ABSTRACT
Background
Patients with cardiovascular disease (CVD), diabetes mellitus (DM) and chronic kidney disease (CKD) often experience fragmented care, which negatively impacts outcomes and health-related quality of life (HRQoL). This study assessed whether multidisciplinary, person-centred care at an integrated clinic improves clinical outcomes and HRQoL.
Methods
This prospective, open, blinded-endpoint trial (CareHND; NCT03362983) included 131 patients with CVD, DM and CKD stages 3–4, most of whom were enrolled during or shortly after acute hospitalization. The intervention group received person-centred care from cardiologists, nephrologists, endocrinologists and specialist nurses at an integrated clinic; the control group received traditional care from separate specialists. Primary disease progression outcome was the composite of major adverse renal and cardiovascular events (MARCE) including death, heart failure (HF) readmission, myocardial infarction, percutaneous coronary intervention/coronary artery bypass graft, acute or end-stage kidney failure, or transient ischaemic attack/stroke at 2 years. Co-primary person-centred outcomes was self-reported HRQoL by RAND-36.
Results
In a pre-specified interim analysis, patients randomized to integrated care had lower estimated glomerular filtration rate and higher NT-proBNP (N-terminal pro brain natriuretic peptide) than traditional care. Follow-up ranged from 2.0 to 5.7 years. Kaplan–Meier analysis showed no difference in MARCE between groups. Cox-regression adjusting for baseline differences, indicated a trend towards reduced HF hospitalizations for integrated care (hazard ratio 0.53; confidence interval 0.28–1.01; P = .054). Integrated care improved role physical and social function scores, and self-rated health (P = .021, P = .019 and P = .011, respectively).
Conclusions
Integrated care improved several dimensions of HRQoL but did not improve MARCE compared with traditional care in this small trial. We observed a trend towards reduced HF hospitalizations. Overall, integrated care presents a promising alternative.
Keywords: heart failure, kidney failure, multimorbidity, patient-centered, person-centered
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Patients with concurrent cardiovascular disease (CVD), chronic kidney disease (CKD) and diabetes mellitus (DM) often receive fragmented care from multiple specialists, potentially negatively impacting their health outcomes and quality of life.
Previous studies suggested that multidisciplinary team care could improve outcomes for patients with heart failure and CKD but there is limited evidence for patients with multimorbidity involving CVD, CKD and DM.
There was a need to assess whether an integrated, person-centred care model could be feasible and offer benefits over traditional, separate specialist care for this complex patient group.
This study adds:
Recently hospitalized patients with concurrent CVD, CKD and DM are at extremely high risk for death or new major adverse renal or cardiovascular events (MARCE), and have low health-related quality of life (HRQoL).
Integrated person-centred care improves several dimensions of HRQoL in patients with concurrent CVD, CKD and DM.
While integrated care did not significantly improve MARCE in this small trial, it showed a trend towards reduced heart failure hospitalizations.
Potential impact:
The findings highlight the importance of integrated, person-centred care models for patients with multimorbid conditions, which could improve their overall quality of life.
Healthcare providers may consider implementing integrated care clinics to provide more cohesive and effective treatment for patients with complex health needs.
INTRODUCTION
Common and shared risk factors contribute to the development and progression of cardiovascular disease (CVD), chronic kidney disease (CKD) and diabetes mellitus (DM) within the cardiovascular, renal and metabolic (CaReMe) disease continuum [1–4]. The most important modifiable risk factors for these include sedentary lifestyle, obesity, hypertension, dyslipidaemia and smoking [1–3], and a multifactorial intervention in these patients has large effects on outcome [5]. Still, age remains a major risk factor, and with an ageing population these comorbidities will escalate [6, 7]. CaReMe diseases are associated with adverse clinical outcomes and poses significant challenges for patients and healthcare providers globally [3]. The intricate nature of these comorbidities necessitates a multidisciplinary approach [4]. At the same time, the healthcare system is locked in a traditional single-disease specialization [4, 6, 7].
It was recognized early that multidisciplinary team care improves patients’ quality of life and outcomes, and reduces hospitalizations for patients with heart failure [8]. The evidence for multidisciplinary care is limited but promising for patients with CKD [9], and in patients with type 2 DM [10]. The definition of ‘multidisciplinary’ vary substantially between studies, and is commonly limited to involve different healthcare professionals, however still within their separate specialties. Ideally, a CaReMe team would include nurses and physician specialized in cardiology, endocrinology, nephrology and primary care, and associated healthcare practitioners including pharmacologists, physiotherapists and nutritionists [4]. There are a limited number of studies on such truly integrated clinics. The first in the CaReMe area is the Integrated Care Clinic at St Paul's Hospital in Vancouver, demonstrating feasibility in a small, randomized trial [11]. While professional associations highlight the importance of an integrated care practice, both from the medical and from the management perspective [4, 6], they also acknowledge a substantial need for further trials.
While integrated care appears attractive from a care-provider perspective, it is also important to acknowledge that CaReMe patients often experience low health-related quality of life (HRQoL) [12, 13], despite receiving abundant care from many providers [14]. Replacing established patient–care provider relationships needs time to develop [15], and requires trust and focus on the patient's needs, abilities and desires [16]. The philosophy of care built around the needs and tailored to the individual patient is commonly termed person-centred care. Person-centred care is described as a partnership between the patient, their relatives and the healthcare professionals, where the decision-making is done together. Person-centred care has been shown to have a positive impact on HRQoL in many diseases, including heart failure [16–18].
An integrated care clinic for the multimorbid patient group with combined CVD, CKD and DM using a person-centred approach appears attractive from several aspects, with potential to improve HRQoL and delay disease progression. We aimed to assess this in a pragmatic, prospective randomized trial.
MATERIALS AND METHODS
This trial was conducted at the heart–nephrology–diabetes (HND) Centre, a multidisciplinary outpatient clinic at Danderyd University Hospital, affiliated with Karolinska Institutet. Established in 2013, the HND Centre manages patients with concomitant CVD, CKD and DM, providing integrated, person-centred care for CaReMe comorbidities [7]. All staff received training in person-centred care upon the clinic's initiation.
The standard protocol begins with an initial consultation with a specialist nurse, followed by evaluation by a physician specialized in cardiology, nephrology or endocrinology. Patients are reviewed as needed at bi-weekly interdisciplinary conferences to develop comprehensive treatment plans. Urgent medical changes are made, and the treatment plan is refined during follow-up visits, typically scheduled 4–6 weeks later. Follow-up care, often managed by specialist nurses, is customized and frequently conducted via telephone, and includes physiotherapists and dieticians as necessary [7].
Patients with less complex conditions typically undergo this intervention for 6–12 months before transitioning to primary care or a specialized outpatient clinic. Those with more advanced diseases continue at the HND Centre until they require dialysis or nursing home placement, or in some cases, until death.
In this prospective randomized open blinded endpoint (PROBE) trial (CareHND; NCT03362983), patients were randomized to integrated care (HND) or traditional care at separate specialist clinics (control). Inclusion criteria included established CVD, CKD, and type 1 or 2 DM. Exclusion criteria included symptomatic dementia, uncontrolled cancer, severe pulmonary disease and significant substance abuse. A short expected lifespan was not an exclusion criterion. In the planning phase of the study, patient participants showed a strong preference for integrated care, and advised to include the possibility to cross-over from standard care to intervention after 1 year.
The primary endpoint for assessing disease progression [major adverse renal or cardiovascular events (MARCE)] was the first occurrence of readmission due to heart failure, myocardial infarction, percutaneous coronary intervention or coronary artery bypass grafting, end-stage kidney disease, acute kidney failure, transient ischaemic attack/stroke, or death within 2 years. The study size was based on a power calculation with a large effect size seen in the STENO-2 trial [hazard ratio (HR) 1.5] [5], with a two-sided significance level of 0.05 and statistical power of 0.80, yielding 260 patients and 2 years follow-up. An interim analysis was planned at 130 patients included. All MARCE outcomes were assessed from printed medical records, blinded to the intervention, separate by two senior medical students following a protocol, and in the few cases not in agreement adjudicated by one senior specialist.
The co-primary person-centred outcome measures were perceived quality of care and patient empowerment assessed by HRQoL questionnaires collected at baseline, 6 and 12 months. Statistical power was calculated for 131 patients at 1 year. The patient-reported outcome measure (PROM) instruments used were RAND-36 and EuroQoL group's EQ-5D-3L (Swedish version). The patient-reported experience measures (PREM) consisted of six questions based on previous research [19]. The RAND-36 comprises 36 questions in eight dimensions: physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), general health (GH), vitality/energy/fatigue (VT), social functioning (SF), mental health/emotional well-being (MH) and role limitations due to mental/emotional problems (RE). One standalone question concern changes in perceived health over the last year [20, 21]. The EQ-5D-3L consist of five questions on mobility, self-care, usual activities, pain/discomfort and anxiety/depression, and one visual analogue scale (VAS). For each dimension, the respondent can select ‘no problems’, ‘some problems’ or ‘extreme problems’ [22]. For the VAS, the respondents score their perceived current state of health from 0 to 100 [23].
Statistical analysis
Variables are reported as mean values with standard deviation, median values with interquartile range or proportions. Differences in baseline characteristics were assessed by Student's t-test for continuous variables and Chi2 test for categorical variables. Skewed variables were log-transformed to improve normality.
MARCE was analysed as intention to treat, using the Kaplan–Meier survival function. The proportional hazards assumptions were assessed by visual inspection of the curves. Outcome was also studied in an explorative analysis by adjusted Cox-regression models.
For the person-centred outcomes, the 36 questions in RAND-36 were grouped into eight dimensions, where each dimension generates a weighted sum score, as stipulated [20]. A lower sum indicates more disabilities and a sum of 100 indicates no disabilities. Each dimension was analysed separately. Analysis of variance (ANOVA) with repeated measurement was used to assess differences over time between groups. For the EQ-5D-3L questions, Chi2 test and a general estimating equation were used to assess differences over time between groups. The VAS scale was analysed by repeated measurement ANOVA with Tukey's post-hoc test.
For the PREM questionnaire, Student's t-test was used. All analyses were performed using IBM SPSS Statistic for Windows, Version 27.0.
RESULTS
The first patient entered the study in March 2015, and the last finished the 2-year follow-up in October 2021. At the interim analysis of MARCE, and the final analysis of the patient centred outcomes, 131 patients were included in the study. Due to the small effect size for the primary outcome, it was decided to halt the trial.
Of the 131 included patients, 73 (56%) were randomized to HND, and 58 (44%) to the control group. Randomization by ballots in sealed envelopes, not in blocks, yielded an uneven distribution for the interim analysis (Table 1).
Table 1:
Patient characteristics.
| Intervention (HND) | Control | |
|---|---|---|
| Included patients, n | 73 | 58 |
| Received intervention, n | 71 | |
| Sex (female), % (n) | 24% (17) | 25% (14) |
| Age, years | 73.4 ± 7.8 | 74.3 ± 7.1 |
| BMI, kg/m2 | 29.90 ± 4.3 | 30.1 ± 6.7 |
| Waist, cm | 108.4 ± 13 | 108.3 ± 17 |
| DM type 1, % (n) | 7.1% (5) | 5.3% (3) |
| DM type 2, % (n) | 93% (66) | 95% (54) |
| Previous stroke, % (n) | 13% (9) | 16% (9) |
| Previous ACS, % (n) | 48% (34) | 54% (31) |
| COPD, % (n) | 20% (14) | 8.8% (5) |
| Atrial fibrillation, % (n) | 58% (41) | 53% (30) |
| Heart failure, % (n) | 73% (52) | 58% (33)a |
| Preserved ejection fraction | 44% (31) | 35% (20) |
| Reduced ejection fraction | 30% (21) | 21% (12) |
| Pacemaker, % (n) | 18% (13) | 23% (13) |
| Current smoking, % (n) | 9.9% (7) | 5.3% (3) |
| Previous smoking, % (n) | 68% (48) | 58% (33) |
| Systolic blood pressure, mmHg | 133.6 ± 20.6 | 137.9 ± 21.3 |
| Diastolic blood pressure, mmHg | 68.8 ± 10.3 | 74.9 ± 13.8 |
| Haemoglobin, g/L | 125.8 ± 22.3 | 128.9 ± 21.1 |
| CRP, mg/L | 3.10 (1.65–9.10) | 2.90 (0.88–5.85) |
| eGFR, mL/min/m2 | 41.6 ± 13.3 | 51.0 ± 17.1b |
| eGFR <30 mL/min/m2 | 17% (12) | 12% (7) |
| Urinary albumin/creatinine, mg/mmol | 7.2 (1.7–29.6) | 5.8 (1.1–15.6) |
| HbA1C (IFCC), mmol/mol | 60.0 ± 13.6 | 59.3 ± 13.3 |
| Cholesterol, mmol/L | 3.57 ± 1.04 | 3.97 ± 1.33 |
| Triglycerides, mmol/L | 1.58 ± 1.00 | 1.83 ± 1.05 |
| LDL, mmol/L | 1.83 ± 0.79 | 2.09 ± 0.97 |
| Albumin, g/L | 34.3 ± 4.20 | 33.5 ± 4.05 |
| Phosphate, mmol/L | 1.14 ± 0.24 | 1.00 ± 0.21 |
| PTH, pmol/L | 8.14 ± 3.59 | 5.73 ± 2.71 |
| Urea, mmol/L | 13.4 ± 7.08 | 9.40 ± 3.18 |
| Uric acid, µmol/L | 479.7 ± 126 | 438.8 ± 122 |
| NT-proBNP, ng/L | 1760 (889–3923) | 971 (261–2535)b |
Mean values ± standard deviation, median (interquartile range) or % (n).
P = .09.
P < .01.
BMI, body mass index; ACS, acute coronary syndrome; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; HbA1C, glycated haemoglobin A1C; IFCC, International Federation of Clinical Chemistry and Laboratory Medicine; LDL, low-density lipoprotein; PTH, parathyroid hormone.
In the HND arm, one patient died prior to the first visit at the HND-centre, and another was admitted for decompensated heart failure at the first visit. These outcomes were counted as intention to treat (Table 2). Two patients withdrew consent shortly after randomization, one in each arm, and were not included in the analyses (Supplementary data, Fig. S2). All but 14 patients were recruited during or shortly after an acute hospitalization or an urgent visit to the heart failure day-care ward. All patients had advanced multimorbidity, and 43 patients (33%) died during the follow-up time (median 2; range 2.0–5.7) years. Of the patients randomized to standard care, 28 elected to cross-over to HND at 1 year. Major changes in medical therapies were noted during the trial, i.e. introduction of sodium–glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP1-RA) in standard care (Table 3).
Table 2:
Outcome events analysed per intention to treat.
| HND 2 years (n = 72) | Control 2 years (n = 57) | P-valueb | HND EOS | Control EOS | |
|---|---|---|---|---|---|
| Crossed over at 1 year, % (n) | 51% (28) | ||||
| Longest follow-up (year) | 5.57 | 5.74 | |||
| Censored at end of follow-up | 38% (27) | 35% (20) | |||
| Patient years in study | 140 | 118 | |||
| First MARCE, % (n) | 46% (33) | 44% (25) | .57 | 61% (44) | 65% (37) |
| Median time to MARCE (days) | 730 | 758 | |||
| Total death, % (n) | 21% (15) | 14% (8) | .29 | 40% (29) | 25% (14) |
| Cardiovascular death, % (n) | 8% (6) | 9% (5) | .95 | 19% (14) | 16% (9) |
| New hospitalization for HF, % (n) | 36% (26) | 19% (11) | .054 | 49% (35) | 33% (19) |
| New AMI, % (n) | 11% (8) | 9% (5) | .69 | 14% (10) | 16% (9) |
| New PCI/CABG, % (n) | 1.4% (1) | 1.7% (1) | .87 | 7% (5) | 11% (6) |
| ESRD, % (n) | 1.4% (1) | 1.7% (1) | .88 | 3% (2) | 2% (1) |
| First new AKIa, % (n) | 17% (12) | 9% (5) | .041 | 18% (13) | 12% (7) |
Increase in S-creatinine ≥50% within 7 days odds ratio ≥26.5 μmol/L within 48 h.
Unadjusted Kaplan–Meier, log-rank test.
EOS, end of study; AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; ESRD, end-stage renal disease; AKI, acute kidney injury.
Table 3:
Medical treatment at baseline and at 1 year.
| Intervention (HND) baseline (n = 71) | Control baseline (n = 57) | P-value | Intervention (HND) 1 year (n = 64) | Control 1 year (n = 53) | P-value | |
|---|---|---|---|---|---|---|
| SU, % (n) | 17% (12) | 12% (7) | .62 | 8% (5) | 9% (5) | .75 |
| Metformin, % (n) | 32% (23) | 42% (24) | .27 | 51% (18) | 49% (17) | .69 |
| DPP4i, % (n) | 21% (15) | 11% (6) | .15 | 23% (15) | 17% (9) | .49 |
| GLP-1-RA, % (n) | 7% (5) | 18% (10) | .10 | 22% (14) | 17% (9) | .64 |
| SGLT-2i, % (n) | 3% (2) | 2% (1) | 1.0 | 9% (6) | 6% (3) | .51 |
| Insulin, % (n) | 63% (44) | 61% (35) | 1.0 | 59% (38) | 47% (34) | .70 |
| Statins, % (n) | 92% (65) | 75% (43) | .02 | 95% (61) | 76% (40) | .002 |
| Ezetimibe, % (n) | 6% (4) | 9% (5) | .51 | 7% (5) | 14% (8) | .24 |
P for Chi2.
SU, sulphonylureas; DPP4i, dipeptidyl peptidase-4 inhibitors.
Visual inspection of the data indicated that the randomization failed to create equal groups, and this was tested for those variables. HND patients had worse kidney function (P = .001), more secondary metabolic disturbances and higher N-terminal pro b-type natriuretic peptide (NT-proBNP) (P = .008), and tended to have more heart failure (P = .090).
Major adverse renal and cardiovascular events
Survival analysis (Kaplan–Meier) for the combined endpoint (MARCE) by intention to treat, showed no differences between the groups (Fig. 1), either at the pre-specified 2-year analysis (P = .57), or at the end of follow-up (P = .99). Explorative analyses adjusting for the significant differences in estimated glomerular filtration rate (eGFR) and NT-proBNP at baseline (factors known to be associated with worse outcome) showed that HND was associated with a similar risk [HR 0.89; confidence interval (CI) 0.56–1.41; P = .61]. Similar results were found when censoring patients that crossed-over, and when restricting the follow-up time to 2 years (HR 0.99; CI 0.59–1.68). The total risk of death was similar in the HND group at 2 years (P = .29), but higher at end of study (P = .045), however non-significant (HR 0.63; CI 0.32–1.22; P = .17) when adjusting for baseline eGFR and NT-proBNP.
Figure 1:
Kaplan–Meier survival curve analysed per intention to treat.
The disease progression outcome was driven mainly by hospitalizations for heart failure. When adjusting for baseline eGFR and NT-proBNP, there was a trend towards lower risk for heart failure hospitalizations in the HND group (HR 0.53; CI 0.279–1.01; P = .054).
An exploratory subgroup analysis of the main outcome stratified by baseline characteristics known to be associated with outcome, suggest that patients with heart failure (65% of all) had reduced risk from the intervention (HR 0.55; CI 0.33–0.91; P = .016 for interaction) (Fig. 2).
Figure 2:
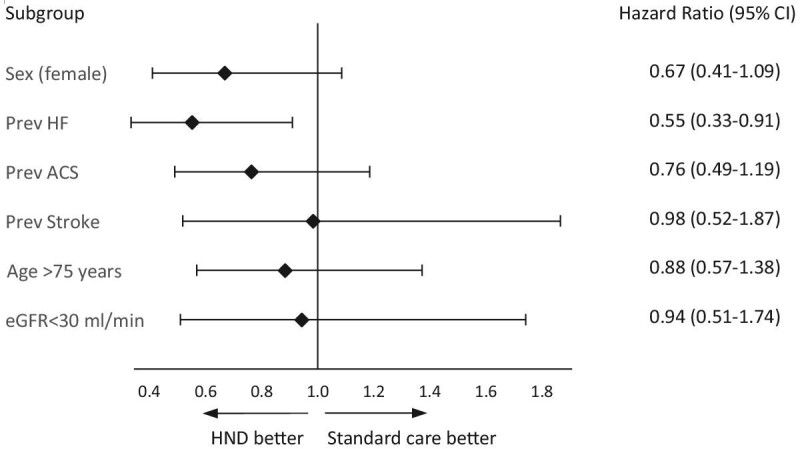
Main outcome MARCE according to subgroup.
Health-related quality of life
For the main person-centred outcome (HRQoL), 128 questionnaires were administered at baseline, of which 113 (86%) were filled. At baseline, in the HND-group (n = 73) there were 7 patients not responding and in the control group (n = 58) there were 10 patients not responding. The RAND-36 results showed that HND patients improved in the sum scores for RP, SF and GH over time, and compared with controls significant improvements were seen for RP and SF (P = .021 and P = .019, respectively) (Fig. 3). The EQ-5D-3L results showed that HND patients improved numerically in Pain and Anxiety over time but not significantly compared with the control group. For VAS, the HND group improved over time (P = .007) and improved, as compared with the control group (P = .011) (Fig. 4). The PREM questions showed that HND patients reported higher satisfaction with given verbal information and staff accessibility (Supplementary data, Fig. S2).
Figure 3:
Patient-reported HRQoL outcomes assessed by RAND-36 at baseline, at 6 months and at 12 months in standard care (control) and integrated care (HND).
Figure 4:
Patient-reported HRQoL outcomes assessed by EQ-5D-3L and VAS at baseline, at 6 months and at 12 months in standard care (control) and integrated care (HND).
DISCUSSION
This randomized controlled trial assessed the impact of an integrated, multi-specialty care model for patients recently hospitalized with concurrent CVD, CKD and DM, compared with traditional care provided at separate specialist clinics in a tertiary teaching hospital. One major finding was that integrated care improved several dimensions of HRQoL. Integrated care did not show a significant difference from traditional care in traditional measures of disease progression in this small trial. This notwithstanding, exploratory analysis suggests that integrated care may reduce hospitalizations in patients with heart failure.
Disease progression and death
We found no significant differences between integrated care and traditional care regarding disease progression or death. This finding suggests that, in terms of MARCE, integrated care was not superior to traditional care models. Our finding aligns with those by Weber et al., on 150 patients attending a kidney care clinic and at least one other specialty clinic (diabetology or cardiology) who were randomized to a combined clinic or standard care [11]. Their primary outcome was hospitalization rate, which did not differ between groups. They did, however, show that an integrated clinic is feasible and attractive in terms of cost savings [11]. Their patient population differed in that they were required to have kidney failure and attending one other clinic, while our cohort was required to have all three disease conditions combined. In addition, most patients in our trial were recruited after an acute hospitalization and were not stable out-patients. This is reflected in the lower mortality (13% after 25 months) in that trial [11], compared with ours (16% after 25 months).
The randomization failed to generate equal groups in the current study, and the intervention group had worse kidney function and higher levels of NT-proBNP, which would likely have influenced these outcomes. Adjusting for these variables did not, however, significantly alter the results. The main outcome was driven by hospitalizations for heart failure, and with adjustment for eGFR and NT-proBNP, a strong trend (P = .054) was seen towards lower risk for heart failure hospitalizations. In recent trials on patients with or without type 2 DM and CKD, the composite outcomes have been driven by heart failure hospitalizations [24]. In the present trial, 85 patients (65%) had a diagnosis of heart failure, and recurrent hospitalizations and mortality are known to be exceedingly high in these patients [25]. Of note, in an exploratory subgroup analysis stratified for heart failure, we observed a potential benefit for heart failure hospitalizations. This finding aligns with previous research suggesting that heart failure patients benefit from a multidisciplinary approach [8]. Easy access to an integrated clinic may ensure correct treatment quicker when experiencing worsening symptoms, preventing worsening decompensation [26]. Patients at the HND centre did indeed report a higher degree of satisfaction with ease of access to staff (Supplementary data, Fig. S2). Additionally, the person-centred approach did improve HRQoL dimensions, which indicate that the patients were more in control of their own disease.
Both SGLT2i and GLP1-RA have revolutionized the treatment of type 2 DM by preventing cardiovascular outcomes and retarding CKD progression [27, 28]. For SGLT2i these effects have been established also in CKD and heart failure [28], and for GLP1-RA also in people with overweight without type 2 DM [29], and in patients with type 2 DM and CKD [30]. However, when the CareHND trial started, in contrast to the situation today, guidelines in Sweden recommended discontinuation of SGLT2i at eGFR <60 mL/min/1.73 m2, and of GLP1-RA at eGFR <30 mL/min/1.73 m2. Accordingly, only a minority were offered these drugs. We can only speculate on the possible effects of these changes in drug use on the outcomes in an integrated clinical setting.
Another challenge during this trial was the coronavirus disease 2019 (COVID-19) pandemic, starting at the interim analysis, which might have influenced the results [31]. These circumstances and the non-significant results for MARCE resulted in the decision to halt the trial. However, the advanced disease and high instability in this patient population, often makes both patient and care provider to prioritize improvements in quality of life and symptom management over MARCE [15, 26], which leads to our patient-centred outcomes.
Health-related quality of life
The integrated care intervention demonstrated improvements in two dimensions of perceived HRQoL (RP and SF) and in self-rated health (as measured by the VAS) at the 6-month follow-up. For this patient population with an advanced stage of multimorbidity, these improvements are particularly meaningful as they directly address daily challenges with significant impact on quality of life. Enhancing physical and social functioning not only helps patients maintain independence and engage in valued activities but also reflects a holistic approach that aligns with their priorities. Furthermore, we observed our patients to report very low HRQoL in all dimensions examined, notably lower than heart failure patients with only one comorbidity [32], and similar to older cancer survivors [33]. Patient perspective and involvement are crucial in all medical treatments, especially so in managing debilitating chronic illnesses, necessitating a tailored approach to each patient's situation, capabilities, resources and needs [34]. The first months of the intervention were therefore important with focus on aligning the care with the patients’ situation, abilities, resources and needs [6, 7]. Most patients experienced major changes in medications and lifestyle interventions within the first 3 months, supported by team visits, nurse consultations, telephone contacts, dietary advice and physiotherapy as required. Potential improvements were anticipated between baseline and the 6-month follow-up, consistent with our findings. The 12-month data indicate that these changes were sustained.
In addition to self-rated health, improvements were observed in RP, which assesses limitations in roles including work and daily activities through four questions, and SF, which evaluates limitations in social activities such as visiting relatives and friends or participating in social events, through two questions. Optimized medical treatment for heart failure has been shown to enhance several HRQoL dimensions, including physical limitations [35]. Moreover, smaller studies in heart failure have suggested that RP can be improved through exercise training [36]. Additionally, in patients with type 2 DM on insulin or sulfonylureas, fear of hypoglycaemia has been related to several HRQoL dimensions, including RP [37]. Therefore, potential mediators of improvement include optimized treatment for heart failure, increased physical activity and improved glucose control.
Limitations
The study was designed to include 260 patients to adequately assess the primary MARCE outcome and 131 for the HRQoL outcome. The interim analysis was conducted with only 131 patients, which limits the robustness of the findings regarding MARCE. The effect size used for the power calculation may not accurately reflect the impact of the interventions in the context of evolving drug treatments. The randomization process did not create provide balanced groups, with the intervention group having lower eGFR and higher NT-proBNP levels. This may, despite adjustments, have influenced outcomes such as disease progression and mortality. The emergence of the COVID-19 pandemic during the trial likely affected both the implementation of the interventions and outcomes.
The exploratory analysis suggesting a potential benefit for patients with heart failure should be interpreted with caution. However, traditional outcomes like MARCE may not fully capture the impact of integrated care in multimorbid patients with late-stage diseases [4, 6]. Additionally, MARCE does not account for potential reductions in healthcare utilization and costs from integrated care [18], and further studies are warranted.
Conclusion
In conclusion, this randomized controlled trial evaluated an integrated care model delivered with a person-centred approach for patients with concurrent CVD, CKD and DM. The study found that integrated care improve several dimensions of HRQoL while it did not improve disease progression, as compared with traditional specialist care in terms of disease progression. However, a trend for benefits for patients with heart failure was observed. Despite a small study population, challenges such as baseline imbalances, evolving medical therapies and the COVID-19 pandemic, the findings highlight the value of integrated, person-centred care and may provide insights for clinical practice. Future research with focus on HRQoL, healthcare resource utilization and efficiency, which align with the goals of integrated care, is warranted.
Supplementary Material
ACKNOWLEDGEMENTS
We extend our gratitude to the colleagues and hospital leadership who enabled the integrated clinic, especially Karin Malmqvist, clinic head, and Camilla Andersson, head nurse. We also thank the staff, particularly Susana Monthan, Magdalena Fröberg and Joachim Lind, and patients, for their contributions to the trial.
Contributor Information
Gudrun Evén, Karolinska Institutet, Department of Clinical Sciences, Danderyd Hospital, Stockholm, Sweden.
Terese Stenfors, Karolinska Institutet, Department of Learning, Informatics, Management and Ethics – LIME.
Stefan H Jacobson, Karolinska Institutet, Department of Clinical Sciences, Danderyd Hospital, Stockholm, Sweden.
Tomas Jernberg, Karolinska Institutet, Department of Clinical Sciences, Danderyd Hospital, Stockholm, Sweden.
Åsa Franzén-Dahlin, Karolinska Institutet, Department of Clinical Sciences, Danderyd Hospital, Stockholm, Sweden.
Susanna Jäghult, Karolinska Institutet, Department of Clinical Sciences, Danderyd Hospital, Stockholm, Sweden; Department of Clinical Science and Education, Södersjukhuset.
Thomas Kahan, Karolinska Institutet, Department of Clinical Sciences, Danderyd Hospital, Stockholm, Sweden.
Jonas Spaak, Karolinska Institutet, Department of Clinical Sciences, Danderyd Hospital, Stockholm, Sweden.
FUNDING
The CareHND trial was supported by grants from the Kamprad Family Foundation for Entrepreneurship, Research & Charity (20180115) and the Region of Stockholm (grant number 955043, 952836, 952431, 951255). G.E. has received support from the Signe and Olof Wallenius Foundation.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author, pending Ethical approval.
CONFLICT OF INTEREST STATEMENT
J.S. declares speaker honoraria from Boehringer Ingelheim, Sanofi, Bayer, AstraZeneca and Novo Nordisk, and shares in Beat Vascular Health AB. S.J. also declares speaker fee from Takeda, Eli Lilly, Mediahuset, Bristol Myers Squibb and ViforPharma. S.H.J. declares speaker and advisory board honoraria from AstraZeneca, Boehringer Ingelheim, Baxter, GlaxoSmithKline GSK and Otsuka.
REFERENCES
- 1. World Health Organization . Cardiovascular Diseases (CVDs). 2021. WHO, Geneva, Switzerland. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/ (15 October 2023, date last accessed). [Google Scholar]
- 2. International Diabetes Federation . IDF Diabetes Atlas. 10th edition 2021. IDF, Brussels, Belgium. Available from: https://www.diabetesatlas.org/en/ (15 October 2023, date last accessed). [Google Scholar]
- 3. Roth GA, Mensah GA, Johnson CO et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vora J, Cherney D, Kosiborod MN et al. Inter-relationships between cardiovascular, renal and metabolic diseases: underlying evidence and implications for integrated interdisciplinary care and management. Diabetes Obesity Metabolism 2024;26:1567–81. 10.1111/dom.15485 [DOI] [PubMed] [Google Scholar]
- 5. Gaede P, Vedel P, Larsen N et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93. 10.1056/NEJMoa021778 [DOI] [PubMed] [Google Scholar]
- 6. Birtcher KK, Allen LA, Anderson JL et al. 2022 ACC expert consensus decision pathway for integrating atherosclerotic cardiovascular disease and multimorbidity treatment: a framework for pragmatic, patient-centered care. J Am Coll Cardiol 2023;81:292–317. 10.1016/j.jacc.2022.08.754 [DOI] [PubMed] [Google Scholar]
- 7. Spaak J. Novel combined management approaches to patients with diabetes, chronic kidney disease and cardiovascular disease. J R Coll Physicians Edinb 2017;47:83–7. 10.4997/jrcpe.2017.118 [DOI] [PubMed] [Google Scholar]
- 8. Holland R, Battersby J, Harvey I et al. Systematic review of multidisciplinary interventions in heart failure. Heart 2005;91:899–906. 10.1136/hrt.2004.048389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang SM, Hsiao LC, Ting IW et al. Multidisciplinary care in patients with chronic kidney disease: a systematic review and meta-analysis. Eur J Intern Med 2015;26:640–5. 10.1016/j.ejim.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 10. Helou N, Dwyer A, Shaha M et al. Multidisciplinary management of diabetic kidney disease: a systematic review and meta-analysis. JBI Database System Rev Implement Rep 2016;14:169–207. 10.11124/JBISRIR-2016-003011 [DOI] [PubMed] [Google Scholar]
- 11. Weber C, Beaulieu M, Djurdjev O et al. Towards rational approaches of health care utilization in complex patients: an exploratory randomized trial comparing a novel combined clinic to multiple specialty clinics in patients with renal disease-cardiovascular disease-diabetes. Nephrol Dial Transplant 2012;27:iii104–10. 10.1093/ndt/gfr292 [DOI] [PubMed] [Google Scholar]
- 12. Lawson KD, Mercer SW, Wyke S et al. Double trouble: the impact of multimorbidity and deprivation on preference-weighted health related quality of life a cross sectional analysis of the Scottish Health Survey. Int J Equity Health 2013;12:67. 10.1186/1475-9276-12-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moradi M, Daneshi F, Behzadmehr R et al. Quality of life of chronic heart failure patients: a systematic review and meta-analysis. Heart Fail Rev 2020;25:993–1006. 10.1007/s10741-019-09890-2 [DOI] [PubMed] [Google Scholar]
- 14. Levin A, Chaudhry MR, Djurdjev O et al. Diabetes, kidney disease and cardiovascular disease patients. Assessing care of complex patients using outpatient testing and visits: additional metrics by which to evaluate health care system functioning. Nephrol Dial Transplant 2009;24:2714–20. 10.1093/ndt/gfp180 [DOI] [PubMed] [Google Scholar]
- 15. Dambha-Miller H, Simpson G, Hobson L et al. Integrated primary care and social services for older adults with multimorbidity in England: a scoping review. BMC Geriatr 2021;21:674. 10.1186/s12877-021-02618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ekman I, Swedberg K, Taft C et al. Person-centered care—ready for prime time. Eur J Cardiovasc Nurs 2011;10:248–51. 10.1016/j.ejcnurse.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization . WHO global strategy on integrated people-centred health services 2016-2026. Executive Summary, WHO, Geneva, Switzerland, 2015. [Google Scholar]
- 18. Sahlen KG, Boman K, Brannstrom M. A cost-effectiveness study of person-centered integrated heart failure and palliative home care: based on a randomized controlled trial. Palliat Med 2016;30:296–302. 10.1177/0269216315618544 [DOI] [PubMed] [Google Scholar]
- 19. Jäghult S, Saboonchi F, Johansson UB et al. Identifying predictors of low health-related quality of life among patients with inflammatory bowel disease: comparison between Crohn's disease and ulcerative colitis with disease duration. J Clin Nurs 2011;20:1578–87. 10.1111/j.1365-2702.2010.03614.x [DOI] [PubMed] [Google Scholar]
- 20. Sullivan M, Karlsson J, Ware JE. The Swedish SF-36 Health Survey—I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med 1995;41:1349–58. [DOI] [PubMed] [Google Scholar]
- 21. Sullivan M, Karlsson J. The Swedish SF-36 Health Survey III. Evaluation of criterion-based validity: results from normative population. J Clin Epidemiol 1998;51:1105–13. 10.1016/S0895-4356(98)00102-4 [DOI] [PubMed] [Google Scholar]
- 22. Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53–72. 10.1016/0168-8510(96)00822-6 [DOI] [PubMed] [Google Scholar]
- 23. Oppe M, Devlin NJ, Szende A. EQ-5D value sets: inventory, comparative review and user guide. Springer, Dordrecht, The Netherlands, 2007. [Google Scholar]
- 24. Heerspink HJL, Stefánsson BV, Correa-Rotter R et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 25. Virani SS, Alonso A, Benjamin EJ et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020;141:e139–596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 26. Whitaker-Brown CD, Woods SJ, Cornelius JB et al. Improving quality of life and decreasing readmissions in heart failure patients in a multidisciplinary transition-to-care clinic. Heart Lung 2017;46:79–84. 10.1016/j.hrtlng.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 27. Holliday MWJr, Frost L, Navaneethan SD. Emerging evidence for glucagon-like peptide-1 agonists in slowing chronic kidney disease progression. Curr Opin Nephrol Hypertens 2024;33:331–6. 10.1097/MNH.0000000000000976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nuffield Department of Population Health Renal Studies Group , SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet North Am Ed 2022;400:1788–801. 10.1016/S0140-6736(22)02074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lincoff AM, Brown-Frandsen K, Colhoun HM et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023;389:2221–32. 10.1056/NEJMoa2307563 [DOI] [PubMed] [Google Scholar]
- 30. Perkovic V, Tuttle KR, Rossing P et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med 2024;391:109–21. 10.1056/NEJMoa2403347 [DOI] [PubMed] [Google Scholar]
- 31. Gerotziafas GT, Catalano M, Colgan MP et al. Guidance for the management of patients with vascular disease or cardiovascular risk factors and COVID-19: position paper from VAS-European Independent Foundation in Angiology/Vascular Medicine. Thromb Haemost 2020;120:1597–628. 10.1055/s-0040-1715798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van den Berge JC, van Vark LC, Postmus D et al. Determinants of quality of life in acute heart failure patients with and without comorbidities: a prospective, observational study. Eur J Cardiovasc Nurs 2022;21:205–12. 10.1093/eurjcn/zvab061 [DOI] [PubMed] [Google Scholar]
- 33. Utley M, Adeyanju T, Bernardo B et al. The association between mental health, social support and physical health outcomes among older female cancer survivors. J Geriatr Oncol 2022;13:834–8. 10.1016/j.jgo.2022.04.001 [DOI] [PubMed] [Google Scholar]
- 34. Andersen-Hollekim T, Solbjør M, Kvangarsnes M et al. Narratives of patient participation in haemodialysis. J Clin Nurs 2020;29:2293–305. 10.1111/jocn.15238 [DOI] [PubMed] [Google Scholar]
- 35. Lewis EF, Claggett BL, McMurray JJV et al. Health-related quality of life outcomes in PARADIGM-HF. Circ Heart Fail 2017;10:e003430. 10.1161/CIRCHEARTFAILURE.116.003430 [DOI] [PubMed] [Google Scholar]
- 36. Savage PA, Shaw AO, Miller MS et al. Effect of resistance training on physical disability in chronic heart failure. Med Sci Sports Exerc 2011;43:1379–86. 10.1249/MSS.0b013e31820eeea1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Li S, Zou Y et al. Fear of hypoglycemia in patients with type 1 and 2 diabetes: a systematic review. J Clin Nurs 2021;30:72–82. 10.1111/jocn.15538 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author, pending Ethical approval.






