Abstract
Objective
To investigate the diagnostic value of serum fibrinogen domain-containing lectin-3 (Ficolin-3) and galectin-3 (Gal-3) in sepsis-associated acute kidney injury (SA-AKI).
Methods
This study retrospectively analyzed 126 SA-AKI patients with SA-AKI and 103 septic patients without AKI as controls. Based on the severity of renal injury, the SA-AKI patients were divided into three groups: mild (41 cases), moderate (53 cases), and severe (32 cases). Serum levels of Ficolin-3 and Gal-3 were measured using ELISA, and their correlation was determined through Pearson analysis. Multivariate logistic regression was used to identify factors associated with the occurrence of SA-AKI.
Results
The serum creatinine (SCr), blood urea nitrogen (BUN), as well as the expression levels of serum Ficolin-3 and Gal-3 in the SA-AKI group were higher than those in the non SA-AKI group (P<0.05). The expression levels of Ficolin-3 and Gal-3 in the serum of the SA-AKI group were also higher than those of the non SA-AKI group (P<0.05). The expression levels of Ficolin-3 and Gal-3 in serum gradually increased with the severity of renal injury in SA-AKI patients (P<0.05). The expression levels of Ficolin-3 and Gal-3 in serum were greatly positively correlated (P<0.001). Elevated levels of BUN, Ficolin-3, and Gal-3 were risk factors affecting the occurrence of SA-AKI (P<0.05). The area under the curve (AUC) of serum Ficolin-3 and Gal-3 for individual diagnosis of SA-AKI was 0.877 and 0.867, respectively, the AUC of their combined diagnosis was 0.953, and the diagnostic sensitivity was higher than that of their individual diagnosis (P<0.001).
Conclusion
The expression levels of serum Ficolin-3 and Gal-3 are closely related to associated with the onset and progression of SA-AKI and hold diagnostic value for its detection. Furthermore, the combined use of both markers provides a more accurate diagnosis than either marker alone.
Keywords: Sepsis complicated with acute kidney injury, Fibrinogen domain-containing lectin-3, Galectin-3
Introduction
Sepsis is a condition triggered by the body’s dysregulated response to infection, resulting in organ dysfunction. In sepsis, increased vascular permeability and altered local blood flow distribution can raise the risk of mortality.1 Acute kidney injury (AKI) is a frequent and complex complication of sepsis. Factors such as infections, urethral obstruction, and certain medications associated with sepsis can contribute to its development. Patients with AKI often exhibit symptoms like reduced or absent urine output, nausea, and generalized edema. Statistics indicate that 50% to 70% of sepsis patients develop sepsis-associated acute kidney injury (SA-AKI), a syndrome marked by impaired renal function. This disease progresses rapidly and can become severe, potentially leading to multi-organ failure and posing a serious threat to the patient’s life, health, and safety.2,3 Therefore, finding specific biomarkers that can assist in the clinical diagnosis of SA-AKI is of great importance.
Fibrinogen domain-containing lectin 3 (Ficolin-3) is one of the important members of the fibrinogen family as well as an important initiator of the complement lectin pathway.4 Research indicates that Ficolin-3 plays a role in inflammation and immune responses by activating specific signaling pathways, thereby exerting pro-inflammatory effects and contributing to the onset and progression of inflammatory diseases.5,6
Galectin-3 (Gal-3) is a member of the lectin family, abundantly present in the extracellular matrix of cardiomyocytes and fibroblasts, and involved in cell differentiation, proliferation, and intercellular communication.7 Recently, scholars have found that Gal-3, as an immune-regulating protein, has a unique structure that enables it to bind extracellular matrix (ECM) glycosylation compounds to the cell surface during tissue remodeling. This interaction stimulates immune cells, such as macrophages, to release chemokines and inflammatory factors, promoting their accumulation and triggering inflammatory responses.8,9
However, there are few studies on the correlation between the expression levels of Ficolin-3 and Gal-3 in serum and the occurrence of SA-AKI. Based on this, this study measured the expression levels of Ficolin-3 and Gal-3 in the serum of SA-AKI patients and analyzed their auxiliary diagnostic value for SA-AKI, aiming to improve the early diagnosis rate of SA-AKI, thereby improving the treatment outcome and prognosis of patients.
Study Subjects and Methods
Study Subjects
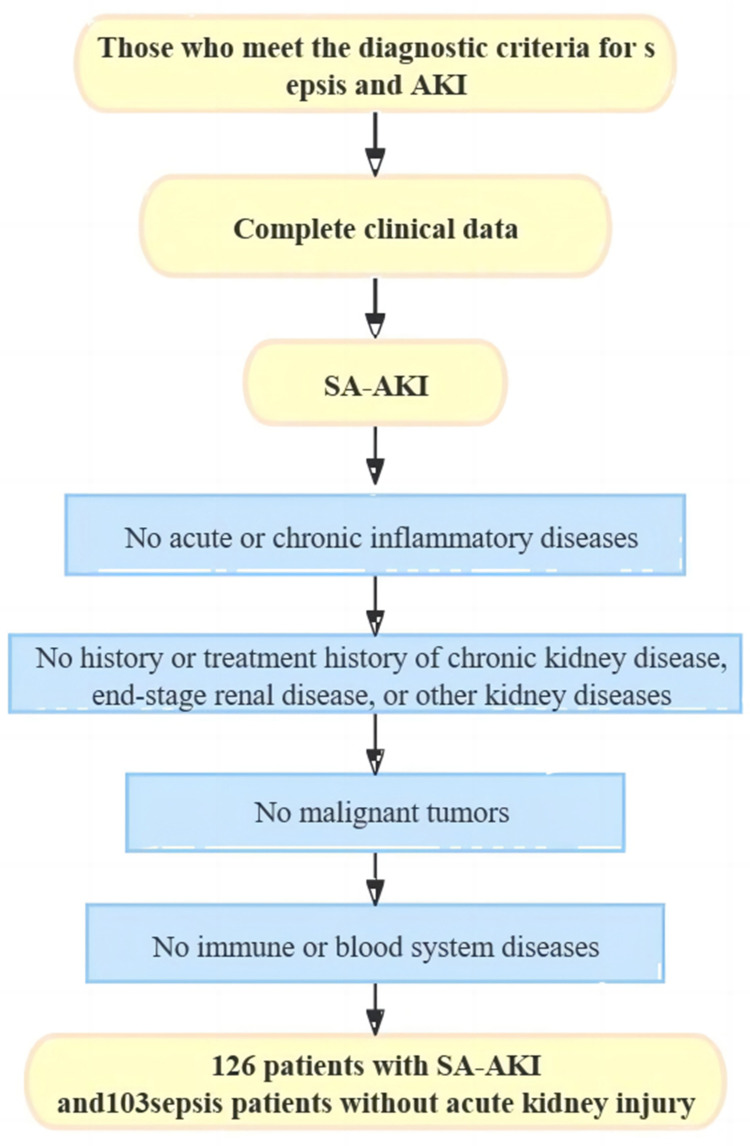
A retrospective selection of 126 SA-AKI patients treated at our hospital between January 2022 and December 2023 was made for the SA-AKI group. The patients were aged between 55 and 75 years, with an average age of 64.79 ± 7.15 years. Inclusion criteria: (1) those who meet the diagnostic criteria for sepsis10 and AKI;11 (2) those with complete clinical data. Exclusion criteria: (1) those with acute or chronic inflammatory diseases such as acute appendicitis, acute enteritis, chronic pancreatitis, and cholecystitis; (2) those with a history of chronic kidney disease, end-stage renal disease, and other kidney diseases and treatments; (3) those with malignant tumors; (4) those with combined heart, lung, or other major organ dysfunction; (5) those with immune system, blood system diseases. Patients in the SA-AKI group were classified into mild (41cases), moderate (53 cases), and severe (32 cases) groups based on the AKI staging criteria.12 Additionally, 103 sepsis patients without acute kidney injury treated in the same period were selected as controls (non-SA-AKI group). The case collection flow chart is shown in Figure 1. All patients and their families provided consent to participate in the study by signing informed consent forms. The study was conducted with the approval of our hospital’s ethics committee.
Figure 1.
Case Collection Process Diagram.
Study Methods
Data Collection
Collected data included age, gender, body mass index (BMI), hypertension, diabetes, and other general information, as well as biochemical indicators such as serum creatinine (SCr) and urea nitrogen (BUN) levels.
Detection of Serum Ficolin-3 and Gal-3 Expression Levels
On the day of admission, 5 mL of fasting venous blood was drawn from all study subjects in the morning. The blood was left to stand for 10 minutes, then centrifuged at 3000 rpm for 10 minutes. The supernatant serum was collected into sterile EP tubes and stored at −80°C for analysis, which was conducted within 24 hours. Serum levels of Ficolin-3 and Galectin-3 (Gal-3) were measured using enzyme-linked immunosorbent assay (ELISA) kits (Ficolin-3 and Gal-3 ELISA kits obtained from Shanghai Enzyme-linked Immunology Company). Serum creatinine (SCr) and blood urea nitrogen (BUN) levels were analyzed using a fully automated biochemical analyzer (purchased from Shanghai Yindu Biological Technology Co., Ltd). All procedures were performed strictly according to the instructions provided with the reagent kits.
Statistical Methods
Data in this article were analyzed using SPSS 25.0 software. Categorical data are presented as n (%), and comparisons between groups were made using the chi-square (χ²) test. Quantitative data are presented as mean ± standard deviation (SD). Comparisons between two groups were conducted using the t-test, while comparisons among multiple groups were further analyzed using the SNK-Q test. The correlation between serum Ficolin-3 and Gal-3 expression levels was analyzed using Pearson’s method. Multifactorial logistic regression analysis was used to identify factors associated with the occurrence of SA-AKI. The diagnostic value of serum Ficolin-3 and Gal-3 for SA-AKI was analyzed using receiver operating characteristic curve (ROC) analysis, with the area under the curve (AUC) comparisons conducted using the Z-test. A P-value of <0.05 was considered statistically significant.
Results
Comparison of General Data Between the Two Groups
There were no statistically significant differences between the two groups in terms of age, gender, BMI, hypertension, diabetes, or coexisting organ dysfunction (P>0.05). However, serum creatinine (SCr) and urea nitrogen (BUN) levels were higher in the SA-AKI group compared to the non-SA-AKI group (P<0.05). See Table 1.
Table 1.
General Information of Two Groups and Serum Ficolin-3 and Gal-3 Expression Levels[( ) /n (%)]
) /n (%)]
| Index | Non SA-AKI group (n=103) | SA-AKIgroup (n=126) | χ2/t | P |
|---|---|---|---|---|
| Age (years) | 65.30±7.30 | 64.79±7.15 | 0.532 | 0.595 |
| Gender | ||||
| Male | 56 (54.36) | 78 (61.90) | 1.326 | 0.250 |
| Female | 47 (45.64) | 48 (38.10) | ||
| BMI (kg/m2) | 23.59±3.03 | 23.72±3.16 | 0.315 | 0.753 |
| Hypertension | 42 (40.78) | 49 (38.89) | 0.084 | 0.771 |
| Diabetes | 28 (27.18) | 29 (23.02) | 0.527 | 0.468 |
| Combined with other organ dysfunction(number) | ||||
| <2 | 82 (79.61) | 91 (72.22) | 1.675 | 0.196 |
| ≥2 | 21 (20.39) | 35 (27.78) | ||
| SCr (μmol/L) | 132.39±16.39 | 147.30±17.22 | 6.661 | <0.001 |
| BUN (μmol/L) | 7.22±1.03 | 4.59±0.81 | 21.628 | <0.001 |
Note: BMI is Body Mass Index; SCr is blood creatinine; BUN stands for urea nitrogen.
Comparison of Serum Ficolin-3 and Gal-3 Expression Levels
The levels of serum Ficolin-3 and Gal-3 in the SA-AKI group were higher than those in the non-SA-AKI group (P<0.05). See Table 2.
Table 2.
Comparison of Serum Ficolin-3 and Gal-3 Expression Levels(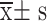 )
)
| Group | Number of cases | Ficolin-3 (mg/L) | Gal-3 (ng/mL) |
|---|---|---|---|
| Non SA-AKI group | 103 | 30.77±4.71 | 4.66±0.69 |
| SA-AKI group | 126 | 39.79±6.00 | 5.97±0.82 |
| T | 12.441 | 12.903 | |
| P | <0.001 | <0.001 |
Note: SA-AKI is sepsis-acute kidney injury; Ficolin-3 is Fibrinogen domain-containing lectin 3; Gal-3 is Galectin-3.
Comparison of Serum Ficolin-3 and Gal-3 Expression Levels Among SA-AKI Patients with Different Degrees of Kidney Injury
The expression levels of serum Ficolin-3 and Gal-3 increased with the severity of kidney injury in SA-AKI patients (P<0.05). See Table 3.
Table 3.
Serum Ficolin-3 and Gal-3 Expression Levels in SA-AKI Patients with Different Degrees of Kidney Injury Severity (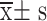 )
)
| Group | Number of cases | Ficolin-3 (mg/L) | Gal-3 (ng/mL) |
|---|---|---|---|
| Mild group | 41 | 36.06±4.97 | 5.32±0.72 |
| Moderate group | 53 | 40.03±6.07* | 6.02±0.83* |
| Severe group | 32 | 44.18±7.22*# | 6.73±0.95*# |
| F | 16.194 | 26.144 | |
| P | <0.001 | <0.001 |
Note: Ficolin-3 is Fibrinogen domain-containing lectin 3; Gal-3 is Galectin-3; Note: Compared with the Mill group, *P<0.05; Compared with the Moderate group, #P<0.05.
Correlation Analysis of Serum Ficolin-3 and Gal-3 Expression Levels
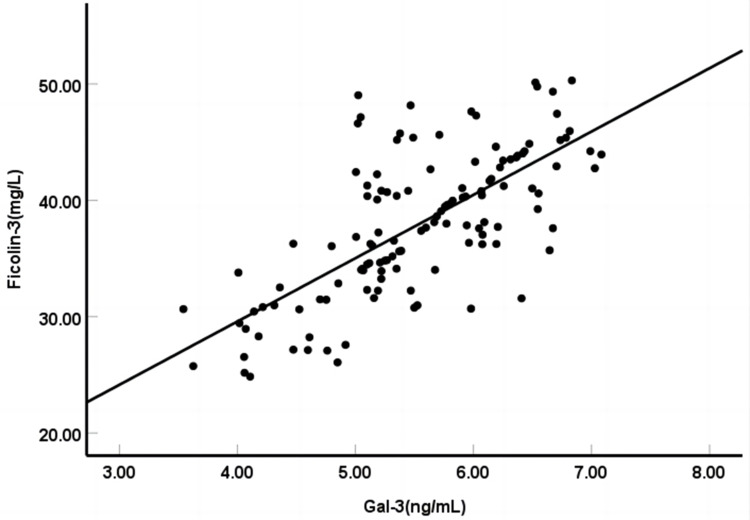
The Pearson correlation analysis showed a positive correlation between the expression levels of serum Ficolin-3 and Gal-3 (r=0.699, P<0.001). See Figure 2.
Figure 2.
Correlation analysis of serum Ficolin-3 and Gal-3 expression levels.
Multifactorial Logistic Regression Analysis of Factors Affecting the Occurrence of SA-AKI
Factors with significant differences (P<0.05) in the general data analysis between the two groups were included in the multifactorial logistic regression analysis. The occurrence of AKI in sepsis patients (yes = 1, no = 0) was used as the dependent variable, with BUN, Ficolin-3, and Gal-3 levels (measured values) as independent variables. Logistic regression analysis revealed that elevated levels of BUN, Ficolin-3, and Gal-3 were significant risk factors for the development of SA-AKI (P < 0.05).See Table 4.
Table 4.
Multivariate Logistic Regression Analysis of the Relevant Factors Affecting the Occurrence of SA-AKI
| Influence factor | B | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| BUN | 0.440 | 0.213 | 4.258 | 0.039 | 1.552 | 1.022~2.356 |
| Ficolin-3 | 1.197 | 0.386 | 9.616 | 0.002 | 3.310 | 1.553~7.053 |
| Gal-3 | 1.026 | 0.344 | 8.896 | 0.003 | 2.790 | 1.422~5.475 |
Note: BUN stands for urea nitrogen; Ficolin-3 is Fibrinogen domain-containing lectin 3; Gal-3 is Galectin-3.
Diagnostic Value Analysis of Serum Ficolin-3 and Gal-3 for SA-AKI
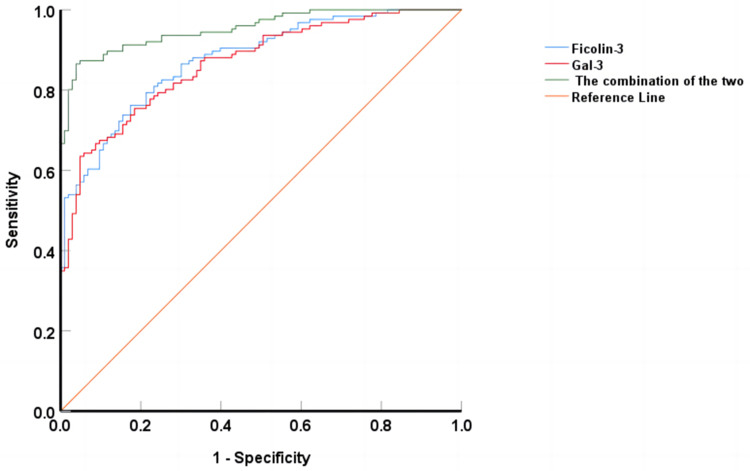
ROC curve analysis of the diagnostic value of serum Ficolin-3 and Gal-3 for SA-AKI showed that the area under the curve (AUC) for diagnosing SA-AKI using serum Ficolin-3 and Gal-3 individually were 0.877 and 0.867, respectively. The combined diagnosis AUC was 0.953, with higher sensitivity, better than individual diagnoses (ZThe combination of the two-Ficolin-3=3.288, ZThe combination of the two-Gal-3=4.434, P<0.001), indicating a certain diagnostic value for the occurrence of SA-AKI. See Figure 3 and Table 5.
Figure 3.
ROC curves of serum Ficolin-3 and Gal-3 for diagnosing SA-AKI.
Table 5.
Analysis of Diagnostic Value of Serum Ficolin-3 and Gal-3 Expression for SA-AKI
| Variable | AUC | Cut off value | 95% CI | Sensitivity (%) | Specificity (%) | Youden index |
|---|---|---|---|---|---|---|
| Ficolin-3 | 0.877 | 35.75 mg/L | 0.827~0.916 | 76.19 | 82.52 | 0.587 |
| Gal-3 | 0.867 | 5.72 ng/mL | 0.816~0.908 | 63.49 | 95.15 | 0.586 |
| The combination of the two | 0.953 | - | 0.917~0.977 | 86.51 | 79.61 | 0.661 |
Note: Ficolin-3 is Fibrinogen domain-containing lectin 3; Gal-3 is Galectin-3.
Discussion
Sepsis is a syndrome resulting from the body’s dysregulated response to infection, leading to immune dysfunction and microcirculatory disturbances, with a high prevalence among the elderly. AKI is a common complication in sepsis patients, marked by a rapid decline or complete loss of renal filtration function. This is mainly due to sepsis damaging vascular endothelial cells, increasing their permeability, and leading to significant proteinuria and renal damage. Epidemiological statistics indicate that nearly 40%–50% of patients with sepsis will develop AKI. The occurrence of SA-AKI not only hinders recovery but also damages other organs, affecting the patient’s life, health, and safety.13,14 Currently, clinical diagnosis and assessment AKI commonly rely on indicators such as serum creatinine (SCr). However, to enhance diagnostic accuracy, many researchers have recently identified specific biomarkers that can aid in clinical diagnosis. For example, Magnusson et al15 found that measuring neutrophil gelatinase-associated lipocalin (NGAL) at the time of patient admission can provide a more accurate diagnosis of early-stage AKI. Therefore, this study aimed to explore indicators that can assist in diagnosing SA-AKI, focusing on the expression levels and clinical significance of serum Ficolin-3 and Gal-3 in SA-AKI patients.
Ficolin-3 is a key recognition molecule in the lectin pathway of the complement system, primarily found in the liver and platelets. It plays a role in regulating cellular proliferation, differentiation, and apoptosis, as well as facilitating the phagocytosis of apoptotic cells by macrophages. Ficolin-3 also influences immune regulation, inflammatory cell infiltration, and the development and malignant progression of inflammatory diseases.16,17 Recent studies have shown that Ficolin-3 can promote the aggregation of inflammatory factors through related signaling pathways, participating in the occurrence and development of diseases such as nephritis and atherosclerosis.18,19 Therefore, it is speculated that the expression level of serum Ficolin-3 might be related to the occurrence of SA-AKI. The results of this study showed that the expression levels of serum Ficolin-3 in the SA-AKI group were higher than in the non-SA-AKI group, and the expression levels of serum Ficolin-3 and Gal-3 increased with the severity of kidney injury. Indicators reflecting renal function, such as SCr and BUN, exhibited a similar trend. This may be attributed to the elevated serum levels of Ficolin-3, which likely enhance inflammatory reactions and tissue damage, thus contributing to the development of SA-AKI. These findings suggest that Ficolin-3 is not only associated with the occurrence of SA-AKI but also that its expression levels correlate with the severity of kidney damage in SA-AKI patients. This indicates that Ficolin-3 could potentially serve as a specific biomarker to aid in the diagnosis of SA-AKI.
Galectin-3 (Gal-3) is a key member of the β-galactoside-binding lectin family. Numerous studies have demonstrated that Gal-3 plays a role in various biological processes, including cell growth and differentiation, and is involved in mediating tissue fibrosis and angiogenesis. Abnormal Gal-3 expression has been observed in patients with conditions such as myocardial infarction and arrhythmias.20,21 Moreover, as one of the receptors for advanced glycation end products, the expression of Gal-3 in the body is also related to the occurrence of various congenital immune responses.22,23 It can regulate diseases such as myocardial ischemia-reperfusion injury by affecting the expression of key immune homeostasis regulatory factors, such as transforming growth factor β1 (TGF-β1) and interleukin-10 (IL-10).24–26Based on this, this study also measured and analyzed the expression of serum Gal-3 in SA-AKI patients, showing that the serum Gal-3 expression level in the SA-AKI group was higher than in the non-SA-AKI group and increased with the severity of kidney injury. An analysis of the correlation between the expression levels of serum Ficolin-3 and Gal-3 showed a positive correlation between them (r=0.699, P<0.001). Further analysis of factors affecting the occurrence of SA-AKI indicated that BUN, Ficolin-3, and Gal-3 are risk factors for SA-AKI. Lastly, ROC curve analysis was used to evaluate the diagnostic value of serum Ficolin-3 and Gal-3 for SA-AKI, showing that the AUCs for diagnosing SA-AKI with serum Ficolin-3 and Gal-3 were 0.877 and 0.867, respectively. There is notable diagnostic value for SA-AKI, and recent studies indicate that the combined detection of biomarkers has significant diagnostic potential in assessing acute kidney injury in patients with ST-segment elevation myocardial infarction.27 Therefore, this study also evaluated the diagnostic value of combining serum Ficolin-3 and Gal-3 for SA-AKI. The results showed that the AUC for the combined diagnosis was 0.953, demonstrating superior accuracy compared to the individual biomarkers.
In conclusion, serum Ficolin-3 and Gal-3 levels are closely associated with the onset and progression of SA-AKI. The combined diagnostic approach offers greater accuracy compared to individual markers, providing valuable diagnostic insight for SA-AKI. However, the limited sample size in this study may introduce some bias into the statistical results. In the future, we plan to gather a broader range of cases to further validate the clinical applicability of our findings. Additionally, we will explore the predictive value of serum Ficolin-3 and Gal-3 for the prognosis of AKI patients and investigate the specific mechanisms through which these biomarkers influence the development of SA-AKI.
Data Sharing Statement
The original contributions presented in the study are included in the article.
Ethics Statement
This study was approved by the Ethics Committee of Ganzhou People’s Hospital and conducted in accordance with the 1964 helsinki Declaration. Informed consent was obtained from the guardian of each subject.
Disclosure
All author(s) declares that they have no Conflict of interest.
References
- 1.C FAN, DING X, SONG Y. A new prediction model for acute kidney injury in patients with sepsis [J]. Ann Palliat Med. 2021;10(2):1772–1778. doi: 10.21037/apm-20-1117 [DOI] [PubMed] [Google Scholar]
- 2.J LIU, H XIE, YE Z, et al. Rates, predictors, and mortality of sepsis-associated acute kidney injury: a systematic review and meta-analysis [J]. BMC Nephrol. 2020;21(1):318. doi: 10.1186/s12882-020-01974-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.JIANG N, HUANG R, ZHANG J, et al. TIMP2 mediates endoplasmic reticulum stress contributing to sepsis-induced acute kidney injury [J]. FASEB J. 2022;36(4):e22228. doi: 10.1096/fj.202101555RR [DOI] [PubMed] [Google Scholar]
- 4.GUIJARRO-BELMAR A, M DOMANSKID, BO X, et al. The therapeutic potential of targeting exchange protein directly activated by cyclic adenosine 3’,5’-monophosphate (Epac) for central nervous system trauma [J]. Neural Regen Res. 2021;16(3):460–469. doi: 10.4103/1673-5374.293256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.S MATZENJ, L KROGHC, FORMAN JL, et al. Lectin complement pathway initiators after subarachnoid hemorrhage - an observational study [J]. J Neuroinflammation. 2020;17(1):338. doi: 10.1186/s12974-020-01979-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Y TERESHCHENKOS, V SMOLNIKOVAM, B FREIDINM. Ficolin-3 and MASP-2 gene variants in Siberian Arctic populations: summarized evidence of selective pressure for the high frequency of lectin complement pathway deficiency [J]. Scand J Immunol. 2023;97(3):e13249. doi: 10.1111/sji.13249 [DOI] [PubMed] [Google Scholar]
- 7.M SREJOVICI, L LUKICM. Galectin-3 in T cell-mediated immunopathology and autoimmunity [J]. Immunol Lett. 2021;233:57–67. doi: 10.1016/j.imlet.2021.03.009 [DOI] [PubMed] [Google Scholar]
- 8.HUTTIN O, MANDRY D, POPOVIC B, et al. Plasma Galectin-3 predicts deleterious vascular dysfunction affecting post-myocardial infarction patients: an explanatory study [J]. PLoS One. 2020;15(5):e0232572. doi: 10.1371/journal.pone.0232572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LI L-C, LI J, GAO J. Functions of galectin-3 and its role in fibrotic diseases [J]. J Pharmacol Exp Ther. 2014;351(2):336–343. doi: 10.1124/jpet.114.218370 [DOI] [PubMed] [Google Scholar]
- 10.SINGER M, S DEUTSCHMANC, W SEYMOURC, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [J]. JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury [J]. Nephron Clin Pract. 2012;120(4):c179–c84. doi: 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 12.V JHA, ARICI M, J COLLINSA, et al. Understanding kidney care needs and implementation strategies in low- and middle-income countries: conclusions from a “Kidney Disease: improving Global Outcomes” (KDIGO) Controversies Conference [J]. Kidney Int. 2016, 90(6): 1164–1174. [DOI] [PubMed] [Google Scholar]
- 13.S GS, L DEIRALORENZOJ, J MBERMEJOM, et al. Survival and renal recovery after acute kidney injury requiring dialysis outside of intensive care units [J]. Int Urol Nephrol. 2020;52(12):2367–2377. doi: 10.1007/s11255-020-02555-2 [DOI] [PubMed] [Google Scholar]
- 14.XU J, RUAN M, WU J, et al. The Role of Renal Pathology in the Prognosis and Recovery of Community-Acquired Acute Kidney Injury [J]. Nephron. 2021;145(4):353–362. doi: 10.1159/000514287 [DOI] [PubMed] [Google Scholar]
- 15.E MAGNUSSONN, FRYDMAN S, FREUND O, et al. Early neutrophil gelatinase-associated lipocalin (NGAL) measurement could rule out future acute kidney injury in patients with acute coronary syndrome-Prospective observational study [J]. Health Sci Rep. 2024;7(7):e2229. doi: 10.1002/hsr2.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkoumi MA, EMAM AA, Allah MAN, et al. Association of ficolin-2 gene polymorphisms and susceptibility to systemic lupus erythematosus in Egyptian children and adolescents: a multicenter study. Lupus. 2019;28(8):995–1002. doi: 10.1177/0961203319856089 [DOI] [PubMed] [Google Scholar]
- 17.DADFAR E, FURUHJELM C, NILSSON J, et al. Fatal pneumococcus meningitis in a child with complement factor ficolin-3 deficiency [J]. J Allergy Clin Immunol Pract. 2020;8(2):778–779. doi: 10.1016/j.jaip.2019.07.039 [DOI] [PubMed] [Google Scholar]
- 18.H GOMAAM, G KHIDRE, ELSHAFEI A, et al. The clinical value of ficolin-3 gene polymorphism in rheumatic heart disease An Egyptian Adolescents Study [J]. BMC Res Notes. 2021;14(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WANG W, D CAI. Complement Components sC5b-9 and CH50 Predict Prognosis in Heart Failure Patients Combined With Hypertension [J]. Am J Hypertens. 2020;33(1):53–60. doi: 10.1093/ajh/hpz140 [DOI] [PubMed] [Google Scholar]
- 20.SEVERINO P, D’amato A, PUCCI M, et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: from Plaque Activation to Microvascular Dysfunction [J]. Int J Mol Sci. 2020;21(21):8118. doi: 10.3390/ijms21218118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Z GAO, LIU Z, WANG R, et al. Galectin-3 Is a Potential Mediator for Atherosclerosis [J]. J Immunol Res. 2020;2020:5284728. doi: 10.1155/2020/5284728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ERDOGAN O, KARAAYVAZ E, ERDOGAN T, et al. A new biomarker that predicts ventricular arrhythmia in patients with ischemic dilated cardiomyopathy: galectin-3. Rev Port Cardiol. 2021;40(11):829–835. doi: 10.1016/j.repc.2020.12.013 [DOI] [PubMed] [Google Scholar]
- 23.Sayed A, MUNIR M, S NABETM, et al. Galectin-3: a Novel Marker for the Prediction of Stroke Incidence and Clinical Prognosis [J]. Mediators Inflamm. 2022;2022:2924773. doi: 10.1155/2022/2924773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.YANG Z, ZINGARELLI B, Szabó C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury [J]. Circulation. 2000;101(9):1019–1026. doi: 10.1161/01.CIR.101.9.1019 [DOI] [PubMed] [Google Scholar]
- 25.A MOHAMMEDD, Mabrouk S, E NAGGARMGM, et al. Interleukin-10: a Potential Prognostic Marker in Patients with Newly Diagnosed Multiple Myeloma [J]. Res Oncol. 2021. [Google Scholar]
- 26.R ABDELHAMMEDM, A AHMEDY, N ADAME, et al. sVCAM-1, and TGFβ1 in chronic phase, chronic myeloid leukemia patients treated with tyrosine kinase inhibitors [J]. Egypt J Immunol. 2022;29(4):163–173. doi: 10.55133/eji.290416 [DOI] [PubMed] [Google Scholar]
- 27.FRYDMAN S, FREUND O, A KATASHH, et al. Combined biomarker testing for the assessment of acute kidney injury among ST-segment elevation myocardial infarction patients [J]. J Nephrol. 2024. doi: 10.1007/s40620-024-02036-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article.