Abstract
Insulin-like growth factor binding protein-2 (IGFBP-2), a binding protein of insulin-like growth factor (IGF) system, regulates the activity of IGFs and also influences cellular function with endogenous activity. In mammals, IGFBP-2 is reported to affect ovarian follicle development and steroidogenesis; however, its role in the chicken ovary is unknown. In this study, we investigated the mRNA expression and function of a novel IGFBP-2 transcript and the effect of reproductive hormones and insulin-like growth factor 1 (IGF1) on its expression in the ovarian granulosa cells of chicken follicles. The mRNA expression of IGFBP-2 was significantly increased in granulosa cells after follicle selection and was higher in hierarchical granulosa cells (Post-GCs) than in pre-hierarchical granulosa cells (Pre-GCs). IGFBP-2 promoted the proliferation and inhibited the apoptosis of both Pre-GCs and Post-GCs, enhanced the mRNA expression of genes involved in progesterone (P4) synthesis in Pre-GCs. However, in Post-GCs, IGFBP-2 inhibited the mRNA expression of these genes and suppressed P4 secretion. The mRNA expression of IGFBP-2 was inhibited by estradiol (E2) and follicle-stimulating hormone (FSH), but enhanced by P4 in Pre-GCs. In Post-GCs, FSH and IGF1 stimulated the mRNA expression of IGFBP-2 synergistically. Knockdown of IGFBP-2 attenuated the stimulatory effect of IGF1 on the mRNA expression of the side chain cleavage enzyme cytochrome P450 family 11 subfamily A member 1 (CYP11A1). These findings indicate that IGFBP-2 is regulated by FSH and IGF1, exerts different functions in Pre-GCs and Post-GCs in regulating IGF1 and plays an important role in chicken follicle development by affecting granulosa cell proliferation and P4 synthesis.
Keywords: Chicken, IGFBP-2, Ovarian follicle, Granulosa cell
Abbreviations
- AKT
protein kinase B
- cAMP
cyclic adenosine monophosphate
- CCND2
cyclin D2
- CDK1
cyclin-dependent kinase 1
- CYP11A1
cytochrome P450 family 11 subfamily A member 1, the side chain cleavage enzyme
- CYP19
cytochrome P450 aromatase
- CYP19A1
cytochrome P450 family 19, subfamily A, polypeptide 1
- E2
estradiol
- EdU
5-Ethynyl-2′-deoxyuridine
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- FSH
follicle-stimulating hormone
- FSHR
FSH receptor
- HSD3B
3β-hydroxysteroid dehydrogenase
- IGF
insulin-like growth factor
- IGF1
insulin-like growth factor 1
- IGF1R
IGF1 receptor
- IGFBP-2
insulin-like growth factor binding protein-2
- IGFBP2-T8
IGFBP-2 transcript 8
- IGFBPs
insulin-like growth factor binding proteins
- LH
luteinizing hormone
- LW
large white follicle
- LY
large yellow follicle
- MAPK
mitogen-activated protein kinase
- NCBI
National Center of Biotechnology Information
- ORF Finder
Open Reading Frame Finder
- P4
progesterone
- PCR
polymerase chain reaction
- Post-GCs
granulosa cells of hierarchical follicles
- Post-TCs
theca cells of hierarchical follicles
- Pre-GCs
granulosa cells of pre-hierarchical follicles
- Pre-TCs
theca cells of pre-hierarchical follicles
- RACE
rapid amplification of cDNA ends
- siRNA
small interfering RNA
- StAR
steroidogenic acute regulatory protein
- SW
small white follicle
- SY
small yellow follicle
Introduction
The development of avian follicles differs from mammals. In chicken follicle development, there is a strict hierarchical system, where follicles undergo selection when they grow to 6-8 mm in diameter. Only the selected follicles enter the hierarchical stage and proceed to further development, maturation, and eventually ovulate. The hierarchical follicles are systematically arranged by size, from F6 to F1, and after ovulation of F1 follicle, a pre-hierarchical follicle is selected into hierarchical follicle in a daily or near-daily rhythm (Johnson, 2015). Follicle selection is an intricate and precise process regulated by reproductive hormones and various secretory factors, and multiple signaling pathways interplay between granulosa cells and oocytes are involved. Follicle selection determines the sequential development of ovarian follicles and enables efficient egg production of hens.
Prior to follicle selection, granulosa cells of chicken pre-hierarchical follicles remain undifferentiated due to the inhibition of mitogen-activated protein kinase (MAPK) signaling transduction, and the cyclic adenosine monophosphate (cAMP) signal stimulated by follicle-stimulating hormone (FSH) is not fully activated, exhibiting no progesterone-producing activity (Woods and Johnson, 2005). After follicle selection, granulosa cells commence differentiation and acquire the capacity to produce progesterone (P4), starting an abundant synthesis of P4, which is regarded as a symbol of follicle selection (Tilly et al., 1991) and is crucial for the development of chicken ovarian follicles and ovulation. The key enzymes or proteins involved in P4 synthesis in the granulosa cells of hierarchical follicles are the steroidogenic acute regulatory protein (StAR), the side chain cleavage enzyme cytochrome P450 family 11 subfamily A member 1 (CYP11A1) and 3β-hydroxysteroid dehydrogenase (HSD3B) (Lee et al., 1998; Johnson and Bridgham, 2001).
FSH is a key hormone in chicken follicle selection process, acting through binding its receptor, FSH receptor (FSHR). The increase of FSHR expression and the activation of the cAMP signaling that follows are markers of follicle selection (Johnson and Woods, 2009). Insulin-like growth factor 1 (IGF1) plays an important role in the development of ovarian follicles. In chicken preovulatory follicles, IGF1 stimulates granulosa cell proliferation, enhances P4 synthesis and potentiates the stimulatory effects of luteinizing hormone (LH) on P4 synthesis (Onagbesan and Peddie, 1995; Onagbesan et al., 1999). IGF1 also promotes granulosa cell proliferation in pre-hierarchical follicles (Zhu et al., 2022), stimulates the expression of genes involved in P4 synthesis and synergizes with FSH (Francoeur et al., 2023). Similarly, the IGF1 receptor (IGF1R) signaling pathway plays a crucial role in the granulosa cells of ovarian follicles (Tosca et al., 2008). In human, rat and mouse granulosa cells, the expression and activation of IGF1R are crucial for the stimulatory effect of FSH on cytochrome P450 aromatase (CYP19) and protein kinase B (PKB, also called AKT) (Zhou et al., 2013).
Insulin-like growth factor binding protein-2 (IGFBP-2), a member of insulin-like growth factor binding proteins (IGFBPs) in insulin-like growth factor (IGF) system, typically functions to inhibit IGFs, but also has enhancing effects (Jones and Clemmons, 1995; Rajaram et al., 1997). In mammals, IGFBP-2 regulates IGFs’ activity (Firth and Baxter, 2002) and modulates cellular function through diverse mechanisms with endogenous activity (Russo et al., 2015; Baxter, 2023). It plays potential regulatory roles in ovary development (Spitschak and Hoeflich, 2018) and is controlled by steroid hormones (Hoeflich et al., 2014). In the granulosa cells of mare ovaries, the expression of IGFBP-2 in large follicles was significantly lower than in small and medium-sized follicles, and was increased by E2 and FSH (Davidson et al., 2002). In the first follicular wave of the bovine estrous cycle, a negative correlation was observed between the level of IGFBP-2 in follicular fluid and follicle diameter (Austin et al., 2001), and in in vitro grown bovine large or dominant follicles, lower level of IGFBP-2 was observed (Rebouças et al., 2014).
The level of IGFBP-2 mRNA expression in several young chicken tissues is regulated by diet (Kita et al., 2002) and IGFBP-2 polymorphisms were reported to be associated with growth, fat metabolism and body composition traits in chickens (Li et al., 2006; Leng et al., 2009). In the chicken ovary, IGF1 is reported to inhibit the IGFBP-2 mRNA expression in primordial follicles (Ahmadi and Ohkubo, 2022); however, the potential regulatory mechanism of IGFBP-2 on ovarian follicle development remains unclear. In this study, we investigated for the first time the effects of a novel IGFBP-2 transcript on granulosa cell proliferation, apoptosis, and differentiation before and after follicle selection in chicken, as well as the regulation of IGFBP-2 by reproductive hormones and IGF1, and the impact of IGFBP-2 on the function of IGF1. Our findings provide new insights into the molecular mechanisms of follicle development and selection in chickens.
Materials and methods
Animals and sample collection
Hy-Line brown laying hens, aged 35-40 weeks, with regular egg-laying pattern, were used in this study. Three chickens were used for each experiment, each experiment was performed with 3 replicates. All hens were housed under the same conditions at a breeding facility in Taian, China, with a photoperiod of 16 h light and 8 h dark, and had access to freely forage and water. Each hen was slaughtered by cervical dislocation 12-14 h before the next expected ovulation. All sized ovarian follicles were removed from each hen and placed in ice-cold saline, including small white follicles (SW, 1-3.9 mm), large white follicles (LW, 4-5.9 mm), small yellow follicles (SY, 6-8.9 mm), large yellow follicles (LY, 9-11.9 mm) and F6-F1 follicles (12-40 mm). SW, LW, SY were categorized into pre-hierarchical follicles, F6-F1 were hierarchical follicles. These follicles were collected for granulosa and theca cell culture or stored in liquid nitrogen for RNA extraction as required. All animal experiments in this study were approved by the Institutional Animal Care and Use Ethic Committee of Shandong Agricultural University (No. SDAUA-2022-36), and conducted in accordance with the “Guidelines for Experimental Animals” issued by the Ministry of Science and Technology of China.
Primary cell isolation and culture
Granulosa and theca cell isolation and culture of chicken ovarian follicles were performed according to reference (Chen et al., 2020). Briefly, the external connective tissue membrane of the follicle was peeled away using forceps, then the outer membrane was pierced to release the follicular contents and transferred into 1% PBS (Solarbio, Beijing, China). After that, granulosa layer cells were separated from the contents by gently shaking, washed with 1% PBS and transferred into a centrifuge tube. The theca layer cells were scissored into a beaker before being transferred into centrifuge tube. Granulosa cells of pre-hierarchical follicles (Pre-GCs) were digested with 1 mg/mL collagenase II (Coolaber, Beijing, China) at 37°C for 5 min and those of hierarchical follicles (Post-GCs) were digested with 0.25% Trypsin-EDTA (Gibco, Grand Island, NY, USA) at 37°C for 8 min to disperse. Theca cells of pre-hierarchical follicles (Pre-TCs) and those of hierarchical follicles (Post-TCs) were respectively digested with collagenase II at 37°C for 30 min, and then, suspensions of Pre-GCs were added with M199 medium (Gibco, Grand Island, NY, USA) containing 1% fetal bovine serum (FBS; Vazyme, Nanjing, China) and 1% antibiotics (1% penicillin and 1% streptomycin mixture; Gibco, Grand Island, NY, USA) to terminate the digestion (For Post-GCs, Pre-TCs and Post-TCs, 5% FBS was used). Subsequently, filtered through a 200-mesh sieve and centrifuged at 2000 rpm/min for 5 min, the cells were suspended with medium mentioned above and planted in a 24-well culture plate, followed by incubation at 39°C with 5% CO2.
RNA extraction and real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from follicles and cells using RNA simple Total RNA Kit (TIANGEN, Beijing, China). The quality and concentration of RNA was assessed with spectrophotometer (Eppendorf, Hamburg, Germany) and agarose gel electrophoresis according to the manufacturer's instructions. The cDNA synthesis was performed using Evo M-MLV RT Mix Kit with gDNA Clean for qPCR (Accurate Biotechnology, Changsha, China). In brief, a mixture of 1 μg of total RNA and gDNA clean reaction mix was incubated at 42°C for 2 min to remove genomic DNA, followed by addition of the reverse transcription reaction mixture and further incubation at 37°C for 15min, 85°C for 5 s to synthesize cDNA. RT-qPCR was carried out in a 20 µL volume reaction containing 10 µL of 2 × SYBR Green Pro Taq HS Premix (Accurate Biotechnology, Changsha, China), 7.2 µL of RNase free water, 0.4 µL each of forward and reverse primers (10 µM, Table 1) that were designed to span intron and specificity checked by BLAST, and 2 µL of cDNA. Reaction for no-template controls (RNase free water replaces template) was performed in duplicate. RT-qPCR was performed using QuantStudioTM 5 System (Thermo Fisher Scientific, Waltham, MA, USA) and LightCycler 96 (Roche, Basel, Switzerland). The amplification efficiency of primers was evaluated by standard curve. The 2−ΔΔCt method was used to calculate the relative gene expression levels, with the β-actin (ACTB) as the reference gene (Zhu et al., 2022). Each sample contains three biological replicates and three technical replicates. Each experiment was independently performed at least three times.
Table 1.
Primers used in this study.
| Genes | Accession number | Strand | Sequence (5′-3′) | Annealing temperature |
|---|---|---|---|---|
| IGFBP-2 | F | ATGAGGGAGAAGGTGAACG | 60°C | |
| R | ATGTGGAGGGAGTAGAGGTG | |||
| ACTB | NM_205518 | F | GTGTGATGGTTGGTATGGGC | 60°C |
| R | CTCTGTTGGCTTTGGGGTTC | |||
| CDK1 | NM_205314 | F | TGGCCTTGAACCACCCATAC | 60°C |
| R | AGGCAGGCAGGCAAAGATAA | |||
| CDK2 | NM_001199857 | F | CCAGAACCTCCTCATCAAC | 64°C |
| R | CAGATGTCCACAGCAGTC | |||
| CCND1 | NM_205381 | F | ATAGTCGCCACTTGGATGCT | 60°C |
| R | AACCGGCTTTTCTTGAGGGG | |||
| CCND2 | NM_204213 | F | TCCGGAAACATGCACAAACG | 60°C |
| R | CCGGACTTGCCTAAGGTTGC | |||
| Caspase3 | NM_204725 | F | TGGTGGAGGTGGAGGAGC | 60°C |
| R | CCTGAGCGTGGTCCATCTTT | |||
| Caspase8 | NM_204592 | F | GCCTTCTTCCAAGCATTACA | 62°C |
| R | TCTCTCTCCATCTCCTCTCG | |||
| Caspase9 | XM_046931415 | F | TCCCGGGCTGTTTCAACTT | 61°C |
| R | CCTCATCTTGCAGCTTGTGC | |||
| StAR | NM_204686 | F | TGCCTGAGCAGCAGGGATTTATCA | 62°C |
| R | TGGTTGATGATGGTCTTTGGCAGC | |||
| CYP11A1 | NM_001001756 | F | TGAATATCATCAGCCCCCGC | 61°C |
| R | GTAGGGCTTGTTGCGGTAGT | |||
| HSD3B | NM_205118 | F | TGGAAGAAGATGAGGCGCTG | 61°C |
| R | GGAAGCTGTGTGGATGACGA | |||
| IGF1R | NM_205032 | F | TTCAGGAACAAAAGGGCGA | 61°C |
| R | TGTAATCTGGAGGGCGATACC | |||
| FSHR | NM_205079 | F | TTCCAGCCTTCCCAAACTA | 61°C |
| R | GCCGTAGAATCACACTTTCAG | |||
| IGFBP2-CDS | F | CCCAAGCTTGGGGCCACCATGGGGGCCGAGGCGTGCGGCG | ||
| R | GGAATTCCCTACTGGCTCCGCAGGGCGTGT | |||
| UPM-L | CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT | |||
| UPM-S | CTAATACGACTCACTATAGGGC | |||
| IGFBP2-5GSP | GGATGTGGAGGGAGTAGAGGTGC | |||
| IGFBP2-5NGSP | TGCTCGTTCACCTTCTCCCTCAT | |||
| IGFBP2-3GSP | CCCATGAGCAGGAGGACCGGGGAGC | |||
| IGFBP2-3NGSP | GAACCAGTCTTTTTTGCTCCTCG |
Rapid amplification of cDNA ends (RACE)
The mRNA sequence of IGFBP-2 was obtained via 5′RACE and 3′RACE using the SMARTer RACE 5′/3′ Kit (Takara, Dalian, China). All PCR amplifications were performed using 2 × Phan-Q5 SuperMix high-fidelity enzyme (Kermey Biotech, Zhengzhou, China). RACE PCR was performed with the Universal Primer Mix (UPM-L, UPM-S) and the IGFBP2-specific primer (Table 1). Reaction volume was 10 µL, containing 5 µL 2 × Phan-Q5, 0.5 µL of forward/reverse primer, 0.5 µL cDNA and 3.5 µL RNase free water. After initial denaturation at 98°C for 30 s, followed by denaturation at 98°C for 10 s, annealing 30 s, extension at 72°C for 30 s, cycling for 30 rounds, and final extension at 72°C for 5 min. PCR products were cloned into the pMD19-T vector (Takara, Dalian, China) and sequenced by Beijing Liuhe Bada Gene Technology Co., LTD.
Overexpression plasmid construction and small interfering RNA (siRNA) synthesis
The sequence obtained from RACE was submitted to the National Center of Biotechnology Information (NCBI) analysis tools, Open Reading Frame Finder (ORF Finder), to predict the coding sequence, resulting in the identification of the longest ORF, which was 702 bp. This ORF was amplified in conjunction with Hind III and EcoR I restriction enzyme sites using the 2 × Phan-Q5 SuperMix high-fidelity enzyme and subsequently ligated into the pcDNA3.1(+) vector to obtain pcDNA3.1-IGFBP2 overexpression plasmid. Plasmid extraction and purification were performed using EndoFree Plasmid Midi kit (Aidlap, Beijing, China), and the purified plasmid was used for subsequent cell transfection. Small interfering RNA (siRNA) were designed and synthesized by RiboBio Co., Ltd (Guangzhou, China). The target sequence of siRNA was listed in the Table 2.
Table 2.
Target sequence of siRNA.
| Name | Target sequence (5′-3′) |
|---|---|
| siRNA-IGFBP2-1 | GGGACTGCTTTCCTATAGT |
| siRNA-IGFBP2-2 | CATGGCTTGTACAATCTCA |
| siRNA-IGFBP2-3 | CACGAGGACTCAAAGAAGT |
| siRNA-FSHR | GCTAGATGTTCAAGACAAT |
| siRNA-IGF1R | GGCTTCACAACTTGAGGAA |
Cell transfection
When cells were grown to 75% confluence, the culture medium was replaced with serum-free medium, and transfected with the pcDNA3.1-IGFBP2 overexpression plasmid or empty pcDNA3.1 vector using the Lipofectamine™ LTX transfection reagent (Thermo Fisher Scientific, Waltham, MA, USA). The siRNA was transfected into cells using Lipofectamine™ RNAiMAX transfection reagent (Thermo Fisher Scientific, Waltham, MA, USA), with the optimal final concentration of 75 nM. Transfection Reagent and Opti-MEM medium (Gibco, Grand Island, NY, USA) were used according to the manufacturer's instructions.
Cell counting Kit-8 (CCK-8) assay
The proliferation activity of granulosa cells was assessed using the Cell Counting Kit-8 (CCK-8; Beyotime, Shanghai, China). Granulosa cells were cultured in 96-well plate, and CCK-8 solution was added at 0, 12, 24, 36 and 48 h after transfection. Then the plates were incubated for 2 h and absorbance was measured at 450 nm using an ELx808 Absorbance Reader (BioTek, Vermont, USA).
5-ethynyl-2′-deoxyuridine (EdU) assay
The proliferative status of the granulosa cells was performed according to the instructions of BeyoClickTM EdU Cell Proliferation Kit with Alexa Fluor 555 (Beyotime, Shanghai, China). Granulosa cells were cultured in 6-well plate. After transfection for 24 h, EdU reagent was added and cells were stained for 2 h, with cell nuclei labeled using Hoechst 33342. Fluorescence microscopy (Olympus, Tokyo, Japan) was used to observe and capture images. ImageJ 1.53e software was employed to count EdU-positive cells and total cell numbers.
Flow cytometry analysis
The apoptosis rate of the granulosa cells was evaluated using an Annexin V-FITC/PI Apoptosis Detection Kit (Vazyme, Nanjing, China). Granulosa cells were cultured in 24-well plate. After transfection for 24 h, the cells were collected and stained according to the manufacturer's instructions, and then analyzed using a Attune NxT Acoustic Focusing Cytometer (Thermo Fisher Scientific). Data were analyzed using AttuneTM Cytometric Software v5.3.0.
Enzyme-linked immunosorbent assay (ELISA)
The granulosa cells were cultured in 24-well plate, and the cell culture medium was collected after transfected for 24 h. The concentration of P4 in the supernatant of culture medium was determined using a chicken P4 (PROG) ELISA kit (Meimian, Jiangsu, China) according to the manufacturer's instructions.
Treatment with reproductive hormones and IGF1
When reached 80% confluence, the cells were cultured with serum-free M199 medium and treated with varying concentrations of estradiol (E2; Sigma-Aldrich, Missouri, USA), P4 (Sigma-Aldrich, Missouri, USA), FSH (MedChemExpress, Shanghai, China) and recombinant human IGF-1 (PeproTech, New Jersey, USA). Following a 24-h treatment period, cells were harvested for RNA extraction. For the co-treatment of hormone stimulation and siRNA transfection, after transfecting with siRNA for 6 h, the cells were cultured to serum-free M199 medium and supplemented with IGF1 and FSH. Subsequently, the cells were collected after 24-h treatment.
Statistical analysis
All data were presented as mean±standard error of the mean. The difference between two groups was compared with Independent Samples t-Test while multiple groups of more than two was analyzed by One-Way ANOVA followed by LSD's multiple comparison using SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and P < 0.05 was considered as significantly different.
Results
Full-length of a novel IGFBP-2 transcript in chicken granulosa cells
By 5′ RACE and 3′ RACE, a 229 bp extension at the 5′ end and a 20 bp extension at the 3′ end of IGFBP-2 transcript 8 (IGFBP2-T8) in chicken granulosa cells was obtained, and its full length was determined to be 1,065 bp (GenBank accession number PQ153874). Comparative analysis with IGFBP-2 sequence on NCBI (NM_205359) indicated that IGFBP2-T8 encompassed a 704 bp of the CDS region alongside a 361 bp segment of the 3′ UTR region (Fig. 1).
Fig. 1.
The comparative analysis between chicken IGFBP2-T8 and IGFBP-2 sequence on NCBI (NM_205359).
Expression of IGFBP-2 mRNA in different chicken ovarian follicles, theca and granulosa cells
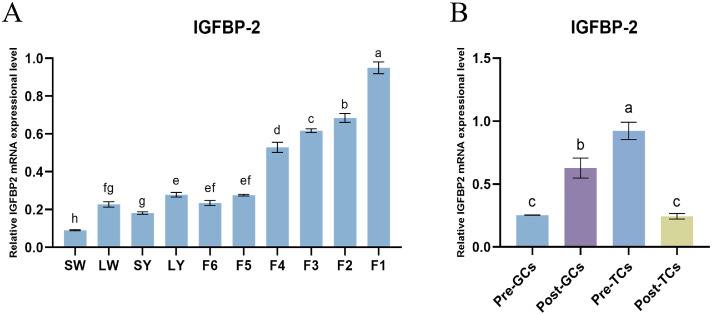
The mRNA expression of IGFBP-2 in ovarian follicles and in follicular theca and granulosa cells of laying hens was measured by RT-qPCR. IGFBP-2 mRNA significantly increased from SW to LW, SY to LY, and F5 to F1 follicles, while not significantly changed from LW to SY and from LY to F5 follicles (Fig. 2A). In theca cells, the mRNA level of IGFBP-2 declined after follicle selection (Post-TCs vs Pre-TCs), however, in granulosa cells, its level significantly increased (Post-GCs vs Pre-GCs) (Fig. 2B).
Fig. 2.
Expression of IGFBP-2 mRNA in different chicken ovarian follicles (A) (n = 3) and in the follicular theca and granulosa cells (B) of chicken ovarian follicles (n = 3). SW, small white follicle; LW, large white follicle; SY, small yellow follicle; LY, large white follicle; F6–F1 represent hierarchical follicles, which are sorted from smallest to largest in diameter. The house-keeping gene β-actin (ACTB) was used as the reference. Results are shown as mean±SEM. Different lowercase letters were used to indicate the level of significance of differences (P < 0.05); the same lowercase letters indicate non-significant differences (P > 0.05).
IGFBP-2 stimulates the proliferation of granulosa cells of chicken follicles
Knockdown of IGFBP-2 with siRNA-IGFBP2 markedly reduced the expression of IGFBP-2 in chicken Post-GCs (P < 0.001, Fig. 3A), resulting in a decrease in the proliferation of both Pre-GCs (P < 0.05, Fig. 3C, D, E), and Post-GCs (P < 0.05, Fig. 3I, J, K). Conversely, overexpression of IGFBP-2 significantly increased the expression of IGFBP-2 in chicken Post-GCs (P < 0.05, Fig. 3B), resulting in an increase in the proliferation of both Pre-GCs (P < 0.05, Fig. 3F, G, H) and Post-GCs (P < 0.01, Fig. 3L, M, N). The impact of IGFBP-2 on the mRNA expression of genes associated with cell proliferation were also analyzed. In Pre-GCs, knockdown of IGFBP-2 significantly reduced the mRNA expression of cyclin-dependent kinase 1 (CDK1) (P < 0.05), while markedly increased the mRNA expression of cyclin D2 (CCND2) (P < 0.01, Fig. 3O). In Post-GCs, knockdown of IGFBP-2 significantly decreased the mRNA expression of CDK1 (P < 0.01), but significantly increased the mRNA expression of CCND2 (P < 0.05, Fig. 3Q). Overexpression of IGFBP-2 significantly reduced the mRNA expression of CCND2 (P < 0.001, Fig. 3P) in both Pre-GCs and Post-GCs (P < 0.001, Fig. 3R).
Fig. 3.
Effect of IGFBP-2 on the proliferation of granulosa cells of chicken ovarian follicles and the mRNA expression of genes associated with cell proliferation. (A) The mRNA expression level of IGFBP-2 after transfection with three siRNAs in GCs (n = 3). (B) The expression of IGFBP-2 after transfection with pcDNA3.1-IGFBP2 in GCs (n = 3). Knockdown of IGFBP-2 on the cell proliferation of Pre-GCs (C, D and E) and Post-GCs (I, J and K). Overexpression of IGFBP-2 on the cell proliferation of Pre-GCs (F, G and H) and Post-GCs (L, M and N). Cell proliferation was determined by 5-ethynyl-2′-deoxyuridine (EdU) after 24 h of transfection with siRNA-IGFBP2 (C, I) (n = 3) and pcDNA3.1-IGFBP2 (F, L) (n = 3) in both Pre-GCs and Post-GCs, scale bar: 100 µm. Cell proliferation at five time points (0, 12, 24, 36, and 48 h) was measured by cell counting kit-8 (CCK-8) following transfection with siRNA-IGFBP2 (E, K) (n = 5) and pcDNA3.1-IGFBP2 (H, N) (n = 5) in both Pre-GCs and Post-GCs. mRNA expression changes of cell proliferation-associated genes in granulosa cells transfected with siRNA-IGFBP2 (O, Q) (n = 3) and pcDNA3.1-IGFBP2 (P, R) (n = 3). Results are shown as mean±SEM. *P < 0.05, **P < 0.01, and ***P < 0.001.
IGFBP-2 inhibits the apoptosis of granulosa cells of chicken follicles
Knockdown of IGFBP-2 significantly increased the apoptosis of both Pre-GCs (P < 0.01, Fig. 4A, 4B, 4C) and Post-GCs (P < 0.05, Fig. 4G, 4H, 4I). On the contrary, overexpression of IGFBP-2 markedly reduced the apoptosis of both Pre-GCs (P < 0.01, Fig. 4D, 4E, 4F) and Post-GCs (P < 0.05, Fig. 4J, 4K, 4L). Moreover, in Pre-GCs, knockdown of IGFBP-2 significantly increased the mRNA expression of caspase3, caspase8, and caspase9 (P < 0.01, Fig. 4M). In Post-GCs, knockdown of IGFBP-2 significantly increased the mRNA expression of caspase9 (P < 0.05, Fig. 4O), while overexpression of IGFBP-2 markedly reduced the mRNA expression of caspase3, caspase8, and caspase9 (P < 0.01, Fig. 4P).
Fig. 4.
Effect of IGFBP-2 on the apoptosis of granulosa cells of chicken ovarian follicles and the mRNA expression of genes associated with cell apoptosis. Knockdown of IGFBP-2 on the cell apoptosis of Pre-GCs (A, B and C) (n = 3) and Post GCs (G, H and I) (n = 3). Overexpression of IGFBP-2 on the cell apoptosis of Pre-GCs (D, E and F) (n = 3) and Post-GCs (J, K and L) (n = 3). mRNA expression changes of cell apoptosis-associated genes in granulosa cells transfected with siRNA-IGFBP2 (M, O) (n = 3) and pcDNA3.1-IGFBP2 (N, P) (n = 3). Results are shown as mean±SEM. *P < 0.05, **P < 0.01, and ***P < 0.001.
Effects of IGFBP-2 on gene expression involved in P4 synthesis and P4 level in chicken granulosa cells
After follicle selection in chicken, the granulosa cells begin to differentiate and produce P4, therefore, we explored the role of IGFBP-2 in regulating P4 production in granulosa cells. In Pre-GCs, knockdown of IGFBP-2 significantly decreased the expression levels of StAR (P < 0.001) and HSD3B (P < 0.01) mRNA, while significantly increased the expression level of CYP11A1 mRNA (P < 0.01, Fig. 5A). Overexpression of IGFBP-2 significantly increased the expression level of HSD3B mRNA (P < 0.01, Fig. 5C). In Post-GCs, knockdown of IGFBP-2 significantly increased the expression level of HSD3B mRNA (P < 0.01, Fig. 5B), however, overexpression of IGFBP-2 significantly decreased the mRNA expression levels of StAR (P < 0.05) and HSD3B (P < 0.05, Fig. 5D). In Post-GCs, knockdown of IGFBP-2 significantly increased the level of P4 (P < 0.05, Fig. 5E), while overexpression of IGFBP-2 significantly decreased the level of P4 (P < 0.05, Fig. 5F).
Fig. 5.
Effects of IGFBP-2 on genes involved in P4 synthesis and P4 production in the granulosa cells of chicken ovarian follicles. (A, B) Expression levels of StAR, CYP11A1 and HSD3B mRNA in Pre-GCs and Post-GCs with IGFBP-2 knockdown (n = 3). (C, D) Expression levels of StAR, CYP11A1 and HSD3B mRNA in Pre-GCs and Post-GCs with IGFBP-2 overexpression (n = 3). Changes in P4 level (pmol/L) in Post-GCs after knockdown (E) (n = 3) and overexpression (F) (n = 3) of IGFBP-2. Results are shown as mean±SEM. *P < 0.05, **P < 0.01, and ***P < 0.001.
Effect of reproductive hormones and IGF1 on the expression of IGFBP-2 mRNA in chicken granulosa cells
Due to the different effects of IGFBP-2 on P4 synthesis in Pre-GCs and Post-GCs, we further investigated the effect of reproductive hormones and IGF1 on IGFBP-2 mRNA expression in chicken granulosa cells. In Pre-GCs, the expression of IGFBP-2 mRNA significantly decreased after treatment with 5 nmol/L E2 (P < 0.05, Fig. 6A), significantly increased after treatment with 50 nmol/L P4 (P < 0.05, Fig. 6B) and significantly decreased after treatment with FSH (P < 0.001, Fig. 6C). In Post-GCs, the expression level of IGFBP-2 mRNA significantly increased after treatment with 5 ng/mL and 50 ng/mL of FSH (P < 0.05, Fig. 6G) and significantly increased by IGF1 in a dose-dependent manner (P < 0.05, Fig. 6H).
Fig. 6.
Changes in expression level of IGFBP-2 mRNA in the granulosa cells of chicken ovarian follicles after treatment with E2 (A, E) (n = 3), P4 (B, F) (n = 3), FSH (C, G) (n = 3) and IGF1 (D, H) (n = 3) in Pre-GCs and Post-GCs. E2, estradiol; P4, progesterone; FSH, follicle stimulation hormone; IGF1, insulin-like growth factor 1. Results are shown as mean±SEM. *P < 0.05, **P < 0.01, and *** P < 0.001.
We further tested the effect of IGF1 in combination with FSH on the expression of IGFBP-2 mRNA in chicken granulosa cells. In Pre-GCs, IGF1 had no effect on the inhibitory action of FSH on IGFBP-2 mRNA expression (Fig. 7A). However, in Post-GCs, co-treatment with FSH and IGF1 further enhanced the expression of IGFBP-2 mRNA compared with FSH or IGF1 alone, and the promoting effects of FSH and IGF1 on IGFBP-2 were abolished upon disruption of FSHR or IGF1R (Fig. 7B).
Fig. 7.
IGFBP-2 expression levels in the granulosa cells of chicken ovarian follicles after combined treatment of IGF1 and FSH. (A) Co-treatment with IGF1 and FSH on the expression of IGFBP-2 mRNA in Pre-GCs (n = 3). (B) Expression level of IGFBP-2 mRNA co-treated with FSH and IGF1 and after being transfected with siRNA-FSHR and siRNA-IGF1R in Post-GCs (n = 3). FSH: 5 ng/mL, IGF1: 10 ng/mL. Results are shown as mean±SEM. Different lowercase letters were used to indicate the level of significance of differences (P < 0.05); the same lowercase letters indicate non-significant differences (P > 0.05).
IGFBP-2 enhances the function of IGF1 in chicken post-GCs
As IGF1 has a synergistic effect with FSH on the expression of steroidogenic genes in chicken Post-GCs, we further examined whether IGFBP-2 affects the stimulatory effect of IGF1 on steroidogenic genes. We found that IGF1 alone promoted the expression of the CYP11A1, and further enhanced the stimulatory effect in collaboration with FSH. Knockdown of IGFBP-2 significantly reduced the stimulatory effect of IGF1 on CYP11A1 (Fig. 8), but it did not affect the synergistic stimulatory effect of FSH and IGF1.
Fig. 8.
Knockdown of IGFBP-2 impaired the stimulatory effect of IGF1 on CYP11A1 mRNA expression in chicken Post-GCs (n = 3). FSH: 5 ng/mL, IGF1: 10 ng/mL. Different lowercase letters were used to indicate the level of significance of differences (P < 0.05); the same lowercase letters indicate non-significant differences (P > 0.05).
Discussion
Follicle selection determines the sequential development of ovarian follicles in chickens and enables efficient egg production for hens. Based on our previous transcriptome sequencing on chicken granulosa cells of ovarian follicles (Li et al., 2022), in this study, we investigated the impact of a novel IGFBP-2 transcript on steroidogenesis in granulosa cells before and after follicle selection, its effect on the proliferation and apoptosis of granulosa cells, the effect of key reproductive hormones in follicle development and IGF1 on the mRNA expression of IGFBP-2 in granulosa cells, and analyzed the influence of IGFBP-2 on the function of IGF1 in Post-GCs.
Our previous sequencing data revealed a novel IGFBP-2 transcript, IGFBP2-T8, that is considerably shorter than that on NCBI (NM_205359), indicating the outcome of alternative splicing. In this study, we first obtained a 1,065 bp full-length sequence of IGFBP2-T8 by 3′ and 5′ RACE that only includes a 704 bp of the CDS region and a 361 bp segment of the 3′ UTR region, encoding 233 aa and lacking 78 aa, compared to the previously identified sequence of IGFBP-2 (Schoen et al., 1995), The absence of these segments may suggest a distinctive role of IGFBP-2 in the chicken ovary.
Studies have reported changes in the expression level of IGFBP-2 in mammalian ovarian granulosa cells, showing that the expression of IGFBP-2 in large or dominant follicles is relatively lower (Austin et al., 2001; Davidson et al., 2002; Rebouças et al., 2014; Spitschak and Hoeflich, 2018). The expression of IGFBP-2 and its function in follicle selection was not reported in poultry species including chicken. In this study, a significant increase in IGFBP-2 mRNA expression was observed in chicken hierarchical follicles from F5 to F1 and also in Post-GCs compared to Pre-GCs of chicken ovarian follicles. The mRNA expression level of IGFBP-2 in chicken ovarian granulosa cells showed a completely different trend compared with that in mammalian ovarian granulosa cells, suggesting that IGFBP-2 may play different roles in chicken ovarian granulosa cells.
To investigate the role of IGFBP-2 in granulosa cells of follicles, we first examined the impact of IGFBP-2 on granulosa cell proliferation and apoptosis. We found that overexpression of IGFBP-2 significantly enhanced granulosa cell proliferation and inhibited apoptosis, while knockdown of IGFBP-2 markedly inhibited granulosa cell proliferation and enhanced apoptosis, also influencing the mRNA expression of genes related to cell proliferation and apoptosis. This marks the first observed influence of IGFBPs on granulosa cell proliferation and apoptosis in chicken follicles. In mammalian systems, particularly in human cancer research, studies have described that IGFBP-2 affected cellular metabolism or growth and development through intrinsic or IGF-dependent mechanisms (Russo et al., 2015). In chickens, IGF1 was also reported to stimulate granulosa cell proliferation (Onagbesan and Peddie, 1995; Onagbesan et al., 1999; Zhu et al., 2022), the activity of which is likely regulated by IGFBP-2.
Within the granulosa cells, cholesterol is transported into the mitochondria by StAR, where it is catalyzed by CYP11A1 to form pregnenolone, which is then catalyzed by HSD3B to produce P4 (Lee et al., 1998; Xiao et al., 2023) that stimulates the release of LH, accelerating follicle maturation and ovulation. In this study, we showed that knockdown or overexpression of IGFBP-2 in Pre-GCs significantly changed the mRNA expression of HSD3B in both Pre-GCs and Post-GCs, suggesting that IGFBP-2 exerted an influence on P4 synthesis during the conversion from pregnenolone to P4. However, IGFBP-2 exhibited opposite effects before and after follicle selection: IGFBP-2 facilitated P4 synthesis in Pre-GCs, whereas inhibited P4 secretion in Post-GCs. Subsequently, we assessed the level of P4 and found that IGFBP-2 significantly inhibited P4 secretion in Post-GCs. In rat granulosa cells, overexpression of IGFBP-2 abolished the stimulatory effects of FSH on CYP19A1 and CYP11A1, however, the effect is reversed by the addition of IGF1 (Zhou et al., 2013), which could be explained by that excessive IGFBP-2 may bind more IGF1, leaving less free IGF1 and thus reducing the effect of IGF1 (Jones and Clemmons, 1995).
Regulation of IGFBP-2 expression by steroid hormones is reported in mammals (Hoeflich et al., 2014). In dominant follicles of heifers, high content of E2 was always accompanied by low concentrations of IGFBP-2 (Austin et al., 2001). In the follicular fluid of cows, the ovarian follicles with high E2 activity also exhibited lower IGFBP-2 binding activity (Echternkamp et al., 1994). E2 slightly diminishes the mRNA levels of IGFBP-2 in the porcine uterus, while P4 elevates the levels of IGFBP-2 mRNA (Simmen et al., 1990). To further investigate whether reproductive hormones and IGF1 play regulatory role in IGFBP-2 expression in chicken ovarian granulosa cells, we employed treatments with E2, P4, FSH, and IGF1 on granulosa cells. In Pre-GCs, E2 suppressed the mRNA expression of IGFBP-2, whereas P4 enhanced its mRNA expression. These findings are similar to reports in mammals. It is noteworthy that FSH significantly suppressed the mRNA expression of IGFBP-2 before follicle selection and promoted its mRNA expression after follicle selection, suggesting that IGFBP-2 may exert distinct roles regulated by FSH in Pre- and Post-GCs, which needs further study.
In mammals and birds, IGF1 plays an important role in the development of ovarian follicles. In chicken follicles, IGF1 can stimulate granulosa cell proliferation, enhance steroid synthesis, and potentiate the stimulatory effects of FSH or LH on granulosa cells (Onagbesan and Peddie, 1995; Onagbesan et al., 1999; Francoeur et al., 2023). IGFBP-2 can regulate the bioavailability of IGFs, typically exhibiting an inhibitory effect on IGF1 (Jones and Clemmons, 1995). In the primary follicles of 7-day-old chicks, IGF1 was found to inhibit the expression of IGFBP-2 (Ahmadi and Ohkubo, 2022). In this study, we found that IGF1 significantly promoted the expression of IGFBP-2 in a dose-dependent manner in Post-GCs. Accordingly, IGF1R was found to be expressed at higher levels specifically in Post-GCs (data not shown), suggesting that IGF1/IGF1R pathway is active in Post-GCs. Similarly, IGF1 specifically increased the mRNA expression levels of IGFBP-2 in Post-GCs. These findings indicated that IGFBP-2 may influence chicken ovarian follicle development through an IGF1-dependent mechanism.
IGF1 and FSH has been shown to enhance the proliferation of granulosa cells and the expression of genes involved in P4 synthesis in chicken pre-hierarchical follicles (Francoeur et al., 2023). The activation of the IGF1R signal is crucial for FSH-stimulated steroidogenic gene expression in granulosa cells in humans, rats, and mice, indicating the existence of overlapping signaling pathways between IGF1 and FSH that synergistically promote follicle development (Zhou et al., 2013). Since both FSH and IGF1 can increase the mRNA expression of IGFBP-2 in granulosa cells, we further investigated whether IGF1 and FSH synergistically regulate the expression of IGFBP-2. In Pre-GCs, IGF1 and FSH had no additional impact on the expression of IGFBP-2, possibly due to the incomplete activation of the IGF1 and FSH signaling pathways. In Post-GCs, IGF1 and FSH act jointly to increase the expression of IGFBP-2. Furthermore, knockdown of FSHR or IGF1R abolished the promoting effects of IGF1 and FSH, suggesting either of IGF1 and FSH pathways is required to regulate the expression of IGFBP-2 mRNA in Post-GCs.
Moreover, we further investigated the impact of IGFBP-2 on the function of IGF1 in modulating steroidogenesis. IGF1 significantly enhanced the expression of CYP11A1, and when combined with FSH, it further stimulated the expression of CYP11A1, consistent with previous findings (Lovell et al., 2002; Francoeur et al., 2023). After knockdown of IGFBP-2, the stimulatory effect of IGF1 on CYP11A1 was significantly reduced, indicating that IGFBP-2 can enhance the function of IGF1 in the granulosa cells of chicken ovarian follicles. However, knockdown of IGFBP-2 did not affect the expression of CYP11A1 stimulated by FSH and IGF1, suggesting that the synergistic increase in the expression of IGFBP-2 by FSH and IGF1 did not promote steroid synthesis, implying that IGFBP-2 may have other functions. We proposed that, on one hand, the increase in IGFBP-2 expression induced by FSH and IGF1 may accelerate granulosa cell proliferation; on the other hand, FSH and IGF1 may indirectly inhibit P4 secretion through IGFBP-2 to prevent the stimulation of LH release and premature ovulation of the follicle, which require further investigations.
In conclusion, in this study, we delineated the effects of a novel IGFBP-2 transcript on the proliferation, apoptosis and P4 secretion of granulosa cells in chicken follicles, and determined the regulation of reproductive hormones and IGF1 on IGFBP-2, suggesting that FSH and IGF1 may modulate the impact of IGFBP-2 on follicular development. We also found that IGFBP-2 as IGFBPs showed different functions in regulating IGF1. These data provide new insights into the molecular mechanisms of follicle development and selection in chickens.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This research was financially supported by grants from the National Key R&D program of China (Grant No. 2021YFD1300100) and the National Natural Science Foundation of China (NSFC 31972545).
References
- Ahmadi S., Ohkubo T. Leptin promotes primordial follicle activation by regulating ovarian insulin-like growth factor system in chicken. Endocrinology. 2022;163:bqac112. doi: 10.1210/endocr/bqac112. [DOI] [PubMed] [Google Scholar]
- Austin E.J., Mihm M., Evans A.C.O., Knight P.G., Ireland J.L.H., Ireland J.J., Roche J.F. Alterations in intrafollicular regulatory factors and apoptosis during selection of follicles in the first follicular wave of the bovine estrous Cycle1. Biol. Reprod. 2001;64:839–848. doi: 10.1095/biolreprod64.3.839. [DOI] [PubMed] [Google Scholar]
- Baxter R.C. Signaling pathways of the insulin-like growth factor binding proteins. Endocr. Rev. 2023;44:753–778. doi: 10.1210/endrev/bnad008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Wang Y., Liu Z., Guo X., Sun Y., Kang L., Jiang Y. Transcriptomic and proteomic analyses of ovarian follicles reveal the role of VLDLR in chicken follicle selection. BMC. Genomics. 2020;21:486. doi: 10.1186/s12864-020-06855-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson T.R., Chamberlain C.S., Bridges T.S., Spicer L.J. Effect of follicle size on in vitro production of steroids and insulin-like growth factor (IGF)-I, IGF-II, and the IGF-binding proteins by equine ovarian granulosa cells1. Biol. Reprod. 2002;66:1640–1648. doi: 10.1095/biolreprod66.6.1640. [DOI] [PubMed] [Google Scholar]
- Echternkamp S.E., Howard H.J., Roberts A.J., Grizzle J., Wise T. Relationships among concentrations of steroids, insulin-like growth factor-I, and insulin-like growth factor binding proteins in ovarian follicular fluid of beef cattle1. Biol. Reprod. 1994;51:971–981. doi: 10.1095/biolreprod51.5.971. [DOI] [PubMed] [Google Scholar]
- Firth S.M., Baxter R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Francoeur L., Scoville D.M., Johnson P.A. Effect of IGF1 and FSH on the function of granulosa cells from prehierarchal follicles in chickens. Biol. Reprod. 2023;109:498–506. doi: 10.1093/biolre/ioad082. [DOI] [PubMed] [Google Scholar]
- Hoeflich A., Wirthgen E., David R., Classen C.F., Spitschak M., Brenmoehl J. Control of IGFBP-2 expression by steroids and peptide hormones in vertebrates. Front. Endocrinol. (Lausanne) 2014;5:43. doi: 10.3389/fendo.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.L. Ovarian follicle selection and granulosa cell differentiation. Poult. Sci. 2015;94:781–785. doi: 10.3382/ps/peu008. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Bridgham J.T. Regulation of steroidogenic acute regulatory protein and luteinizing hormone receptor messenger ribonucleic acid in hen granulosa cells. Endocrinology. 2001;142:31616–31624. doi: 10.1210/endo.142.7.8240. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Woods D.C. Dynamics of avian ovarian follicle development: Cellular mechanisms of granulosa cell differentiation. Gen. Comp. Endocrinol. 2009;163:12–17. doi: 10.1016/j.ygcen.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Jones J.I., Clemmons D.R. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kita K., Nagao K., Taneda N., Inagaki Y., Hirano K., Shibata T., Yaman M.A., Conlon M.A., Okumura J. Insulin-like growth factor binding protein-2 gene expression can be regulated by diet manipulation in several tissues of young chickens. J. Nutr. 2002;132:145–151. doi: 10.1093/jn/132.2.145. [DOI] [PubMed] [Google Scholar]
- Lee K.A., Volentine K.K., Bahr J.M. Two steroidogenic pathways present in the chicken ovary: theca layer prefers delta 5 pathway and granulosa layer prefers delta 4 pathway. Domest. Anim. Endocrinol. 1998;15:1–8. doi: 10.1016/s0739-7240(97)00057-x. [DOI] [PubMed] [Google Scholar]
- Leng L., Wang S., Li Z., Wang Q., Li H. A polymorphism in the 3’-flanking region of insulin-like growth factor binding protein 2 gene associated with abdominal fat in chickens. Poult. Sci. 2009;88:938–942. doi: 10.3382/ps.2008-00453. [DOI] [PubMed] [Google Scholar]
- Li Z.H., Li H., Zhang H., Wang S.Z., Wang Q.G., Wang Y.X. Identification of a single nucleotide polymorphism of the insulin-like growth factor binding protein 2 gene and its association with growth and body composition traits in the chicken. J. Anim. Sci. 2006;84:2902–2906. doi: 10.2527/jas.2006-144. [DOI] [PubMed] [Google Scholar]
- Li D., Zhong C., Sun Y., Kang L., Jiang Y. Identification of genes involved in chicken follicle selection by ONT sequencing on granulosa cells. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.1090603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell T.M., Gladwell R.T., Groome N.P., Knight P.G. Modulatory effects of gonadotrophins and insulin-like growth factor on the secretion of inhibin A and progesterone by granulosa cells from chicken preovulatory (F1-F3) follicles. Reproduction. 2002;123:291–300. [PubMed] [Google Scholar]
- Onagbesan O.M., Peddie M.J. Effects of insulin-like growth factor I and interactions with transforming growth factor alpha and LH on proliferation of chicken granulosa cells and production of progesterone in culture. J. Reprod. Fertil. 1995;104:259–265. doi: 10.1530/jrf.0.1040259. [DOI] [PubMed] [Google Scholar]
- Onagbesan O.M., Vleugels B., Buys N., Bruggeman V., Safi M., Decuypere E. Insulin-like growth factors in the regulation of avian ovarian functions. Domest. Anim. Endocrinol. 1999;17:299–313. doi: 10.1016/s0739-7240(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Rajaram S., Baylink D.J., Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- Rebouças E.L., Costa J.J.N., Passos M.J., Silva A.W.B., Rossi R.O.D.S., Van Den Hurk R., Silva J.R.V. Expression levels of mRNA for insulin-like growth factors 1 and 2, IGF receptors and IGF binding proteins in in vivo and in vitro grown bovine follicles. Zygote. 2014;22:521–532. doi: 10.1017/S0967199413000166. [DOI] [PubMed] [Google Scholar]
- Russo V.C., Azar W.J., Yau S.W., Sabin M.A., Werther G.A. IGFBP-2: the dark horse in metabolism and cancer. Cytokine Growth Factor Rev. 2015;26:329–346. doi: 10.1016/j.cytogfr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Schoen T.J., Mazuruk K., Waldbillig R.J., Potts J., Beebe D.C., Chader G.J., Rodriguez I.R. Cloning and characterization of a chick embryo cDNA and gene for IGF-binding protein-2. J. Mol. Endocrinol. 1995;15:49–59. doi: 10.1677/jme.0.0150049. [DOI] [PubMed] [Google Scholar]
- Simmen R.C.M., Simmen F.A., Hofig A., Farmer S.J., Bazer F.W. Hormonal regulation of insulin-like growth factor gene expression in pig uterus*. Endocrinology. 1990;127:2166–2174. doi: 10.1210/endo-127-5-2166. [DOI] [PubMed] [Google Scholar]
- Spitschak M., Hoeflich A. Potential functions of IGFBP-2 for ovarian folliculogenesis and steroidogenesis. Front. Endocrinol. (Lausanne) 2018;9:119. doi: 10.3389/fendo.2018.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly J.L., Kowalski K.I., Johnson A.L. Stage of ovarian follicular development associated with the initiation of steroidogenic competence in avian granulosa cells. Biol. Reprod. 1991;44:305–314. doi: 10.1095/biolreprod44.2.305. [DOI] [PubMed] [Google Scholar]
- Tosca L., Chabrolle C., Crochet S., Tesseraud S., Dupont J. IGF-1 receptor signaling pathways and effects of AMPK activation on IGF-1-induced progesterone secretion in hen granulosa cells. Domest. Anim. Endocrinol. 2008;34:204–216. doi: 10.1016/j.domaniend.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Woods D.C., Johnson A.L. Regulation of follicle-stimulating hormone-receptor messenger RNA in hen granulosa cells relative to follicle selection1. Biol. Reprod. 2005;72:643–650. doi: 10.1095/biolreprod.104.033902. [DOI] [PubMed] [Google Scholar]
- Xiao C., Wang J., Zhang C. Synthesis, regulatory factors, and signaling pathways of estrogen in the ovary. Reprod. Sci. 2023;30:350–360. doi: 10.1007/s43032-022-00932-z. [DOI] [PubMed] [Google Scholar]
- Zhou P., Baumgarten S.C., Wu Y., Bennett J., Winston N., Hirshfeld-Cytron J., Stocco C. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol. Endocrinol. 2013;27:511–523. doi: 10.1210/me.2012-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Wang D., Zou K., Wang F., Zhang Z., Song X., Jia C., Wei Z. Insulin-like growth factor-1 regulates follicle selection of hens by promoting proliferation and inhibiting apoptosis of granulosa cells in prehierarchical follicles in vitro. Anim. Reprod. Sci. 2022;247 doi: 10.1016/j.anireprosci.2022.107091. [DOI] [PubMed] [Google Scholar]