Key Points
Question
What proportion of adults with respiratory syncytial virus (RSV) infections initially diagnosed in the outpatient setting were hospitalized within 28 days during the 2016 to 2022 RSV seasons?
Findings
In this cohort study of 67 239 outpatient medically attended RSV infections from 3 large US databases across 6 RSV seasons, 1 in 20 adults experienced all-cause hospitalization within 28 days.
Meaning
This cohort study quantified real-world risk for hospitalization among adults diagnosed with RSV in outpatient settings to inform clinical risk assessment and development of outpatient interventions that aim to prevent hospitalization.
This cohort study assesses absolute risk of 28-day, all-cause hospitalization following outpatient medically attended respiratory syncytial virus (RSV) infections in adults during the 2016 to 2022 RSV seasons in the US.
Abstract
Importance
Respiratory syncytial virus (RSV) is a leading cause of acute respiratory tract infections among adults and is estimated to cause approximately 159 000 hospitalizations among adults aged 65 years and older in the US each year. Estimates of hospitalization among adults with outpatient medically attended RSV (MA-RSV) infections are required to design interventional studies that aim to prevent hospitalization.
Objective
To assess absolute risk of 28-day, all-cause hospitalization following outpatient MA-RSV infections in adults.
Design, Setting, and Participants
In this cohort study, data from 3 different deidentified databases containing electronic health records (EHR) linked to closed claims data (Optum’s deidentified Integrated Claims-Clinical dataset, TriNetX Linked, and Veradigm Network EHR [VNEHR] database linked with claims) were analyzed separately across 6 RSV years (October 1, 2016, to September 30, 2022) in adults with commercial or government insurance. Outpatient (eg, clinics and emergency departments) MA-RSV infections were identified based on clinical laboratory data or RSV-specific International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes. Data were analyzed from March 2023 to April 2024.
Main Outcomes and Measures
The main outcome was all-cause 28-day hospitalization following outpatient MA-RSV infections among all adults and a high-risk subgroup (defined as age ≥65 years or with asthma, chronic obstructive pulmonary disease [COPD], or congestive heart failure [CHF]).
Results
In this cohort study of 67 239 MA-RSV infections in adults (2771 from Optum, 7442 from TriNetX, and 57 026 from VNEHR), most occurred among females (62%-67%) and comorbidity prevalences were 20.0% to 30.5% for COPD, 14.6% to 24.4% for CHF, 14.6% to 24.4% for asthma; 14.0% to 54.5% of individuals were aged 65 years or older. The proportion hospitalized was 6.2% (95% CI, 5.3%-7.1%) in Optum, 6.0% (95% CI, 5.4% to 6.5%) in TriNetX, and 4.5% (95% CI, 4.3%-4.6%) in VNEHR. Among the high-risk subgroup, the proportion hospitalized was 7.6% (95% CI, 6.5%-8.9%) in Optum, 8.5% (95% CI, 7.6%-9.4%) in TriNetX, and 6.5% (95% CI, 6.2%-6.8%) in VNEHR.
Conclusions and Relevance
In this cohort study of adults with outpatient MA-RSV infections from 3 large deidentified US databases across 6 RSV seasons, approximately 1 in 20 adults experienced all-cause hospitalization within 28 days. The results of this study highlight the public health need for RSV prevention and treatment.
Introduction
Respiratory syncytial virus (RSV) has been identified as an important cause of acute respiratory tract infections among adults.1 In the US, the RSV season usually spans from October to March and peaks in December, following a similar pattern as influenza, with regional variation.2 Adult patients infected with RSV often develop an upper respiratory tract infection, with symptoms such as nasal congestion and rhinorrhea; however, a proportion of patients may progress to a lower respiratory tract infection (LRTI).1,3 RSV is associated with a considerable cost burden, estimated at almost (2020 USD) $1.2 billion annually in the US.4
Individuals with chronic health conditions are particularly vulnerable to RSV. RSV infection can contribute to the exacerbation of respiratory conditions, such as asthma and chronic obstructive pulmonary disease (COPD). Additionally, patients with 1 or more comorbid conditions (eg, cardiac, pulmonary, or kidney disease; diabetes; and immunocompromising conditions) are more likely to develop severe RSV illness5 and have increased risk for developing life-threatening infection.6 RSV also poses considerable risk for older adults, contributing to increased health care utilization.5,7 In a meta-analysis of studies reporting medically attended RSV incidence, McLaughlin et al8 reported a pooled annual incidence rate of 178 hospitalizations per 100 000 US adults aged 65 years and older compared with 67 and 13 hospitalizations per 100 00 for those aged 50 to 64 years and aged younger than 50 years, respectively.8 After adjustment for test sensitivity, the estimate increased to 267 hospitalizations per 100 000 for those aged 65 years and older. This increase in health care utilization extends to outpatient settings. From the same meta-analysis, pooled annual rates of RSV-associated outpatient visits were estimated to range from 934 to 1148 (1401 to 1722 adjusted) visits per 100 000 patients among patients younger than 50 years in a recent meta-analysis, while adults aged 65 years and older accounted for 1519 (2278 adjusted) outpatient visits per 100 000 patients.8 A systematic review of studies that have quantified the relative risk of severe RSV among adults in high-income countries observed that increasing age was consistently associated with increased risk of RSV-related hospitalization, death following hospitalization, and RSV-related LRTI.5
Despite the demonstrated burden of RSV among older adults and individuals with chronic medical conditions, infection rates have been underestimated, in part due to low rates of testing.9 Low testing rates are possibly influenced by limited management options for patients infected with RSV. While 2 vaccines were recently licensed for RSV prevention among older adults,10 there are limited available approved treatments for RSV. Currently, limited estimates exist to determine the risk of hospitalization following outpatient RSV disease diagnoses in the US. Therefore, this study was conducted to address this gap in the literature and inform development of clinical trials that will assess efficacy of an outpatient RSV antiviral treatment in preventing RSV-related hospitalization within 28 days among adults with high risk of progression to severe illness. The primary objective of this analysis is to quantify the absolute risk of 28-day, all-cause hospitalization following an outpatient RSV diagnosis among US adults in clinical practice.
Methods
Data Sources
This retrospective cohort study used previously collected deidentified health care data from 3 different deidentified US databases containing electronic health care records (EHR) linked to closed claims data (Optum’s deidentified Integrated Claims-Clinical dataset, TriNetX Linked, and Veradigm Network EHR [VNEHR] linked with claims) between April 1, 2016, and October 31, 2022. Closed insurance claims data, which contain nearly all health care interactions for a given patient, were selected to better ensure capture of the patient journey; open claims were not used for this study. Each deidentified database was analyzed separately for RSV outpatient encounters occurring between October 1, 2016, to September 30, 2022. Since this study was conducted under a data use agreement using a limited deidentified dataset that did not constitute human participants research as defined by the Common Rule (45 CFR 46.102), institutional review board approval and informed consent were not required. The closed claims in these datasets are fully adjudicated by third-party payers, which ensures data quality. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
The Optum deidentified Integrated Claims-Clinical dataset combines adjudicated claims data (Medicare Advantage and commercial payers) with Optum’s EHR data (demographic characteristics, diagnoses, procedure codes, and laboratory results) from clinicians across the continuum of care. The TriNetX Linked Network provides access to deidentified EHRs for more than 13.2 million patients across 21 health care organizations linked with closed insurance claims from more than 100 commercial and government payers. These data contain information on encounters, diagnoses, procedures, laboratory results, and treatments from the EHR and claims. The VNEHR consists of health care information derived from ambulatory care practices that have partnered with Veradigm to use their EHR platform. The EHR network covers an estimated 20% of US ambulatory physicians, spanning multiple specialties and care settings. The linked insurance claims data contain deidentified inpatient, outpatient, and pharmacy claims. The linked dataset contained 65.2 million individuals who were seen in 43 800 practices contributing to the EHR.
Study Design and Population
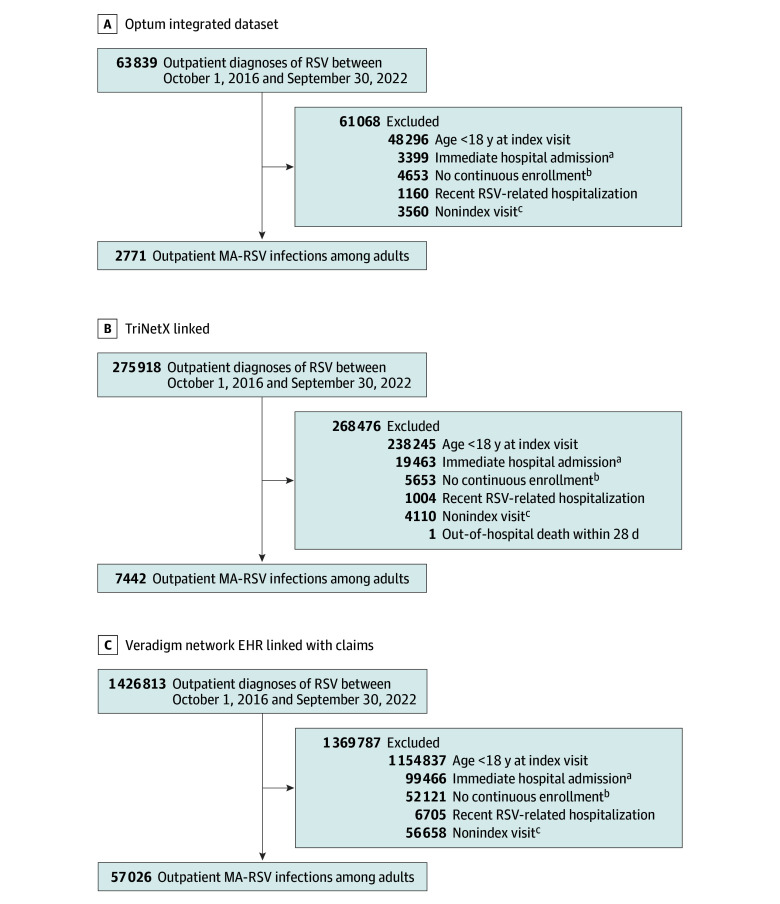
Adults aged 18 years and older with a medically attended RSV (MA-RSV) infection identified from outpatient settings (office, urgent care, and telehealth visits), including emergency departments (EDs), were included. The MA-RSV infection was defined by the documentation of an RSV diagnosis either by a positive RSV test result (polymerase chain reaction, antigen, or culture) within the EHR data or by 1 of the specified RSV-related (International Statistical Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM]) diagnosis codes (B97.4, J12.1, J20.5, or J21.0) in any position within the claims or EHR data. MA-RSV infections occurring in each study year (defined as October 1 through September 30 to capture complete RSV seasons) were evaluated for eligibility (Figure 1). Individuals were also required to have continuous enrollment in their health insurance plan for at least 180 days before the RSV index visit and through 28 days after index. Only the first observed eligible MA-RSV infection for each adult within an RSV season was included; however, an individual patient could contribute multiple infections in subsequent RSV seasons during the study period. MA-RSV infections diagnosed in the ED and resulting in hospitalization within 1 day were excluded. Any MA-RSV infection with an RSV-related hospitalization in the previous 14 days was excluded, as this was a potential follow-up visit and not initial diagnosis. Infections among patients who disenrolled from their health insurance plan or experienced an out-of-hospital death prior to hospital admission within the 28-day period after the RSV index date were also excluded.
Figure 1. Study Design.
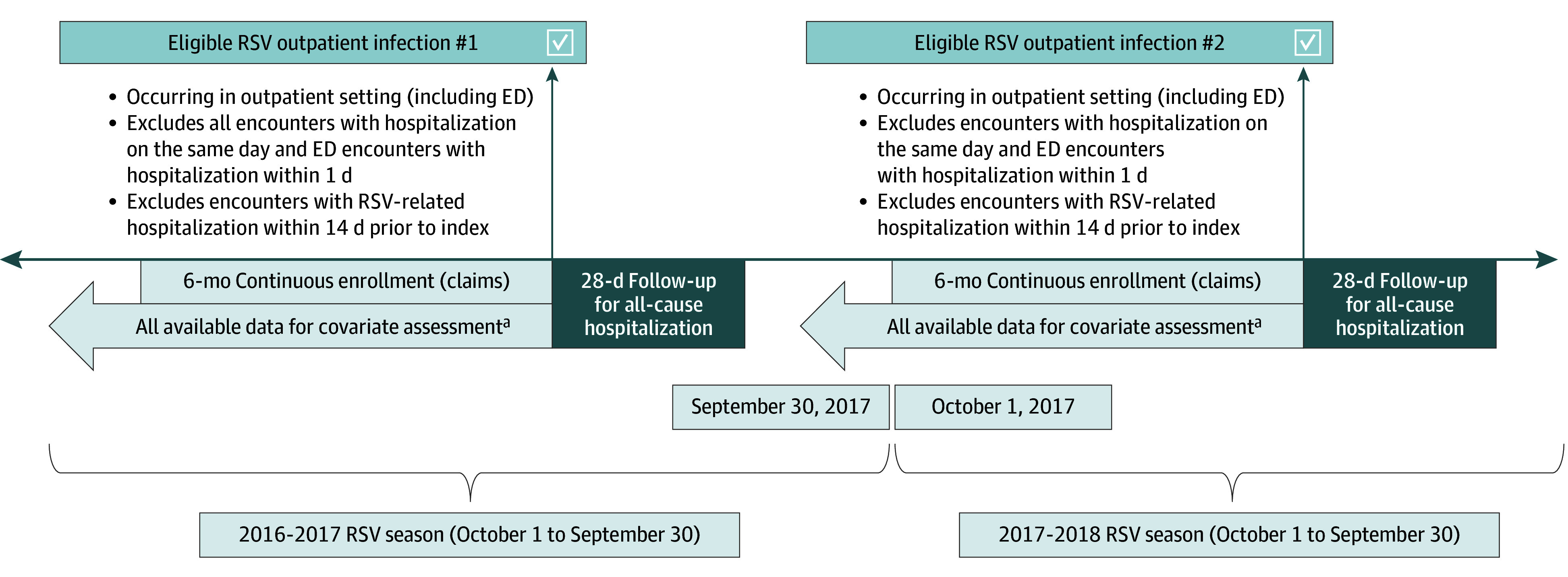
The same methods were used to identify eligible respiratory syncytial virus (RSV) outpatient infections for the 2018 to 2019, 2019 to 2020, 2020 to 2021, and 2021 to 2022 RSV seasons. ED indicates emergency department.
aAll-available data is defined as using all observable data present in the database for a patient at the time of outpatient infection.
High-risk comorbidities, as identified by prior publications, were defined in this study based on the presence of ICD-10-CM diagnosis codes in the EHR or claims data for COPD, CHF, moderate to severe asthma, type 2 diabetes, cystic fibrosis, chronic kidney disease (CKD), cancer, pulmonary fibrosis, or immunocompromised status,5,11,12 using all available historical data for each patient prior to the index date (eAppendix in Supplement 1). A high-risk subgroup was identified to capture outcomes of patients at highest risk of severe RSV outcomes or condition exacerbation based on Centers for Disease Control and Prevention guidance (age ≥65 or ≥75 years, asthma, COPD, or CHF).13 MA-RSV infections were classified as presenting with LRTI at the time of outpatient RSV diagnosis if an LRTI diagnosis code was observed on the same day (eAppendix in Supplement 1).
Outcomes
The outcome of interest was all-cause hospitalization occurring within 28 days following the MA-RSV infection. Given that RSV can exacerbate chronic conditions that lead to hospitalization14 and may not be recorded as the final clinical diagnosis for hospitalization due to its underdetection, the outcome of all-cause hospitalizations was selected rather than hospitalizations identified with RSV-specific codes.
Statistical Analysis
Absolute risk was calculated as the proportion of outpatient MA-RSV infections with a hospitalization observed within a 28-day period. Each MA-RSV infection was only eligible for 1 hospitalization during the 28-day observation period, and subsequent hospitalizations during this period were not counted. Absolute risk of hospitalization was calculated for each database and also was calculated for subgroups based on year, age groups (≥65 and ≥75 years), and high-risk comorbidities (asthma, COPD, CHF, type 2 diabetes, cystic fibrosis, CKD, cancer, pulmonary fibrosis, or immunocompromised status). All descriptive statistics are presented as frequencies for the categorical variables, or mean with SD or median with IQR for the numerical (continuous) variables, as appropriate. Data analysis was conducted using the Snowflake platform version 8.30.2 (Snowflake) for VNEHR data and using SAS software version 9.4 (SAS Institute) for Optum and TriNetX data. R statistical programing language version 4.1.0 (R Project for Statistical Computing) was used to calculate 95% CIs. Any comparisons of proportions hospitalized between seasons or databases were informal and qualitative. This study was descriptive, and no formal hypotheses were tested. Data were analyzed from March 2023 to April 2024.
Results
Baseline Demographics and Clinical Characteristics
In this cohort study that spanned 6 RSV seasons, we identified 67 239 outpatient MA-RSV infections in adults (2771 from Optum, 7442 from TriNetX, and 57 026 from VNEHR) that met all inclusion and exclusion criteria (Figure 2). Baseline demographics and clinical characteristics are reported in Table 1. Across all 3 databases, female patients made up more than one-half of the study sample (62.0% to 67.1%). A high variability in age distribution was observed between databases, as individuals aged 65 years and older accounted for 54.5% (1510 of 2771 patients) in the Optum database, 14.0% (1042 of 7442 patients) in the TriNetX database, and 21.0% (11 979 of 57 026 patients) in the VNEHR database. Outpatient MA-RSV infections in the high-risk subgroup accounted for more than half of RSV infections across TriNetX (52.4%), VNEHR (55.2%), and Optum (73.2%) databases. LRTI was present at the time of MA-RSV index visit in 58.5% to 67.7% of observed RSV infections across all seasons and all database.
Figure 2. Study Inclusion Flowcharts.
aAn immediate hospital admission occurring on the same day as the index visit (or 1 day after an emergency department [ED] index visit) did not allow for adequate time to communicate the respiratory syncytial virus (RSV) diagnosis to the patient and consider outpatient management.
bContinuous enrollment for at least 180 days before the visit date and at least 28 days continuous enrollment after the visit date (censored for death) was required for inclusion into the study.
cOnly the first outpatient visit for RSV per person per RSV season was included as an index visit for this study.
Table 1. Baseline Patient Demographics and Clinical Characteristics.
| Characteristic | MA-RSV infections, No. (%) | ||
|---|---|---|---|
| Optum integrated dataset (n = 2771) | TriNetX Linked (n = 7442) | Veradigm network EHR linked with claims (n = 57 026) | |
| Age, y | |||
| 18-49 | 627 (22.6) | 4182 (56.2) | 27 917 (49.0) |
| 50-64 | 634 (22.9) | 2218 (29.8) | 17 130 (30.0) |
| 65-74 | 587 (21.2) | 575 (7.7) | 5781 (10.1) |
| ≥75 | 923 (33.3) | 467 (6.3) | 6198 (10.9) |
| Sex | |||
| Male | 1054 (38.0) | 2451 (32.9) | 18 827 (33.0) |
| Female | 1716 (61.9) | 4991 (67.1) | 38 126 (66.9) |
| Unknown | 1 (<0.1) | 0 | 73 (0.1) |
| Payera | |||
| Commercial | 1259 (45.4) | 3721 (50.0) | 27 206 (47.7) |
| Medicare Advantage | 1480 (53.4) | 747 (10.0) | 8376 (14.7) |
| Medicaid | 63 (2.3) | 3084 (41.4) | 20 877 (36.6) |
| Other or unknown | 94 (3.4) | 143 (1.9) | 567 (1.0) |
| Index visit type | |||
| ED | 515 (18.6) | 3060 (41.1) | 20 267 (35.5) |
| Other outpatient | 2256 (81.4) | 4382 (58.9) | 36 759 (64.5) |
| RSV seasons | |||
| 2016-2017 | 344 (12.4) | 625 (8.4) | 6883 (12.1) |
| 2017-2018 | 453 (16.3) | 834 (11.2) | 8683 (15.2) |
| 2018-2019 | 520 (18.8) | 1096 (14.7) | 10 168 (17.8) |
| 2019-2020 | 598 (21.6) | 1240 (16.7) | 9660 (16.9) |
| 2020-2021 | 334 (12.1) | 1644 (22.1) | 7858 (13.8) |
| 2021-2022 | 522 (18.8) | 2003 (26.9) | 13 774 (24.2) |
| Presence of LRTI at Index | |||
| Yes | 1757 (63.4) | 4350 (58.5) | 38 612 (67.7) |
| No | 1014 (36.6) | 3092 (41.6) | 18 414 (32.3) |
| High-risk comorbidities | |||
| Asthma | 832 (30.0) | 2557 (34.4) | 19 081 (33.5) |
| COPD | 846 (30.5) | 1485 (20.0) | 13 159 (23.1) |
| CHF | 675 (24.4) | 1084 (14.6) | 9556 (16.8) |
| Immunocompromised statusb | 552 (19.9) | 1778 (23.9) | 8312 (14.6) |
| Cancer | 529 (19.1) | 1049 (14.1) | 6510 (11.4) |
| Type 2 diabetes | 935 (33.7) | 1989 (26.7) | 17 533 (30.7) |
| Chronic kidney disease | 677 (24.4) | 1118 (15.0) | 9953 (17.5) |
| Pulmonary fibrosis | 143 (5.2) | 230 (3.1) | 1665 (2.9) |
| Cystic fibrosis | 5 (0.2) | 68 (0.9) | 155 (0.3) |
| High-risk subgroup | |||
| Age ≥65 y, asthma, COPD, or CHF | 2027 (73.2) | 3902 (52.4) | 31 497 (55.2) |
| Age ≥75 y, asthma, COPD, or CHF | 1804 (65.1) | 3707 (49.8) | 29 600 (51.9) |
Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; LRTI, lower respiratory tract infection; MA-RSV, medically attended respiratory syncytial virus.
Payer type was assessed on the date of the index visit. Some infections occurred among patients who had more than 1 payer type active on the index visit date.
Immunocompromised status includes diagnoses, procedures, and medications for hematologic cancers, conditions of immunodeficiency (including HIV), and organ or stem cell transplants.
Hospitalization
The overall proportion of MA-RSV infections with hospitalization within 28 days was 6.2% (171 of 2771 infections; 95% CI, 5.3%-7.1%) in Optum, 6.0% (443 of 7442 infections; 95% CI, 5.4%-6.5%) in TriNetX, and 4.5% (2544 of 57 026 infections; 95% CI, 4.3%-4.6%) in VNEHR. Of these hospitalizations, a high proportion occurred following MA-RSV infections in the high-risk subgroup of patients aged 65 years or older or with COPD, CHF, or asthma (155 of 171 hospitalizations [90.6%] in Optum; 330 of 443 hospitalizations [74.5%] in TriNetX; 2046 of 2544 hospitalizations [80.4%] in VNEHR). Among this high-risk subgroup, the proportion hospitalized was 7.6% (155 of 2027 infections; 95% CI, 6.5%-8.9%) in Optum, 8.5% (330 of 3902 infections; 95% CI, 7.6%-9.4%) in TriNetX, and 6.5% (2046 of 31497 infections; 95% CI, 6.2%-6.8%) in VNEHR. Among a second high-risk subgroup (age ≥75 years, asthma, COPD, or CHF), the proportion hospitalized was 8.1% (147 of 1804 infections; 95% CI, 6.9%-9.5%) in Optum, 8.7% (322 of 3707 infections; 95% CI, 7.8%-9.7%) in TriNetX, and 6.7% (1988 of 29 600 infections; 95% CI, 6.4%-7.0%) in VNEHR. A summary of absolute risk of hospitalization by comorbidities of interest is presented in Table 2.
Table 2. Absolute Risk of 28-Day, All-Cause Hospitalization of RSV Episodes, Overall and by Medically Attended RSV Infection Characteristicsa.
| Characteristic | Hospitalizations, No./total No. (%) | ||
|---|---|---|---|
| Optum integrated dataset (n = 2771) | TriNetX Linked (n = 7442) | Veradigm network EHR linked with claims (n = 57 026) | |
| All adults | 171/2771 (6.2) | 443/7442 (6.0) | 2544/57 026 (4.5) |
| RSV season | |||
| 2016-2017 | 23/344 (6.7) | 29/625 (4.6) | 232/6883 (3.4) |
| 2017-2018 | 34/453 (7.5) | 71/834 (8.5) | 403/8683 (4.6) |
| 2018-2019 | 43/520 (8.3) | 80/1096 (7.3) | 482/10 168 (4.7) |
| 2019-2020 | 35/598 (5.9) | 83/1240 (6.7) | 486/9660 (5.0) |
| 2020-2021 | 9/334 (2.7) | 72/1644 (4.4) | 297/7858 (3.8) |
| 2021-2022 | 27/522 (5.2) | 108/2003 (5.4) | 644/13 774 (4.7) |
| Age category, y | |||
| ≥65 | 135/1510 (8.9) | 107/1042 (10.3) | 943/11 979 (7.9) |
| ≥75 | 92/923 (10.0) | 62/467 (13.3) | 567/6198 (9.1) |
| High-risk comorbid condition | |||
| Asthma | 58/832 (7.0) | 167/2557 (6.5) | 1097/19 081 (5.7) |
| COPD | 89/846 (10.5) | 172/1485 (11.6) | 1222/13 159 (9.3) |
| CHF | 88/675 (13.0) | 163/1084 (15.0) | 1073/9556 (11.2) |
| Immunocompromised status | 47/552 (8.5) | 163/1778 (9.2) | 743/8312 (8.9) |
| Cancer | 56/529 (10.6) | 104/1049 (9.9) | 533/6510 (8.2) |
| Type 2 diabetes | 77/935 (8.2) | 200/1989 (10.1) | 1261/17 533 (7.2) |
| Chronic kidney disease | 72/677 (10.6) | 141/1118 (12.6) | 1032/9953 (10.4) |
| Pulmonary fibrosis | 13/143 (9.1) | 32/230 (13.9) | 168/1665 (10.1) |
| Cystic fibrosis | NAb | 6/68 (8.8) | 12/155 (7.7) |
| High-risk subgroup | |||
| Age ≥65 y, asthma, COPD, or CHF | 155/2027 (7.6) | 330/3902 (8.5) | 2046/31 497 (6.5) |
| Age ≥75 y, asthma, COPD, or CHF | 147/1804 (8.1) | 322/3707 (8.7) | 1988/29 600 (6.7) |
Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; EHR, electronic health record; NA, not available; RSV, respiratory syncytial virus.
Absolute risk was calculated as the unadjusted proportion of medically attended RSV infections with any hospitalization observed within a 28-day period.
Not reported due to small sample size.
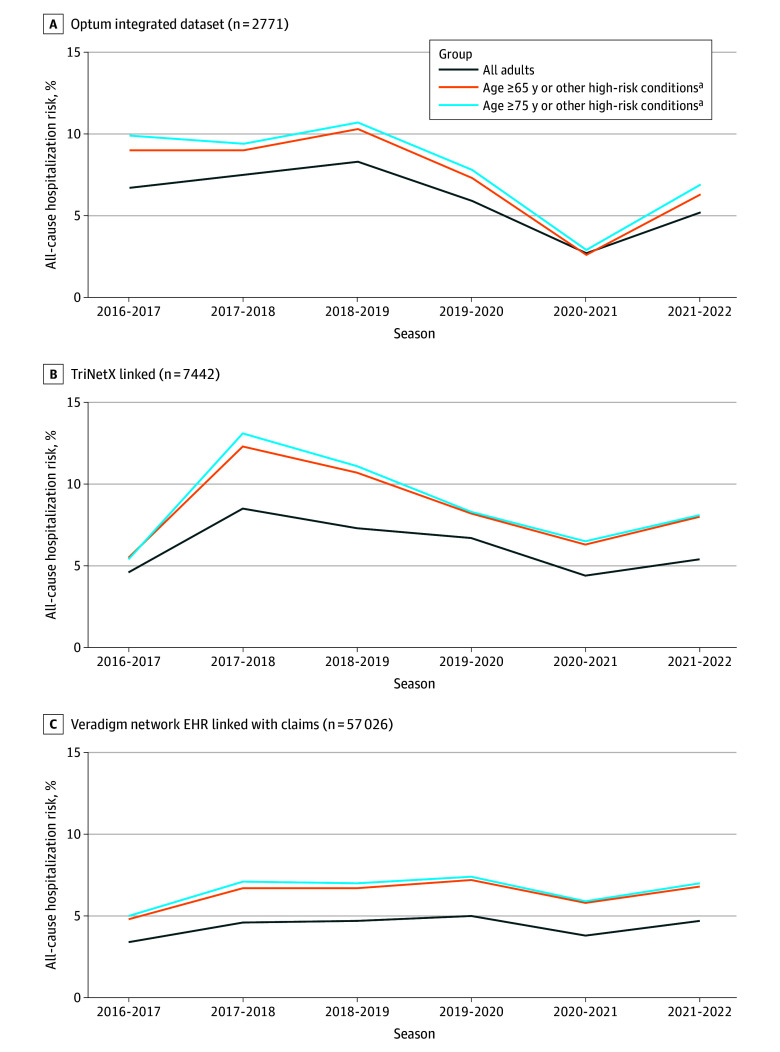
Figure 3 highlights absolute risk of hospitalization across the 6 years and across each database separately. The absolute risk of hospitalization among all adults across unique RSV seasons ranged from 2.7% to 8.3% in Optum, 4.4% to 8.5% in TriNetX, and 3.4% to 5.0% in VNEHR. A potential decrease in proportion hospitalized was noted in Optum for the 2020 to 2021 RSV season, coinciding with the COVID-19 pandemic, but this trend was not as distinct in TriNetX or VNEHR (Figure 3).
Figure 3. Absolute Risk of All-Cause Hospitalization Stratified by Year and High-Risk Subgroup Among Adults With Outpatient Medically Attended Respiratory Syncytial Virus .
EHR indicates electronic health record.
aConditions included asthma, chronic obstructive pulmonary disease, and congestive heart failure
If the index visit was in the ED, the proportion hospitalized was 8.5% (44 of 515 infections) in Optum, 7.5% (228 of 3060 infections) in TriNetX, and 6.3% (1282 of 20 267 infections) in VNEHR. If the index visit was not in the ED, the proportion hospitalized was 5.6% (127 of 2256 infections) in Optum, 4.9% (215 of 4382 infections) in TriNetX, and 3.4% (1262 of 36 759 infections) in VNEHR.
Among MA-RSV infections with a hospitalization within 28 days, the mean (SD) time from index visit to hospitalization was 8.9 (7.7) days in Optum, 11.0 (7.9) days in TriNetX, and 9.9 (8.4) days in VNEHR, with a median (IQR) of 6 (3-14) days in Optum, 10 (4-17) days in TriNetX, and 7 (2-16) days in VNEHR. Among MA-RSV infections with a hospitalization within 28 days, the mean (SD) hospital length of stay was 5.8 (5.5) days in Optum, 10.0 (18.1) days in TriNetX after excluding 2 extreme outliers, and 6.7 (6.0) days in VNEHR, with a median (IQR) of 4 (3-7) days in Optum, 5 (2-9) days in TriNetX, and 5 (3-8) days in VNEHR. The proportion of hospitalizations with a length of stay 7 days or longer was 27.5% in Optum, 35.4% in TriNetX, and 32.3% in VNEHR.
Discussion
Our cohort study was conducted across 3 unique US-based EHR deidentified databases linked with claims. We assessed a large number of MA-RSV infections among adults across 6 consecutive RSV seasons from 2016 to 2022 and have provided new insights into the absolute risk of hospitalization following MA-RSV in an outpatient setting. To our knowledge, this work represents the largest effort to date to quantify the absolute risk of hospitalization following outpatient diagnosis of RSV. The study had several notable strengths. A common protocol was executed across 3 different deidentified US databases to minimize the risk of selection bias that may have been inherent regarding the composition of each individual database. This study leveraged deidentified EHR data plus linked claims data to more accurately and completely capture the patient journey over the 28-day period. The study included information for 6 years of RSV seasons, allowing for additional clarity on the variability of absolute risk of hospitalization between seasons, especially during the disruptions in RSV epidemiology and health care delivery that occurred during the COVID-19 pandemic.
The literature on hospitalization following an RSV index visit is limited and largely outdated. Estimates of hospitalization following RSV range from 16% to 29% among adults at high risk, although there are variations in methods, and more recent time periods are not included.7,13,15,16 Using administrative claims data from a sample of the Medicare Fee-for-Service population (ie, US adults aged ≥65 years), Wyffels et al13 observed that 16.8% of patients diagnosed with RSV in outpatient settings were hospitalized within 180 days between January 2011 and December 2015.13 Our findings build on this work by leveraging linked claims and deidentified EHR data and implementing a common protocol across each data source for a comprehensive estimate of absolute risk of hospitalization. A high and clinically significant absolute risk of hospitalization following MA-RSV infection in the outpatient setting was observed in the oldest age groups and individuals with COPD, CHF, CKD, pulmonary fibrosis, or cancer.
As previously noted by McLaughlin et al8 and Njue et al,5 chronic conditions, such as diabetes and chronic cardiopulmonary, kidney, and immunocompromising conditions, contribute to hospitalizations and severe outcomes among younger adults. In our analysis of outpatient RSV episodes, chronic lung disease, CHF, type 2 diabetes, CKD, and immunocompromised status were among the most common conditions observed. Absolute risks of hospitalization for those with CKD or type 2 diabetes were higher than asthma, but similar to COPD. Older patients (aged ≥65 or ≥75 years) and conditions that may lead to severe infection, such as asthma, COPD, or CHF,17 accounted for most RSV hospitalizations across databases. Wyfells et al13 reported similar comorbidity prevalence estimates to those observed in our study, including hypertension (76.3%), COPD (53.7%), high cholesterol (52.0%), diabetes (41.1%), CHF (41.1%), coronary artery disease (39.8%), and CKD (31.0%), associated with patients admitted to the hospital following an RSV infection.13 Additionally, RSV-related morbidity and mortality has been observed in patients with diseases that can lead to an immunocompromised state.5
Our analysis showed an absolute risk of all-cause hospitalization ranging from 4.5% to 6.2% among adults diagnosed with RSV in the outpatient setting. Prior cohort studies have suggested that most all-cause hospitalizations (82%-90%) observed within 28 to 30 days of RSV diagnosis in adults are related to acute respiratory infection.15,18 The literature suggests that RSV-associated hospitalizations are associated with substantial health care burden.19,20,21,22,23,24 RSV-related hospitalizations can result in a prolonged hospital durations of almost a week.19,20 In addition, Walsh et al19 reported that 28% of patients hospitalized for RSV were later admitted to an ICU for respiratory difficulty, respiratory rate outside reference range, or low blood oxygen level.19 Further literature highlights the severity of RSV compared with influenza. A 2017 study by Kwon et al21 found a 2-fold increase (adjusted hazard ratio, 2.32; 95% CI, 1.17-4.58) in the risk of all-cause mortality among patients hospitalized with RSV (18.4%) compared with those hospitalized with influenza (6.7%). Older adults hospitalized with RSV have a higher likelihood of requiring longer hospital stays and more intensive interventions compared with older adults hospitalized with either influenza or COVID-19.22,23 In a study by Choi et al,4 RSV hospitalization among patients aged 18 to 49 years was associated with a mean total cost of hospitalization of (2020 USD) $11 124, with mean cost as high as $20 577 in patients admitted to the ICU.4 In addition, a model that assessed the association of vaccinations with RSV outcomes in US adults aged at least 60 years during 1 RSV season found that a vaccine with 50% efficacy and coverage would prevent an estimated 43 700 to 81 500 RSV hospitalizations and 8000 to 14 900 RSV-associated deaths.24 Therapeutic and preventive measures to reduce hospitalizations following RSV infection would provide major benefits to patients in the US health care system.
Limitations
This study has some limitations. The study used secondary data that were originally collected for routine medical care. RSV testing is not consistently performed in health care settings.25,26 The MA-RSV infections identified within these databases via a positive laboratory test result or a diagnosis code underrepresent the true number of RSV events during the time period.8 For this reason, study findings can only be applied to RSV infections that are identified in an outpatient setting and cannot be used to estimate the true burden of RSV infections in the larger population. Additionally, not all patients had laboratory test data available in their EHR to confirm an RSV infection. Definitions of comorbidities relied on the presence of an accurate and relevant diagnosis or procedure code, and diagnosis codes may not accurately distinguish disease severity.
Patient overlap between databases was not assessed due to data privacy concerns, and an RSV infection may have been observed in more than 1 database. This study did not perform any modeling to estimate adjusted risks or control for within-person correlation for patients contributing multiple instances of MA-RSV infection.
The outcome of all-cause hospitalization was selected to capture a broad range of events that may be influenced by RSV infection (eg, exacerbation of chronic conditions). This method may include hospitalizations that are unrelated to RSV. However, a similar approach using all-cause hospitalization within a 14-day observation period following a positive RSV test result is used by the Centers of Disease Control and Prevention RSV Hospitalization Surveillance Network.27
Conclusions
In this cohort study of outpatient MA-RSV infections among adults, 3 large US databases of EHR data linked with claims were used to generate generalizable estimates across 6 RSV seasons (2016-2022). Approximately 5% of adults with outpatient MA-RSV infections experienced an all-cause hospitalization within 28 days. These results highlight the unmet medical need for outpatient interventions and preventive measures that can reduce hospitalizations.
eAppendix. Code List
Data Sharing Statement
References
- 1.Nam HH, Ison MG. Respiratory syncytial virus infection in adults. BMJ. 2019;366:l5021. doi: 10.1136/bmj.l5021 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . RSV HHS regional trends. Updated February 1, 2024. Accessed February 8, 2024. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/surveillance/nrevss/rsv/hhsregion.html
- 3.Jain H, Scheitzer J, Justice N. Respiratory Virus Infection in Children. In: StatPearls. StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 4.Choi Y, Hill-Ricciuti A, Branche AR, et al. Cost determinants among adults hospitalized with respiratory syncytial virus in the United States, 2017-2019. Influenza Other Respir Viruses. 2022;16(1):151-158. doi: 10.1111/irv.12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Njue A, Nuabor W, Lyall M, et al. Systematic literature review of risk factors for poor outcomes among adults with respiratory syncytial virus infection in high-income countries. Open Forum Infect Dis. 2023;10(11):ofad513. doi: 10.1093/ofid/ofad513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SY, Kim T, Jang YR, et al. Factors predicting life-threatening infections with respiratory syncytial virus in adult patients. Infect Dis (Lond). 2017;49(5):333-340. doi: 10.1080/23744235.2016.1260769 [DOI] [PubMed] [Google Scholar]
- 7.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749-1759. doi: 10.1056/NEJMoa043951 [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin JM, Khan F, Schmitt HJ, et al. Respiratory syncytial virus-associated hospitalization rates among US infants: a systematic review and meta-analysis. J Infect Dis. 2022;225(6):1100-1111. doi: 10.1093/infdis/jiaa752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozenbaum MH, Begier E, Kurosky SK, et al. Incidence of respiratory syncytial virus infection in older adults: limitations of current data. Infect Dis Ther. 2023;12(6):1487-1504. doi: 10.1007/s40121-023-00802-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801. doi: 10.15585/mmwr.mm7229a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad N, Walker TA, Waite B, et al. Respiratory syncytial virus-associated hospitalizations among adults with chronic medical conditions. Clin Infect Dis. 2021;73(1):e158-e163. doi: 10.1093/cid/ciaa730 [DOI] [PubMed] [Google Scholar]
- 12.Branche AR, Saiman L, Walsh EE, et al. Incidence of respiratory syncytial virus infection among hospitalized adults, 2017-2020. Clin Infect Dis. 2022;74(6):1004-1011. doi: 10.1093/cid/ciab595 [DOI] [PubMed] [Google Scholar]
- 13.Wyffels V, Kariburyo F, Gavart S, Fleischhackl R, Yuce H. A real-world analysis of patient characteristics and predictors of hospitalization among US Medicare beneficiaries with respiratory syncytial virus infection. Adv Ther. 2020;37(3):1203-1217. doi: 10.1007/s12325-020-01230-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branche AR, Falsey AR. Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs Aging. 2015;32(4):261-269. doi: 10.1007/s40266-015-0258-9 [DOI] [PubMed] [Google Scholar]
- 15.Belongia EA, King JP, Kieke BA, et al. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults ≥60 years old. Open Forum Infect Dis. 2018;5(12):ofy316. doi: 10.1093/ofid/ofy316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falsey AR, Walsh EE, Esser MT, Shoemaker K, Yu L, Griffin MP. Respiratory syncytial virus-associated illness in adults with advanced chronic obstructive pulmonary disease and/or congestive heart failure. J Med Virol. 2019;91(1):65-71. doi: 10.1002/jmv.25285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention . RSV in older adults and adults with chronic medical conditions. Updated January 18, 2024. Accessed February 8, 2024. https://www.cdc.gov/rsv/older-adults/?CDC_AAref_Val=https://www.cdc.gov/rsv/high-risk/older-adults.html
- 18.Sundaram ME, Meece JK, Sifakis F, Gasser RA Jr, Belongia EA. Medically attended respiratory syncytial virus infections in adults aged ≥ 50 years: clinical characteristics and outcomes. Clin Infect Dis. 2014;58(3):342-349. doi: 10.1093/cid/cit767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh E, Lee N, Sander I, et al. RSV-associated hospitalization in adults in the USA: a retrospective chart review investigating burden, management strategies, and outcomes. Health Sci Rep. 2022;5(3):e556. doi: 10.1002/hsr2.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falsey AR, Walsh EE, House S, et al. Risk factors and medical resource utilization of respiratory syncytial virus, human metapneumovirus, and influenza-related hospitalizations in adults—a global study during the 2017-2019 epidemic seasons (Hospitalized Acute Respiratory Tract Infection [HARTI] Study). Open Forum Infect Dis. 2021;8(11):ofab491. doi: 10.1093/ofid/ofab491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon YS, Park SH, Kim MA, et al. Risk of mortality associated with respiratory syncytial virus and influenza infection in adults. BMC Infect Dis. 2017;17(1):785. doi: 10.1186/s12879-017-2897-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackerson B, Tseng HF, Sy LS, et al. Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin Infect Dis. 2019;69(2):197-203. doi: 10.1093/cid/ciy991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surie D, Yuengling KA, DeCuir J, et al. ; IVY Network . Disease severity of respiratory syncytial virus compared with COVID-19 and influenza among hospitalized adults aged ≥60 years—IVY Network, 20 U.S. states, February 2022-May 2023. MMWR Morb Mortal Wkly Rep. 2023;72(40):1083-1088. doi: 10.15585/mmwr.mm7240a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herring WL, Zhang Y, Shinde V, Stoddard J, Talbird SE, Rosen B. Clinical and economic outcomes associated with respiratory syncytial virus vaccination in older adults in the United States. Vaccine. 2022;40(3):483-493. doi: 10.1016/j.vaccine.2021.12.002 [DOI] [PubMed] [Google Scholar]
- 25.Ramirez J, Carrico R, Wilde A, et al. Diagnosis of respiratory syncytial virus in adults substantially increases when adding sputum, saliva, and serology testing to nasopharyngeal swab RT-PCR. Infect Dis Ther. 2023;12(6):1593-1603. doi: 10.1007/s40121-023-00805-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onwuchekwa C, Moreo LM, Menon S, et al. Underascertainment of respiratory syncytial virus infection in adults due to diagnostic testing limitations: a systematic literature review and meta-analysis. J Infect Dis. 2023;228(2):173-184. doi: 10.1093/infdis/jiad012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization . Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic. Accessed May 29, 2023. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Code List
Data Sharing Statement