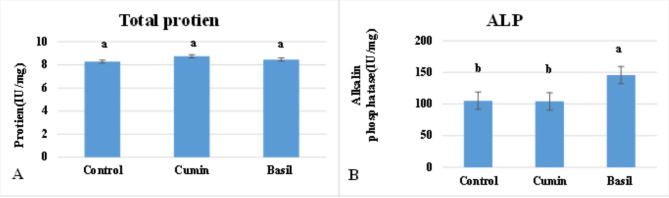
The results of the present study showed that there was no significant difference in S. oryzae total protein content compared to the control (Fig. 2A). Where it recorded 8.74 g/dl for cumin oil and 8.46 g/dl for basil oil compared to the control (8.29 g/dl). On the other hand, alkaline phosphatase (ALP) showed a significant difference between the control and basil treatments, where there was an increase in basil treatment by (38%) compared to the control treatment. While cumin treatment showed no significant difference compared with control, recording 104.02 IU/L compared to 105.17 IU/L for control (Fig. 2B).
Fig. 2.
In vivo protein (A) and Alkalin phosphatase activity (B) in Sitophilus oryzae exposed to LC50 of cumin and basil oil after 24 h of exposure. Values are represented as mean ± SEM of 3 independent experiments.
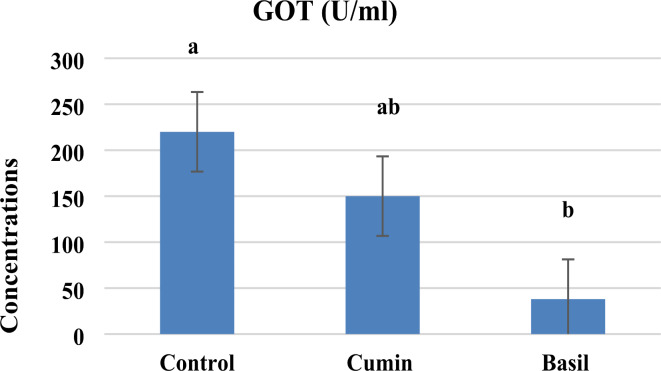
In addition, glutamic oxaloacetic transaminase (GOT) showed significant inhibition between control and treatments, where it recorded 150 U/L for cumin and 38 U/L for basil treatment compared to 220 U/L in control, indicating inhibition rats by (– 68%) and (− 83%) for both tested essential oils respectively (Fig. 3).
Fig. 3.
In vivo GOT activity in Sitophilus Oryzae exposed to LC50 of cumin and basil oil after 24 h of exposure. Values are represented as mean ± SEM of three independent experiments.
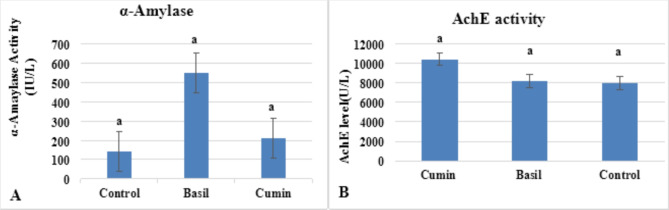
On the other hand, there was an increase in alpha-amylase and acetylcholinesterase (AchE) activity in both cumin and basil treatments, but the increase was not significantly different from the control treatment. Where alpha amylase recorded 213.46 U/L for cumin and 552.63 U/L for basil treatment compared to 142.307 U/L in control (Fig. 4A). Acetyl cholinesterase activity recorded 10425.78 and 8186.95 U/L for cumin and basil treatment respectively compared to 7976.88 U/L in the control treatment (Fig. 4B).
Fig. 4.
In vivo α-amylase (A) and acetylcholine esterase activity (B) in Sitophilus oryzae exposed to LC50 of cumin and basil oil after 24 h of exposure. Values are represented as mean ± SEM of three independent experiments.
Discussion
Evidently, the toxicity of essential oils to stored products is influenced by the chemical composition of the oil. Essential oils are considered very promising for the development of botanical insecticides and have received much attention in the scientific field, especially with stored grain insect pest management programs, as also confirmed by our results and mentioned by40–43. Many studies have shown that plant extracts are effective in combating various insect pests, giving them an advantage as an alternative to traditional pesticides44,45. Essential oils have contains several components which may act as active ingredients, which prevent or at least delay the insect resistance to these essential oils. Several previous studies mentioned that the insecticidal activities of essential oils may refers to it is fumigant and repellent effect and the effect on acetylecoleneesterase4.
The potential of cumin and basil EOs as ingredients in botanical insecticides is supported by a substantial scientific background. Indeed, cumin EO exhibited strong toxicity and fumigant effects, as well as ovicidal and insecticidal effects on Sitophilus zeamais41. Khalil et al.46 studied the chemical composition of Ocimum basilicum and its insecticidal activities against stored product insects, and their results were in line with those of the current study. They reported that linalool was the main component of O. basilicum essential oil with 54.8%.
From our results level of total protein in S. oryza showed a slight increase with no significant changes compared to control. Sahayaraj47 reported that several reports show that botanical compounds increased protein levels may be due to the conversion of carbohydrates and lipids to proteins, or the appearance of new peptides in hemolymph upon botanical treatment, may liberate free radicals, which affect nitrogenous compounds directly. Abo El Makarem et al.48 suggested that the increase in protein content in basil- and anise-oil-treated weevils could be the result of an elevation in tissue metabolic activity to compensate for the stress caused by the essential oils.
Alkaline phosphatase (ALP) is primarily found in the intestinal epithelium of animals, and its major function is to provide phosphate ions from mononucleotides and ribonucleoproteins for a variety of metabolic processes. Sahayaraj47 found that ALP plays a role in the maintenance of the energy supply of the living cells by catalysing the phosphate esters and releasing the high-energy phosphate bonds, which are utilized for revealing metabolic processes. Our results are in line with those of El-Gizawy et al.49, who reported that there was no significant increase in alkaline phosphatase activity of T. castaneum larvae treated with garlic oils compared to the control treatment. In agreement with the current results, Askar et al.50 reported that the AST activities of S. zeamais were decreased after 7 days of treatment with anise oil. a-amylase is one of the key enzymes involved in carbohydrate metabolism in insects. Khater and El-Shafiey51 reported that changes in α-amylase activity in T. castaneum larvae after treatments with the LC50 of two oils could be due to the presence of plant defense compounds such as inhibitors, which act on insect gut enzymes such hydrolases, α-amylase, and proteinases. In the same trend, El-Gizawy et al.49 reported that the increase in alpha-amylase activity was not significant with garlic oil treatments compared to the control treatment, where the increase was 9.00%. Our results are in line with those of Ahmed et al.31,52, who reported that the α-amylase enzyme activity was increased by 63.63% in Sitophilus granarius treated with Valeriana jatamansi.
AChE has been used as a sensitive biomarker for toxicity evaluation of synthetic insecticides against a variety of invertebrate species. The present study is in line with that of Rajkumar et al.53 who reported that Mentha piperita essential oil compounds, menthone and menthol, have weak AChE inhibitors against S. oryzae and T. castaneum, similar to the results reported by Park et al.54. In contrast, Bhavya et al.30 reported that Ocimum tenuiflorum oil had significantly inhibited AChE activity in S. oryzae adults treated with fumigation. Therefore, the effects of essential oils at lethal doses were studied to understand the mechanism of toxicity.
Conclusion
Our results emphasize that botanical insecticides, such as essential oils of both cumin (C. cyminum) and basil (O. basilicum) may provide a complementary management and eco-friendly biodegradable product method to limit chemicals in the control of stored grain pests with the superiority of cumin oil over basil. In addition, the tested essential oil has approved toxicity potential against S. oryzae insects associated with affecting certain metabolic enzymes (ALP, GOT, Alpha Amylase, Total Protein and AChE) that are responsible for the vital process of insects. However, further studies on the safety of human and non-target organisms and mode of action are necessary for the practical use of botanical essential oils and their components as novel insect control agents.
Acknowledgements
We acknowledge Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R221), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khaled University for funding this work through the Large Research Project (grant number RGP2/486/45).
Author contributions
Ahmed Fayez Omar, Fatma Mohamed Ameen Khalil, Tamer Ismail, Ahmed M. Abouelatta design the experiment, analyse the data and wrote the main manuscript text, Maryam M. Alomran, Tamer Ismail, Ahmed I. El‐Tokhy, Khaled Abdelaal, Fatehia N. Gharsan, Reem Nasser Almozini, prepared figures. All authors reviewed the manuscript.
Funding
This work was supported by Princess Nourah bint Abdulrahman University Researchers Sup-porting Project number (PNURSP2024R221), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia and funded by the Deanship of Research and Graduate Studies at King Khaled University for funding this work through the Large Research Project under grant number RGP2/486/45.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel, F. R. & Siegel, F. R. Food 2050: More Mouths to Feed-Food Availability and Access the Earth’s Human Carrying Capacity: Limitations Assessed, Solutions Proposed. 87–107. (2021).
- 2.World Health Organization. Report of the 12th FAO/WHO Joint Meeting on Pesticide Management: 19–22 November 2019 World Health Organization. (2020).
- 3.Nayak, M. K. & Daglish, G. J. Importance of stored product insects In Recent Advances in Stored Product Protection. Springer Berlin Heidelberg, 1-17. (2018). [Google Scholar]
- 4.Abouelatta, A. M., Keratum, A. Y., Ahmed, S. I. & El-Zun, H. M. Repellent, contact and fumigant activities of geranium (Pelargonium graveolens L’Hér) essential oils against Tribolium castaneum (Herbst) and Rhyzopertha dominica (F). Int. J. Trop. Insect. Sci. 40, 1021–1030. (2020).
- 5.Seada, M. A. & Abouelatta, A. M. Potential for Using Four Plant Essential Oils to Protect Stored Products against Tribolium castaneum (Herbst) (Coleoptera:Tenebrionidae). Fine Chem. Eng., 154–171. (2024).
- 6.Seada, M. A., Hamza, A. M. & Abouelatta, A. M. Chemical characterization, fumigant toxicity and antifeedant activity of essential oils of four indigenous plants against Rhyzopertha dominica (Coleoptera: Bostrychidae. Delta J. Sci.48 (1), 13–32 (2024). [Google Scholar]
- 7.Majd-Marani, S., Naseri, B., Hassanpour, M., Razmjou, J. & Jalaeian, M. Life history and life table parameters of the rice weevil, Sitophilus oryzae L (Coleoptera: Curculionidae) fed on 10 rice cultivars and lines in Iran. J. Stored Prod. Res.102, 102118 (2023). [Google Scholar]
- 8.Abo Arab, H. R. et al. Fumigant and contact toxicity of some essential components against three stored product insects. Fresenius Environ. Bull.31 (10), 10136–10143 (2022). [Google Scholar]
- 9.El-Tokhy, A. I. A., Ali, M. M., Hafez, Y. & Abdelaal, K. Impacts of different insecticides on Tuta absoluta (Meyrick) larvae with their effects on physiological characteristics and fruits yield of stressed tomato plants. J. Plant Prot. Pathol. 11(6), 269–277. (2020).
- 10.Mohamed, N. E., Keratum, A. Y., Ismail, A., Hafez, Y. & Abdelaal, K. Laboratory evaluation of some environmentally safe chemicals against the two spotted spider mite, Tetranychus Urtıcae and its predator insect, Stethorus Gilvifrons. Fresenius Environ. Bull.31 (8B), 8904–8913 (2022). [Google Scholar]
- 11.Taha, Y. N., El-Zahi, S. E., Keratum, A., Ismail, T. & Abdelaal Kh, Hafez, Y. Comparative efficacy of salicylic acid and some systemic insecticides against Bemisia tabaci (Genn): and their impact on cotton defense response. Fresenius Environ. Bull.29 (8), 6707–6713 (2020). [Google Scholar]
- 12.Manivannan, S., Swati, A. P., Hemalatha, P., Gisha, E. K. & Roopa, R. S. Phosphine gas generated from an aluminium phosphide tablet exhibits early knock down effects on tamarind pod borer. RSC Adv.6 (93), 90024–90030 (2016). [Google Scholar]
- 13.Bomzan, D. P. et al. Potential of pyrethroid-synergised pyrethrum on stored product insects and implications for use as prophylactic sprays. J. Food Sci.Technol.55(6), 2270–2278. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Essawy, M. M. et al. Susceptibility of some faba bean varieties to infestation with the main insect pests associated with physiological, biochemical and yield characters. Fresenius Environ. Bull.29(7A), 6147–6158. (2020). [Google Scholar]
- 15.Ismail, T. et al. Efficiency of Foliar fertilizers and growth regulators on Cowpea Productivity and Control of Cowpea Weevil, Callosobruchus maculatus (Coleoptera: Bruchidae). Pol. J. Environ. Stud.32 (5), 4607–4615 (2023). [Google Scholar]
- 16.Perumal, V., Kannan, S., Pittarate, S., Chinnasamy, R. & Krutmuang, P. Essential oils from Acacia nilotica (Fabales: Fabaceae) seeds: may have insecticidal effects? Heliyon, 9(4), e14808 (2023). [DOI] [PMC free article] [PubMed]
- 17.Derbalah, A. et al. The efficacy of some synthetic monoterpenes and Yucca extract for controlling Tribolium castaneum (Herbst) in wheat grain. J. Plant. Prot. Res.64 (1), 2 (2024). [Google Scholar]
- 18.Elmadawy, A. A., Omar, A. F. & Ismail, T. Bags impregnated with garlic (Allium sativum L.) and parsley (Petroselinum crispum (Mill.) Fuss) essential oils as a new biopesticide tool for Trogoderma granarium Everts, 1898 pest control. Acta Agriculturae Slov.119 (1), 1–13 (2023). [Google Scholar]
- 19.Pavela, R. & Benelli, G. Essential oils as ecofriendly biopesticides. Chall. Constraints Trends Plant. Sci.21 (12), 1000–1007 (2016). [DOI] [PubMed] [Google Scholar]
- 20.da Silva Acácio, R. et al. Dataset of Schinus terebinthifolius essential oil microencapsulated by spray-drying. Data Brief 47 108927 (2023). [DOI] [PMC free article] [PubMed]
- 21.Ma, S., Jia, R., Guo, M., Qin, K. & Zhang, L. Insecticidal activity of essential oil from Cephalotaxus sinensis and its main components against various agricultural pests’. Ind. Crops Prod.150, 112403 (2020). [Google Scholar]
- 22.Singh, J., Gupta, S. & Khoshe, P. In vitro regeneration of pomegranate (Punicagranatum L.) from nodal explant. Int. J. Adv. Pharm. Biol. Chem.3 (3), 734–736 (2014). [Google Scholar]
- 23.NascimentoAFD, da CamaraCA & MoraesMMD Fumigant activity of Schinus terebinthifolius essential oil and its selected constituents against Rhyzopertha dominica. Rev. Fac. Nac. Agron. Medellín. 71 (1), 8359–8366 (2018). [Google Scholar]
- 24.Bernardi, J. L. et al. Potential agrochemical applications of Schinus terebinthifolius essential oil. J. Stored Prod. Res.105, 102260 (2024). [Google Scholar]
- 25.El-Talpanty, D. M. et al. Chemical analysis of Viloa Odorata L. (Fam. Violaceae) and the efficacy of its essential oil against some stored product insects. Plant. Sci. Today. 11 (4), 287–294 (2024). [Google Scholar]
- 26.Vivekanandhan, P., Alharbi, S. A. & Ansari, M. J. Toxicity, biochemical and molecular docking studies of Acacia nilotica L., essential oils against insect pests. Toxicon243, 107737 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Vivekanandhan, P., Alahmadi, T. A., Ansari, M. J. & Subala, S. P. Biocontrol efficacy of cajeput oil against Anopheles Stephensi L. mosquito and its effect on non-target species. Front. Physiol.15, 1357411 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiran, S. & Prakash, B. Assessment of toxicity, antifeedant activity, and biochemical responses in stored-grain insects exposed to lethal and sublethal doses of Gaultheria procumbens L essential oil. J. Agric. Food Chem.63 (48), 10518–10524 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Omar, A. F., El-Ebiary, M. E., Nasr, G. M. & Hassan, H. M. Toxicity and biochemical effects of Cumin and Basil essential oils on Tribolium Castaneum. Sci. Agric. Bohem. 52 (3), 39–48 (2021). [Google Scholar]
- 30.Bhavya, M. L., Chandu, A. G. S. & Devi, S. S. Ocimum tenuiflorum oil, a potential insecticide against rice weevil with anti-acetylcholinesterase activity. Ind. Crops Prod.126, 434–439 (2018). [Google Scholar]
- 31.Ahmed, S. et al. Change in Malate Dehydrogenase and Alpha Amylase Activities in Rubus fruticosus and Valeriana Jatamansi Treated Granary Weevil, Sitophilus Granaries Braz. J. Biol. (2020). [DOI] [PubMed]
- 32.Swigar, A. A. & Silverstein, R. M. Monoterpenes WI: Aldrich Chemical Company Publ. Milwaukee, javascript:void (0) (1981).
- 33.Adams, R. P. Identification of essential oil components by gas chromatography/mass spectroscopy Allured publishing Co Carol Stream. Illinois, javascript:void (0) (1995).
- 34.Abbott, W. S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol.18, 265–267 (1925). [Google Scholar]
- 35.Reitman, S. & Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol.28 (1), 56–63 (1957). [DOI] [PubMed] [Google Scholar]
- 36.Belfield, A. & Goldberg, D. M. Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme12, 561–573 (1971). [DOI] [PubMed] [Google Scholar]
- 37.Caraway, W. T. A stable starch substrate for the determination of amylase in serum and other body fluids. Am. J. Clin. Pathol.32, 97–99 (1959). [DOI] [PubMed] [Google Scholar]
- 38.Gornall, A. G., Bardawill, C. J. & David, M. M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem.177 (2), 751–766 (1949). [PubMed] [Google Scholar]
- 39.Knedel, M. & Böttger, R. Eine kinetische Methode zur Bestimmung der Aktivität der Pseudocholinesterase (Acylcholin-acythydrolase 3.1.1.8.) [A kinetic method for determination of the activity of pseudocholinesterase (acylcholine acyl-hydrolase 3.1.1.8.)]. Klin. Wochensch.45(6), 325–327 (German) (1967). [DOI] [PubMed] [Google Scholar]
- 40.Abo Arab, H. R. et al. Fumigant and mixing with medium effect of Citrus reticulata and Tagetes minuta essential oil against adults of Sitophilus oryzae (L) and Tribolium castaneum (Herbst). Fresenius Environ. Bull.31 (11), 10758–10766 (2022b). [Google Scholar]
- 41.Chaubey, M. K. Evaluation of insecticidal properties of Cuminum cyminum and Piper nigrum essential oils against Sitophilus zeamais. J. Entomol.14, 148–154 (2017). [Google Scholar]
- 42.Fadl, H. et al. Monitoring of the toxicity and repellency of some essential oils against Solenopsis invicta (Hymenoptera: Formicidae). Fresenius Environ. Bull.31 (11), 10928–10933 (2022). [Google Scholar]
- 43.Sheasha, A. et al. Efficacy of some plant essential oils against two spotted spider mite tetranychus urticae under Laboratory conditions. Pol. J. Environ. Stud.32 (4), 1–8 (2023). [Google Scholar]
- 44.Pratheeba, T., Vivekanandhan, P., Faeza, A. N. & Natarajan, D. Chemical constituents and larvicidal efficacy of Naringi crenulata (Rutaceae) plant extracts and bioassay guided fractions against Culex quinquefasciatus mosquito (Diptera: Culicidae). Biocatal. Agric. Biotechnol. 19, 101137. (2019).
- 45.Vivekanandhan, P., Senthil-Nathan, S. & Shivakumar, M. S. Larvicidal, pupicidal and adult smoke toxic effects of Acanthospermum hispidum (DC) leaf crude extracts against mosquito vectors. Physiol. Mol. Plant Pathol.101, 156–162 (2018). [Google Scholar]
- 46.Khalil, F. M. A., ALshahari, E. A. A., Abo Arab, R. B. & Abouelatta, A. M. Toxicoligical study of Ocimum basilicum and Jasminum grandiflorum essential oils against Rhyzopertha dominica and Tribolium castaneum Ama Agric. Mech. Asia Africa Lat. Am.53(10), 10017–10031. (2022).
- 47.Sahayaraj, K. Modulation of botanicals on Pest’s Biochemistry. Short. Views Insect Biochem. Mol. Biology. 1, 57–74 (2014). [Google Scholar]
- 48.Makarem, A. E., Kholy, H. A., Abdel-Latif, S. E., Seif, A. & AI Physiological and biochemical effects of some essential oils on the granary weevil, Sitophilus granarius (L) (Coleoptera: Curculionidae). Egypt. J. Exp. Biol.. 11, 117–123 (2015). [Google Scholar]
- 49.El-Gizawy, K. K. H., Halawa, S. M. I., Mehany, A. L. & Mohamed, S. A. Toxicity of some essential oils and its biochemical effect against Red Flour Beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Egypt. Acad. J. Biol. Sci. F Toxicol. Pest. Control11 (1), 27–38 (2019). [Google Scholar]
- 50.Askar, S. I., Al-Assaal, M. S. & Nassar, A. M. Efficiency of some essential oils and insecticides in the control of some Sitophilus insects (Coleoptera: Curculionidae). Egypt. J. Plant. Prot. Res.4, 39–55. (2016). [Google Scholar]
- 51.Khater, K. S. & El-Shafiey, S. N. Insecticidal effect of essential oils from two aromatic plants against Tribolium castaneum (Herbst): (Coleoptera: Tenebrionidae. Egypt. J. Biol. Pest. Control25(1), 129. (2015). [Google Scholar]
- 52.Arab, H. R. A. et al. Fumigant and mixing with medium effect of Citrus reticulata and Tagetes minuta essential oil against adults of Sitophilus oryzae (l) and Tribolium castaneum (herbst). Fresenius Environ. Bull. 31 (11), 10942–10949. (2022).
- 53.Rajkumar, V. et al. Toxicity, antifeedant and biochemical efficacy of Mentha piperita L essential oil and their major constituents against stored grain pest. Pest. Biochem. Physiol.156, 138–144 (2019). [DOI] [PubMed]
- 54.Park, C. G., Jang, M., Yoon, K. A. & Kim, J. Insecticidal and acetylcholinesterase inhibitory activities of Lamiaceae plant essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Ind. Crops Prod.89, 507–513. (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.