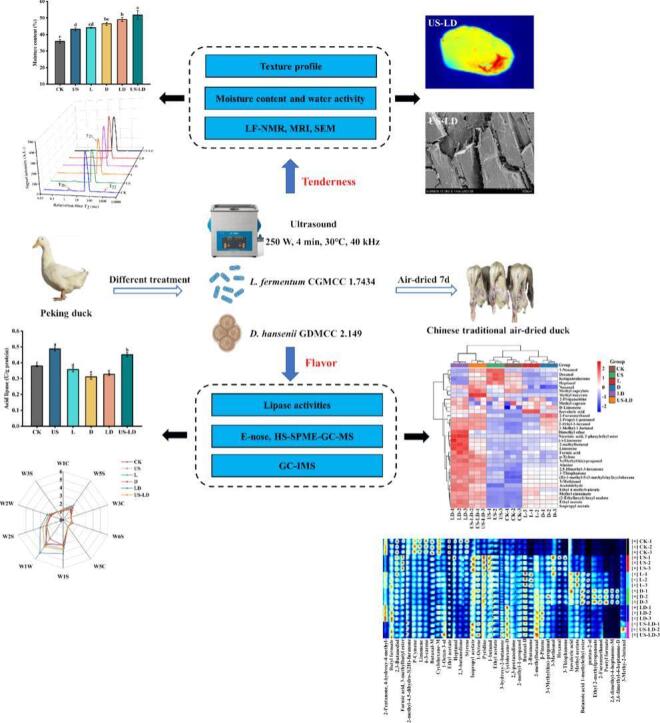
Abstract
The tenderness and flavor of meat products are critical factors influencing consumers' purchasing decisions. This study investigated the effects of ultrasonic pretreatment with synergistic microbial strain fermentation on tenderness and flavor of air-dried duck under low nitrite process. The results demonstrated that ultrasonic pretreatment combined with microbial strain fermentation improved water retention and tenderness of duck meat by disrupting the muscle microstructure, increasing muscle fiber spacing, and facilitating water migration and distribution. This primarily concerns the cavitation and mechanical effects of ultrasound and the role of lactic acid bacteria and yeasts in muscle protein hydrolysis. A total of 34 and 55 volatile flavor compounds were detected by HS-SPME-GC–MS and GC-IMS, respectively. The results indicated that acetaldehyde (stimulating, fruity, green apple), ethyl acetate (sweet, fruity, pineapple), and 3-hydroxy-2-butanone (sweet, creamy) were responsible for the improved flavor during this process, which was primarily related to the increased activity of neutral lipase (0.38 U/g protein), acidic lipase (0.48 U/g protein), and phospholipase (0.09 U/g protein). This study provides valuable insights into the synergistic effects of ultrasonic pretreatment and microbial co-fermentation, offering a theoretical basis for optimizing air-dried duck production and enhancing flavor quality.
Keywords: Air-dried duck, Ultrasonic pretreatment, Lactic acid bacteria, Tenderness, Flavor
Graphical abstract
Effect of ultrasonic pretreatment with synergistic bacterial fermentation on tenderness and flavor of air-dried duck under low nitrite process.
Highlights
-
•
Ultrasound disrupts muscle microstructure and promotes water migration.
-
•
Ultrasound's cavitation and mechanical effects disrupt myofibrillar protein structure.
-
•
Acetaldehyde, ethyl acetate, and pentan-2-ol the cause of the flavor improvement.
1. Introduction
Air-dried duck is a traditional Chinese fermented meat product that has a rich historical background. It is produced from fresh or thawed ducks through a meticulous process involving trimming, curing, air-drying, and packaging (Li, Nie, et al., 2024; Li, Yang, et al., 2024). Known for its high protein content, low fat, and rich nutritional value, air-dried duck remains popular among consumers for its unique flavor, reddish-brown color, and fresh taste. The natural fermentation that occurs during the drying process is heavily reliant on microbial communities, which play a crucial role in determining the quality and safety of the product (Li, Al-Dalali, et al., 2022; Li, Deng, et al., 2022). However, these microbial communities can be heavily influenced by different environmental conditions, equipment and raw materials, resulting in variations in product quality, such as flavor, color, and texture (Li, Al-Dalali, et al., 2022). In addition, Conventional fermented air-dried meat products typically exhibit low water activity (aw 0.70–0.85) and a moisture to protein ratio of 0.75 (Hu et al., 2023), resulting in a product that is often dry and tough. As meat tenderness and flavor are closely related to consumer satisfaction, addressing the texture and characteristic flavor of air-dried duck is a top priority for the meat industry to meet the growing consumer demand for high-quality products.
In recent years, several techniques have been explored to accelerate mass transfer and improve meat tenderization, including electrical stimulation, ultrahigh pressure (Picouet et al., 2012), vacuum (Cheng & Sun, 2006) and ultrasound (Llull et al., 2002). Ultrasound, as a novel food processing technology, offers many advantages such as high efficiency, safety, high penetration, and low energy consumption, making it widely used in meat processing (Xiong et al., 2020). Ultrasonic treatment has been widely applied in meat products to improve tenderness, enhance water-holding capacity, reduce microbial load, and promote flavor development. Mechanistically, ultrasound induces cavitation, which generates localized high pressures and temperatures, resulting in the disruption of muscle fibers, protein denaturation, and enhanced water migration, all of which contribute to improved meat texture and quality (Bian et al., 2022; Qiu et al., 2020). For example, Luo et al. (2021) used ultrasound to treat infant puree and found that the hardness and viscosity of the puree decreased when the ultrasound power was at 400 W, which could improve the texture of the puree. Hu et al. (2018) found that at an ultrasound power of 200 W, a treatment time of 9.6 min, and a temperature of 45 °C, muscle structure was disrupted and tenderness increased significantly. Lactic acid bacteria (LAB) and yeast are considered “safe” microorganisms widely used in fermented meat products. Numerous studies have shown that co-fermentation with LAB and yeast can enhance the flavor profile of meat products and promote the production of nutrients (He et al., 2022). For instance, Cai et al. (2020) found that cured duck legs fermented with Lactobacillus plantarum and Saccharomyces cerevisiae produced a more diverse array of volatile compounds. Lv et al. (2023) found that the inoculation of Saccharomyces cerevisiae LXPSC1 into sour meat significantly promoted the release of free amino acids (FAA) by the hydrolysis of myofibrillar proteins, with the levels of valine, isoleucine, and leucine being 1.44, 1.33, and 1.22 times higher than those of the CK group, respectively. The fermentation group had significantly higher levels of alcohols, esters, aromatic hydrocarbons, and aromatic compounds compared to the control group. However, the effect of ultrasonic pretreatment combined with LAB and yeast symbiotic fermentation on the tenderness and flavor of air-dried duck has not yet been reported.
High intake of nitrite in food can induce cancer. Excessive residual nitrite during processing and digestion can react with secondary amines to form nitrosamines, which possess strong carcinogenic, mutagenic, and teratogenic properties (Wakamatsu et al., 2020). In recent years, research efforts have increased to find ways to reduce or eliminate nitrite. For example, microbial degradation and the use of natural compounds have been explored as alternatives to nitrites (Bedale et al., 2016). Ultrasonic treatment has been shown to promote microbial fermentation by enhancing the bioavailability of nutrients and facilitating the growth of beneficial microorganisms (Zhao et al., 2024). In the preliminary stage of this study, it was found that ultrasonic pretreatment (250 W, 4 min, 30 °C, 40 kHz) and combined with inoculated fermentation (Limosilactobacillus fermentum CGMCC 1.7434 and Debaryomyces hansenii GDMCC 2.149) synergistically reduced the nitrite content of air-dried ducks. After air-drying for 7 days, the nitrite residue was 0.91 mg/kg, and the degradation rate reached 84.55 %. However, nitrite is essential for the quality of meat products. Therefore, the impact of ultrasonic pretreatment combined with the symbiotic fermentation of L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 on the quality of air-dried duck needs further investigation.
Therefore, the aim of this study was to investigate the effect of ultrasonic pretreatment combined with the symbiotic fermentation of L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149, on the tenderness and flavor of air-dried ducks under a low-nitrite process. To elucidate the microstructure of air-dried duck meat, moisture distribution and migration were determined by low-field nuclear magnetic resonance (LF-NMR) and magnetic resonance imaging (MRI), while the microstructure was observed by scanning electron microscopy (SEM). Additionally, volatile flavoring substances were analyzed using electronic nose (E-nose), headspace-solid phase microextraction-gas chromatography, and GC-IMS techniques to provide comprehensive and reliable information. Key flavoring substances and biomarkers were then screened using PCA and PLS-DA. This study aims to provide a new theoretical basis for improving the quality of air-dried duck meat.
2. Materials and methods
2.1. Materials
The strain Debaryomyces hansenii GDMCC 2.149 was acquired from the Guangdong Bacterial Strain Preservation Centre (GDMCC). Limosilactobacillus fermentum CGMCC 1.7434 was isolated in our laboratory and subsequently deposited in the China General Microbiological Culture Collection Centre (CGMCC). Peking ducks, each weighing approximately 2.5 kg, were procured from Ningbo Langde Company (Ningbo, Zhejiang, China). Sodium nitrite (99 % purity), 2-methyl-3-heptanone (used as internal standard, 99 % purity) and 4-Methylumbelliferyl Oleate (95 % purity), Triton X-100 (98 % purity), Bovine Serum Protein (98 % purity), and Sodium Fluoride (98 % purity) were purchased from Sinopharm (Shanghai, China), Merck (Darmstadt, Germany), Macklin (Shanghai, China), YeaSen (Shanghai, China), Sangon Biotech (Shanghai, China), respectively.
2.2. Sample preparation
Both strains were activated twice, in MRS broth at 37 °C and YPD broth at 28 °C, respectively. After 20 h, the cells were harvested by centrifugation at 10,000g for 5 min at 4 °C. The precipitated cells were washed twice with sterile saline solution (0.9 %, w/v), and the cell concentration was adjusted to 107 CFU/g. Freshly slaughtered Peking ducks were selected and rinsed thoroughly to remove any residual blood. The ducks were then marinated in an 8 % brine solution with 0.01 % NaNO2 at 4 °C for 24 h. The microbial strains were sprayed evenly on the surfaces and ventral cavities of the duck embryos using a sterile alcohol sprayer. This study examined the effects of ultrasound treatment (250 W, 4 min, 30 °C, 40 kHz, US) alone, LAB inoculation alone (L. fermentum CGMCC 1.7434, inoculum size of 7 log CFU/g, L), yeast inoculation alone (D. hansenii GDMCC 2.149, inoculum size of 7 log CFU/g, D), co-inoculation (L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149, inoculum size of 7 log CFU/g, 1:1 ratio, LD), and their combination (US-LD) on the quality of air-dried ducks. The treated ducks were then air-dried at 16 °C and 68 % relative humidity for 7 d. After air drying, breast and thigh muscle were collected and stored at −80 °C for further analysis of physicochemical properties and flavor profiles.
2.3. Texture profile determination
At the end of air-drying, the air-dried duck meat was cut into pieces measuring 2 cm × 2 cm × 2 cm. Measurements were performed using a texture analyzer (Stable Micro Systems Corporation, England) in TPA mode with the following parameters: probe model P50, trigger force of 5 g, and pre-test, mid-test, and post-test velocities of 2 mm/s, 2 mm/s, and 10 mm/s, respectively, with a strain factor of 50 % (Gao et al., 2024).
2.4. Moisture content and water activity
Moisture content was determined by measuring the rate of weight loss after drying at 105 °C until a constant weight (≤2 mg), following the procedure outlined in GB 5009.3-2016. Aw was measured using a moisture meter (Shanghai Youke Instrumentation Co., Ltd., China).
2.5. Low-field nuclear magnetic resonance (LF-NMR) determination
The distribution and proportion of moisture in air-dried duck meat samples were determined using a low-field NMR analyzer (Neumay, Suzhou, China) according to the method described by Guo et al. (2023). After the instrument was calibrated, 4 g of the sample was placed into a 25 mm NMR tube, and the spin-spin relaxation time (T2) was measured using a Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence. Typical pulse parameters were set as follows: spectral width of 200 kHz, wait time of 1500 ms, RF delay time of 0.002 ms, 16 scans, 10,000 echoes, and an echo time of 0.4 ms.
2.6. Magnetic resonance imaging (MRI) measurement
The hydrogen proton densities and distributions of air-dried duck meat samples were analyzed using MRI techniques according to the method described by Li et al. (2023). The samples were cut into 1 cm × 1 cm × 1 cm cubes and placed into an NMR tube with an inner diameter of 25 mm. The proton density images of the air-dried duck meat samples were simulated using an MRI system in a low-frequency MRI analyzer. Imaging tests were performed using a spin-echo sequence with parameters of TR = 2750 ms, TE = 27.36 ms, and a mean of 8.
2.7. Scanning electron microscope (SEM) observation
The ultrastructure of the air-dried duck meat samples was observed using a scanning electron microscope (S-3400 N, Hitachi, Tokyo, Japan) according to the method described by Fan et al. (2023). The samples were cut into 5 mm × 5 mm × 5 mm squares, fixed with 2.5 % (v/v) glutaraldehyde for 48 h, and then dehydrated with a gradient of 50 %, 70 %, 90 %, 95 %, and 100 % (v/v) ethanol. Micrographs of the ultrastructure of muscle bundles exposed on the sample surface were taken after vacuum freeze-drying for 48 h and gold coating.
2.8. Lipase extraction and activity determination
2.8.1. Crude lipase solution extraction
The crude lipase solution was extracted according to the method described by Huang et al. (2014) with appropriate modifications. A 200 mg sample was homogenized in 3 mL of lysis buffer (50 mM phosphate buffer, pH 7.5, 5 mM EGTA) at 8000 rpm for 3 × 0.3 min, centrifuged at 10,000g for 20 min at 4 °C, and the supernatant was used to determine the protein concentration using the bisulfite method.
2.8.2. Determination of neutral lipase, acidic lipase, phospholipase activity
Neutral, acidic, and phospholipase activities were determined according to the method described by Chen et al. (2023). Firstly, 10 μL of crude enzyme solution was added to 280 μL of reaction buffer, and the fluorescence values were measured at 350 nm excitation and 445 nm emission wavelengths, respectively, after the reaction at 37 °C for 30 min.
2.9. Determination of E-nose and HS-SPME-GC–MS
2.9.1. Determination of E-nose
The odor of the sample was determined using an electronic nose (PEN3, AirSense Analytics GmbH, Schwerin, Germany) according to the method described by Shi et al. (2020). A 5 g sample of duck meat was accurately weighed and heated in a water bath at 50 °C for 20 min. The parameters of the electronic nose system were set to a chamber flow rate of 200 mL/min and an injection flow rate of 200 mL/min. Each measurement lasted for 150 s.
2.9.2. Determination of volatile flavor compounds based on HS-SPME-GC–MS
A headspace solid-phase microextraction (SPME) coupled with gas chromatography–mass spectrometry (GC–MS) (Agilent, Santa Clara, CA) was employed for the extraction and determination of volatile compounds in air-dried duck meat samples. The chromatographic column used was a VOCOL capillary column (60 m × 0.32 mm × 1.8 μm) with helium as the carrier gas at a flow rate of 1.0 mL/min. The volatile compounds were identified using the NIST14.L (NIST, Gaithersburg, MD, USA) database. Additionally, the internal standard 2-methyl-3-heptanone was added to the samples at a concentration of 10 ppm. The quantity of volatile compounds was calculated by dividing the area of each compound by the area of the internal standard (Chen et al., 2023).
2.10. Determination of GC-IMS
Gas chromatography ion mobility spectrometry (GC-IMS, FlavorSpec® Flavor Analyzer, Anyut, Berlin, Germany) was conducted following the method of Fan, Gao, et al. (2024) and Fan, Liu, et al. (2024) with minor adjustments. An accurately weighed 3.0 g duck mince sample was placed in a 20 mL headspace vial and incubated at 60 °C for 15 min. 500 μL of the headspace sample was then automatically injected into the GC-IMS system. The preheated minced meat samples were analyzed using an MXT-5 column (15 m × 0.53 mm, 1 μm) at 40 °C for 30 min. Nitrogen was used as the carrier gas and was flowed according to the following procedure: initially at a rate of 2 mL/min for 2 min, then increased to 10 mL/min for 10 min, and finally increased to 100 mL/min for 25 min until the end. The spectra were analyzed using VOCal software.
2.11. Statistical analyses
All experiments were conducted with a minimum of 3 repetitions independently for data analysis. Findings are expressed as the mean ± standard deviation (SD). Data were analyzed by one-way analysis of variance (ANOVA) using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA), with significant differences determined at P < 0.05. Heatmap visualizations were generated using the Biodeep Cloud Platform system (https://v2.biodeep.cn). The odors of various flavor compounds were described using a flavor database (https://flavornet.org/flavornet.html) in combination with Baidu (https://www.baidu.com). Multivariate statistical analysis was performed using principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA). Variable importance in projection (VIP) scores were used to assess the significance of volatiles, with scores greater than 1 considered statistically significant. Graphs were generated using Origin 8.0.
3. Results and discussion
3.1. Texture profile analysis
Texture is an organoleptic attribute that refers to the state of organization, sensory quality, and physical properties of food perceived through the sense of touch, and is considered one of the most critical quality attributes of food (Inguglia et al., 2019). Hardness, the force required to change the shape of an object, is a physical indicator used to describe the amount of force needed to chew food, while chewiness reflects the energy expended to grind solid food into a swallowable state. Table 1 presents the TPA parameters of air-dried duck meat samples under different treatments. Compared with the CK group, both LD and US-LD groups significantly reduced the hardness and chewiness of air-dried duck meat (p < 0.05). The hardness of the US-LD group (1258.98 g) and chewiness of the LD group (369.71) were the lowest, which were 24.97 % and 63.75 % lower than those of the CK group (1678.07 g and 1019.93, respectively). This may be due to ultrasonic treatment increasing the moisture content in the duck meat through the cavitation effect, thus softening the meat, whereas L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 may break down the fat and collagen in the duck meat, resulting in a softer meat and ultimately improving the tenderness of the duck meat (Zou et al., 2019).
Table 1.
Effect of different treatments on textural characteristics of air-dried duck meat. Data are expressed as mean ± standard deviation. Different letters (a-d) in each column indicate statistically significant differences (p < 0.05). CK: untreated; US: ultrasonication (250 W, 4 min, 30 °C, 40 kHz); L: inoculation with L. fermentum CGMCC 1.7434; D: inoculation with D. hansenii GDMCC 2.149; LD: inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149; US-LD: inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 after ultrasonic pretreatment (250 W, 4 min, 30 °C, 40 kHz).
| Group | Hardness (g) | Springiness | Cohesiveness | Chewiness | Resilience |
|---|---|---|---|---|---|
| CK | 1678.07 ± 89.28a | 0.79 ± 0.04a | 0.64 ± 0.03a | 1019.93 ± 138.98a | 0.27 ± 0.01ab |
| US | 1562.46 ± 178.18ab | 0.64 ± 0.05bc | 0.67 ± 0.05a | 754.42 ± 28.20b | 0.29 ± 0.04a |
| L | 1656.02 ± 68.19a | 0.72 ± 0.05ab | 0.64 ± 0.01a | 523.72 ± 65.64c | 0.28 ± 0.01ab |
| D | 1576.19 ± 20.75ab | 0.66 ± 0.08b | 0.66 ± 0.05a | 451.39 ± 29.26cd | 0.27 ± 0.02ab |
| LD | 1433.67 ± 33.33b | 0.63 ± 0.04bc | 0.56 ± 0.01b | 369.71 ± 24.49d | 0.24 ± 0.01b |
| US-LD | 1258.98 ± 96.59c | 0.55 ± 0.01c | 0.49 ± 0.01c | 412.56 ± 20.16cd | 0.17 ± 0.01c |
Springiness is the ability of an object to recover its original shape immediately after the withdrawal of an external force that has caused deformation. The springiness of the US (0.64), L (0.72), D (0.66), LD (0.63), and US-LD (0.55) groups was significantly reduced (p < 0.05) compared to the CK group (0.79). It is noteworthy that the US-LD group may exhibit a synergistic effect in reducing the springiness of air-dried duck meat. Ultrasonication, inoculated fermentation, and their combination may cause significant changes in the structure of myogenic fibers. Previous studies have suggested that springiness is related to the mechanical properties of connective tissues. Hu et al. (2023) noted that the myofibrillar alignment of beef jerky was disorganized, and the structural integrity was significantly loosened by ultrasonication at 300 W for 30 min. Wang et al. (2022) suggested that inoculation with LAB and yeasts promotes the release of certain histone proteases from the meat, which in turn accelerates the breakdown of myofibrillar proteins during fermentation, leading to myofibrillar breakage. Ultrasound synergized with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 fermentation resulted in the fracture, depolymerization, and lysis of myogenic fibers in duck meat, weakening the mechanical strength and resistance of the tissues, and ultimately reducing springiness.
Cohesiveness is the amount of internal binding force required to maintain the structural integrity of food and is usually associated with the structural integrity and recovery of meat tissue. In the present study, cohesiveness and resilience showed similar trends to springiness. Both cohesiveness and resilience were significantly lower in the US-LD group compared to the CK group (p < 0.05).
3.2. Moisture content and water activity analysis
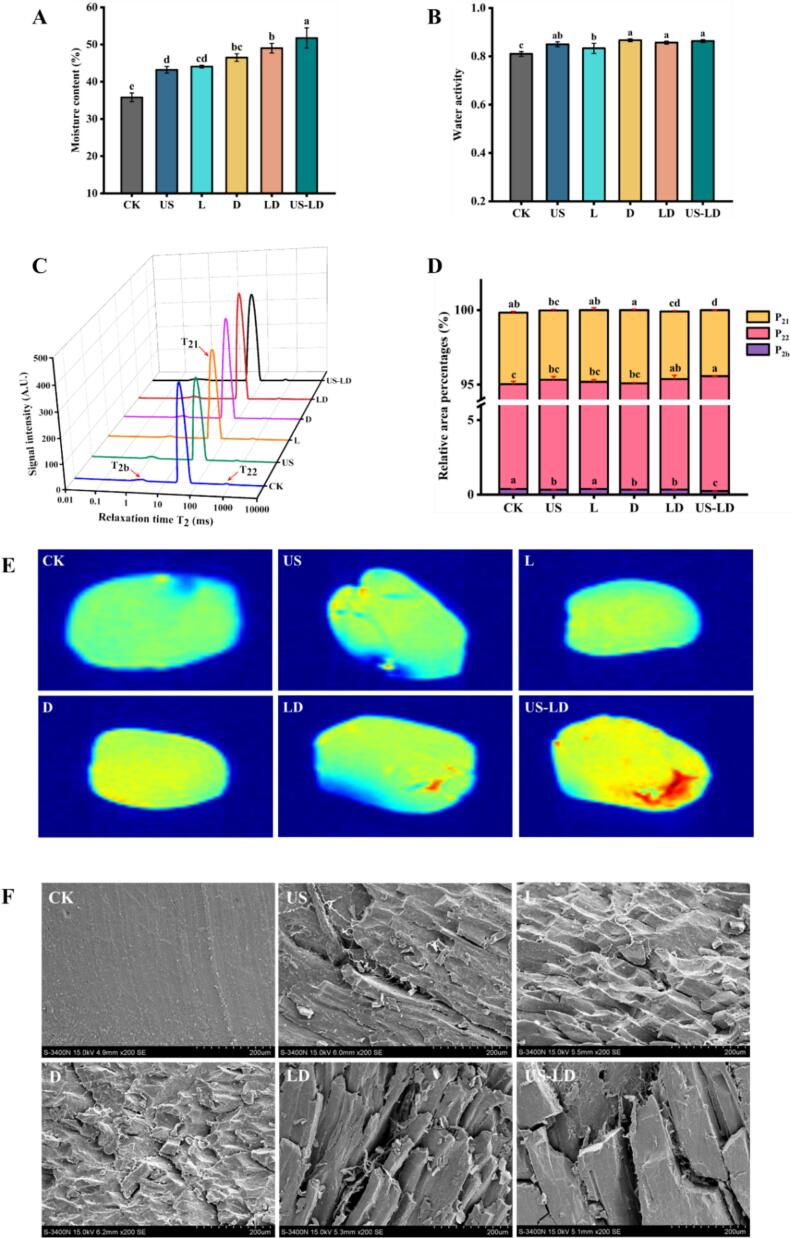
The changes in texture and weight loss of air-dried duck meat were closely related to moisture content and water activity. As shown in Fig. 1A, B, the moisture content and Aw in duck meat of all treatment groups were significantly higher than in the CK group (p < 0.05). The US-LD group showed the most significant increase in moisture content, reaching 51.76 %, followed by the LD group with 49.01 %. An increase in moisture content generally results in a more tender texture, which positively impacts the palatability and overall mouthfeel of meat products. This is mainly because the increase in water content promotes protein hydration and reduces the tight junctions between muscle fibers, resulting in a looser texture (Fan, Gao, et al., 2024). This is consistent with the aforementioned results for textural characterization. This indicates that ultrasonic treatment combined with the fermentation of L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 could be more effective in improving the water retention and maintaining the tenderness of air-dried duck meat. This may be due to ultrasonic waves using the cavitation effect to create tiny air bubbles in the meat. When these bubbles burst, they generate strong shock waves, leading to changes in the meat's microstructure. This structural change can increase porosity and surface area, facilitating water penetration and retention (Barekat & Soltanizadeh, 2017). Additionally, L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 may have decomposed large molecules such as proteins and fats into smaller amino acids and short-chain fatty acids during fermentation, which are highly hydrophilic and retain water more effectively. This supports previous findings by Zhao et al. (2022), who observed that using Lactobacillus paracasei in the production of fermented chorizo rabbit meat increased the free amino acid content (from 205.1 mg/100 g to 505.6 mg/100 g) and effectively improved tenderness.
Fig. 1.
Migration and distribution of moisture and microstructure in air-dried duck meat under different treatments. Moisture content (A); water activity (B); T2 relaxation spectra (C); relative peak area percentages for different water states (D); magnetic resonance images (E); scanning electron microscope images (magnification 200×) (F). Data are expressed as mean ± standard deviation. Different letters (a-e) indicate statistically significant differences (p < 0.05). CK: untreated; US: ultrasonication (250 W, 4 min, 30 °C, 40 kHz); L: inoculation with L. fermentum CGMCC 1.7434; D: inoculation with D. hansenii GDMCC 2.149; LD: inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149; US-LD: inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 after ultrasonic pretreatment (250 W, 4 min, 30 °C, 40 kHz).
3.3. Moisture migration and distribution
The effects of different treatments on the state and mobility of water molecules in duck meat samples were analyzed by measuring the proton transverse relaxation time (T2) using low-field nuclear magnetic resonance (LF-NMR). As shown in Fig. 1C, three peaks appeared within the range of 0.01–10,000 ms, representing the three states of water in duck meat. This result is consistent with the water distribution observed in beef jerky by Gao et al. (2024). According to research, based on the length of the relaxation time, T2b (0.1–10 ms) represents bound water, which is tightly bound to macromolecules such as proteins; T21 (10–100 ms) represents fixed water, which does not easily flow between the epimysium, perimysium and endomysium; and T22 (100–1000 ms) represents free water, which is located on the extracellular surface or meat surface. As shown in Fig. 1C and Table 2, T2b and T22 were significantly reduced and T21 was significantly prolonged (p < 0.05) in the US, L, D, LD, and US-LD groups compared to the CK group, with the most significant effect in the US-LD group. The shorter relaxation time of T2b indicated that the binding of bound water was more tightly bound, and the degree of freedom of water in the corresponding state was lower, and the water retention of meat was better (Yang et al., 2016). Although T21 was prolonged, it remained within the range of fixed water (10–100 ms). Tong et al. (2022) found that ultrasonic treatment shortened the relaxation times of T2b and T21 and increased the relaxation time of T22, which is consistent with the results of the present study. Ultrasonic pretreatment combined with the fermentation of L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 may have increased the solubility of muscle fiber proteins, disrupted the spatial structure of muscle fibers, facilitated the release of salt-soluble proteins, exposed hydrophobic groups, and improved water retention in the meat (Hu et al., 2023; Li et al., 2020).
Table 2.
Effect of different treatments on relaxation time of air-dried duck meat. Data are expressed as mean ± standard deviation. Different letters (a-f) in each column indicate statistically significant differences (p < 0.05). CK: untreated; US: ultrasonication (250 W, 4 min, 30 °C, 40 kHz); L: inoculation with L. fermentum CGMCC 1.7434; D: inoculation with D. hansenii GDMCC 2.149; LD: inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149; US-LD: inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 after ultrasonic pretreatment (250 W, 4 min, 30 °C, 40 kHz).
| Treatments | T2b (ms) | T21 (ms) | T22 (ms) |
|---|---|---|---|
| CK | 2.03 ± 0.02a | 35.50 ± 0.97e | 267.58 ± 10.32a |
| US | 1.28 ± 0.02e | 37.40 ± 1.13d | 248.42 ± 10.36b |
| L | 1.72 ± 0.01c | 39.61 ± 0.44c | 249.63 ± 9.42b |
| D | 1.81 ± 0.03b | 39.54 ± 0.62c | 249.47 ± 10.01b |
| LD | 1.59 ± 0.02d | 41.66 ± 0.68b | 231.74 ± 3.49c |
| US-LD | 1.07 ± 0.01f | 49.46 ± 0.63a | 214.27 ± 5.10d |
As shown in Fig. 1D, the proportion of P2b in the US, D, LD, and US-LD treatment groups was significantly lower than that in the CK group (p < 0.05). The changes in the protein part of P2b were mainly attributed to protein denaturation and modification (McDonnell et al., 2013). In the LD and US-LD treatments, the proportions of P21 and P22 were significantly increased and decreased (p < 0.05), respectively. This indicates that the migration of free water to fixed water may have occurred in the duck meat, thereby improving the water retention performance of the duck meat. This result is consistent with the above findings regarding water content.
3.4. Magnetic resonance imaging (MRI) analysis
MRI can visualize the internal structure of food matrices, as well as hydrogen proton density and moisture distribution (Yang et al., 2022). Fig. 1E shows the MRI images of air-dried duck meat under different treatments. The change in hydrogen proton density is indicated by colors ranging from blue to red; the deeper the red, the stronger the hydrogen proton signal and the higher the corresponding water content. As shown in Fig. 1E, the signal intensity at the edge of each sample was significantly lower than that in the interior, mainly because air could only effectively remove water from the sample surface during the air-drying process. The signal intensity of hydrogen protons in the CK group was more uniform and mainly showed blue-green colors. After ultrasonic pretreatment and fermentation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149, the intensity of hydrogen proton signals in the samples increased significantly, with the most significant effect in the LD and US-LD groups. Some areas showed red color, indicating that the water content in the muscle increased significantly after ultrasonication pretreatment and fermentation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149. The combined action had a better effect than single microbial strain fermentation and sonication. Therefore, the combination of T2 chromatograms and magnetic resonance imaging results indicated that the combination of ultrasonic pretreatment and fermentation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 could be more effective in promoting the migration and distribution of water, improving the water retention of air-dried duck meat, and maintaining its tenderness.
3.5. SEM analysis
SEM was used to observe the microstructure of air-dried duck meat. The arrangement and integrity of the muscle fiber microstructure can directly reflect the texture of the meat product and therefore determine its tenderness (Du et al., 2021). Fig. 1F shows the muscle fiber microstructure of air-dried duck meat under different treatments. The muscle fibers of air-dried duck meat in the CK group were tightly arranged and orderly, with a flat surface and no obvious inter-fiber gaps. In the US, L, and D treatment groups, especially the LD and US-LD treatment groups, the myofibrils were disorganized, mostly fractured, and the structural integrity was obviously loose. This result can be explained by the cavitation and mechanical effects of ultrasound, as well as the role of LAB and yeast in the hydrolysis of muscle proteins (Hu et al., 2023). Barekat and Soltanizadeh (2017) reported that ultrasound can disrupt the muscle fiber structure, a result attributed to the cavitation and mechanical effects of ultrasound, which enlarge the gaps and voids between the muscle fibers, consequently loosening the muscular structure. In addition, ultrasound treatment may also increase meat tenderization by altering protein structure to promote the separation of muscle membranes.
3.6. Lipase activities analysis
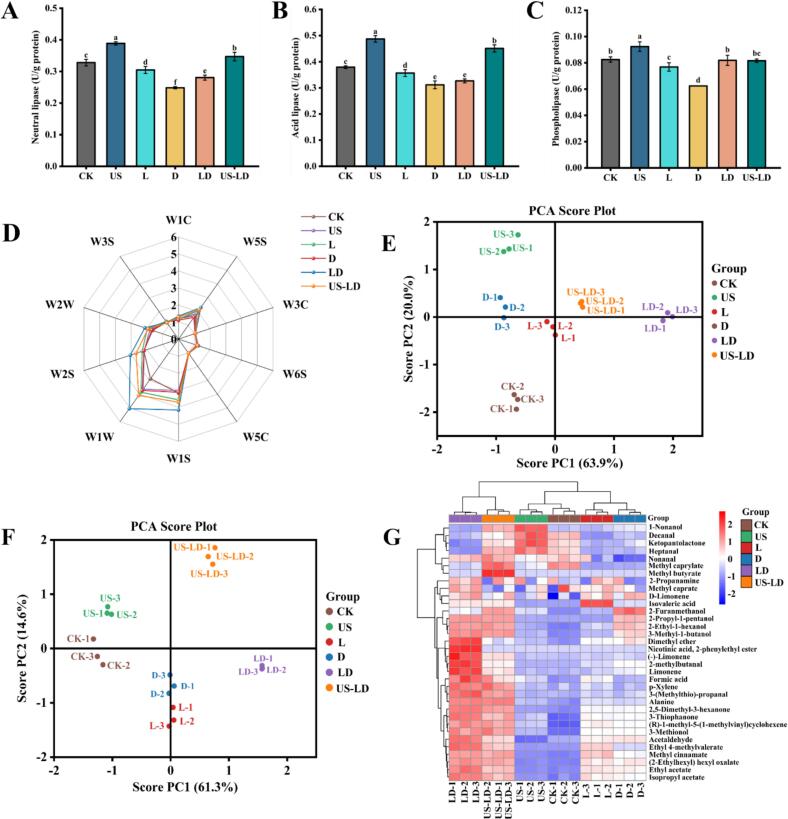
Lipase is the key enzyme that catalyzes lipolysis, and the free fatty acids and other metabolites produced by lipolysis are important precursors of volatile flavor substances (Jin et al., 2010). Fig. 2A, B, C shows the effect of different treatments on the lipolytic enzyme activities in air-dried duck meat. Among the three enzymes, acid lipase had the highest activity (Fig. 2B), indicating that it was the main enzyme in the fat oxidation process, followed by neutral lipase (Fig. 2A). The enzyme activities in the US group were significantly higher than those in the CK group (p < 0.05), with activities of neutral, acidic, and phospholipase being 0.38, 0.48, and 0.09 U/g protein, respectively. This may be due to the fact that ultrasound destroys the cellular structure in the meat products, releasing more enzymes. Additionally, ultrasound promotes the transfer of substances and improves the chances of contact between the substrate and the enzyme, thereby increasing the overall activity (Costello et al., 2021). Unlike the US group, the enzyme activities in both the L and D groups were significantly lower than those in the CK group (p < 0.05). This may be attributed to certain metabolites secreted by L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149, such as organic acids and ethanol, which inhibited the lipase activity. Huang et al., 2014 found that the activity of acidic lipase was significantly greater than that of neutral lipase and phospholipase throughout the process, which is consistent with the results of the present study.
Fig. 2.
Lipase activity and flavor of air-dried duck meat under different treatments. Neutral lipase (A); acidic lipase (B); phospholipase (C). Radar plots of E-nose response data (D); E-nose principal component analysis score plots (E); GC–MS principal component analysis score plots (F); volatile flavor substances heat map (G). Data are expressed as mean ± standard deviation. Different letters (a-f) indicate statistically significant differences (p < 0.05). CK: untreated; US: ultrasonication (250 W, 4 min, 30 °C, 40 kHz); L: inoculation with L. fermentum CGMCC 1.7434; D: inoculation with D. hansenii GDMCC 2.149; LD: inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149; US-LD: inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 after ultrasonic pretreatment (250 W, 4 min, 30 °C, 40 kHz).
3.7. Determination of flavor compounds in air-dried duck using molecular sensory techniques
3.7.1. Electronic nose analysis
The E-nose was used to collect and differentiate the volatile compounds present in air-dried duck meat samples subjected to different treatments (Yakubu et al., 2022). As shown in Fig. 2D, among all treatments, W5S (nitrogen oxides), W1S (methyl groups), W1W (inorganic sulfides), W2S (p-alcohols, aldehydes, and ketones), and W2W (aromatic constituents, organosulfides) exhibited the most significant changes. The most significant increase in values was observed in the LD group, followed by the US-LD group, compared to the CK group. W1W and W2W detected sulfur-containing compounds, which enhance the flavor of the meat and thus contribute to the overall flavor of air-dried duck meat (Li, Nie, et al., 2024; Li, Yang, et al., 2024). Dimensionality reduction was performed using Principal Component Analysis (PCA) to highlight changes and visualize patterns in the dataset. The PCA results of the E-nose are shown in Fig. 2E. The combined variance explained by the first principal component (PC1: 63.9 %) and the second principal component (PC2: 20.0 %) was 83.9 %. The CK group was clearly separated from the other groups, with the differences between the samples primarily centered on PC1. The data points for the six groups of samples were distinctly different, suggesting that the E-nose was capable of completely separating the samples, and that the samples in each group possessed distinct aroma profiles.
3.7.2. GC–MS analysis
To gain a deeper understanding of the flavor profile of air-dried duck meat, its volatile components were analyzed using gas chromatography–mass spectrometry (GC–MS). A total of 34 volatile compounds were identified in the air-dried duck meat, including 3 ketones, 6 aldehydes, 4 terpenoids, 9 esters, 2 acids, 6 alcohols, and 4 others (Table S1). These volatile compounds are typically generated through amino acid metabolism, carbohydrate metabolism, fat oxidation, substrate esterification, and the addition of spices (Hu et al., 2020). According to Fig. 2F, the PCA results demonstrated that GC–MS effectively distinguished air-dried duck meat samples with different treatments. The data points of LD and US-LD were relatively distant from the other samples, whereas the data points of CK and US, L, and D were relatively close to each other. These results may be attributed to significant changes in the microbial community composition. Fig. 2G depicts heat maps of the volatile components that contributed significantly to the differences between treatments, providing a clearer, simpler visualization of the different treatments on volatile components in air-dried duck meat. The heat map clusters the six treatments into three groups (CK and US; L and D; LD and US-LD) and distinguishes two separate clusters of volatile components. In this study, high variability in ester, aldehyde, and alcohol contents may be the main cause of flavor differences in air-dried duck meat.
Esters are primarily produced through esterification reactions between carboxylic acids and alcohols, contributing to pleasant fruity and floral flavors while attenuating the pungency of fatty acids and the bitterness of amino acids. In LD and US-LD treated air-dried duck meat, the levels of Ethyl 4-methylvalerate, (2-Ethylhexyl) hexyl oxalate, Ethyl acetate, Methyl cinnamate, and Isopropyl acetate were significantly higher (p < 0.05) than in the other treatment groups. Notably, the content of Ethyl acetate was significantly higher than the combined sum of all other esters (p < 0.05). Additionally, Isopropyl acetate was detected only in the US-LD group, and Nicotinic acid, 2-phenylethyl ester was detected only in the LD group.
Aldehydes primarily derive from lipid oxidation and significantly contribute to the formation of flavor in air-dried duck meat due to their low odor threshold (Nie et al., 2022). Acetaldehyde and 3-(Methylthio)-propanal were the predominant aldehydes detected in the duck meat samples. Duck meat samples from the LD group contained significantly lower levels of Decanal (sweet, fruity, citrus) and Heptanal (intense, fatty, almond) than the other samples, suggesting that inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 inhibited lipid oxidation. Decanal and Heptanal contents were the highest in the US group (p < 0.05). This indicates that ultrasonic treatment could promote lipid oxidation in air-dried duck meat samples (Kang et al., 2016). These results showed a high correlation with lipase activity. Aldehyde content was mainly affected by fat oxidation and degradation, and the lipase activity was lower in the L, D, and LD groups than in the CK group. This was primarily due to the limitation of fat oxidation by the inoculation of LAB and yeast, whereas the US group, which promoted fat oxidation, exhibited the opposite result.
The alcohols detected in air-dried duck meat were primarily 2-Ethyl-1-hexanol (fresh, slight, fruity) and 2-Propyl-1-pentanol (slightly sweet, alcohol), with the highest levels detected in samples from the LD group, followed by samples from the US-LD group (p < 0.05). In addition, terpenoids, ketones, and other compounds (e.g., limonene, ketopantolactone, isovaleric acid, 2-propanamine) conferred a pleasant sweet and fruity odor to air-dried duck meat and were primarily derived from amino acid metabolism and fat oxidation.
3.8. GC-IMS analysis
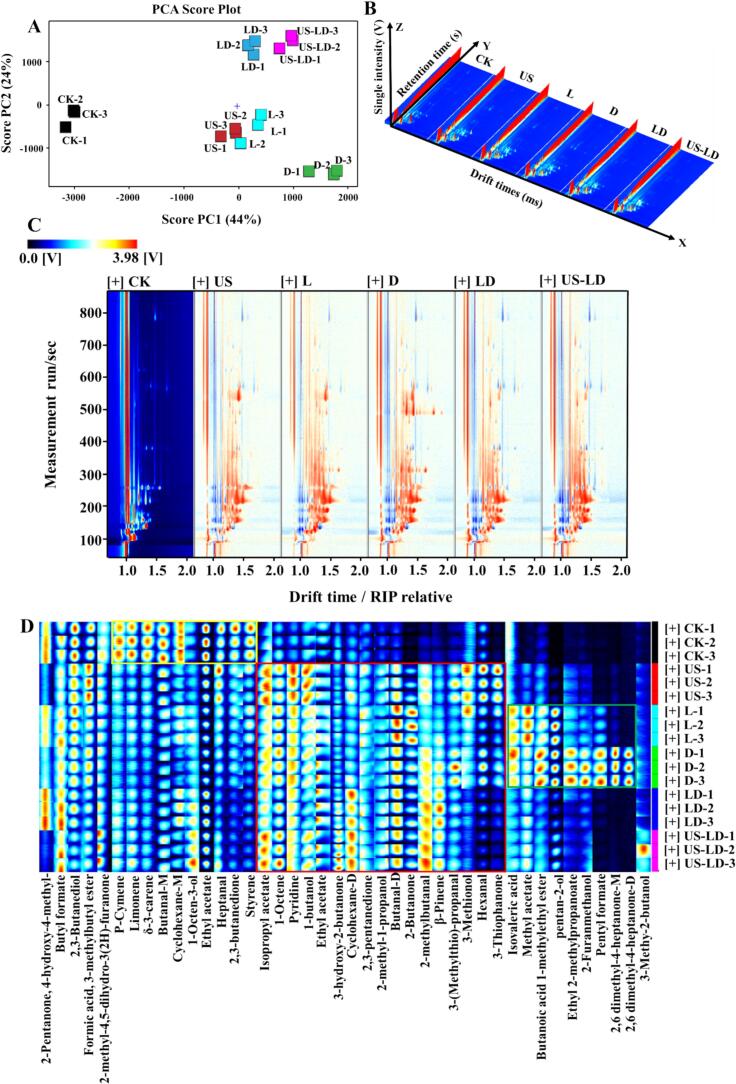
To further examine the flavor compounds composition in air-dried duck meat, gas chromatography-ion mobility spectrometry (GC-IMS) was utilized to identify the flavor substances in air-dried duck meat subjected to different treatments. The PCA analysis results are shown in Fig. 3A, with the combined variance explained by the first principal component (PC1: 44 %) and the second principal component (PC2: 24 %) being 68 %. This indicates that the first two principal components effectively capture most of the information in the samples. The clear separation between the six sample groups observed in the two-dimensional plots suggests significant differences in the overall aroma profiles of the duck meat samples subjected to the different treatments. Fig. 3B illustrates the three-dimensional spectra of flavor compounds in duck meat samples subjected to different treatments, where the X, Y, and Z axes represent ion migration time, GC retention time, and ion peak intensity, respectively. As observed in the figure, the differences in the flavor compounds of duck meat samples subjected to different treatments were mainly reflected in the position, number, intensity, and time of the ion peaks. A top view of the GC-IMS spectra was obtained by normalizing the ion migration time and the position of the reactive ion peak (RIP) (Fig. 3C). The entire spectrum represents the total flavor compounds, and the individual dots to the right of the RIP indicate the VOCs detected from the sample. The colors indicate the signal intensity of the individual compounds, with red indicating high intensity and blue indicating low intensity. A deeper color signifies a higher intensity. The results show that most signals appeared within a retention time of 100–800 s and a drift time of 1.0–1.7 s. The top view of the CK group was used as a reference. For VOCs with the same concentration in the reference and analyte, the background appears white. Blue indicates the compound concentration is below the reference value, while red indicates it is above. As shown in Fig. 3C, the signal intensities in the samples from the US, L, D, LD, and US-LD groups are higher than those from the CK group. The retention time of most flavor ions ranged from 100 to 600 s, and the drift time ranged from 1.0 to 1.5 s.
Fig. 3.
GC-IMS analysis of air-dried duck meat under different treatments. Plot of principal component analysis scores (A); 3D topography (B); difference spectrum (C); fingerprinting (D). CK: untreated; US: ultrasonication (250 W, 4 min, 30 °C, 40 kHz); L: inoculation with L. fermentum CGMCC 1.7434; D: inoculation with D. hansenii GDMCC 2.149; LD: inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149; US-LD: inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 after ultrasonic pretreatment (250 W, 4 min, 30 °C, 40 kHz).
To identify changes in flavor substances in air-dried duck meat under different treatments and to visually and quantitatively analyze these differences, fingerprints were generated using the Gallery drawing plug-in in VOCal software. Fig. 3D shows that the X-axis represents the detected flavor substances, while the Y-axis represents the sample names. Each row corresponds to a signal peak identified in a particular treated sample, whereas each column represents a signal peak for the same flavor substance across different treated samples. Each highlight denotes a flavor substance, with the brightness of the highlight indicating the amount of that substance. Monomers and dimers of the same substance are denoted by “M” and “D,” respectively. A total of 55 VOCs (contains 13 unidentified substances that have been ignored), including 9 ketones, 8 esters, 8 alcohols, 1 furan, 4 terpenes, 6 aldehydes, 1 acid, and 5 other compounds, were detected in air-dried duck meat under different treatments, as shown in Fig. 3D. In the area marked by the red rectangle, the levels of Isopropyl acetate, 1-Octene, Pyridine, 1-butanol, Ethyl acetate, 3-hydroxy-2-butanone, Cyclohexane-D, 2,3-pentanedione, 2-methyl-1-propanol, Butanal-D, 2-Butanone, 2-methylbutanal, β-Pinene, 3-(Methylthio)-propanal, 3-Methionol, Hexanal, and 3-Thiophanone were significantly increased compared to the CK group. The levels of Isopropyl acetate (sweet, fruity aroma), Ethyl acetate (sweet, fruity aroma), Cyclohexane-D (light, fruity flavor), and 2-methylbutanal (fruity flavor) were significantly higher in the LD and US-LD groups compared to the ultrasound treatment alone and the fermentation of L. fermentum CGMCC 1.7434 with D. hansenii GDMCC 2.149. These findings suggest that the combined fermentation of L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149, enhanced by ultrasound, can effectively improve the flavor of air-dried duck meat. In the area marked by the green rectangle, the levels of Isovaleric acid, Methyl acetate, Butanoic acid 1-methylethyl ester, pentan-2-ol, Ethyl 2-methylpropanoate, 2-Furanmethanol, Pentyl formate, 2,6-dimethyl-4-heptanone-M, 2,6-dimethyl-4-heptanone-D, and 3-Methyl-2-butanol were significantly increased in the L and D groups. These compounds were mainly produced by amino acid metabolism, esterification, and carbohydrate metabolism facilitated by L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149. Meanwhile, terpenoids such as P-Cymene, Limonene, and δ-3-carene were more abundant in the CK group, as indicated in the yellow-labelled region.
3.9. Identification of volatile flavor compounds using GC–MS and GC-IMS
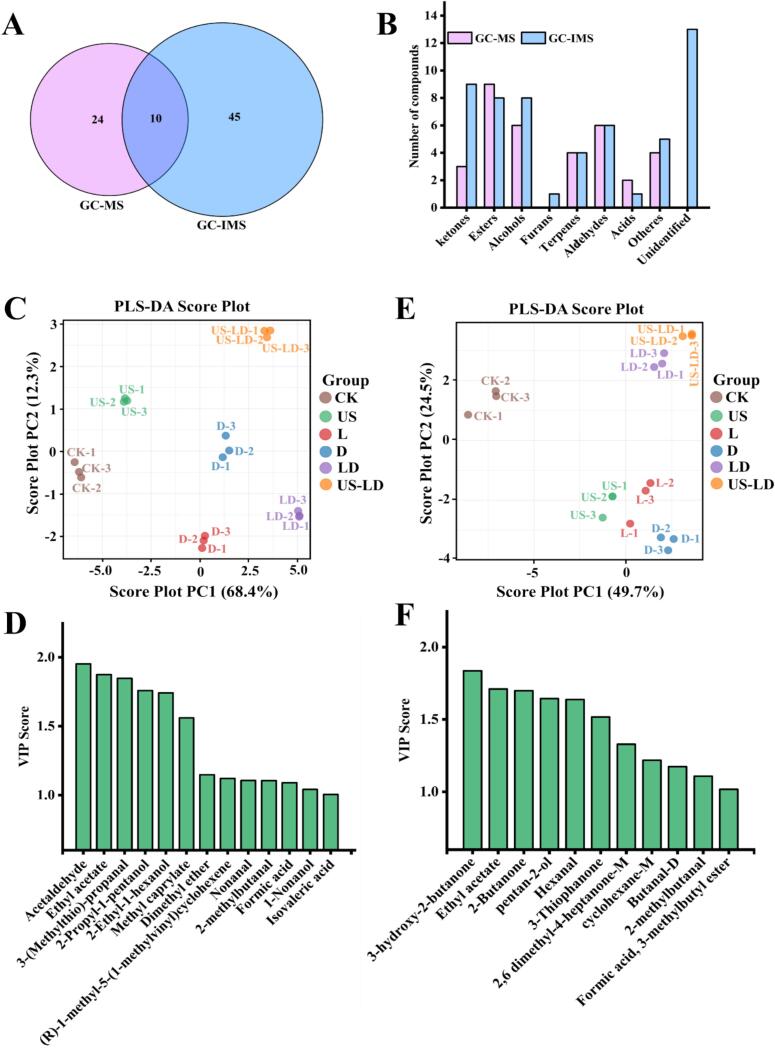
A combined analysis revealed that a total of 79 volatile flavor compounds, including 11 ketones, 15 esters, 12 alcohols, 1 furan, 7 terpenoids, 9 aldehydes, 2 acids, 9 other compounds, and 13 unidentified compounds, were detected in the air-dried duck meat samples using GC–MS and GC-IMS. Ten of these compounds were detected using both GC–MS and GC-IMS (Fig. 4A), namely, 3-Thiophanone, 2-methylbutanal, Heptanal, 3-(Methylthio)-propanal, Limonene, Ethyl acetate, Isopropyl acetate, Isovaleric acid, 2-Furanmethanol, and 3-Methionol. More volatile flavor compounds were detected by GC-IMS compared to GC–MS, and GC-IMS showed higher sensitivity in identifying a variety of flavor compounds (e.g., ketones, alcohols, and furans). As shown in Fig. 4B, nine ketones were identified by GC-IMS, but only three were detected by GC–MS. In addition, GC-IMS detected the only furan compound (2-methyl-4,5-dihydro-3(2H)-furanone, sweet aroma) in the sample. This result is similar to the findings of Li, Al-Dalali, et al. (2022), where 31 and 51 volatile flavor compounds were detected in brine ducks using GC–MS and GC-IMS, respectively.
Fig. 4.
Combined analysis of volatile flavor substances by GC–MS and GC-IMS. Wayne diagram of volatile flavor substances (A); bar chart of volatile flavor substance types (B); PLS-DA score plot based on GC–MS (C); VIP score plot based on GC–MS (D); PLS-DA score plot based on GC-IMS (E); VIP score plot based on GC-IMS (F). CK: untreated; US: ultrasonication (250 W, 4 min, 30 °C, 40 kHz); L: inoculation with L. fermentum CGMCC 1.7434; D: inoculation with D. hansenii GDMCC 2.149; LD: inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149; US-LD: inoculation with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 after ultrasonic pretreatment (250 W, 4 min, 30 °C, 40 kHz).
3.10. Identification of volatile flavor markers based on PLS-DA
To clarify the reasons for the differences between the six samples, multivariate statistical analyses of the GC–MS and GC-IMS data were performed using the supervised PLS-DA method, which screens biomarker metabolites from a large dataset and develops accurate discriminative models (Chen et al., 2024). Fig. 4C and E show the score plots of PLS-DA for GC–MS and GC-IMS volatile flavor compounds, respectively. PC1 and PC2 explained 80.7 % and 74.2 % of the total variance for GC–MS and GC-IMS, respectively. The six samples were completely separated, indicating a difference in flavor among the samples, consistent with the E-nose results. The PLS-DA model uses Variable Importance in Projection (VIP) scores to identify potential flavor markers in air-dried duck meat, which significantly influence the flavor of the samples when VIP > 1. Higher VIP values indicate stronger differences. In Fig. 4D, 13 volatile flavor substances based on GC–MS had VIP scores greater than 1, namely Acetaldehyde (Stimulating, fruity, green apple), Ethyl acetate (Sweet, fruity, pineapple), 3-(Methylthio)-propanal (Intense, onion, garlic), 2-Propyl-1-pentanol (Slightly sweet, alcohol), 2-Ethyl-1-hexanol (Fresh, slight, fruity), Methyl caprylate (Sweet, fruity, coconut), Dimethyl ether (Slight, etheric odor), (R)-1-methyl-5-(1-methylvinyl)cyclohexene (Intense, citrus flavor, orange), Nonanal (Sweet, fruity, rose), 2-methylbutanal (Sweet, fruity, fermented aroma), Formic acid (Intense, sour taste, anthranilic acid), 1-Nonanol (Sweet, floral, rose), and Isovaleric acid (Sour, sweaty, cheese). These 13 compounds were identified as biomarkers in the PLS-DA model developed by GC–MS. In the VIP score plot derived from GC-IMS data (Fig. 4F), 3-hydroxy-2-butanone (Sweet, fruity, creamy), Ethyl acetate (Sweet, fruity, pineapple), 2-Butanone (Etheric odor), pentan-2-ol (Fresh, alcohol-like odor), Hexanal (Fruity, fatty, green leafy), 3-Thiophanone (Sweet, sulphurous, onion), 2,6-dimethyl-4-heptanone-M (Intense, fatty, citrusy aroma), cyclohexane-M (Fresh, lemon, orange), Butanal-D (Stimulating, fruity, green apple), 2-methylbutanal (Sweet, fruity, apple), Formic acid (Intense, sour taste, anthranilic acid), and 3-methylbutyl ester (Sweet, fruity, citrus) exhibited VIP scores exceeding 1. Therefore, these 11 compounds were identified as biomarkers in the PLS-DA model based on GC-IMS. In conclusion, these biomarkers play a crucial role in determining the flavor of air-dried duck meat. Compared to GC-IMS, GC–MS identified additional biomarker metabolites that were not recognized by GC-IMS, suggesting these biomarkers could serve as unique indicators of the flavor of traditional air-dried duck.
4. Conclusion
In conclusion, the tenderness and flavor of air-dried duck meat were improved by ultrasonic pretreatment combined with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 fermentation. This was mainly because the mechanical and cavitation effects of ultrasound synergized with the symbiotic fermentation of L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149, disrupting the muscle fiber structure and effectively improving the water-holding capacity and textural properties of the air-dried duck meat by facilitating the migration of free water to fixed water. Regarding volatile flavor substances, results from E-nose, GC–MS, and GC-IMS indicated that ultrasonic pretreatment synergized with L. fermentum CGMCC 1.7434 and D. hansenii GDMCC 2.149 symbiotic fermentation, which promoted lipid oxidation, amino acid metabolism, and substrate esterification by increasing lipase activity, thereby affecting the content of acetaldehyde (stimulating, fruity, green apple), ethyl acetate (sweet, fruity, pineapple), 3-hydroxy-2-butanone (sweet, creamy), and pentan-2-ol (fresh, alcohol-like odor), which enhanced the aroma of air-dried duck. Therefore, ultrasonic pretreatment synergistic with fermentation of microbial strains can be considered a promising technique as an effective way to improve the tenderness and flavor quality of air-dried ducks.
CRediT authorship contribution statement
Weitao Zhao: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Xiankang Fan: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation. Zihang Shi: Visualization, Formal analysis. Yangying Sun: Formal analysis. Zhen Wu: Methodology. Ming Huang: Investigation, Data curation. Daodong Pan: Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key R & D Program of China (2021YFD2100104), Science and Technology Programs of Zhejiang (2019C02085), and Ningbo (202002N3076), and China Agricultural Research System of MOF and MARA(CARS-42-25).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101946.
Contributor Information
Xiankang Fan, Email: fanxiankang2022@163.com.
Daodong Pan, Email: daodongpan@163.com.
Appendix A. Supplementary data
Table S1. Volatile flavoring substances in air-dried duck meat were analyzed qualitatively and quantitatively (ng/g) by GC-MS.
Data availability
Data will be made available on request.
References
- Barekat S., Soltanizadeh N. Improvement of meat tenderness by simultaneous application of high-intensity ultrasonic radiation and papain treatment. Innovative Food Science & Emerging Technologies. 2017;39:223–229. doi: 10.1016/j.ifset.2016.12.009. [DOI] [Google Scholar]
- Bedale W., Sindelar J., Milkowski A. Dietary nitrate and nitrite: Benefits, risks, and evolving perceptions. Meat Science. 2016;120:85–92. doi: 10.1016/j.meatsci.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Bian C., Cheng H., Yu H., Mei J., Xie J. Effect of multi-frequency ultrasound assisted thawing on the quality of large yellow croaker (Larimichthys crocea) Ultrasonics Sonochemistry. 2022;82 doi: 10.1016/j.ultsonch.2021.105907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z., Ruan Y., He J., Dang Y., Cao J., Sun Y., Pan D., Tian H. Effects of microbial fermentation on the flavor of cured duck legs. Poultry Science. 2020;99(9):4642–4652. doi: 10.1016/j.psj.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Fan X., Hu Y., Zhou C., Sun Y., Du L., Pan D. Effect of different salt substitutions on the decomposition of lipids and volatile flavor compounds in restructured duck ham. LWT. 2023;176 doi: 10.1016/j.lwt.2023.114541. [DOI] [Google Scholar]
- Chen J., Wang W., Jin J., Li H., Chen F., Fei Y., Wang Y. Characterization of the flavor profile and dynamic changes in Chinese traditional fish sauce (Yu-lu) based on electronic nose, SPME-GC-MS and HS-GC-IMS. Food Research International. 2024;192 doi: 10.1016/j.foodres.2024.114772. [DOI] [PubMed] [Google Scholar]
- Cheng Q., Sun D. Improving the quality of pork ham by pulsed vacuum cooling in water. Journal of Food Process Engineering. 2006;29(2):119–133. doi: 10.1111/j.1745-4530.2006.00052.x. [DOI] [Google Scholar]
- Costello K., Velliou E., Gutierrez-Merino J., Smet C., Kadri H., Impe J., Bussemaker M. The effect of ultrasound treatment in combination with nisin on the inactivation of Listeria innocua and Escherichia coli. Ultrasonics Sonochemistry. 2021;79 doi: 10.1016/j.ultsonch.2021.105776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Li H., Nuerjiang M., Shi S., Kong B., Liu Q., Xia X. Application of ultrasound treatment in chicken gizzards tenderization: Effects on muscle fiber and connective tissue. Ultrasonics Sonochemistry. 2021;79 doi: 10.1016/j.ultsonch.2021.105786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Gao X., Li R., Pan D., Zhou C. Myofibrillar proteins’ intermolecular interaction weakening and degradation: Are they mainly responsible for the tenderization of meat containing l–arginine, l–lysine, or/and NaCl? Food Chemistry. 2024;441 doi: 10.1016/j.foodchem.2023.138318. [DOI] [PubMed] [Google Scholar]
- Fan X., Ling N., Liu C., Liu M., Xu J., Zhang T., Zeng X., Wu Z., Pan D. Screening of an efficient cholesterol-lowering strain of Lactiplantibacillus plantarum 54-1 and investigation of its degradation molecular mechanism. Ultrasonics Sonochemistry. 2023;101 doi: 10.1016/j.ultsonch.2023.106698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Liu M., Shi Z., Zhang T., Du L., Wu Z., Zeng X., Wu X., Pan D. Binary probiotic fermentation promotes signal (cyclic AMP) exchange to increases the number of viable probiotics, anthocyanins and polyphenol content, and the odor scores of wolfberry fermented beverages. Food Chemistry. 2024;448 doi: 10.1016/j.foodchem.2024.139085. [DOI] [PubMed] [Google Scholar]
- Gao J., Cheng S., Zeng X., Sun X., Bai Y., Hu S., Yue J., Yu X., Zhang M., Xu X., Han M. Effects of contact ultrasound coupled with infrared radiation on drying kinetics, water migration and physical properties of beef during hot air drying. Ultrasonics Sonochemistry. 2024;108 doi: 10.1016/j.ultsonch.2024.106978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Xu X., Zhang X., Chen Z., He R., Ma H. Application of simultaneous ultrasonic curing on pork (longissimus dorsi): Mass transport of NaCl, physical characteristics, and microstructure. Ultrasonics Sonochemistry. 2023;92 doi: 10.1016/j.ultsonch.2022.106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Chen Y., Zhang H., Wang H., Chen S., Liu S., Liu A., Li Q., Ao X., Liu Y. Isolation and identification of Lactobacillus and yeast species and their effect on the quality of fermented rice cakes. Innovative Food Science & Emerging Technologies. 2022;77 doi: 10.1016/j.ifset.2022.102984. [DOI] [Google Scholar]
- Hu J., Ge S., Huang C., Cheung P., Lin L., Zhang Y., Zheng B., Lin S., Huang X. Tenderization effect of whelk meat using ultrasonic treatment. Food Science & Nutrition. 2018;6(7):1848–1857. doi: 10.1002/fsn3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Dong Z., Wen R., Kong B., Yu W., Wang J., Liu H., Chen Q. Combination of ultrasound treatment and starter culture for improving the quality of beef jerky. Meat Science. 2023;204 doi: 10.1016/j.meatsci.2023.109240. [DOI] [PubMed] [Google Scholar]
- Hu Y., Zhang L., Liu Q., Wang Y., Chen Q., Kong B. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from Northeast China. Food Microbiology. 2020;91 doi: 10.1016/j.fm.2020.103505. [DOI] [PubMed] [Google Scholar]
- Huang Y., Li H., Huang T., Li F., Sun J. Lipolysis and lipid oxidation during processing of Chinese traditional smoke-cured bacon. Food Chemistry. 2014;149:31–39. doi: 10.1016/j.foodchem.2013.10.081. [DOI] [PubMed] [Google Scholar]
- Inguglia E., Burgess C., Kerry J., Tiwari B. Foods. Vol. 8. 2019. Ultrasound-assisted Marination: Role of frequencies and treatment time on the quality of sodium-reduced poultry meat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G., Zhang J., Yu X., Zhang Y., Lei Y., Wang J. Lipolysis and lipid oxidation in bacon during curing and drying–ripening. Food Chemistry. 2010;123(2):465–471. doi: 10.1016/j.foodchem.2010.05.031. [DOI] [Google Scholar]
- Kang D., Zou Y., Cheng Y., Xing L., Zhou G., Zhang W. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrasonics Sonochemistry. 2016;33:47–53. doi: 10.1016/j.ultsonch.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Li C., Al-Dalali S., Wang Z., Xu B., Zhou H. Investigation of volatile flavor compounds and characterization of aroma-active compounds of water-boiled salted duck using GC–MS–O, GC–IMS, and E-nose. Food Chemistry. 2022;386 doi: 10.1016/j.foodchem.2022.132728. [DOI] [PubMed] [Google Scholar]
- Li H., Wang L., Wang J., Li X., Li J., Cui F., Yi S., Xu Y., Zhu W., Mi H. Effects of ultrasound-assisted freezing on the quality of large yellow croaker (Pseudosciaena crocea) subjected to multiple freeze-thaw cycles. Food Chemistry. 2023;404(Pt A) doi: 10.1016/j.foodchem.2022.134530. [DOI] [PubMed] [Google Scholar]
- Li W., Yang X., Wang J., Dong Y., Xu X., Wang H. Water holding-capacity and flavor improvement of prepared meat patties induced by magnetic field-assisted marinating and preheating. Journal of Food Engineering. 2024;381 doi: 10.1016/j.jfoodeng.2024.112194. [DOI] [Google Scholar]
- Li X., Deng J., Wu Y., Nie W., Wang Z., Zhou H., Xu B. Insight into the correlation between microbial diversity and flavor profiles of traditional dry-cured duck from the metabolomic perspective. Food Research International. 2022;156 doi: 10.1016/j.foodres.2022.111349. [DOI] [PubMed] [Google Scholar]
- Li X., Nie W., Wu Y., Li P., Li C., Xu B. Insight into the dynamic change of flavor profiles and their correlation with microbial community succession and lipid oxidation during the processing of dry-cured duck. LWT. 2024;198 doi: 10.1016/j.lwt.2024.115966. [DOI] [Google Scholar]
- Li Z., Wang J., Zheng B., Guo Z. Impact of combined ultrasound-microwave treatment on structural and functional properties of golden threadfin bream (Nemipterus virgatus) myofibrillar proteins and hydrolysates. Ultrasonics Sonochemistry. 2020;65 doi: 10.1016/j.ultsonch.2020.105063. [DOI] [PubMed] [Google Scholar]
- Llull P., Simal S., Benedito J., Rosselló C. Evaluation of textural properties of a meat-based product (sobrassada) using ultrasonic techniques. Journal of Food Engineering. 2002;53(3):279–285. doi: 10.1016/S0260-8774(01)00166-2. [DOI] [Google Scholar]
- Luo M., Shan K., Zhang M., Ke W., Zhao D., Nian Y., Wu J., Li C. Application of ultrasound treatment for improving the quality of infant meat puree. Ultrasonics Sonochemistry. 2021;80 doi: 10.1016/j.ultsonch.2021.105831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J., Lin X., Liu M., Yan X., Liang H., Ji C., Li S., Zhang S., Chen Y., Zhu B. Effect of Saccharomyces cerevisiae LXPSC1 on microorganisms and metabolites of sour meat during the fermentation. Food Chemistry. 2023;402 doi: 10.1016/j.foodchem.2022.134213. [DOI] [PubMed] [Google Scholar]
- McDonnell C., Allen P., Duggan E., Arimi J., Casey E., Duane G., Lyng J. The effect of salt and fibre direction on water dynamics, distribution and mobility in pork muscle: A low field NMR study. Meat Science. 2013;95(1):51–58. doi: 10.1016/j.meatsci.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Nie S., Li L., Wang Y., Wu Y., Li C., Chen S., Zhao Y., Wang D., Xiang H., Wei Y. Discrimination and characterization of volatile organic compound fingerprints during sea bass (Lateolabrax japonicas) fermentation by combining GC-IMS and GC-MS. Food Bioscience. 2022;50 doi: 10.1016/j.fbio.2022.102048. [DOI] [Google Scholar]
- Picouet P., Sala X., Garcia-Gil N., Nolis P., Colleo M., Parella T., Arnau J. High pressure processing of dry-cured ham: Ultrastructural and molecular changes affecting sodium and water dynamics. Innovative Food Science & Emerging Technologies. 2012;16:335–340. doi: 10.1016/j.ifset.2012.07.008. [DOI] [Google Scholar]
- Qiu L., Zhang M., Chitrakar B., Bhandari B. Application of power ultrasound in freezing and thawing processes: Effect on process efficiency and product quality. Ultrasonics Sonochemistry. 2020;68 doi: 10.1016/j.ultsonch.2020.105230. [DOI] [PubMed] [Google Scholar]
- Shi J., Nian Y., Da D., Xu X., Zhou G., Zhao D., Li C. Characterization of flavor volatile compounds in sauce spareribs by gas chromatography–mass spectrometry and electronic nose. LWT. 2020;124 doi: 10.1016/j.lwt.2020.109182. [DOI] [Google Scholar]
- Tong H., Cao C., Du Y., Liu Y., Huang W. Ultrasonic-assisted phosphate curing: A novel approach to improve curing rate and chicken meat quality. International Journal of Food Science & Technology. 2022;57(5):2906–2917. doi: 10.1111/ijfs.15597. [DOI] [Google Scholar]
- Wakamatsu J., Kawazoe H., Ohya M., Hayakawa T., Kumura H. Improving the color of meat products without adding nitrite/nitrate using high zinc protoporphyrin IX-forming microorganisms. Meat Science. 2020;161 doi: 10.1016/j.meatsci.2019.107989. [DOI] [PubMed] [Google Scholar]
- Wang D., Cheng F., Wang Y., Han J., Gao F., Tian J., Zhang K., Jin Y. The changes occurring in proteins during processing and storage of fermented meat products and their regulation by lactic acid bacteria. Foods. 2022;11(16) doi: 10.3390/foods11162427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong G., Fu X., Pan D., Qi J., Xu X., Jiang X. Influence of ultrasound-assisted sodium bicarbonate marination on the curing efficiency of chicken breast meat. Ultrasonics Sonochemistry. 2020;60 doi: 10.1016/j.ultsonch.2019.104808. [DOI] [PubMed] [Google Scholar]
- Yakubu H., Kovacs Z., Toth T., Bazar G. Trends in artificial aroma sensing by means of electronic nose technologies to advance dairy production – A review. Critical Reviews in Food Science and Nutrition. 2022;63(2):234–248. doi: 10.1080/10408398.2021.1945533. [DOI] [PubMed] [Google Scholar]
- Yang H., Zhang W., Li T., Zheng H., Khan M., Xu X., Sun J., Zhou G. Effect of protein structure on water and fat distribution during meat gelling. Food Chemistry. 2016;204:239–245. doi: 10.1016/j.foodchem.2016.01.053. [DOI] [PubMed] [Google Scholar]
- Yang W., Huang J., Zhu Z., Lei Y., Zhou X., Huang M. Changes in nitrosohemachrome lead to the discoloration of spiced beef during storage. Food Chemistry. 2022;394 doi: 10.1016/j.foodchem.2022.133449. [DOI] [PubMed] [Google Scholar]
- Zhao W., Fan X., Shi Z., Sun Y., Wu Z., Zeng X., Wang W., Zhou C., Xia Q., Wang Z., Pan D. Limosilactobacillus fermentum CGMCC 1.7434 and Debaryomyces hansenii GDMCC 2.149 synergize with ultrasound treatment to efficiently degrade nitrite in air-dried ducks. Poultry Science. 2024 doi: 10.1016/j.psj.2024.104395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Li S., Liu Y., He Y., Hu R., Yang J., Wang Q. Production of fermented spicy rabbit meat using Lactobacillus paracasei. Bioprocess and Biosystems Engineering. 2022;45(1):87–95. doi: 10.1007/s00449-021-02642-3. [DOI] [PubMed] [Google Scholar]
- Zou Y., Shi H., Xu P., Jiang D., Zhang X., Xu W., Wang D. Combined effect of ultrasound and sodium bicarbonate marination on chicken breast tenderness and its molecular mechanism. Ultrasonics Sonochemistry. 2019;59 doi: 10.1016/j.ultsonch.2019.104735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Volatile flavoring substances in air-dried duck meat were analyzed qualitatively and quantitatively (ng/g) by GC-MS.
Data Availability Statement
Data will be made available on request.