Highlights
-
•
Interstitial HDR has local control, cosmetic, and functional results similar to periocular surgery.
-
•
This study studies periocular and orbital tumor clinicopathology and post-operative interventional RT therapy.
-
•
Local and distant eyelid malignancies are controlled with post-operative HDR-IRT.
-
•
HDR-IRT catheters may be less effective for controlling diseases in OSSNs with a ≥T3, particularly for conjunctival melanoma.
Keywords: Eyelid tumors, Ocular surface tumors, Eyelid tumors treatments, Ocular surface tumors treatments, Brachytherapy, Interventional radiotherapy
Abstract
Purpose
To evaluate the clinicopathological characteristics and the effectiveness of post-operative high-dose-rate (HDR) interventional radiotherapy (IRT - brachytherapy) in managing advanced ocular surface squamous neoplasia (OSSN) and eyelid tumors.
Methods
Nineteen patients with advanced malignancies affecting the ocular surface (stage ≥ T2) and eyelids (staging ≥ T3) were enrolled. Post-operative HDR-IRT treatment followed surgery after multidisciplinary discussion. In our series a total dose of 49 Gy was administered in 14 fractions of 3.5 Gy each, 2 doses per day. Local disease control is the study's main outcome. Death rate, total survival, disease-free survival, and toxicity are secondary outcomes.
Results
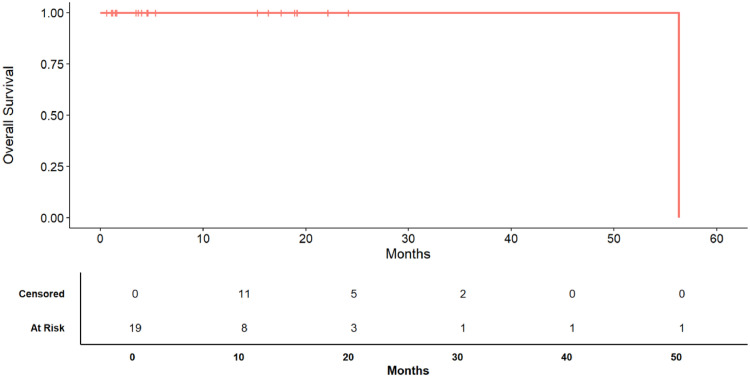
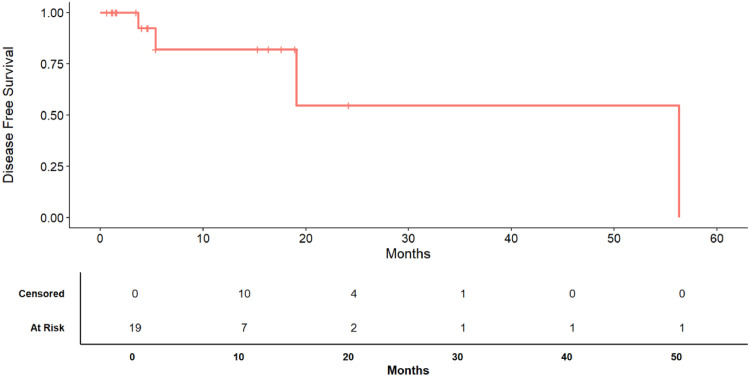
Local recurrence was observed in 4 cases, 2 were conjunctival melanomas and 2 were conjunctival squamous cell carcinoma. The median OS was 56.3 months. The 12, 24 and 36 months survival rate was respectively 100.00% (IQR: 100.00% - 100.00%), 100.00% (IQR: 100.00% - 100.00%), 100.00% (IQR: 100.00% - 100.00%) respectively . The median DFS was 56.3 months. The 12, 24 and 36 months disease survival rate was respectively 85.71% (IQR: 69.21% - 100.00%), 68.57% (IQR: 42.11% - 100.00%), 68.57% (IQR: 42.11% - 100.00%) respectively. In eyelid tumors, madarosis and eyelid abnormalities are the main side effects, while in OSSNs, dry eye symptoms are frequently reported.
Conclusion
Postoperative HDR-IRT has been effective in advanced eyelid cancers control. More challenging appears instead an effective treatment of advanced OSSNs, particularly conjunctival melanomas. Multicenter studies are needed to get a larger patient sample and to evaluate different radiotherapy dosages by different histologic and T types of tumors.
Introduction
Ocular surface and periocular region malignant tumors, which can spread to periocular tissues and orbit, are neoplasms that have various clinicopathological subgroups. Primary surgery or chemotherapy is typically necessary as the initial treatment for tumors, taking into account their anatomical extension and histological subtype. However, performing major surgery or retreatments on advanced tumors can be challenging, ineffective and can lead to both significant functional and aesthetic impairment. More conservative and combined approaches are progressively gaining prominence in the field of oncology and ocular oncology alongside the improvements in radiotherapy techniques and the advances in medical oncology.
Ocular Surface Squamous Neoplasia (OSSN) refers to a range of cancerous conditions characterized by the abnormal growth of dysplastic squamous epithelial cells on the surface of the eye. This spectrum includes conjunctival and corneal intraepithelial neoplasia (CIN), squamous cell carcinoma in situ, and squamous cell carcinoma (SCC), which, by definition, invades through the epithelial basement membrane. OSSN commonly occurs in the area between the eyelids known as the interpalpebral limbal zone. The typical presentation is a vascularized, gray, gelatinous limbal mass that is positioned either medially or laterally in the sun-exposed interpalpebral fissure. The lesion may also expand onto the peripheral cornea. Additional characteristics, such as the presence of leukoplakia (a white plaque) and twisted dilated "cork-screw" feeder arteries, may also be observed.
Conjunctival melanoma originates from the mucous membrane of the eye. It can be categorized as primary, which means it arises without a preexisting lesion, or secondary, which typically occurs from a primary acquired melanosis (PAM) with atypia or, less frequently, from the malignant transformation of a conjunctival nevus. Melanomas typically present as nodular growths and can affect any part of the conjunctiva. The nodule might have pigmentation or lack pigmentation, with amelanotic nodules accounting for 15 % to 25 % of conjunctival melanomas. Conjunctival melanomas have a metastasis rate of 25 % to regional lymph nodes, as well as to the lungs, liver, brain, bone, and skin. The mortality rate in these instances varies between 15 % and 30 %. Adverse prognostic indicators include the presence of the tumor in nonbulbar conjunctival locations, tumor thickness above 1.8 mm and involvement of the outermost part of the eyelid.
The predominant neoplasms affecting the eyelids are of epidermal origin. Within this group, we recognize Basal Cell Carcinoma (BCC), Squamous Cell Carcinoma (SCC) and keratoacanthoma. Basal Cell Carcinoma (BCC) is the most prevalent cancer in the periocular skin region, specifically the eyelids. It accounts for around 90 % of all cutaneous eyelid tumors, with 50 % of these tumors originating from the lower eyelid. Following BCC, Squamous Cell Carcinoma (SCC) and sebaceous carcinoma (SC) are the next most common malignancies in this area. Cutaneous melanoma, sebaceous carcinoma (SC) and Merkel cell carcinoma are rare, however they have a much poorer prognosis due to a relatively high incidence of distant metastases and mortality.
When the pathology appears as locally advanced, coordinated planning for the management of the tumor by a multidisciplinary team is advisable [1,2].
OSSN has traditionally been managed through surgical excision with approximately 4 mm wide margins and adjuvant cryotherapy [3]. However, fibrosis, irregularity of the ocular surface, and limbal stem cell deficiency may result from an the extensive excision site. Recently, topical chemotherapy—specifically mitomycin C (MMC), 5-fluorouracil (5-FU), or interferon alpha-2b (IFN-a2b)—has surfaced as a viable alternative for primary treatment [4,5]. Brachytherapy with Ruthenium106-plaques provides an additional treatment option for adjunctive treatment of limbal and bulbar OSSN that exhibits focal spreading [6].
Adjuvant radiotherapy has been advised for patients with high-risk basal cell carcinoma; however, no randomized controlled study has yet to demonstrate its efficacy [7,8]. For both curing the patient and preventing the return of the condition, Mohs surgery remains the optimal option [9,10]. Advancements in surgical devices, including microscopes and 3D assisted surgery, along with the use of adjuvant treatments like radiotherapy and/or chemotherapy, have led to the development of more effective and less invasive therapeutic strategies. These methods have resulted in improved prognostic outcomes, with a success rate of approximately 90 % in controlling these tumors [11].
Unlike traditional External Beam Radiation Therapy (EBRT), Interventional Radiotherapy (IRT- Brachytherapy) offers the significant benefit of delivering a concentrated dose of radiation directly to the main tumor, resulting in maximum radiation absorption within the tumor mass. This approach minimizes the radiation exposure to surrounding healthy tissue by utilizing a rapid fall-off mechanism. Interstitial high-dose-rate (HDR-IRT) is a highly effective, brief, and relatively low-burden treatment for skin malignancies of the upper and lower eyelid, medial and lateral cants, with infiltration of ocular structures [12].
IRT entails the temporary implantation of radioactive sources directly into a tumor (interstitial) or at superficial tumors, directly adjacent to the tumor, laid over the lesion (contact). It can be administered through a variety of methods, including needles, plaques, or dedicated applicators that can be tailored to the specific characteristics of the tumor, depending on the type of implant [13]. The implantation of needles and catheters for interstitial HDR treatments has been demonstrated to produce local control, cosmetic and functional results comparable to those of surgery of the periocular region [7,14,15].
This study aims to analyze the clinicopathological characteristics of cancer in the periocular and/or orbital tissues by assessing the effectiveness of post-operative (adjuvant or salvage) IRT in controlling local disease. The objectives of this study are to assess the disease-free survival (DFS) and overall survival (OS) of patients with ocular surface squamous neoplasia (OSSN) and eyelid tumors, and to identify factors associated with higher recurrence rates that necessitate more intensive follow-up in clinical practice.
Methods
We enrolled 19 patients affected advanced tumors affecting the OSSN and eyelid carcinoma admitted in our Ocular Oncology Unit. These patients were referred to the Ocular Oncology Unit of Fondazione Policlinico Universitario A. Gemelli IRCCS and treated at our Interventional Oncology Center (IOC) using Interstitial HDR-IRT from June 2019 to February 2024.
The study examined the location of the tumor, individual risk factors, a thorough ophthalmological assessment (including visual acuity, visual fields, clinical photographies and intraocular pressure). All the tumors were staged according to the American Joint Committee on Cancer Classification (AJCC) staging system (including prognostic staging). Moreover, we evaluated histological features, timing of post-surgery and post-IRT follow-up and rate of recurrence. The primary outcome of the study is local control of the disease. The secondary outcomes are the mortality rate (MR), overall survival (OS) and Disease Free Survival (DFS).
The study was approved by the Catholic University/Fondazione Policlinico Universitario A. Gemelli IRCCS Institutional Ethics Committee (protocol ID number: 5572; ClinicalTrials ID: NCT05797415). A signed informed consent was obtained by each enrolled patient, after a full explanation of the target protocol. The study was carried out in conformity to the Declaration of Helsinki.
Inclusion criteria and patient enrollment
Patients with diagnosed with advanced OSSN (≥T2,N0,M0) and periocular/eyelid tumors (≥T3,N0,M0) were evaluated by a team of specialist experts in ocular and periocular cancers, referred to as a multidisciplinary ocular and periocular Tumor Board [2]. Patients were identified using our electronic database which enables doctors to retrieve anonymised patient data retroactively, ensuring compliance with the General Data Protection Regulations (GDPR) [16].
Following the procedure of surgical excision, additional treatment with post-operative interstitial HDR-IRT was therefore recommended in the presence of the following anatomo-pathological features:
-
-
positive margin was present HDR IRT;
-
-
negative margins but with high risk features for further recurrences: several previous recurrences, poor differentiation, peri-neural invasion, immunosuppression, large primary tumor diameters and thickness.
Patients were required to give informed consent for both the procedure and the treatment, as well as for the utilization of personal data for research objectives.
Exclusion criteria
Exclusion criteria included patients under the age of 18, those who had not provided informed consent, and those who had received radiation treatment or topical chemotherapy treatment. Patients with OSSN<T2 and eyelid cancer <T3, as well as individuals with lymph node involvement and metastatic disease, were not included in the study.
Surgical procedure
All patients included in this report underwent a standardized surgical procedure both for OSSN and eyelid carcinoma.
Eyelid carcinoma surgical procedure
The visible boundaries of the lesion were identified under microscopic examination, and a surgical removal of the tumor was performed with a 3-mm margin. Different colored sutures were used to mark the borders for orientation. Postoperative histological assessment was conducted on every specimen using permanent paraffin sections. An intraoperative histological frozen section of the margins was obtained in the majority of patients, which included poorly defined clinical margins and large and recurring tumors. After obtaining a favorable result, an additional removal of tissue from the edge was performed and the paraffin sections were analyzed after the surgery. Subsequent eyelid reconstruction techniques were carried out.
OSSN surgical procedure
Regarding tumors originating from the conjunctiva,we followed the Shields ‘no-touch’ technique with wide margins [17]. Visible limits of the tumor are delineated with wide margins of 3–4 mm. In cases where tumors involve both the limbal and corneal areas, the corneal epitheliectomy procedure is carried out by using 100% alcohol for an amount of 1 min. The lesion is subsequently removed intact. Particular emphasis is placed on manipulating only within the designated boundaries and maintaining a dry surgical area to avoid the potential spread of tumor cells. The cryotherapy was performed on the conjunctival margins of the excised area, as well as the limbus and cornea if necessary, to a double freeze-slow thaw approach. Closure of the wound with amniotic membrane graft was applied. In cases of extra-conjunctival extension, the technique used was similar to invasive tumors on the eyelids.
Post-surgical follow-up
Rigorous monitoring following surgery involved regular ocular clinical examinations, photographic imaging, and radiological imaging as necessary. The monitoring occurred every 3 weeks for 4 months, and then every 2 months thereafter. The time interval between surgery and HDR-IRT was determined by talks at our multidisciplinary meetings. It was decided that the procedure should be conducted 4 to 6 weeks after the main malignancy surgery to allow sufficient time for the lesions to heal and regain tissue integrity through scarring and wound healing.
Interstitial HDR-IRT procedure
Head-and-neck Magnetic Resonance Imaging (MRI) or PET-CT scans were performed to rule out any visible remaining malignancy, and a pre-implantation assessment was conducted to determine the optimal number and placement of catheters. Interstitial HDR-IRT is performed under local or general anesthesia and aseptic conditions. Metallic needles were inserted into the tumor bed, spaced approximately 10 mm apart, to ensure sufficient coverage of the Clinical Target Volume (CTV) while minimizing the risk of harm to nearby delicate structures. inserted into the treatment area, parallel and equidistant to each other in a single plan. The implant geometry mainly aimed at following the Paris- System geometry rules for monoplanar implants, however, in several circumstances also anatomical implantion was perfomed because modern IRT allows for intensity modulation as already described in head and neck district [18,19].
The implant procedure consisted of three distinct phases: pre-planning, implantation, and therapy planning/delivery.
-
•Pre-planning:
-
-PET-CT or Head-and-neck MRI to exclude any gross residual tumor
-
-Pre-implant definition of the ideal number of the catheters
-
-Pre-implant definition of the ideal position of the catheters
-
-
-
•Step 2: Implant Technique
-
-Metallic needle insertion carefully avoiding injury
-
-Metallic needle substitution with plastic tubes
-
-Plastic tubes sewn to the skin for stability
-
-
-
•Step 3: Treatment planning and delivery
-
-CT definition of the actual plastic tube position for 3D treatment planning
-
-CT-based HDR IRT planning and optimization
-
-Treatment delivery
-
-
The metallic needles were later replaced by flexible 6 Fr plastic tubes, which were secured using plastic buttons that were stitched onto the skin. CT simulation was consistently conducted the day following the plastic tube implantation to facilitate the reduction of swelling caused by the interventional technique. This procedure induces edema due to the insertion of needles and the infusion of local anesthetic into the interstitial space. Following CT simulation, the clinical target volume (CTV) was delineated, and the catheters were digitally rebuilt. The treatment plan was computed using the Oncentra Brachy (Elekta)The actual therapy was carried out using a high-dose rate afterloader (Elekta Flexitron).
The treatment protocol used in our study involved administering a total dose of 49 Gy in 14 fractions, with each fraction delivering 3.5 Gy. The treatment schedule remained the same, with 2 fractions per day. The catheter was removed without any complications after the completion of treatment.
Post-HDR IRT follow-up
The patients underwent weekly reviews for one month to observe acute radiation reactions, followed by monthly assessments for six months to evaluate response and any problems connected to the treatment. Subsequently, they underwent bi-monthly evaluations for a duration of one year, followed by quarterly evaluations for the subsequent 36 months. Follow-up patients underwent clinical examination and routine ocular check-up to assess any vision decline or lid complications. The ocular adverse effects were categorized using the Toxicity criteria of the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC).
Statistical analysis
Disease-free survival was determined by measuring the time from the conclusion of treatment till the most recent follow-up. The duration of the follow-up was 36 months. Patients who did not have any remaining or recurring local lesions or distant metastases until their last follow-up were considered to be clear of illness. The statistical analysis was conducted using Stata 11. The length for disease-free survival was estimated from the date when radiotherapy was completed to the occurrence of the event. To describe the distribution of demographic characteristics and clinical data in the study population, numerical variables were presented as median (interquartile range) and categorical variables as count (percentage). Moreover, Overall Survival (OS) and Disease Free Survival (DFS) were analyzed using Kaplan-Meier survival curves.
Results
A total of 19 patients were included in the study, consisting of 8 (42,11 %) individuals with Basal Cell Carcinoma (BCC), 2 (10,53 %) with cutaneous squamous cell carcinoma (SCC), 4 (21,53 %) with sebaceous gland carcinoma (SGC), 3 (15,79 %) with squamous cell carcinoma (SCCc) of the conjunctiva, and 2 (10,53 %) with conjunctival melanoma (CM). The predominant histotype was BCC (8.00, 42.11 %), while the prevalent T staging value was T3c (7.00, 36.84 %).
Mean Age at presentation was 75.30±9.03 (IQR: 82.02 – 67.33). Ten (52,63 %) were female and 9 (47,37 %) male.
Out of the total, 5 individuals, which accounts for 26.32 % of the sample, were exposed to cigarette smoke. Out of the total sample, 2 individuals (10.53 %) had a previous record of being exposed to heat sources, 6 individuals (31.58 %) had exposure to the sun, and 2 individuals (10.53 %) had a family history of malignant neoplasms.
Only 4 cases showed local recurrence of disease, of which 2 (100 % of the total number of cases of this type of tumour) were conjunctival melanomas and 2 (66.6 % of the total number of cases of this type of tumour) were squamous cell carcinoma of the conjunctiva. Conjunctival squamous cell carcinoma cases exhibited local recurrence after 23.3 and 16.4 weeks, while conjunctival melanoma cases exhibited recurrence after 40.86 and 83 weeks, respectively. Concurrently, the latter instance demonstrated a lymph node recurrence.
Out of the total, just one patient, accounting for 5.26 % of the sample, had a documented record of past malignancies.
Table 1 provides a summary of the demographic and staging features of the tumors.
Table 1.
Demographic characteristics and clinical data of the population. Numerical variables are presented as median (IQR), categorical variables as count (percentage).
| Overall Population (N = 19) | ||
|---|---|---|
| Mean Age at presentation | Years | 75.30 (82.02 - 67.33) |
| Gender | Male | 9.00 (47.37%) |
| Female | 10.00 (52.63%) | |
| Smoking exposure | Yes | 5.00 (26.32%) |
| No | 14.00 (73.68%) | |
| Heat exposure | Yes | 2.00 (10.53%) |
| No | 17.00 (89.47%) | |
| Sun exposure | Yes | 6.00 (31.58%) |
| No | 13.00 (68.42%) | |
| Family history of malignancy | Yes | 2.00 (10.53%) |
| No | 17.00 (89.47%) | |
| Light phenotype | Yes | 1.00 (5.26%) |
| No | 18.00 (94.74%) | |
| Previous malignancies | Yes | 1.00 (5.26%) |
| No | 18.00 (94.74%) | |
| Histotype | BCC | 8.00 (42.11%) |
| MC | 2.00 (10.53%) | |
| SCC | 2.00 (10.53%) | |
| SCCc | 3.00 (15.79%) | |
| SGC | 4.00 (21.05%) | |
| T staging | T2c | 5.00 (26.32%) |
| T3b | 1.00 (5.26%) | |
| T3c | 7.00 (36.84%) | |
| T4a | 6.00 (31.58%) | |
| Time between histology and IRT (weeks) | 21.43 (38.72–12.24) | |
| Time between IRT and follow-up (weeks) | 30.00 (89.64–15.64) | |
| Mortality rate | Yes | 1.00 (5.26%) |
| No | 18.00 (94.74%) | |
The median time between histology and IRT was 21.43 (IQR 38.72 – 12.24) weeks while the median time between IRT and follow-up was 30.00 (IQR 89.64 – 15.64) weeks. During the follow-up period, only one patient (5.26 %) died, and this individual was one of the two cases of conjunctivitis melanoma, after 244,72 weeks from IRT.
Furthermore, disease control at the local level was successfully established in 15 (79%) cases, specifically in all cases (100%) of eyelid tumors. However, there were differences observed in OSSNs, where disease recurrence was observed in 4 (80%) out of 5 cases.
The median DFS was 56.3 months (Fig. 2). The 12, 24 and 36 months disease survival rate was respectively 85.71% (IQR: 69.21% - 100.00%), 68.57% (IQR: 42.11% - 100.00%), 68.57% (IQR: 42.11% - 100.00%) respectively (Fig. 2) .
Fig. 2.
Kaplan-Meier survival curve of overall survival (OS). The bottom table displays the number of patients at risk and the number of censored patients at each time step.
The median OS was 56.3 months (Fig. 1). The 12, 24 and 36 months survival rate was respectively 100.00% (IQR: 100.00% - 100.00%), 100.00% (IQR: 100.00% - 100.00%), 100.00% (IQR: 100.00% - 100.00%) respectively (Fig. 1) and the mortality rate, during the observation period has been 5.26% (Table 1) .
Fig. 1.
Kaplan-Meier survival curve of disease free survival (DFS). The bottom table displays the number of patients at risk and the number of censored patients at each time step.
Out of the 14 patients diagnosed with an eyelid tumor, 10 (71%) exhibited madarosis and 8 (57%) had an eyelid deformity. Only 4 (28%) of the patients experienced dry eye condition. Out of the 5 patients receiving OSSN treatment, 3 (60%) experienced dry eye syndrome and 1(20%) developed cataracts.
Discussion
Our findings demonstrated a significant disparity in treatment responsiveness between eyelid tumors and OSSNs. Although there are just a few cases to evaluate, it is evident that HDR-IRT provides satisfactory local disease control. This means that it is effective in treating all instances of basal cell carcinoma, cutaneous squamous cell carcinoma, and sebaceous gland tumors. The evidence about eyelid tumors aligns with the findings of Laskar et al., who also saw a 100% recurrence control rate using HDR-IRT with Ir192 [1,20]. On the other hand, Azad had determined a disease control rate of 75.6% while considering a longer period of observation, specifically 5 years [15].
Although OSSN generally has a favorable prognosis, with a low likelihood of spreading to other parts of the body and a low death rate, it is frequently associated with the spread of cancer cells to nearby or distant areas or invasion into the brain [21,22]. Furthermore, the literature has indicated a recurrence risk of up to 39 % following therapy, which increases to 43% in cases treated only with surgery or purely with topical medications [[23], [24], [25], [26], [27]]. This evidence prompted the development of a more effective treatment for the management of advanced disease, such as HDR-IRT.
Nevertheless, the most intriguing finding was an 80% recurrence rate of OSSN. Indeed, all previous studies have reported a disease control rate of no <79%, as confirmed by a 5-year follow-up [28]. Therefore, the utilization of HDR-IRT with catheters is evidently not the most efficient method for achieving local disease management in cases of conjunctiva-invasive squamous cell melanomas. Brouwer et al. conducted a study on 58 patients with conjunctival melanoma, comparing the efficacy of Strontium90 (Sr-90) and Ruthenium 106 (Ru-106) as treatments [28]. They found that the recurrence rate at 5 years was 19% with Sr-90 and 23% with Ru-106.However, in that particular instance, the lesions were exclusively epibubar, unlike our cases where the lesions were were located at the superior tarsal fornix, which is a significant factor contributing to the increased likelihood of disease recurrence. 28Furthermore, that study lacks any mention of the variable T, thereby restricting the ability to compare it with other studies.
A more valuable comparison can be conducted with the data of Damato et al., who categorized patients with conjunctival melanoma based on TMN and identified 8 cases with a T=3 classification [29]. However, only 4 patients did not exhibit disease recurrence, indicating a recurrence risk of approximately 50% [29]. However, in that instance, radiation therapy was administered using ruthenium plaques instead of catheters.
Furthermore, we assessed the overall disease-free survival (DFS) and overall survival (OS) of all the cases under consideration. More precisely, we have discovered the following The median duration of disease-free survival (DFS) was 56.3 months. The disease's survival rates at 12, 24, and 36 months were 85.71% (IQR: 69.21% - 100.00%), 68.57% (IQR: 42.11% - 100.00%), 68.57% (IQR: 42.11% - 100.00%). The median overall survival (OS) was 56.3 months, while the survival rates at 12, 24, and 36 months were all 100.00%. The mortality rate was 94.74%, with only one patient deceased (5.26%).
The findings on treatment toxicity accord with existing literature. In situations of eyelid tumors, the predominant observation are madarosis and eyelid deformity, whereas in cases of OSSNs, the most significant risk is the development of dry eye symptoms [12,30]. The absence of significant toxicity is due to the fact that IRT achieves a balance by delivering a high dosage of radiation to the tumor while safeguarding adjacent healthy tissues. The main factor contributing to this condition is the rapid decrease in the dosage of IRT [8,31]. In our trial, we adopted a treatment procedure that involved giving a total dose of 49 Gy in 14 fractions, with each fraction delivering 3.5 Gy. The treatment schedule remained same, with a total of 2 portions administered daily [8,32]. Nevertheless, it is evident from the literature that current treatment regimens lack complete standardization, hence complicating the comparison of the published results.
However, the main limitation of the study is the significantly small sample size, which hinders the ability to evaluate different malignancies and compute disease-free survival (DFS) and overall survival (OS) based on tumor type. Furthermore, the lack of other radioisotopes precluded a comparison from being conducted.
Conclusions
Post-operative HDR-IRT is a highly effective approach for managing eyelid tumors, achieving complete control of the disease both locally and at distant sites. Nevertheless, the use of catheters in HDR-IRT seems to be less efficient in controlling diseases for OSSNs with a ≥T3, particularly in the case of conjunctival melanoma. It is desirable to perform multicenter studies in order to obtain a more extensive patient sample treated using the same HDR-IRT schedule.
CRediT authorship contribution statement
Bruno Fionda: Data curation, Conceptualization. Monica Maria Pagliara: Investigation, Formal analysis. Maria Grazia Sammarco: Supervision, Software, Resources, Methodology. Francesco Pastore: Writing – review & editing, Writing – original draft, Visualization. Federico Giannuzzi: Writing – review & editing, Writing – original draft, Visualization, Validation. Giovanni Cuffaro: Writing – review & editing, Writing – original draft, Visualization. Flavia Quaranta Leoni: Writing – review & editing, Writing – original draft. Luca Tagliaferri: Supervision, Software, Project administration. Gustavo Savino: Project administration, Methodology, Investigation, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.102160.
Appendix. Supplementary materials
References
- 1.Vavassori A., et al. Mould-based surface high-dose-rate brachytherapy for eyelid carcinoma. J. Contemp. Brachytherapy. 2019;11:443–448. doi: 10.5114/jcb.2019.88619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savino G., et al. Multidisciplinary ocular and periocular cancers meetings: implementation in a tertiary referral center and analysis over a 12-months period. BMC Ophthalmol. 2022;22:497. doi: 10.1186/s12886-022-02694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanji A.A., et al. Surgical versus medical treatment of ocular surface squamous neoplasia: a comparison of recurrences and complications. Ophthalmology. 2014;121:994–1000. doi: 10.1016/j.ophtha.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeoh C.H.Y., et al. The management of ocular surface squamous neoplasia (OSSN) Int. J. Mol. Sci. 2022;24 doi: 10.3390/ijms24010713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monroy D., Serrano A., Galor A., Karp C.L. Medical treatment for ocular surface squamous neoplasia. Eye. 2023;37:885–893. doi: 10.1038/s41433-023-02434-x. (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahal A., et al. Brachytherapy as a curative option for ocular surface squamous neoplasia. Int. Ophthalmol. 2023;43:1861–1865. doi: 10.1007/s10792-022-02585-y. [DOI] [PubMed] [Google Scholar]
- 7.Krengli M., et al. Interstitial brachytherapy for eyelid carcinoma. Outcome analysis in 60 patients. Strahlenther. Onkol. 2014;190:245–249. doi: 10.1007/s00066-013-0495-y. [DOI] [PubMed] [Google Scholar]
- 8.Tagliaferri L., et al. ORIFICE (interventional radiotherapy for face aesthetic preservation) study: results of interdisciplinary assessment of interstitial interventional radiotherapy (Brachytherapy) for periorificial face cancer. J. Pers. Med. 2022;12 doi: 10.3390/jpm12071038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindgren G., Lindblom B., Larkö O. Mohs' micrographic surgery for basal cell carcinomas on the eyelids and medial canthal area. II. Reconstruction and follow-up. Acta Ophthalmol. Scand. 2000;78:430–436. doi: 10.1034/j.1600-0420.2000.078004430.x. [DOI] [PubMed] [Google Scholar]
- 10.Tildsley J., Diaper C., Herd R. Mohs surgery vs primary excision for eyelid BCCs. Orbit. 2010;29:140–145. doi: 10.3109/01676830903421218. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra R., Huilgol S.C., Huynh N.T., Selva D. The Australian Mohs database: periocular squamous cell carcinoma. Ophthalmology. 2004;111:617–623. doi: 10.1016/j.ophtha.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Cisek P., et al. Interstitial HDR brachytherapy in the treatment of non-melanocytic skin cancers around the eye. Cancers. 2021;13 doi: 10.3390/cancers13061425. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagliara M.M., et al. Interventional radiotherapy (Brachytherapy) in eyelid and ocular surface tumors: a review for treatment of naïve and recurrent malignancies. Neurosignals. 2022;30:1–10. doi: 10.33594/000000505. [DOI] [PubMed] [Google Scholar]
- 14.Cuffaro G., et al. Post-operative interventional radiotherapy (brachytherapy) in advanced ocular surface and eyelid tumors as an alternative to surgical retreatment. Eur. J. Ophthalmol. 2023 doi: 10.1177/11206721231215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azad S., Choudhary V. Treatment results of high dose rate interstitial brachytherapy in carcinoma of eye lid. J. Cancer Res. Ther. 2011;7:157–161. doi: 10.4103/0973-1482.82922. [DOI] [PubMed] [Google Scholar]
- 16.Lancellotta V., et al. SKIN-COBRA (Consortium for Brachytherapy data Analysis) ontology: the first step towards interdisciplinary standardized data collection for personalized oncology in skin cancer. J. Contemp. Brachytherapy. 2020;12:105–110. doi: 10.5114/jcb.2020.94579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shields J.A., Shields C.L., De Potter P. Surgical management of conjunctival tumors. The 1994 Lynn B. McMahan lecture. Arch. Ophthalmol. 1997;115:808–815. doi: 10.1001/archopht.1997.01100150810025. [DOI] [PubMed] [Google Scholar]
- 18.Pierquin B., et al. The Paris system in interstitial radiation therapy. Acta Radiol. Oncol. Radiat. Phys. Biol. 1978;17:33–48. doi: 10.3109/02841867809127689. [DOI] [PubMed] [Google Scholar]
- 19.Fionda B., et al. Interventional radiotherapy (Brachytherapy) for nasal vestibule: novel strategies to prevent side effects. J. Clin. Med. 2023;12 doi: 10.3390/jcm12196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laskar S.G., et al. Postoperative interstitial brachytherapy in eyelid cancer: long term results and assessment of Cosmesis After Interstitial Brachytherapy scale. J. Contemp. Brachytherapy. 2015;6:350–355. doi: 10.5114/jcb.2014.46693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman Y.G., Lauer S.A., Rosenbaum P.S. Mortality due to conjunctival squamous cell carcinoma; A clinicopathologic case report. Invest. Ophthalmol. Vis. Sci. 2004;45 3928-3928. [Google Scholar]
- 22.Tabbara K.F., Kersten R., Daouk N., Blodi F.C. Metastatic squamous cell carcinoma of the conjunctiva. Ophthalmology. 1988;95:318–321. doi: 10.1016/s0161-6420(88)33180-5. [DOI] [PubMed] [Google Scholar]
- 23.Frucht-Pery J., Rozenman Y., Pe'er J. Topical mitomycin-C for partially excised conjunctival squamous cell carcinoma. Ophthalmology. 2002;109:548–552. doi: 10.1016/s0161-6420(01)00967-8. [DOI] [PubMed] [Google Scholar]
- 24.Frucht-Pery J., et al. Mitomycin C treatment for conjunctival-corneal intraepithelial neoplasia: a multicenter experience. Ophthalmology. 1997;104:2085–2093. doi: 10.1016/s0161-6420(97)30055-4. [DOI] [PubMed] [Google Scholar]
- 25.Midena E., Angeli C.D., Valenti M., de Belvis V., Boccato P. Treatment of conjunctival squamous cell carcinoma with topical 5-fluorouracil. Br. J. Ophthalmol. 2000;84:268–272. doi: 10.1136/bjo.84.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shields C.L., et al. Conjunctival tumors in 5002 cases. Comparative analysis of benign versus malignant counterparts. The 2016 james D. Allen lecture. Am. J. Ophthalmol. 2017;173:106–133. doi: 10.1016/j.ajo.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 27.Tabin G., Levin S., Snibson G., Loughnan M., Taylor H. Late recurrences and the necessity for long-term follow-up in corneal and conjunctival intraepithelial neoplasia. Ophthalmology. 1997;104:485–492. doi: 10.1016/s0161-6420(97)30287-5. [DOI] [PubMed] [Google Scholar]
- 28.Brouwer N.J., et al. Management of conjunctival melanoma with local excision and adjuvant brachytherapy. Eye. 2021;35:490–498. doi: 10.1038/s41433-020-0879-z. (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damato B., Coupland S.E. An audit of conjunctival melanoma treatment in liverpool. Eye. 2009;23:801–809. doi: 10.1038/eye.2008.154. (Lond) [DOI] [PubMed] [Google Scholar]
- 30.Mareco V., et al. Interstitial high-dose-rate brachytherapy in eyelid cancer. Brachytherapy. 2015;14:554–564. doi: 10.1016/j.brachy.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Guinot J.L., et al. GEC-ESTRO ACROP recommendations in skin brachytherapy. Radiother. Oncol. 2018;126:377–385. doi: 10.1016/j.radonc.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 32.The GEC ESTRO Handbook of Brachytherapy - ch.29: skin cancer (2nd edition - 2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.