Abstract
Background
Myocardial ischemia/reperfusion injury (MI/RI) can lead to impaired cardiac function. Quercetin (Que) has a positive effect and improves MI/RI. Sirtuin-3 (Sirt3) is a deacetylase that ameliorates oxidative stress and is associated with MI/RI. This study aimed to investigate the molecular mechanism by which Que protects cardiac function against MI/RI through the Sirt3 signaling pathway.
Methods
We conducted experiments by constructing hypoxia/reoxygenation (H/R) cardiomyocytes and MI/RI rat models. H9C2 cells were transfected with siRNA-Sirt3. Cardiomyocyte apoptosis was examined by TUNEL and Western blotting. The oxidative stress index was also determined. Mitochondrial reactive oxygen species (ROS) activity assays, ATP assays and mitochondrial membrane potential assays were performed. Evans Blue/TTC staining was used to examine surviving myocardial tissue.
Results
In the constructed H/R cells and MI/RI animal models, it was found that myocardial cell apoptosis increased (Bcl-2 expression was downregulated; Bax and cleaved caspase-3/8/9 expression were upregulated). In addition, oxidative stress levels increased (MDA levels increased; SOD, CAT, GSH-Px levels decreased), myocardial tissue was damaged (LDH, CK content increased), Sirt3 expression was downregulated, acetylation levels of superoxide dismutase 2 (SOD2) increased (AC-SOD2), and mitochondrial ROS increased. Que treatment alleviated the effects of MI/RI on cardiomyocytes and rats. Sirt3 expression and activity were upregulated, SOD2 acetylation was decreased, and mitochondrial ROS production was reduced by Que treatment. After Sirt3 was knocked down, we found that AC-SOD2 expression was upregulated and mitochondrial ROS were increased in H/R cardiomyocytes, further increased the degree of injury, while Que treatment attenuated the effect of Sirt3 knockdown on H/R cardiomyocytes.
Conclusion
Que inhibits cardiomyocyte apoptosis, reduces oxidative stress levels, protects mitochondrial function and prevents the impairment of cardiac function during MI/RI via the Sirt3/SOD2/mitochondrial ROS pathway.
Keywords: Myocardial ischemia/reperfusion injury, Quercetin, Sirt3, SOD2, Mitochondrial ROS, Apoptosis
1. Introduction
Myocardial ischemia/reperfusion injury (MI/RI) is a serious condition caused by coronary blood flow interruption and recovery, which can lead to cardiac insufficiency, myocardial stunning, reperfusion arrhythmias, no-reflow phenomenon and irreversible myocardial cell death [1,2]. Studies have shown that the occurrence of MI/RI is accompanied by increased cardiomyocyte apoptosis, elevated inflammatory cytokine levels, ferroptosis, and increased mitochondrial dysfunction [[3], [4], [5], [6]]. Among them, oxidative stress promotes apoptosis, myocardial hypertrophy and fibrosis [7]. Mitochondrial dysfunction leads to abnormal mitochondrial dynamics, uncontrolled increases in ROS, an impaired electron transport chain, and exacerbated MI/RI [8]. In recent years, mortality and morbidity due to MI/RI have increased [9]. However, there is currently no effective treatment method for MI/RI in clinical practice. It is critical to identify potential molecules that can effectively inhibit cardiac dysfunction and structural damage caused by MI/RI and their mechanisms to improve the cardiac function injury caused by it.
Quercetin (Que) is a flavonoid that exist extensively in fruits and vegetables and has antioxidant, antiviral, antibacterial and anti-inflammatory properties [10,11]. Que can mitigate oxidative stress damage by scavenging reactive oxygen species (ROS) [12]. In recent years, numerous studies have shown that Que has cardioprotective properties and exerts positive effects on MI/RI. For example, Que significantly reduces the levels of ROS and oxidative stress and improves cardiac pathomorphology [13]. Que regulates the MAPK signaling pathway through ROS and inhibits myocardial fibrosis [14]. Low concentrations of Que inhibit oxidative stress by modulating ERK1/2-DRP1 signaling, improve mitochondrial function, and alleviate MI/RI [15]. These findings suggest that the cardioprotective effect of Que on MI/RI may occur mainly by reducing the level of oxidative stress, improving mitochondrial function, and inhibiting cardiomyocyte apoptosis. Que has great potential as an alternative treatment for MI/RI, and it is critical to explore the specific molecular mechanism of its effects in multiple directions for the development of a clinical treatment.
As a deacetylated mitochondrial enzyme in the mitochondrial matrix, Sirtuin 3 (Sirt3) plays an important role in ameliorating oxidative stress and mitochondrial dysfunction [16]. As a manganese peroxidase in mitochondria, superoxide dismutase 2 (SOD2) can eliminate ROS to protect cells from oxidative stress, and deacetylation of two key lysine residues on SOD2 (lysine 68 and lysine 122) promotes Sirt3 antioxidant activity [17]. In MI/RI, Sirt3 can decrease the deleterious effects of ROS during MI/RI through SOD2, CYP-D and HIF-1α mediated pathways [18]. Melatonin can improve MI/RI by activating the SIRT3 signaling pathway to reduce oxidative stress and apoptosis [19]. In addition, previous studies have shown that the SIRT3-SOD2 pathway regulates mitochondrial ROS production and affects the progression of liver injury [20]. However, studies on the effect of the Sirt3/SOD2/mitochondrial ROS pathway on MI/RI have not been reported. Therefore, this study aimed to explore the protective effect of Que on MI/RI-induced cardiomyocytes through the Sirt3/SOD2/mitochondrial ROS pathway by reducing oxidative stress levels, improving mitochondrial function and inhibiting cardiomyocyte apoptosis.
2. Materials and methods
2.1. Cell culture and treatments
In this study, H9C2 (GNR 5) rat cardiomyocytes were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco's Modified Eagle's Medium (DMEM, 10566016, Gibco, Grand Island, NY, USA) for 12 h with 10 % fetal bovine serum (FBS, 10099141C, Gibco, Grand Island, NY, USA). The culture conditions were 37 °C and 5 % CO2. Based on previous treatment of H9C2 cells with Que at a concentration [21], we used different concentrations of Que and treated cardiomyocytes to identify the most suitable drug concentration. Cells in the logarithmic growth stage were treated with 0, 10, 20, 30, 40, 50 and 60 μM Que (1592409, Merck Millipore, Billerica, MA, USA) for 24 h.
2.2. Cell transfection
H9C2 cells were cultured in antibiotic-free extracellular matrix for 24 h. Subsequently, H9C2 cells were transfected with the target siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), and the cells were collected 48 h after being transfected. The siRNA sequences are shown in Table 1. All siRNAs used in the experiments were synthesized by GenePharma (Shanghai, China).
Table 1.
siRNA primer sequences.
| Target gene | Sequences |
|---|---|
| si-Sirt3-1 | F: 5′-UUCCGAACGUGUUCACGUTT-3′ |
| R: 5′-ACGUGACGUUCGGAGAATT-3′ | |
| si-Sirt3-2 | F:5′-GACGGAUAAGACAGACUAUGG-3′ |
| R: 5′-AUAGUCUGUCUUAUCCGUCCU-3′ | |
| si-NC | F: 5′-GGUGGAAGAAGGUCCAUAUTT-3′ |
| R: 5′-AUAUGGGACCUUCUCACCT-3′ |
2.3. CCK-8 assay
In this study, cardiomyocytes were placed on 96-well plates and incubated with different concentrations of Que for 24 h. Ten microliters of CCK-8 reagent (C0037, Beyotime, Shanghai, China) was added to the cells and incubated for 4 h at 37 °C. Finally, the absorbance was measured at 450 nm using an Microplate Reader (ELX800, BioTeK, UK). The cell proliferation rate= (experimental group absorption value - blank control absorption value)/(control group absorption value - blank control absorption value) × 100 %.
2.4. Isolation and extraction of cell mitochondria and cytoplasm
H9C2 cells were washed with PBS and digested with Trypsin-EDTA Solution (C0201, Beyotime, Shanghai, China) at 100–200 g and centrifuged at room temperature for 5–10 min to collect cells. The cell precipitates were lightly re-suspended with 4 °C PBS. Centrifuge at 4 °C for 5 min to precipitate cells. Discard the supernatant. Then mitochondria were extracted according to the instructions of the cell mitochondrial isolation kit (C3601, Beyotime, Shanghai, China).
H9C2 cells were washed with PBS, the cells were scraped off with cell scrapers, and the cells were blown down with a pipette. Centrifuge the cells, discard the supernatant, and leave the cells to precipitate for later use. Then the cytoplasm was extracted according to the cytoplasmic protein extraction kit (P0028, Beyotime, Shanghai, China).
2.5. Construction of the H/R model in cardiomyocytes
Cardiomyocytes experience a sharp loss of nutrients under ischemic (no oxygen, no nutrients) conditions, similar to what happens in serum-free media. In addition, nutritional deprivation can activate stress responses within cells to maintain cell survival under stressful conditions. First, the medium of H9C2 cells was changed. The hypoxia/reoxygenation (H/R) injured group was placed in sugar-free and serum-free DMEM, while the normal control group was placed in complete DMEM. The cells in each group were cultured in a 37 °C constant humidity incubator containing 94 % N2, 5 % CO2 and 1 % O2 for 3 h (simulating the process of cardiomyocyte ischemia). After anoxic culture, the culture medium was replaced with DMEM complete culture medium, and the cells were placed in a 5 % CO2, 95 % O2, and 37 °C constant temperature and humidity cell incubator for 16 h (simulating cardiomyocyte reperfusion process) to complete the construction of the H/R cell model. Cells in the H/R + Que group were incubated with Que (50 μM) for 4 h, cells in the H/R + Que group were transfected with si-Sirt3, and cells in the H/R + Que + si-Sirt3 group were transfected with si-Sirt3 and incubated with Que. Then, hypoxia/reoxygenation treatment was performed.
2.6. Construction of the rat MI/RI model
Sixty male Sprague–Dawley rats (3 months old, weighing 220 ± 20 g) were purchased from Experimental Animal Center of Kunming Medical University (Kunming, China). All rats were kept at a temperature of 22 ± 2 °C and humidity of 50 ± 10 %, with alternating light/darkness for 12 h. After one week of adaptive feeding, the animal experiments began. According to the literature [22], a rat model of myocardial ischemia‒reperfusion injury was constructed by ligating the left anterior descending coronary artery (LAD) of the heart. The rats were induced with 40 mL/L isoflurane (R510-22-10, Reward, Shenzhen, China) followed by endotracheal intubation and isoflurane (20 mL/L) maintenance anesthesia. The skin was first cut from the left anterior thorax with ophthalmic scissors, the muscle was separated with forceps, and then the pericardium was punctured between the fourth and fifth ribs with microscissors to expose the heart. Ligation of the coronary artery was performed with a 6-0 silk thread (approximately 2 mm at the lower edge of the left atrial appendage). After 30 min of ligation, the filament was removed to allow reperfusion for 2 h (Western blot analysis, oxidative stress assay, ATP assay, and mitochondrial ROS assay) or 24 h (TUNEL assay, cardiac function assay, and myocardial infarction area assay). Then the rats were sacrificed by cervical dislocation at corresponding time.
According to the experimental needs, the rats were randomly divided into the sham-operated group (sham), MI/RI group (model), MI/RI+3-TYP (Sirt3 inhibitor, HY-108331, MedChem Express, Monmouth Junction, NJ, USA) group, MI/RI + Que group, and MI/RI + Que+3-TYP group. (I) In the sham group, silk wire was only passed through LAD, but it was not ligated, and 1 % ethanol was administered as a control. (II) In the MI/RI group, 1 % ethanol was administered after complete LAD MI/RI surgery. (III) In the MI/RI+3-TYP group, before MI/RI surgery, inject 3-TYP intraperitoneally at a dose of 50 mg/kg every 2 days for a total of three times. (IV) In the MI/RI + Que group, rats were subjected to MI/RI before perfusion for 10 min and were intraperitoneally injected with quercetin (20 mg/kg). (V) In the MI/RI + Que+3-TYP group, the rats were subjected to MI/RI after 3-TYP pretreatment, and 20 mg/kg Que was intraperitoneally injected 10 min before perfusion.
2.7. TUNEL assay
After 24 h of reperfusion, myocardial tissue was collected, fixed in 4 % formalin, paraffin-embedded and sectioned. The sections were dewaxed and then labeled and stained according to the TUNEL kit instructions (C1089, Beyotime, Shanghai, China). A 4 % paraformaldehyde solution was used to fix the treated cells at 25 °C for 30 min. Subsequently, a 0.3 % H2O2 methanol solution was used to block the cells. For permeabilization, the samples were resuspended in a solution of 0.1 % sodium citrate containing 0.1 % Triton X-100. A fluorescence microscope was used to photograph the tissues and cells (400857, Nikon, Japan).
2.8. Western blot analysis
Total protein was isolated from myocardial tissue and H9C2 cells using a Radioimmunoprecipitation assay buffer (R0278, Sigma-Aldrich, St. Louis, MO, USA) containing 1 % protease inhibitor (R8340, Sigma-Aldrich, St. Louis, MO, USA), and a BCA protein assay kit (P0012, Beyotime, Shanghai, China) was used to determine the total protein concentration. After the total proteins were separated by SDS‒PAGE (10 %), the separated proteins were transferred to polyvinylidene fluoride membranes (FFP19, Beyotime, Shanghai, China), which were then blocked with 5 % skim milk for 3 h at 25 °C and then incubated with diluted primary antibodies against Bcl-2 (1:2000, ab59348, Abcam, Cambridge, UK), Bax (1:2000, ab182733, Abcam, Cambridge, UK), cleaved caspase-3 (1:5000, ab214430, Abcam, Cambridge, UK), cleaved caspase-8 (1:1000, #9496, Cell Signaling Technology, Danvers, MA, USA), cleaved caspase-9 (1:1000, #9505, Cell Signaling Technology, Danvers, MA, USA), SOD2 (1:500, ab68155, Abcam, Cambridge, UK), AC-SOD2 (1:1000, ab137037, Abcam, Cambridge, UK), Sirt3 (1:1000, ab246522, Abcam, Cambridge, UK), Cyto-c (1:1000, #4272, Cell Signaling Technology, Danvers, MA, USA), β-actin (1:1000, ab8227, Abcam, Cambridge, UK), and VDAC1 (1:1000, ab306581, Abcam, Cambridge, UK) overnight at 4 °C. Next, the membranes were incubated with secondary antibody horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (ab205718, 1:20000, Abcam, UK) dilution for 1 h at 25 °C and detected with enhanced chemiluminescence reagents. Finally, the bands were semiquantitatively analyzed using ImageJ software.
2.9. Oxidative stress index test
Firstly, the samples were processed in the following way: the myocardial tissue block (0.2–1 g) was rinsed with cold normal saline, and the weight of the animal tissue was accurately weighed and homogenized by adding 9 times the volume of homogenate medium according to the ratio of weight (g): volume (mL) = 1:9. H9C2 cells were collected into the centrifuge tube, and the supernatant was discarded after centrifugation to collect the precipitated cells. Then 0.5 mL of isotonic PBS buffer was added to suspend the cells, and the cells were broken by ultrasound. Tissue homogenates or broken cell suspensions were centrifuged and supernatant was obtained. Subsequently, GSH-Px activity, MDA levels, SOD activity and CAT activity in the supernatant were measured by a spectrophotometer according to the relevant kit (GSH-Px: A005; MDA: A003-1; SOD: A001-1-1; CAT: A007-1-1; Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).
2.10. Measurement of lactate dehydrogenase (LDH) and creatine phosphokinase (CK) levels
To characterize the extent of myocardial injury in rats, cell supernatants and blood were collected. Sample supernatants and serum were assayed for LDH and CK levels according to the manufacturer's instructions (LDH: A020-1; CK: A032-1-1, Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).
2.11. H & E staining
Myocardial tissue sections were fixed in formalin, sectioned in paraffin, treated with hematoxylin for 5 min, stained with eosin for 2 min, and observed under a light microscope (Olympus Optical Co., Ltd., Tokyo, Japan).
2.12. ATP assay
A total of 4 × 103 cells and 100 μL of reagents were seeded in each well of a 96-well plate, and the results were measured using EnVision (PerkinElmer, Waltham, MA, USA) approximately 30 min later. For tissue, supernatant specimens of myocardial tissue were isolated and obtained, and ATP levels were measured according to the ATP kit (S0026, Beyotime, Shanghai, China) instructions.
2.13. Mitochondrial ROS activity assay
Mitomycin red reagent (S0061S, Beyotime, Shanghai, China) was used to measure ROS activity in mitochondria. MitoSOX Red (5 μM) was incubated with the cells for 15 min at 37 °C, and PBS was used to wash the cells 3 times at 25 °C. After the freshly isolated, frozen, unfixed rat hearts were sectioned with a 10 μm cryostat, the tissues were incubated with MitoSOX Red (5 μM) under the same conditions, and the solution on the slides was removed. Then, the fluorescence levels of tissues and cells were examined using a NovoCyte flow cytometer (ACEA Biosciences, USA).
2.14. Mitochondrial membrane potential assay
According to the instructions of the JC-1 kit (C2006, Beyotime, Shanghai, China), after the cells were incubated with JC-1 solution for 15 min at 37 °C, they were washed with PBS 3 times. Five fields were selected from each group, and the results were photographed and analyzed under a confocal microscope.
2.15. Evans blue/TTC double staining
After 24 h of reperfusion, the aortic arch was injected with 2 % Evans blue. Subsequently, the myocardium was cut into 4 transverse sections and soaked with 1 % TTC in the dark for 30 min at 37 °C. Finally, the sections were placed in 10 % formalin and incubated for 24 h at 25 °C. In the images, the surviving myocardial tissue is red, and the infarcted myocardial tissue is white.
2.16. Statistical analysis
GraphPad Prism 8.0 software was used to analyze and plot the experimental results. To statistically analyze the data, student-t tests and one-way analysis of variance were used for comparisons between two groups and multiple groups, respectively. P < 0.05 indicated a significant difference, and for each experimental result, we performed three independent replicates.
3. Results
3.1. Que inhibits apoptosis induced by H/R and reduces oxidative stress levels in cardiomyocytes
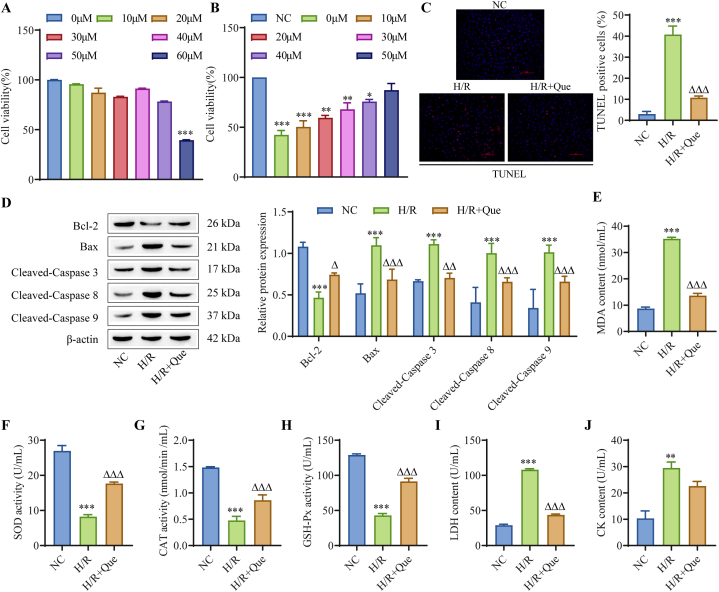
To determine the optimal treatment concentration of Que, we first treated normal H9C2 cells with seven concentrations (0, 10, 20, 30, 40, 50, and 60 μM). The results showed that 0, 10, 20, 30, 40, and 50 μM quercetin had no effect on normal H9C2 cells, but cell viability was inhibited by 60 μM (Fig. 1A). In addition, we treated H/R-induced cardiomyocytes with 0, 10, 20, 30, 40, and 50 μM quercetin and found that the viability of H9C2 cells was gradually increased with increasing concentrations (Fig. 1B). Therefore, we chose a concentration of 50 μM as the optimal quercetin concentration for subsequent experiments. After the hypoxia/reoxygenation model (H/R) in cardiomyocytes was constructed, we observed that compared with that in the NC group, cardiomyocyte apoptosis in the H/R group was increased, and Que treatment alleviated cardiomyocyte apoptosis to a certain extent (Fig. 1C). In addition, Western blot analysis of apoptosis related protein expression showed that compared with that in the NC group, the expression of Bcl-2 in the H/R group was downregulated, and the expression of Bax, cleaved caspase-3, cleaved caspase-8 and cleaved caspase-9 was upregulated. Que treatment reversed the changes in the expression of these proteins to some extent (Fig. 1D). Next, we detected the relevant indicators of oxidative stress and found that compared with those in the NC group, the levels of MDA in the H/R group were increased, while the activities of SOD, CAT and GSH-Px were decreased (Fig. 1E–H). Moreover, the release of the myocardial injury indicators LDH and CK was significantly increased (Fig. 1I–J). Similarly, treatment with Que alleviated H/R-induced oxidative stress and cardiomyocyte damage (Fig. 1I–J). Que inhibited H/R-induced cardiomyocyte apoptosis and reduced the level of cellular oxidative stress.
Fig. 1.
Que inhibits H/R-induced cardiomyocyte apoptosis and reduces cellular oxidative stress levels. (A) The effect of different concentrations of Que (0, 10, 20, 30, 40, 50, 60 μM) on the proliferation of normal cardiomyocytes was detected by CCK-8 assays. (B) The effects of different concentrations of Que (0, 10, 20, 30, 40, 50 μM) on cardiomyocyte proliferation in the H/R model were detected by CCK-8 assays (NC stands for normal H9C2). (C) TUNEL analysis of cardiomyocyte apoptosis (scale bar = 100 μm). (D) Western blot analysis of apoptosis-related proteins. (E) Detection of MDA levels by a kit. (F) Detection of SOD activity by a kit. (G) CAT activity was detected by a kit. (H) GSH-Px activity was detected by a kit. (I) Detection of LDH levels in cell supernatants. (J) Detection of CK levels in cell supernatants. ∗∗∗P < 0.001 vs. NC; △P < 0.05, △△P < 0.01, △△△P < 0.001 vs. H/R, n = 3. For the uncropped protein band plot, please see Fig. 1D in the Supplementary Material.
3.2. Que inhibits apoptosis and reduces oxidative stress in MI/RI rats
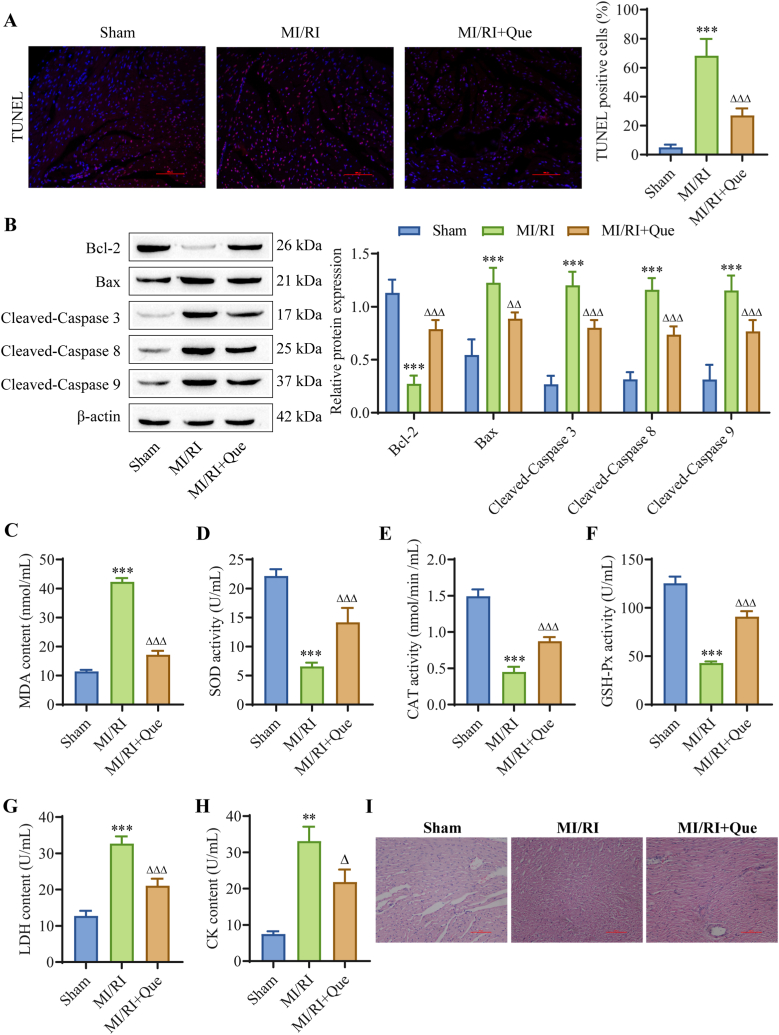
We further determined the effect of Que on myocardial injury in MI/RI rats. The TUNEL results showed that compared with that in the sham group, myocardial apoptosis was increased in the MI/RI group and decreased by Que treatment (Fig. 2A). The Western blot results showed that compared with that in the sham group, the expression of the apoptosis-related proteins Bax, cleaved caspase-3, cleaved caspase-8 and cleaved-caspase 9 was upregulated in the MI/RI group, and the expression of Bcl-2 was downregulated. Que treatment reversed these effects (Fig. 2B). The results of oxidative stress index analysis showed that compared with those in the sham group, MDA levels and the oxidative stress index were increased in the MI/RI group, and the activities of SOD, CAT and GSH-Px were decreased. After Que treatment, these changes in the oxidative stress index were reversed (Fig. 2C–F). In addition, serum concentrations of the myocardial injury indicators LDH and CK in rats were detected. Compared with those in the sham group, the concentrations of LDH and CK in the MI/RI group were increased, while the concentrations of LDH and CK in the MI/RI + Que group were decreased compared with those in the MI/RI group (Fig. 2G–H). Finally, changes in myocardial tissue were observed by H & E staining, and it was found that the myocardium in the MI/RI group had an irregular arrangement of muscle fibers and blurred boundaries between cells. In the MI/RI + Que group, the myocardial fibers were arranged more neatly, and the necrotic area of myocardial cells was significantly reduced (Fig. 2I). These findings indicate that Que treatment can effectively reduce the oxidative stress level of myocardial tissue in MI/RI rats, reduce cell apoptosis, and ameliorate pathomorphological status caused by MI/RI.
Fig. 2.
Que reduces oxidative stress and apoptosis in rat myocardial tissues after MI/RI. (A) Injury was detected using the TUNEL assay to examine apoptosis (scale bar = 100 μm). (B) The expression levels of apoptosis-related proteins were detected in tissues. (C–F) An oxidative stress kit was used to detect the changes in tissues (MDA, SOD, CAT and GSH-Px). (G) LDH levels in rat serum. (H) CK levels in rat serum. (I) H & E staining was used to evaluate the pathological changes in tissue (scale bar = 100 μm). ∗∗P < 0.01, ∗∗∗P < 0.001 vs. sham; △P < 0.05, △△P < 0.01, △△△P < 0.001 vs. MI/RI, n = 4. For the uncropped protein band plot, please see Fig. 2B in the Supplementary Material.
3.3. Que affects the expression of Sirt3 and SOD2 and mitochondrial function in cardiomyocytes
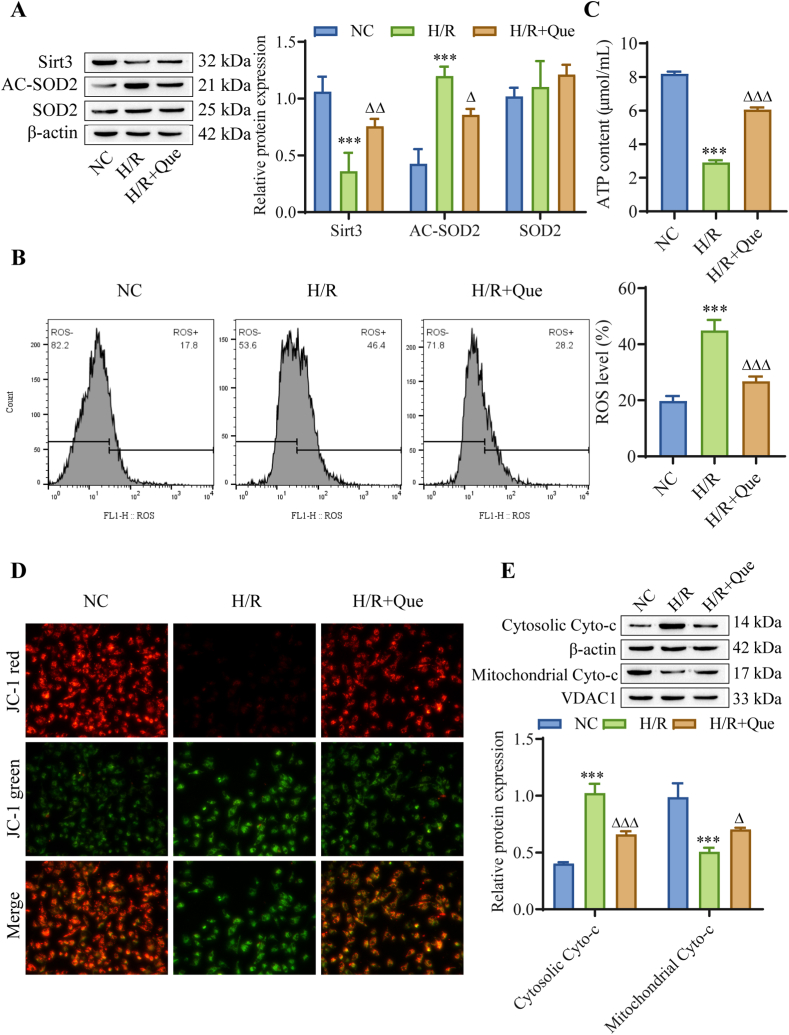
Mitochondrial damage and dysfunction are important causes of MI/RI, and we observed the effect of Que on mitochondrial function in H/R-induced cardiomyocytes. Western blot analysis showed that compared with that in the NC group, the protein expression of Sirt3, which regulates mitochondrial metabolic enzyme activity, was downregulated in the H/R group, and the protein expression of AC-SOD2 (the form of SOD2 with no antioxidant activity) was upregulated, while Que treatment promoted the expression of Sirt3 and inhibited the expression of AC-SOD2. There was no significant change in SOD2 expression among the groups (Fig. 3A). Mitochondrial dysfunction can lead to an increase in mitochondrial ROS. Flow cytometry showed that ROS levels in the H/R group were significantly increased compared with those in the NC group, and Que treatment significantly reduced mitochondrial ROS levels in H/R-induced cardiomyocytes (Fig. 3B). The decrease in ATP partly reflects mitochondrial dysfunction. The level of ATP in cardiomyocytes was detected, and compared with those in the NC group, ATP levels in the H/R group were reduced, and ATP levels were restored to a certain extent by Que treatment (Fig. 3C). Then, by measuring the mitochondrial membrane potential, we found that the mitochondrial membrane potential of cardiomyocytes in the H/R group was depolarized, and after Que treatment, mitochondrial membrane potential polarization was increased (Fig. 3D). Finally, we examined the expression of Cyto-c and found that the release of Cyto-c from the mitochondria to the cytoplasm in cardiomyocytes was significantly increased in the H/R group compared to the NC group, while the release of Cyto-c from mitochondria to the cytoplasm was significantly reduced by Que treatment (Fig. 3E). These result indicate that Que affects the mitochondrial function in cardiomyocytes and is related to the proteins Sirt3 and SOD2.
Fig. 3.
Que affects the expression of Sirt3 and SOD2 and mitochondrial function in cardiomyocytes (A) Detection of Sirt3, SOD2 and AC-SOD2 protein expression by Western blot analysis. (B) Mitochondrial ROS levels were detected by flow cytometry. (C) ATP levels. (D) Mitochondrial membrane potential. (E) The expression of Cyto-c in mitochondria and cytoplasm was detected by Western blotting. ∗∗∗P < 0.001 vs. NC; △P < 0.05, △△P < 0.01, △△△P < 0.001 vs. I/R, n = 3. For the uncropped protein band plot, please see Fig. 3A–E in the Supplementary Material.
3.4. Effect of Que on myocardial mitochondrial function in MI/RI rats
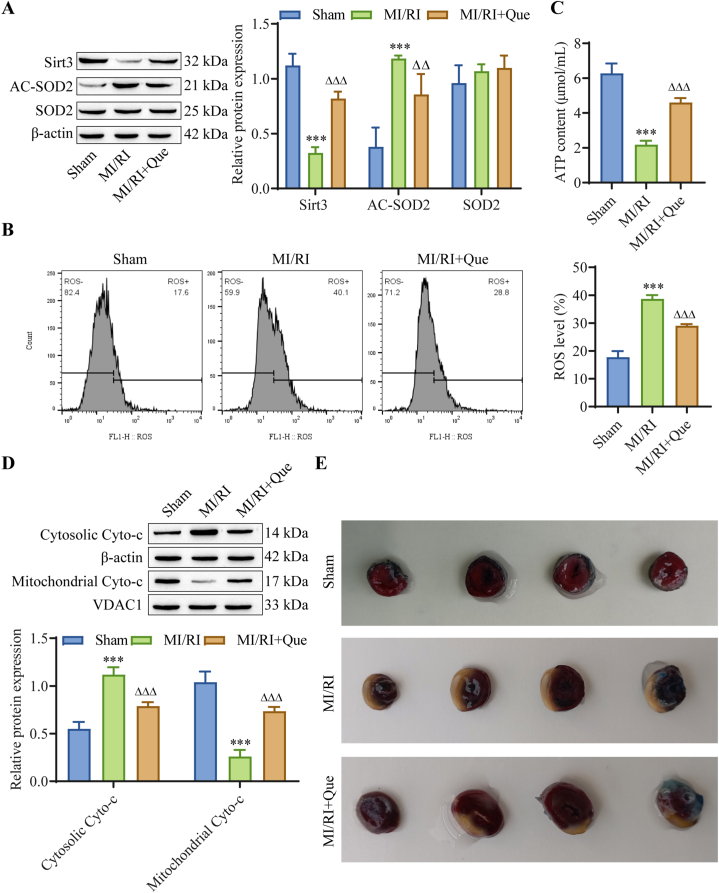
To further determine the effect of Que on mitochondrial function in cardiomyocytes, we conducted in vivo experiments. The Western blot results showed that compared with that in the sham group, the expression of Sirt3 was downregulated and the expression of AC-SOD2 was upregulated in the MI/RI group. However, compared with those in the MI/RI group, Que treatment reversed these changes in expression. Similarly, there was no significant difference in the expression of SOD2 among the groups (Fig. 4A). The flow cytometry results showed that compared with those in the sham group, ROS levels in the MI/RI group were significantly increased, and Que treatment significantly decreased ROS levels (Fig. 4B). Compared with those in the sham group, ATP levels in the myocardial tissue of the MI/RI group were decreased, and Que treatment increased ATP levels (Fig. 4C). The Western blot results showed that Cyto-c released from mitochondria to the cytoplasm was significantly increased in the MI/RI group. However, compared with that in the MI/RI group, Cyto-c released from the mitochondria into the cytoplasm was significantly decreased by Que treatment (Fig. 4D). The Evans blue/TTC staining results showed that the myocardial infarction area in the MI/RI group was significantly increased compared with that in the sham group, while Que treatment significantly reduced the myocardial infarction area in rats (Fig. 4E). These findings suggest that Que can alleviate myocardial damage in MI/RI rats and has a protective effect on normal mitochondrial function in MI/RI rats.
Fig. 4.
Effect of Que on myocardial mitochondrial function in MI/RI rats. (A) Western blot analysis of tissue Sirt3, SOD2 and AC-SOD2 protein expression. (B) Mitochondrial ROS levels were detected by flow cytometry. (C) ATP levels. (D) Cyto-c expression was detected by Western blotting. (E) Evans blue/TTC double staining to assess myocardial infarct size in rats. ∗∗∗P < 0.001 vs. sham; △P < 0.05, △△P < 0.01, △△△P < 0.001 vs. MI/RI. For the uncropped protein band plot, please see Fig. 4A–D in the Supplementary Material.
3.5. Que protects mitochondrial function in cardiomyocytes by regulating Sirt3/SOD2 signaling
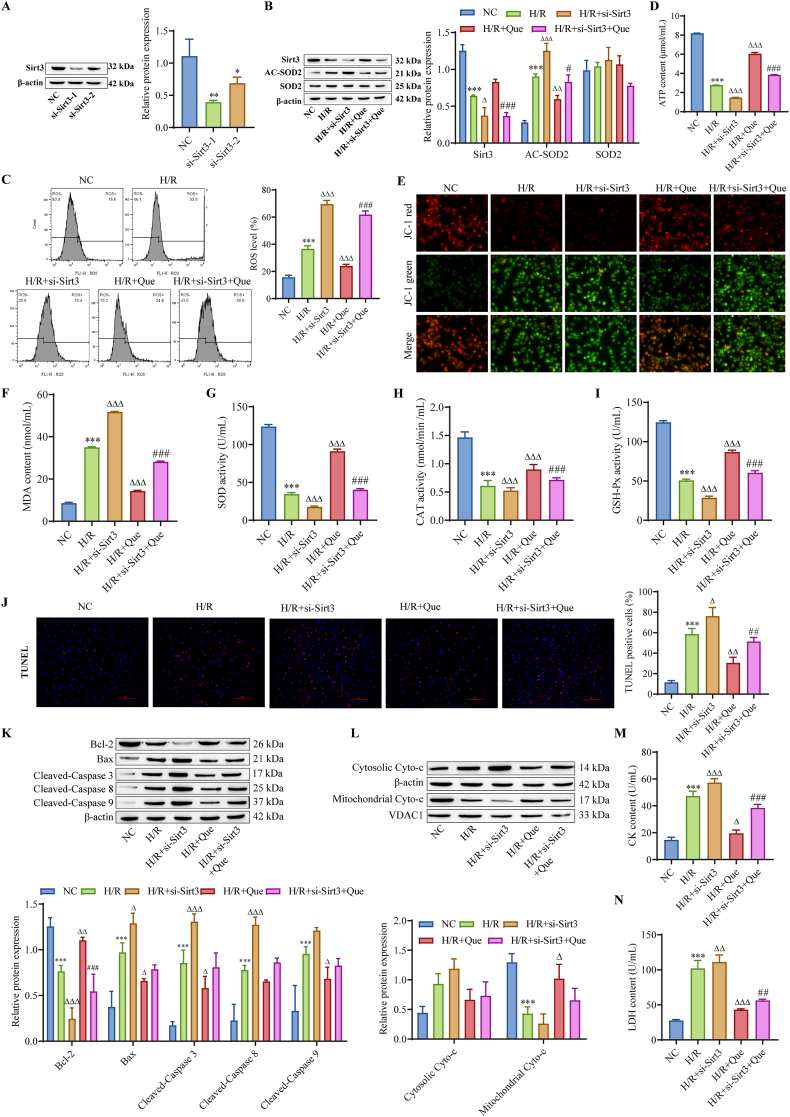
To investigate the potential signaling pathways in which Que is involved, Sirt3 siRNA was transfected into cardiomyocytes. As shown in Fig. 5A, si-Sirt3-1 had better transfection efficiency than the other sequences; therefore, we used si-Sirt3-1 to knock down Sirt3 for subsequent experiments. SOD2 is localized in mitochondria and helps eliminate mitochondrial ROS, protect against oxidative stress, and exert antiapoptotic effects [24]. The Western blot results showed that compared with that in the H/R group, Sirt3 knockdown further downregulated the expression of Sirt3 and upregulated the expression of AC-SOD2, indicating that Sirt3 regulated the deacetylation of SOD2, while Sirt3 knockdown and Que treatment reversed this effect (Fig. 5B). Flow cytometry showed that compared with those in the H/R group, Sirt3 knockdown increased mitochondrial ROS levels, while Sirt3 knockdown and Que treatment decreased ROS levels (Fig. 5C). Compared with those in the H/R group, Sirt3 knockdown further reduced ATP levels, while Sirt3 knockdown and Que treatment increased ATP levels (Fig. 5D). In addition, compared with that in the H/R group, mitochondrial membrane potential in the H/R + si-Sirt3 group was further decreased, while mitochondrial membrane potential in the H/R+ si-Sirt3+Que group was increased to a certain extent compared with that in the H/R+ si-Sirt3 group. si-Sirt3 transfection further enhanced the effect of H/R treatment, which led to an increase in MDA levels and a decrease in SOD, CAT and GSH-Px activity, while Que treatment reversed the effect of si-Sirt3 to some extent (Fig. 5F–I). TUNEL detected apoptosis and showed that the effect of si-Sirt3 transfection on promoting cardiomyocyte apoptosis was reversed to some extent by Que treatment (Fig. 5J). Western blot analysis showed that si-Sirt3 transfection further inhibited the expression of Bcl-2 and further promoted the expression of Bax, cleaved caspase-3, cleaved caspase-8 and cleaved caspase-9. In addition, si-Sirt3 transfection further promoted the release of Cyto-c from mitochondria to the cytoplasm. Treatment with Que reversed the effect of si-Sirt3 to some extent (Fig. 5K–L). The levels of CK and LDH, which are indicators of myocardial injury, were detected. Compared with those in the H/R group, the levels of CK and LDH in the H/R + Sirt3 group were further increased, while the levels of CK and LDH in the H/R + Sirt3+Que group were decreased compared with those in the H/R + Sirt3 group (Fig. 5M–N). These findings indicate that Que inhibits mitochondrial ROS production through Sirt3/SOD2 signaling, protects mitochondrial function in cardiomyocytes and inhibits cardiomyocyte apoptosis.
Fig. 5.
Que inhibits mitochondrial ROS production via Sirt3/SOD2 signaling and suppresses I/R-induced cardiomyocyte apoptosis. (A) Western blot analysis of Sirt3 transfection efficiency. (B) Detection of cellular Sirt3, SOD2 and AC-SOD2 expression by Western blot analysis. (C) Mitochondrial ROS levels. (D) Cellular ATP levels. (E) Mitochondrial membrane potential. (F–I) Changes in oxidative stress indicators. (J) TUNEL assay to detect apoptosis (scale bar = 100 μm). Western blot analysis of the expression of apoptosis-related proteins (K) and Cyto-c (L). (M − N) LDH and CK levels in cell supernatants. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 vs. NC; △P < 0.05, △△P < 0.01, △△△P < 0.001 vs. H/R; #P < 0.05, ##P < 0.01, ###P < 0.01 vs. H/R + si-Sirt3, n = 3. For the uncropped protein band plot, please see Fig. 5A,B,K,L in the Supplementary Material.
3.6. Que protects cardiac function by inhibiting the Sirt3/SOD2 mitochondrial ROS pathway in MI/RI-induced apoptotic cardiomyocytes in rats
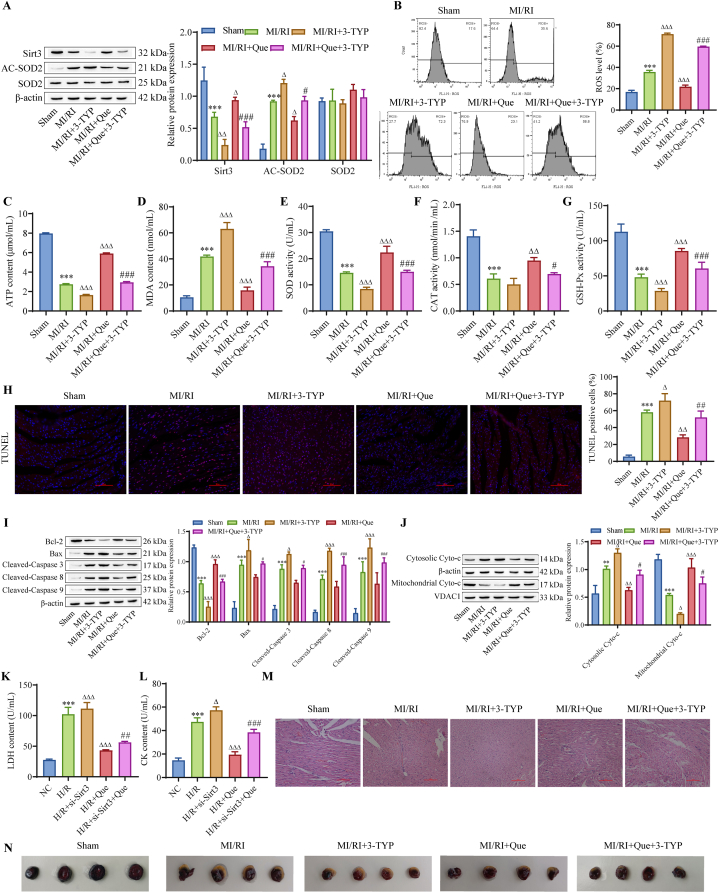
To verify that Que affects the development of MI/RI through the Sirt3/SOD2/mitochondrial ROS pathway, we performed in vivo experiments and inhibited Sirt3 expression with 3-TYP. MI/RI rats were treated with Que or the Sirt3 inhibitor 3-TYP or with Que + 3-TYP. The Western blot results showed that compared with that in the MI/RI group, the expression of Sirt3 was downregulated and the expression of AC-SOD2 was upregulated in the MI/RI+3-TYP group, and the MI/RI + Que group showed the opposite results. Que treatment further weakened the effect of 3-TYP (Fig. 6A). Flow cytometry showed that mitochondrial ROS levels were increased after Sirt3 inhibition and that the ROS levels were decreased after Sirt3 inhibition and Que treatment in MI/RI rats (Fig. 6B). Compared with those in the MI/RI group, ATP levels in the MI/RI group were further decreased after Sirt3 inhibition, and ATP levels were increased after Sirt3 inhibition and Que treatment (Fig. 6C). Analysis of oxidative stress indicators showed that the oxidative stress level in the MI/RI + 3-TYP group was higher than that in the MI/RI group, and the oxidative stress level was reduced by inhibiting Sirt3 and administering Que to MI/RI rats (Fig. 6D–G). Compared with that in the MI/RI group, apoptosis was increased after Sirt3 inhibition, and apoptosis was decreased after Sirt3 inhibition and Que treatment in the MI/RI group (Fig. 6H–I). Compared with that in the MI/RI group, inhibiting Sirt3 aggravated myocardial injury, while inhibiting Sirt3 and addition of Que alleviated myocardial injury in MI/RI rats (Fig. 6J–L). In addition, H & E and Evans blue/TTC staining showed that inhibiting Sirt3 further aggravated myocardial cell necrosis and expanded the myocardial infarction area, while inhibiting Sirt3 and administering Que ameliorated myocardial tissue damage (Fig. 6M–N). These results showed that Que inhibited myocardial cell apoptosis in MI/RI rats and protected cardiac function through the Sirt3/SOD2 mitochondrial ROS pathway.
Fig. 6.
Que protects cardiac function through the Sirt3/SOD2 mitochondrial ROS pathway to inhibit cardiomyocyte apoptosis in MI/RI rats. (A) Western blot analysis of Sirt3, SOD2 and AC-SOD2 protein levels in tissue. (B) Mitochondrial ROS levels. (C) ATP levels in tissues. (D–G) Changes in oxidative stress indicators. (H) Detection of apoptosis by TUNEL assays (scale bar = 100 μm). Western blot analysis of apoptosis-related proteins (I) and Cyto-c (J) in tissues. (K–L) LDH and CK levels in rat serum. (M) H & E staining to assess myocardial histopathological changes (scale bar = 100 μm). (N) Assessment of the myocardial infarction area in rats by Evans/TTC double staining. ∗∗P < 0.01, ∗∗∗P < 0.001 vs. sham, △P < 0.05, △△P < 0.01, △△△P < 0.001 vs. MI/RI, #P < 0.01, ###P < 0.001 vs. MI/RI+3-TYP. For the uncropped protein band plot, please see Fig. 6A–I,J in the Supplementary Material.
4. Discussion
MI/RI can seriously affect the prognosis of a variety of cardiac diseases. Both oxidative stress and inflammatory reactions can cause MI/RI, and MI/RI can also promote oxidative stress and inflammatory reactions. This vicious cycle further lead to cardiomyocyte apoptosis and necrosis, thereby leading to unavoidable cardiac injury [23]. The development of MI/RI is a complex process that mainly involves mitochondrial dysfunction. Impaired mitochondrial function leads to ROS accumulation, decreases in ATP levels, and cell membrane ion gradient collapse, which lead to oxidative stress, inflammatory reactions and eventually cell death [8,24]. Wang et al. [25] found that homocysteine induced mitochondrial dysfunction and oxidative stress by stimulating ROS production and the ERK1/2 signaling pathway and promoted MI/RI. Consistent with previous findings, oxidative stress levels and cardiomyocyte apoptosis were increased, and mitochondrial function in this study was impaired in both cellular H/R and animal MI/RI models. Yang et al. [26] found that vitexin regulates mitochondrial dysfunction through EPAC1-RAP1 signaling and alleviates MI/RI in rats. Yu et al. [27] found that Elabela treatment reduced I/R-induced cardiomyocyte fibrosis and apoptosis, improved mitochondrial dysfunction in animals and cells, reduced oxidative stress levels, and alleviated MI/RI. Therefore we hypothesized that regulating oxidative stress and improving mitochondrial dysfunction may improve MI/RI symptoms.
Que is a bioactive compound with strong antioxidant activity that is derived from plants. Que can significantly reduce heart rate, ROS levels, serum levels of myocardial enzymes and other inflammatory markers, and improve pathological morphology of heart tissue [13,28]. In recent years, many studies have shown that Que protects against MI/RI and regulates the progression of MI/RI through a variety of pathways. Li et al. found that low concentrations of Que can alleviate I/R injury by regulating ERK1/2-DRP1 signaling, inhibiting oxidative stress and improving mitochondrial function [15]. Que can prevent MI/RI-induced myocardial infarction by upregulating antioxidants and activating STAT3 [29]. We constructed animal and cell models and found that Que could reduce oxidative stress in cells and tissues, reduce the production of mitochondrial ROS, improve mitochondrial dysfunction, reduce MI/RI, and protect cardiac function. This evidence provides a new theoretical basis and research direction for the treatment of MI/RI. However, the specific mechanism by which Que regulates oxidative stress and mitochondrial function is still unclear, and further experiments are needed to verify this hypothesis. In addition, Que effectively inhibited the occurrence of cardiomyocyte apoptosis. Que can promote the expression of the antiapoptotic protein Bcl-2 and inhibit the expression of the proapoptotic proteins Bax, cleaved caspase-3, cleaved caspase-8, and cleaved caspase-9. Caspase-3 is the main executor of endogenous and exogenous apoptosis in cells. When activated by the apoptosis complex, caspase-9 is activated to form cleaved caspase-9, which further activates caspase-3 and generates cleaved caspase-3, resulting in apoptosis [30]. Similarly, activated caspase-8 further activates caspase-3 to induce apoptosis [31]. These findings indicate that Que can inhibit the occurrence of cardiomyocyte apoptosis. Other studies have shown that Que can protect chondrocytes and effectively inhibit the occurrence of apoptosis [32].
Sirt3 is a deacetylated mitochondrial enzyme that can directly or indirectly regulate downstream effectors to improve oxidative stress and mitochondrial dysfunction and regulate the progression of various diseases [16]. In colorectal cancer, Sirt3 can inhibit oxidative stress [33]. In liver ischemia‒reperfusion injury, Sirt3 attenuates the harmful effects of ROS through SOD2-, CYP-D- and HIF-1α-mediated pathways [18]. In MIRI, Sirt3 attenuates MI/R injury by regulating cell apoptosis, autophagy, ROS scavenging and other cellular mechanisms [34]. A lack of Sirt3 worsens myocardial dysfunction, including systolic dysfunction, distolic dysfunction, and abnormal cardiomyocyte calcium flux during stress [35]. We found that inhibiting Sirt3 aggravated mitochondrial dysfunction, increased ROS levels, increased apoptosis, and thus aggravated the progression of MI/RI. Furthermore, after the treatment of cells and tissues with a Sirt3 inhibitor, TYP-3, the therapeutic effect of Que on MI/RI was weakened, indicating that Que inhibits cardiomyocyte apoptosis by regulating Sirt3 expression. Que can reduce oxidative stress levels, protect mitochondrial function and alleviate MI/RI. In addition, studies have shown that Sirt3 regulates the deacetylation of SOD2, which is a key enzyme that can reduce mitochondrial ROS levels [36]. The Sirt3/SOD2/mitochondrial ROS signaling pathway is associated with oxidative stress [37], autophagy [38], mitochondrial dysfunction [39] and apoptosis [40] in diseases. We found that knockdown of Sirt3 and AC-SOD2 increased the expression of mitochondrial ROS and that Que affected the progression of MI/RI through the SIRT3/SOD2/mitochondrial ROS signaling pathway. However, there are still some shortcomings in this study. First, the experimental cells are single, and different cells have different tolerance to stress and drugs, as well as different metabolism, so primary cardiomyocytes or HL1 cell lines should be supplemented for the experiment. Second, the regulatory mechanism of a signaling pathway is complex, and we can only verify this mechanism from one aspect. Therefore, there is still a lack of sufficient and reliable evidence to develop new MI/RI treatment methods based on this evidence, which still has a long way to go.
5. Conclusions
This study found that Que treatment upregulated Sirt3 expression and activity, reduced SOD2 and AC-SOD2 levels, and reduced mitochondrial ROS production. In addition, knockdown of Sirt3 experiments have shown that the upregulation of SOD2 and AC-SOD2 expression and increased mitochondrial ROS in H/R cardiomyocytes exacerbate myocardial cell damage, while Que treatment weakens this effect, indicating that Que affects the progression of MI/RI through the Sirt3/SOD2/mitochondrial ROS pathway. These findings provide new insights into the clinical treatment of MI/RI, revealing the potential of Que as a drug for heart disease, and delving into its molecular mechanisms.
Funding
The Yunnan Fundamental Research Project (202301AT070094), Foreign Talent Introduction Program: Intellectual Achievements Promotion Project (202305AQ350008), the Emergency Medicine Open Project of The First People's Hospital of Yunnan Province (2024JZKFKT-04), and the Applied Basic Research Joint Project of Yunnan Provincial Science & Technology Department and Kunming Medical University (202001AY070001‐117).
Ethics statement
All animal experiments were carried out in accordance with the U.K. Animals (Scientific Procedures) Act 1986, associated guidelines, and the European Communities Council Directive 2010/63/EU. All animal studies complied with the ARRIVE guidelines. The animal study protocol was approved by the Ethics Committee of First People's Hospital of Yunnan Province (YYLH072).
Data availability statement
Data will be made available upon request.
CRediT authorship contribution statement
Da Xiong: Validation, Methodology, Conceptualization. Xin Wang: Writing – original draft, Visualization, Methodology, Formal analysis, Data curation. Haiyu Wang: Methodology. Xia Chen: Writing – original draft, Methodology. Hongrong Li: Supervision. Yongwu Li: Methodology, Formal analysis. Minghua Zhong: Visualization, Data curation. Jingcheng Gao: Data curation. Zicong Zhao: Validation. Wenjun Ren: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e39031.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
References
- 1.Zhou M., Yu Y., Luo X., Wang J., Lan X., Liu P., et al. Myocardial ischemia-reperfusion injury: therapeutics from a mitochondria-centric perspective. Cardiology. 2021;146:781–792. doi: 10.1159/000518879. [DOI] [PubMed] [Google Scholar]
- 2.Deng J. Advanced research on the regulated necrosis mechanism in myocardial ischemia-reperfusion injury. Int. J. Cardiol. 2021;334:97–101. doi: 10.1016/j.ijcard.2021.04.042. [DOI] [PubMed] [Google Scholar]
- 3.Huang Z.Q., Xu W., Wu J.L., Lu X., Chen X.M. MicroRNA-374a protects against myocardial ischemia-reperfusion injury in mice by targeting the MAPK6 pathway. Life Sci. 2019;232 doi: 10.1016/j.lfs.2019.116619. [DOI] [PubMed] [Google Scholar]
- 4.Li Q., Li Z., Fan Z., Yang Y., Lu C. Involvement of non-coding RNAs in the pathogenesis of myocardial ischemia/reperfusion injury. Int. J. Mol. Med. 2021;47 doi: 10.3892/ijmm.2021.4875. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lv Z., Wang F., Zhang X., Zhang X., Zhang J., Liu R. Etomidate attenuates the ferroptosis in myocardial ischemia/reperfusion rat model via Nrf2/HO-1 pathway. Shock. 2021;56:440–449. doi: 10.1097/SHK.0000000000001751. [DOI] [PubMed] [Google Scholar]
- 6.Man-Li Z., Yu F., Shu-le Q., Xiao-Xin L., Si-Qi X.U., Wei-Xiong J. [Research progress on potential targets-mitochondrial dynamics-related proteins in treatment of myocardial ischemia-reperfusion injury] Zhongguo Zhongyao Zazhi. 2020;45:4183–4195. doi: 10.19540/j.cnki.cjcmm.20200623.602. [DOI] [PubMed] [Google Scholar]
- 7.Kura B., Szeiffova Bacova B., Kalocayova B., Sykora M., Slezak J. Oxidative stress-responsive MicroRNAs in heart injury. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paradies G., Paradies V., Ruggiero F.M., Petrosillo G. Mitochondrial bioenergetics and cardiolipin alterations in myocardial ischemia-reperfusion injury: implications for pharmacological cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2018;315:H1341–h1352. doi: 10.1152/ajpheart.00028.2018. [DOI] [PubMed] [Google Scholar]
- 9.Peng K., Liu H., Yan B., Meng X.W., Song S.Y., Ji F.H., et al. Inhibition of cathepsin S attenuates myocardial ischemia/reperfusion injury by suppressing inflammation and apoptosis. J. Cell. Physiol. 2021;236:1309–1320. doi: 10.1002/jcp.29938. [DOI] [PubMed] [Google Scholar]
- 10.Di Petrillo A., Orrù G., Fais A., Fantini M.C. Quercetin and its derivates as antiviral potentials: a comprehensive review. Phytother Res. 2022;36:266–278. doi: 10.1002/ptr.7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseini A., Razavi B.M., Banach M., Hosseinzadeh H. Quercetin and metabolic syndrome: a review. Phytother Res. 2021;35:5352–5364. doi: 10.1002/ptr.7144. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y.M., Zhang Z.Y., Wang R.X. Protective mechanisms of quercetin against myocardial ischemia reperfusion injury. Front. Physiol. 2020;11:956. doi: 10.3389/fphys.2020.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang Y., Zhang Y., Liu M., Han X., Zhang J., Zhang X., et al. Protective effect of quercetin against myocardial ischemia as a Ca(2+) channel inhibitor: involvement of inhibiting contractility and Ca(2+) influx via L-type Ca(2+) channels. Arch Pharm. Res. (Seoul) 2020;43:808–820. doi: 10.1007/s12272-020-01261-y. [DOI] [PubMed] [Google Scholar]
- 14.Min Z., Yangchun L., Yuquan W., Changying Z. Quercetin inhibition of myocardial fibrosis through regulating MAPK signaling pathway via ROS. Pak. J. Pharm. Sci. 2019;32:1355–1359. [PubMed] [Google Scholar]
- 15.Li F., Li D., Tang S., Liu J., Yan J., Chen H., et al. Quercetin protects H9c2 cardiomyocytes against oxygen-glucose deprivation/reoxygenation-induced oxidative stress and mitochondrial apoptosis by regulating the ERK1/2/DRP1 signaling pathway. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/7522175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng J., Shi L., Liang F., Xu W., Li T., Gao L., et al. Sirt3 ameliorates oxidative stress and mitochondrial dysfunction after intracerebral hemorrhage in diabetic rats. Front. Neurosci. 2018;12:414. doi: 10.3389/fnins.2018.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma C., Sun Y., Pi C., Wang H., Sun H., Yu X., et al. Sirt3 attenuates oxidative stress damage and rescues cellular senescence in rat bone marrow mesenchymal stem cells by targeting superoxide dismutase 2. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katwal G., Baral D., Fan X., Weiyang H., Zhang X., Ling L., et al. SIRT3 a major player in attenuation of hepatic ischemia-reperfusion injury by reducing ROS via its downstream mediators: SOD2, CYP-D, and HIF-1α. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/2976957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai M., Li B., Duan W., Jing L., Zhang B., Zhang M., et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J. Pineal Res. 2017;63 doi: 10.1111/jpi.12419. [DOI] [PubMed] [Google Scholar]
- 20.Li D.P., Chen Y.L., Jiang H.Y., Chen Y., Zeng X.Q., Xu L.L., et al. Phosphocreatine attenuates Gynura segetum-induced hepatocyte apoptosis via a SIRT3-SOD2-mitochondrial reactive oxygen species pathway. Drug Des. Dev. Ther. 2019;13:2081–2096. doi: 10.2147/DDDT.S203564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo X., Chen M., Zeng H., Liu P., Zhu X., Zhou F., et al. Quercetin attenuates ethanol-induced iron uptake and myocardial injury by regulating the angiotensin II-L-Type calcium channel. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700772. [DOI] [PubMed] [Google Scholar]
- 22.Xu Z., McElhanon K.E., Beck E.X., Weisleder N. A murine model of myocardial ischemia-reperfusion injury. Methods Mol. Biol. 2018;1717:145–153. doi: 10.1007/978-1-4939-7526-6_12. [DOI] [PubMed] [Google Scholar]
- 23.Wu D., Gu Y., Zhu D. Cardioprotective effects of hydrogen sulfide in attenuating myocardial ischemia-reperfusion injury. Mol. Med. Rep. 2021;24 doi: 10.3892/mmr.2021.12515. submitted for publication. [DOI] [PubMed] [Google Scholar]
- 24.Sánchez G., Chalmers S., Ahumada X., Montecinos L., Olmedo I., Eisner V., et al. Inhibition of chymotrypsin-like activity of the proteasome by ixazomib prevents mitochondrial dysfunction during myocardial ischemia. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L., Niu H., Zhang J. Homocysteine induces mitochondrial dysfunction and oxidative stress in myocardial ischemia/reperfusion injury through stimulating ROS production and the ERK1/2 signaling pathway. Exp. Ther. Med. 2020;20:938–944. doi: 10.3892/etm.2020.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H., Xue W., Ding C., Wang C., Xu B., Chen S., et al. Vitexin mitigates myocardial ischemia/reperfusion injury in rats by regulating mitochondrial dysfunction via epac1-rap1 signaling. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/9921982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu P., Ma S., Dai X., Cao F. Elabela alleviates myocardial ischemia reperfusion-induced apoptosis, fibrosis and mitochondrial dysfunction through PI3K/AKT signaling. Am J Transl Res. 2020;12:4467–4477. [PMC free article] [PubMed] [Google Scholar]
- 28.Patel R.V., Mistry B.M., Shinde S.K., Syed R., Singh V., Shin H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018;155:889–904. doi: 10.1016/j.ejmech.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 29.Albadrani G.M., Binmowyna M.N., Bin-Jumah M.N., El-Akabawy G., Aldera H., Al-Farga A.M. Quercetin protects against experimentally-induced myocardial infarction in rats by an antioxidant potential and concomitant activation of signal transducer and activator of transcription 3. J. Physiol. Pharmacol. 2020;71 doi: 10.26402/jpp.2020.6.11. [DOI] [PubMed] [Google Scholar]
- 30.Asadi M., Taghizadeh S., Kaviani E., Vakili O., Taheri-Anganeh M., Tahamtan M., et al. Caspase-3: structure, function, and biotechnological aspects. Biotechnol. Appl. Biochem. 2022;69:1633–1645. doi: 10.1002/bab.2233. [DOI] [PubMed] [Google Scholar]
- 31.Mandal R., Barrón J.C., Kostova I., Becker S., Strebhardt K. Caspase-8: the double-edged sword. Biochim. Biophys. Acta Rev. Canc. 2020;1873 doi: 10.1016/j.bbcan.2020.188357. [DOI] [PubMed] [Google Scholar]
- 32.Feng K., Chen Z., Pengcheng L., Zhang S., Wang X. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell. Physiol. 2019;234:18192–18205. doi: 10.1002/jcp.28452. [DOI] [PubMed] [Google Scholar]
- 33.Paku M., Haraguchi N., Takeda M., Fujino S., Ogino T., Takahashi H., et al. SIRT3-Mediated SOD2 and PGC-1α contribute to chemoresistance in colorectal cancer cells. Ann. Surg Oncol. 2021;28:4720–4732. doi: 10.1245/s10434-020-09373-x. [DOI] [PubMed] [Google Scholar]
- 34.Zheng Y., Shi B., Ma M., Wu X., Lin X. The novel relationship between Sirt3 and autophagy in myocardial ischemia-reperfusion. J. Cell. Physiol. 2019;234:5488–5495. doi: 10.1002/jcp.27329. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J., He Z., Fedorova J., Logan C., Bates L., Davitt K., et al. Alterations in mitochondrial dynamics with age-related Sirtuin1/Sirtuin3 deficiency impair cardiomyocyte contractility. Aging Cell. 2021;20 doi: 10.1111/acel.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T., Ma X., Ouyang T., Chen H., Xiao Y., Huang Y., et al. Efficacy of 5-aminolevulinic acid-based photodynamic therapy against keloid compromised by downregulation of SIRT1-SIRT3-SOD2-mROS dependent autophagy pathway. Redox Biol. 2019;20:195–203. doi: 10.1016/j.redox.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Z.H., Wang S.F., Liao W. Zoledronic acid accelerates osteogenesis of bone marrow mesenchymal stem cells by attenuating oxidative stress via the SIRT3/SOD2 pathway and thus alleviates osteoporosis. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2095–2101. doi: 10.26355/eurrev_202002_20389. [DOI] [PubMed] [Google Scholar]
- 38.Zhou S., Sun L., Qian S., Ma Y., Ma R., Dong Y., et al. Iron overload adversely effects bone marrow haematogenesis via SIRT-SOD2-mROS in a process ameliorated by curcumin. Cell. Mol. Biol. Lett. 2021;26:2. doi: 10.1186/s11658-020-00244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L., Du Z., He L., Liang W., Liu K., Gong S. ROS-induced oxidative damage and mitochondrial dysfunction mediated by inhibition of SIRT3 in cultured cochlear cells. Neural Plast. 2022;2022 doi: 10.1155/2022/5567174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y., Yang X., Ge X., Zhang F. Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed. Pharmacother. 2019;109:726–733. doi: 10.1016/j.biopha.2018.10.161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request.