Abstract
Fragmentation, disorganization, and depletion of the collagen-rich dermal extracellular matrix (ECM) are hallmarks of aged human skin. These deleterious alterations are thought to critically mediate many of the prominent clinical attributes of aged skin including thinning, fragility, impaired wound healing, and propensity for carcinoma. Matrix metalloproteinase-1 (MMP1), initiates cleavage of collagen fibrils and is significantly increased in dermal fibroblasts in aged human skin. To investigate the role of elevated MMP1 in skin aging, we generated a conditional bitransgenic mouse (Col1a2;hMMP1) that expresses full-length, catalytically-active human MMP1 (hMMP1) in dermal fibroblasts. hMMP1 expression is activated by a tamoxifen-inducible Cre recombinase that is driven by the collagen1A2 (Col1a2) promoter and upstream enhancer. Tamoxifen induced hMMP1 expression and activity throughout the dermis in Col1a2 in hMMP1 mice. At six months of age, Col1a2;hMMP1 mice displayed loss and fragmentation of dermal collagen fibrils, which was accompanied by many of the features of aged human skin, such as contracted fibroblast morphology, reduced collagen production, increased expression of multiple endogenous MMPs and proinflammatory mediators. Interestingly, Col1a2;hMMP1 mice displayed substantially increased susceptibility to skin papilloma development. These data demonstrate that fibroblast expression of hMMP1 is a critical mediator of dermal aging and creates a dermal microenvironment that promotes keratinocyte tumor development.
Introduction
The dermal extracellular matrix (ECM) is primarily composed of type I collagen fibrils. These fibrils provide structural/mechanical support and play essential roles in skin biology. Fragmentation and disorganization of the collagen fibrils are the hallmarks of aged human skin (dermal aging) (Fisher et al., 2008). Age-related degeneration of the collagen-rich dermal ECM is a major contributing factor to the functional decline and fragility of aged skin and creates a tissue microenvironment more prone to skin disorders, such as poor wound healing (Wells et al., 2016) and cancer development (Fane and Weeraratna, 2020).
We previously reported that matrix metalloproteinases-1 (MMP1), which initiates collagen fragmentation, is significantly elevated in aged human skin dermal fibroblasts in vivo (Fisher et al., 1996, Fisher et al., 2009). To address the role of MMP1 in human skin dermal aging, we have generated a genetically modified humanized mouse model that conditionally expresses a full-length, catalytically-active form of human MMP1 (hMMP1) (Xia et al., 2013), in dermal fibroblasts, the source of the elevated MMP1 in aged human skin. We report that expression of hMMP1 in mouse fibroblasts causes phenotypic, cellular, and molecular alterations that closely mimic those observed in aged human skin. Interestingly, expression of hMMP1 in mouse fibroblasts displayed substantially increased susceptibility to skin papilloma development in the two-stage chemical carcinogenesis model. The data presented in this report provides proof of concept that MMP1 is a key mediator of dermal aging and MMP1-induced alterations in the dermal microenvironment promote epithelial skin tumor development.
Results
Establishment of a humanized mouse model of dermal aging: fibroblast-selective expression of human MMP1.
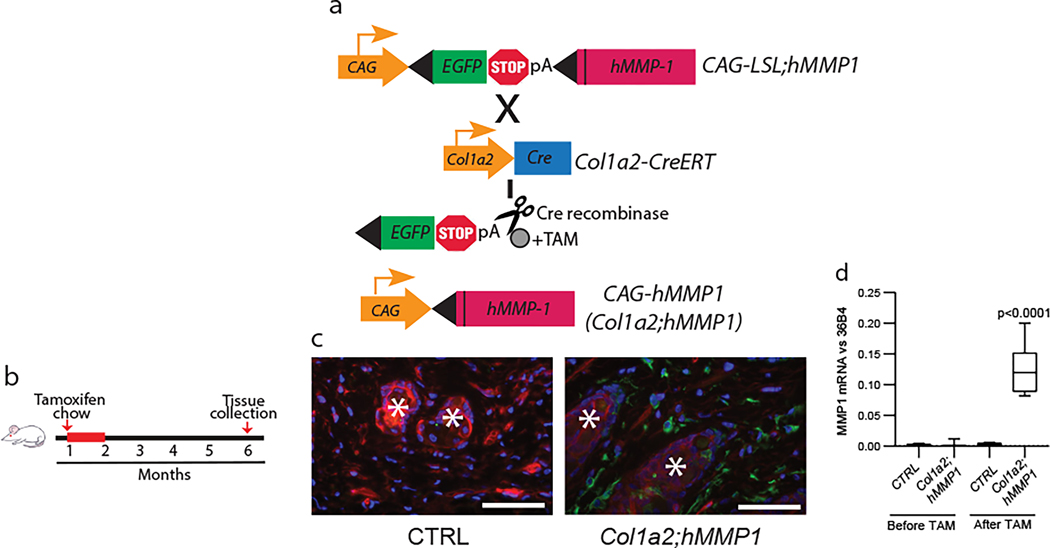
To investigate the role of MMP1 in dermal aging, we generated transgenic mice (CAG-LSL-hMMP1) that contain a Cre recombinase inducible MMP1 expression cassette. This cassette consisted of three elements; 1) a ubiquitous CAG promoter, 2) transcriptional stop sequences flanked by loxP sites (LSL), and 3) catalytically active, full-length, human MMP1 (hMMP1) (Xia et al., 2013). Two transgenic founders were bred with pure C57BL/6 mice for multiple generations to produce CAG-LSL-hMMP1 mice with a C57BL/6 genetic background (Fig 1a). These mice were bred with mice that express tamoxifen-activated Cre recombinase (Cre-ERT2) under the control of the fibroblast-specific promoter and upstream enhancer of the collagen 1A2 (Col1a2) gene (Sonnylal et al., 2010), (Fig 1a). The resulting bitransgenic mice (Col1a2-CreER;CAG-LSL-hMMP1, abbreviated Col1a2;hMMP1) and control littermates (containing a single or neither transgene) were fed tamoxifen-containing chow (400mg/kg) for one month during the second month of life and harvested for analyses at six months of age (Fig 1b). We confirmed that this Cre activation protocol induced fibroblast-specific recombination, using mTmG reporter mice, which express a green fluorescent protein (GFP), under the control of the fibroblast-specific promoter and upstream enhancer of the Col1a2 gene (Li et al., 2017). At six months of age, dermal fibroblasts in reporter mice constitutively expressed GFP throughout the dermis(Fig 1c). Following the above tamoxifen induction protocol, the skin of Col1a2;hMMP1 mice displayed substantial levels of hMMP1 gene expression (Fig 1d). hMMP1 expression was near the limit of detection in control littermates. Two Col1a2;hMMP1lines with similar hMMP1 expression were used for the studies presented in this report.
Figure 1. Generation of a humanized mouse model of dermal aging by fibroblast-selective expression of hMMP1. MMP1.
(a) Schematic representation of the generation of a mouse model of fibroblast expression of full-length, catalytically-active human MMP1 (hMMP1, Col1a2;hMMP1 mice). hMMP1 expression is activated by tamoxifen (TAM)-inducible Cre recombinase under the control of the fibroblast-specific Col-1a2 promoter and upstream enhancer. (b) Schematic diagrams depicting the initial feeding of one-month-old mice with TAM-containing chow (400mg/kg) for one month and back skin samples were collected at six months of age for analyses. (c) Cre recombinase activity (GFP) in the dermal fibroblasts of Col1a2-Cre(ER)T;ROSA26mTmG reporter mice. One-month-old mice were fed TAM chow for one month and the back skin was harvested at six months of age. The mTmG transgene confers expression of membrane-targeted tandem timer (td)Tomato prior to Cre-mediated excision and membrane-targeted green fluorescent protein (GFP) after excision. Tissue was counterstained with DAPI to allow visualization of nuclei. Representative images are shown. *Hair follicles. Bar=100 μm. (d) hMMP1 transgene mRNA expression in Col1a2;hMMP1 mice. The back skin samples were collected from one-month-old mice without tamoxifen administration (Before TAM) or six months after tamoxifen administration (After TAM). hMMP1 mRNA levels were determined by RT-PCR (normalized to 36B4, housekeeping gene internal control). N=5 for each group. Statistical analyses (t-test) were performed to evaluate the significance between the two groups. All P-values are considered significant when <0.05.
Fibroblast-selective expression of human MMP1 in mouse skin causes age-related dermal collagen alterations.
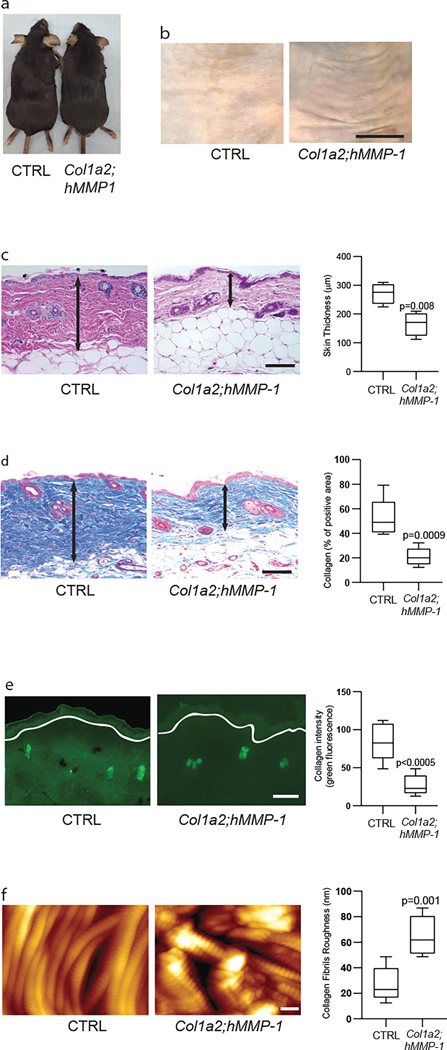
Newborn and adult Col1a2;hMMP1 mice were grossly normal in appearance (Fig 2a) however, after tamoxifen administration, at six months of age Col1a2;hMMP1 mice revealed skin wrinkles (Fig 2b right panel) and histological alterations (Figs 2c and 2d) that closely resembled those seen in aged human skin (Fisher et al., 2008). Dermal thickness was reduced by 39%, compared to sex and age-matched control littermates (Fig 2c right panel). Masson’s trichrome staining, which visualizes collagen fibrils, revealed a 60% reduction of collagen fibril density in tamoxifen-treated Col1a2;hMMP1 mice, compared to control littermates (Fig 2d ). To assess MMP enzymatic activity we used in situ zymography (Fisher et al., 2009) to detect the endogenous capacity of skin sections to degrade exogenous fluorescently labeled collagen, which is coated on the surface of slides (Fig. 2e). Skin sections from Col1a2;hMMP1 mice exhibited a 2.4-fold lower fluorescence than skin sections from control mice, demonstrating elevated collagen-degrading MMP protease activity. This collagen-degrading activity was localized in the dermis. Atomic force microscopy (AFM), which visualizes individual collagen fibrils (Quan et al., 2013), indicated significant fibril fragmentation and disorganization in Col1a2;hMMP1 mice (Fig 2f). Quantitative analysis demonstrated that the average roughness of collagen fibrils, an indicator of collagen fibril organization, was 3.2-fold greater in Col1a2;hMMP1 mice, compared to control mice (26.9 nm vs. 64.9 nm). Taken together, the above data indicate that fibroblast-specific expression of hMMP1 in Col1a2;hMMP1 mice causes dermal ECM alterations that closely mimic those observed in aged human skin (Fisher et al., 2008). The dermal ECM phenotype in Col1a2;hMMP1 mice strongly supports the concept that elevated MMP1 functions as a key driver of characteristic features of dermal aging.
Figure 2. Fibroblast-selective expression of human MMP1 in mouse skin causes age-related dermal collagen alterations.
(a) Representative gross appearance of control (left) and Col1a2;hMMP1 mice (right). (b) Representative images of skin wrinkles in control (left) and Col1a2;hMMP1 mice (right). N=5 per group. Bar=5mm. (c) Representative H&E staining of back skin from control (left panel) and Col1a2;hMMP1 (right panel) mice. The dermal thickness was quantified by Image J software (NIH, Bethesda, MD). Note thinness of dermis in Col1a2;hMMP1 mouse. N=5 per group. Bar=100 μm. (d) Representative Mason’s Trichrome staining of 6 months old control (left panel) and Col1a2;hMMP1 (right panel) mice. Cells stain red and collagen fibrils stain blue. The dermal collagen density (blue) was quantified by Image J software (NIH, Bethesda, MD). Note fewer collagen fibril bundles in Col1a2;hMMP1 mice. N=5 per group. Bar=100 μm. (e) Representative images of in situ zymography, which assesses collagenase activity, in control (left panel) and Col1a2;hMMP1 (right panel) mice. Loss of green fluorescence in Col1a2;hMMP1 mice indicates degradation of fluorescein-labeled collagen substrate. The fluorescein collagen signals were quantified by Image J software (NIH, Bethesda, MD). White lines indicate the boundary between the epidermis and dermis. N=5 per group. Bar=100 μm. (f) Representative nanoscale atomic force microscopy images of collagen fibrils from control (left panel) and Col1a2;hMMP1 (right panel) mice. Note intact densely-packed collagen fibrils in control, compared to fragmented, disorganized collagen fibrils in Col1a2;hMMP1 mice. Dermal collagen fibril organization, measured as collagen fibril average roughness (Ra, nm), was quantified using Nanoscope Analysis software (Nanoscope_Analysis_v120R1sr3, Bruker-AXS, Santa Barbara, CA). N=5 per group. Bar=100 μm.
Fibroblast-selective expression of human MMP1 in mouse skin causes age-related deleterious alterations of the dermal microenvironment.
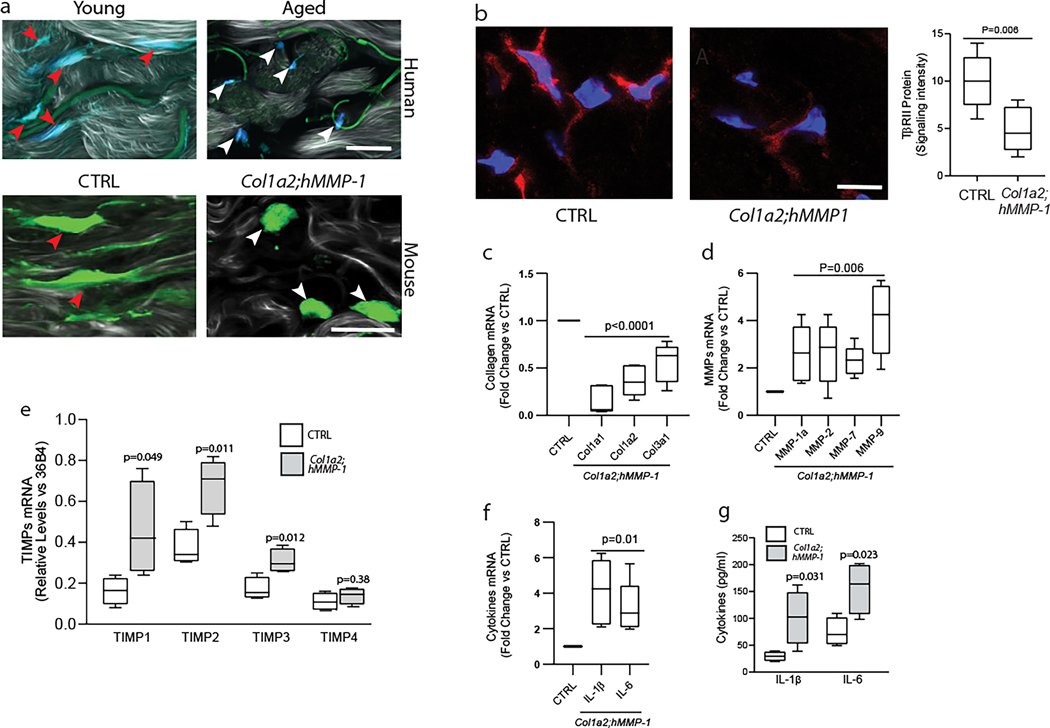
Dermal fibroblasts reside within the collagen-rich ECM environment. Integrin-mediated attachment to collagen fibrils allows fibroblasts to achieve a spread morphology, which fundamentally regulates fibroblast function (Zeltz and Gullberg, 2016). We previously reported that in aged skin, fragmentation of collagen fibrils prevents fibroblast attachment resulting in a contracted “collapsed” fibroblast morphology (Fisher et al., 1997, Fisher et al., 2009, Fisher et al., 2008). Functional adaptation of fibroblasts to this state causes an imbalance in ECM homeostasis, with increased production of ECM-degrading enzymes and reduced expression of ECM proteins (Fisher et al., 2008). Similar to human skin (Fig 3a upper panels), dermal fibroblasts in Col1a2;hMMP1 mice show significantly collapsed morphology and reduced size (Fig 3a lower panels).
Figure 3. Fibroblast-selective expression of human MMP1 in mouse skin causes age-related deleterious alterations of the dermal microenvironment.
(a) Upper panels: representative images of human skin dermal fibroblasts in young (26 years, left panel) and aged (78 years, right panel) human skin. Dermal fibroblasts were identified by immunostaining with collagen chaperone heat shock protein 47 (blue). Green signals indicate elastin autofluorescence. Lower panels: representative images of mouse skin dermal fibroblasts in control (lower left panel) and Col1a2;hMMP1 mice (lower right panel). Dermal cells in frozen skin sections were stained with HCS CellMask™ Deep Red Stain (green). SHG imaging of collagen fibrils is shown in shades of gray. Note stretched fibroblasts (red arrowheads) in young human skin and control mice versus contracted fibroblasts (white arrowheads) in aged human skin and Col1a2;hMMP1 mice. Images were obtained by multiphoton laser scanning fluorescence microscopy. N=6 for each group. Bars=25μm. (b) Reduced TβRII protein in the dermis of Col1a2;hMMP1 mice. TβRII protein was identified by immunofluorescence staining (red), and nuclei were stained with DAPI (blue). The fluorescence signals were quantified by Image J software (NIH, Bethesda, MD). Representative images are shown, N=5 for each group. (c) Reduced expression of major collagens (Col1a1, Col1a2, and Col1a3), (d) Increased expression of multiple collagen-degrading MMPs; (e) Increased expression of TIMPs, and (f, g) Increased expression of inflammaging-related cytokines (IL-1β, and IL-6) in the skin of Col1a2;hMMP1 mice. mRNA levels were determined by RT-PCR (normalized to 36B4, housekeeping gene internal control). N=5 for each group. Mouse skin homogenates were used for the ELISA (IL-1β, and IL-6) analysis. N=4 for each group. Statistical analyses (t-tests) were performed to evaluate the significance between the two groups. All P-values are considered significant when <0.05.
The TGF-β pathway is a major mediator of ECM production in human skin (Quan and Fisher, 2015, Quan et al., 2013). TGF-β type-II receptor (TβRII) expression is reduced in fibroblasts in aged human skin, and this reduction is consistent with the age-related decline of collagen production (Fisher et al., 2016, Quan and Fisher, 2015). We observed that TβRII expression is reduced by 52% in Col1a2;hMMP1 mice, compared to matched littermate controls (Fig 3b).
Furthermore, we determined that gene expression of the major collagens (Col1a1, Col1a2, and Col3a1) was decreased (Fig 3c), and multiple collagen-degrading MMPs (MMP1a, MMP-2, MMP-7, and MMP-9) were increased (Fig 3d) in Col1a2;hMMP1 mice. MMPs and tissue inhibitors of metalloproteinases (TIMPs) are often coordinately regulated to control excess MMP activity. We found that TIMP1–4, but not TIMP4, were upregulated in Col1a2;hMMP1 mice (Fig 3e). These data suggest that upregulation of TIPMs may limit MMP activities, which may partially explain the time dependence (six months after tamoxifen treatment) for the overt development of the dermal aging phenotype in Col1a2;hMMP1 mice.
In addition, both mRNA (Fig 3f) and protein (Fig 3g) levels of the inflammaging-related cytokines IL-1β and IL-6 were elevated in Col1a2;hMMP1 mice, as observed in aged human skin (Quan et al., 2011). These data demonstrate that fibroblast expression of human MMP1 in mouse skin alters fibroblast morphology and ECM homeostasis thereby creating a dermal microenvironment that displays many of the hallmarks of aged human skin.
MMP1-mediated dermal aging microenvironment promotes skin papilloma formation.
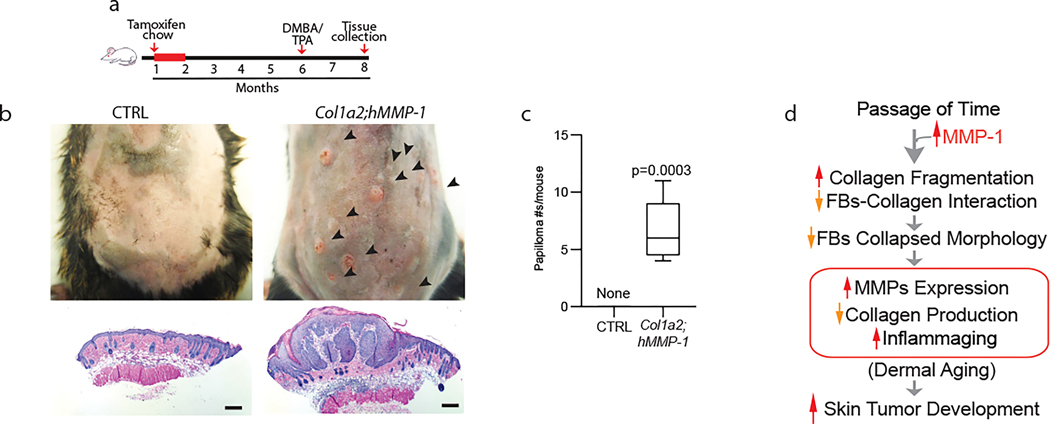
Finally, we investigated the impact of the age-related dermal microenvironment on skin tumor development in Col1a2;hMMP1 mice. We performed cutaneous two-stage chemical carcinogenesis studies, which employ a single topical exposure to the mutagen dimethylbenz[a]anthracene (DMBA), followed by repeated applications of a phorbol ester (TPA) (Fig 4a) (Abel et al., 2009). Interestingly, Col1a2;hMMP1 mice displayed substantially increased susceptibility to skin papilloma development (Fig 4b and 4c). Notably, sex and aged-matched littermates showed no evidence of skin papillomas, consistent with the known resistance of the parent mouse strain (C57BL/6) (Abel et al., 2009). These data support the concept that elevated dermal expression of MMP1 induces alterations in the dermal microenvironment that promote epithelial skin tumor development.
Figure 4. MMP1-mediated dermal aging promotes skin papilloma formation.
(a) Schematic diagrams depicting the initial feeding of one-month-old mice with TAM-containing chow (400mg/kg) and two-stage carcinogenesis. Back skin from Col1a2;hMMP1 and littermate hMMP1 negative control (six months after TAM treatment) mice were treated with a single dose of DMBA (100μg/100μl acetone) followed by biweekly applications of TPA (25μg/100μl acetone) for 8 weeks (two-stage chemical carcinogenesis). (b) Representative images of mice from each group are shown. Multiple skin tumors (black arrowheads) are seen in Col1a2;hMMP1 mice (upper right panel), while no tumors were observed in the control mice (upper left panel). Representative histology of treated back skin from control (lower left panel) and Col1a2;hMMP1 (lower right panel) mice. Note severe epidermal papillomatous dysplasia in Col1a2;hMMP1 mice. Bars=200μm (c) Quantitation of tumor numbers in control and Col1a2;hMMP1, following chemical carcinogenesis treatment. N=5 for each group. Statistical analyses (t-tests) were performed to evaluate the significance between the two groups. The P-value is considered significant when <0.05. (d) Proposed model for MMP1 mediated dermal aging and skin tumor formation: Age-related elevation of MMP1 activity degrades collagen fibrils thereby disrupting fibroblast-collagen interactions, resulting in reduced spreading and adaptive functional alterations that perpetuate further dermal ECM fragmentation (dermal aging) and creates a dermal microenvironment that is conducive to skin tumor development.
Discussion
Fragmentation and disorganization of collagen fibrils, the major structural component in the dermis, are the hallmarks of the aged human dermis (dermal aging). Collagen fibril fragmentation is initiated by MMP1 (Brennan et al., 2003), which is very low in healthy young skin, but significantly increased in aged dermal fibroblasts in vivo (Fisher et al., 2009, Qin et al., 2017). However, whether elevated MMP1 expression by fibroblasts is sufficient to drive the aged dermal phenotype that is observed in aged human skin is not known. We generated and characterized Col1a2;hMMP1 to address this question. The data presented above provide proof of concept that expression of human MMP1 in dermal fibroblasts, the source of elevated MMP1 in aged human skin, leads to the development of the characteristic features of aged human dermis and thus accelerates is a key mediator of the dermal aging process (Fig 2). In addition to the fragmentation and disorganization of collagen fibrils, Col1a2;hMMP1 mice developed age-related deleterious alterations of the dermal microenvironment including contracted “collapsed” fibroblast morphology (Fig 3a), increased expression of multiple MMPs (Fig 3d), impaired TGF-β signaling and reduced collagen production (Figs 3b and 3c), and increased expression of pro-inflammatory cytokines (inflammaging) (Figs 3f and 3g). We reported that the accumulation of fragmented collagen fibrils and subsequent alterations in fibroblast morphology leads to functional impairment of collagen homeostasis (Fisher et al., 2009, Fisher et al., 2008, Qin et al., 2017, Quan and Fisher, 2014).
Accumulating evidence demonstrates that the association of cells with the ECM provides important instructions for the regulation of cell function (Chaudhuri et al., 2020, Chen et al., 1997). Microenvironmental cues from the ECM affect intracellular signaling networks and control cellular activities including adhesion, migration, proliferation, differentiation, and survival (Humphrey et al., 2014, Iskratsch et al., 2014, Mammoto et al., 2013). In the young human dermis, fibroblasts interact with collagen fibrils through collagen-binding cell surface integrins. Integrins interact intracellularly with the cellular actin cytoskeleton. This integrin-mediated attachment of fibroblasts to the ECM, in conjunction with the cytoskeleton, establishes mechanical forces and a stretched morphology. However, in aged human dermis, fragmented collagen fibrils cannot interact with the integrins, and fibroblasts become rounded (Fisher et al., 2008, Qin et al., 2017, Qin et al., 2014). Loss of dermal fibroblast stretching, caused by MMP1-mediated fragmentation of collagen fibrils, significantly inhibits the production of collagen by fibroblasts (Qin et al., 2014), as observed in our Col1a2;hMMP1 mice (Fig 2c). Mechanistically, reduced stretching of fibroblasts significantly leads to impairment of the TGF-β pathway, the major regulator of s collagen/ECM production. This impairment is due to decreased expression of TβRII, which is reduced in the aged human dermis (Fisher et al., 2016) and in Col1a2;hMMP1 mice (Fig 3b). Additionally, reduced dermal fibroblast stretch caused by MMP1-mediated collagen fibril fragmentation significantly up-regulates the expression of several age-associated MMPs. This upregulation is mediated by activation of the transcription AP-1 (Qin et al., 2017), which is composed of Jun and Fos proteins. AP-1 binds to the proximal promoter regions of multiple MMPs, thereby stimulating gene transcription (Eferl and Wagner, 2003). As such, the age-related reduction of fibroblast stretch is a critical marker of dermal aging and a mediator of the aberrant ECM homeostasis that is observed in aged skin.
Importantly, we found that MMP1-induced age-related alteration of the dermal microenvironment substantially increased the susceptibility to skin papilloma initiation and development. Keratinocyte skin cancer is very common in the elderly, but is rare in young healthy skin (Rogers et al., 2015), although the critical oncogenic mutations, such as Notch and p53, are readily detected in physiologically healthy young normal skin (Jonason et al., 1996, Martincorena et al., 2015). Our data suggest that age-related alterations of the dermal ECM microenvironment, such as composition, structural integrity, organization, and mechanical properties, as well as dermal inflammaging, are likely to contribute to skin tumor initiation and promotion. This concept may partially explain why keratinocyte cancer is so common in the elderly.
D’armiento et al reported that keratinocyte expression of human MMP1 in mouse skin led to epidermal hyperplasia and increased papilloma development following two-stage chemical carcinogenesis (D’Armiento et al., 1995). Papilloma formation in this model likely stems from abnormal keratinocyte proliferation since epidermal hyperplasia increases both keratinocyte tumor initiation and promotion (DiGiovanni, 1992). In addition, it is also conceivable that epidermal MMP1 protein is secreted and transits to the dermis where it degrades collagen fibrils in the dermis (Xia et al., 2015) and turn contributes to increased tumor susceptibility, as observed in Col1a2;hMMP1 mice (Fig 3).
In aged human skin, elevated MMP1 is expressed in the dermal fibroblasts, not in the epidermal keratinocytes (Fisher et al., 2009). Therefore, our Col1a2;hMMP1 mice model of dermal aging is relevant to the pathophysiology status of aged human skin. Interestingly, the dermal fibroblast-specific elevation of MMP1 is also observed in patients with segmental progeroid syndrome trichothiodystrophy (TTD) (Arseni et al., 2015). Primary dermal fibroblasts, but not keratinocytes, cultured from TTD patients have elevated expression of MMP1. interestingly, TTD patients display increased dermal collagen degradation and reduced expression of type I collagen, as observed in our Col1a2;hMMP1 mice and aged human skin. These observations in TTD patients provide further support that elevated expression of MMP1 by dermal fibroblasts in human skin leads to increased collagen degradation and decreased collagen deposition.
Expression of hMMP1 under the control of the Col1a2 promoter and upstream enhancer (Fig 1a) could lead to the expression of MMP1 in collagen-producing cells in tissues other than the skin. We investigated the effects of hMMP1 transgene expression on the mouse major organs in Col1a2;hMMP1. Histopathological examination did not reveal significant alterations in the connective tissues and other major organ tissues in the brain, heart, liver, spleen, pancreas, stomach, and intestine. Minor inflammatory cell infiltration was observed in the lung, and kidney (Supplementary table 1).
MMP1 can cleave proteins in addition to collagen (Hegedus et al., 2008, Klein and Bischoff, 2011, Page-McCaw et al., 2007, Young et al., 2019). It has been proposed that, in addition to its role in ECM turnover, MMP1 may regulate signaling pathways in a nonproteolytic manner (Dufour and Overall, 2013, Kessenbrock et al., 2010, Parks et al., 2004). Since mice lack a clear ortholog of human MMP1, defining the physiological role of MMP1 by creating knockout mice is not possible. Our Col1a2;hMMP1 mice may be useful for understanding novel aspects of MMP1. Our Col1a2;hMMP1 mice are a practical mouse model of skin dermal aging to determine the role of MMP1 in dermal aging processing, as well as in the development of age-related skin diseases such as keratinocyte tumor formation and impaired wound healing, which are common in elderly.
In summary, the above data reveal that fibroblast-specific expression of human MMP1 in mouse skin generates a phenotype that closely resembles dermal aging in human skin. These data support the concept that the observed age-related elevation of MMP1 in human skin leads to the fragmentation of collagen fibrils, which disrupts fibroblast-collagen interactions (Fig 4d). This disruption results in reduced fibroblast spreading, which alters fibroblast function, shifting collagen homeostasis in favor of collagen fibril degradation and reduction of collagen fibril production. The fragmented collagen fibril ECM microenvironment also induces the expression of pro-inflammatory mediators that contribute to the aging process. Significantly, we observed that the age-related dermal microenvironment promotes skin tumor development. Our data provide insight that partially explains the strong connection between aging and keratinocyte skin cancer incidence.
Methods and materials
Mice.
Col1a2;hMMP1 mice:
The transgenic construct was generated by cloning an auto-activating mutant human MMP1 (hMMP1/V94G) cDNA (Xia et al., 2013) into the pCLEX vector (Pasca di Magliano et al., 2006). This vector contains a highly active CAG promoter, followed by a green fluorescent protein (EGFP) cDNA with a polyA signal and strong termination sequence flanked by loxP sites, which prevents transcription of the downstream hMMP1/V94G transgene. Upon tamoxifen-inducible Cre recombination, the EGFP sequences are excised and transcription of the hMMP1/V94G is irreversibly activated in Cre-expressing cells and their progeny (Fig 1a). All cloning was verified by sequencing. The hMMP1/V94G expression cassette was purified and injected into (C57BL/6 X SJL)F2 mouse eggs by the University of Michigan Transgenic Core. The transgenic founders (CAG-LSL-hMMP1 mice) were identified by PCR genotyping using hMMP1-specific primers (5’-AACCTGAAGAATGACGGCA-3, 5’-ATACCAGGTCCAGGCTGAA-3’). CAG-LSL-hMMP1 mice were crossed six or more generations with pure C57BL/6 mice to reduce the variability of the genetic background. The resultant hMMP1 transgenic mice were mated with Col1a2-CreER mice (The Jackson Laboratory, stock number 029235), with Cre expression under the control of a stromal cell-specific regulatory sequence from the collagen 1a2 (Col1a2) promoter and enhancer. This crossing yielded 25% Col1a2;hMMP1 (experimental mice) and 75% of mice designated as control mice (25% hMMP1, 25% Col1a2-CreER, 25% C57BL/6 background). To activate hMMP1 transgene in mouse skin dermal fibroblasts, tamoxifen (TAM) was systemically delivered by TAM chow (400mg/kg) for one month (Fig 1b), which sufficiently activates transgene in Cre-expressing cells and their progeny (Fig 1c) (Li et al., 2017, Verhaegen et al., 2015). There was no evidence of embryonic lethality in Col1a2;hMMP1 mice. Tissue samples were fixed in neutral-buffered formalin at room temperature overnight and embedded in paraffin and stained with H&E or Mason’s Trichrome. Col1a2-Cre(ER)T;ROSA26mTmG reporter mice: Mice hemizygous for a tamoxifen-dependent Cre recombinase under the control of the fibroblast-specific promoter and upstream enhancer of the collagen 1A2 (Col1a2) gene (Sonnylal et al., 2010) were bred with mice harboring a double-fluorescent reporter transgene (mTmG) integrated into the Gt(ROSA)26Sor locus (strain #:007576, Jackson Lab) (Muzumdar et al., 2007) to generate Col1a2-Cre(ER)T;ROSA26mTmG mice, as previously described (Li et al., 2017). Reporter mice expressing a tamoxifen-inducible Cre recombinase under the control of a Col1a2 promoter/enhancer show expression of membrane-targeted tdTomato (tdTom) prior to Cre-mediated recombination and membrane-targeted GFP following Cre-mediated recombination. All experiments performed with the mice followed the standards of care approved by the University of Michigan Unit for Laboratory Animal Medicine (ULAM). Protocols for mouse experimentation were approved by the University of Michigan Institutional Animal Care and Use Committee.
Histology, Immunohistology, and second harmonic generation microscopy.
For H&E and Masson’s trichrome staining, the skin samples were fixed in 10% neutral buffered formalin, -embedded in paraffin, and stained by the standard procedures. The dermal thickness and collagen density (blue in Masson’s trichrome) were quantified by Image J software. Immunohistology was performed as described previously (Fisher et al., 2009). Briefly, skin OCT-embedded cryo-sections (7μm thickness) were fixed in 2% paraformaldehyde for two hours. Subsequently, the slides were incubated for 1 hour at room temperature with normal control serum followed by incubation of anti-TβRII antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). All sections were lightly counterstained with hematoxylin and were mounted with mounting media (Vector, Laboratories, CA, USA). Images were obtained using Zeiss fluorescence microscopy. Second harmonic generation microscopy was performed using a Leica SP8 Confocal Microscope with 2-Photon, at the University of Michigan Microscopy and Image Analysis Laboratory. Dermal cells were stained with heat shock protein 47 (a marker of dermal fibroblasts, Cat#:sc-5293, Santa Cruz Biotechnology, Dallas, TX) or HCS CellMask™ Deep Red Stain (green) (Invitrogen-ThermoFisher).
RNA isolation and quantitative real-time PCR.
Total RNA was isolated from mouse skin using a commercial kit (RNeasy mini kit, Qiagen, Chatsworth, CA) according to the manufacturer’s protocol. PCR template was prepared by reverse transcription using TaqMan Reverse Transcription kit (Applied Biosystems, Foster City, CA). All PCR primers were purchased from RealTimePrimers.com (Elkins Park, PA). PCR was performed in duplicate with 2μl of cDNA for the genes of interest using TaqMan Universal PCR Master Mix kit (Applied Biosystems) and a 7700 sequence detector system (Applied Biosystems). PCR procedures were performed with a robotic workstation (Biomek 2000; Beckman Coulter, Inc., Hialeah, FL) to ensure accuracy and reproducibility. Target gene mRNA levels were normalized to the mRNA levels of the housekeeping gene, 36B4 (internal control). All primers were obtained from RealTimePrimers.com (Real Time Primers, LLC Elkins Park, PA).
Zymography and Enzyme-Linked Immunosorbent Assay.
In situ zymography was performed as described previously (Fisher et al., 1997, Fisher et al., 2009). Briefly, FITC-labeled calf skin type I collagen (Elastin products, Owensville, MO) was coated onto glass slides. Cryostat skin sections (5 μm) were placed on top of the collagen coating, and incubated for 24 hours in a sealed, humidified chamber at 37°C. Sections were visualized by fluorescence microscopy. Mouse skin homogenates were used for the analysis of cytokines (IL-1β and IL-6) levels, using Quantikine enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN) following the manufacturer’s protocol.
Atomic force microscopy (AFM) imaging.
Fresh skin biopsies were embedded in OCT and cryo-sections (15μm) were mounted on microscope cover glass (1.2 mm diameter, Fisher Scientific Co., Pittsburgh, PA). AFM images of the collagen fibrils were acquired using ScanAsyst mode, an optimized PeakForce Tapping technique that provides high-resolution AFM images (Quan et al., 2013). ScanAsyst mode visualizes automatically and continuously monitors image quality and makes appropriate parameter adjustments. For each subject, AFM images were obtained from 6 different regions of each skin section (2×2μm scan size), which included collagen fibrils in both the reticular and papillary dermis. AFM images were obtained with a 512 × 512-pixel resolution. The surface roughness of the scanned regions was calculated as the roughness average (Ra), which is typically used to describe the roughness of materials’ surfaces and is calculated by a surface’s measured microscopic peaks and valleys. The Ra of the scanned regions was quantified from raw data, without modifications, such as cleaning, flattening, filtering, or plane fitting, using Nanoscope Analysis software (Nanoscope Analysis v120R1sr3, Bruker-AXS, Santa Barbara, CA, USA). AFM images were obtained from the Electron Microbeam Analysis Laboratory (EMAL), University of Michigan College of Engineering, and analyzed using Nanoscope Analysis software (Bruker, Santa Barbara, CA).
Two-stage carcinogenesis.
Cutaneous two-stage chemical carcinogenesis was performed as described elsewhere (Abel et al., 2009). Briefly, mice were treated with a single dose of DMBA (30μg/100μl acetone) followed by biweekly applications of TPA (25μg/100μl ethanol). Skin papillomas were analyzed 8 weeks following TPA administration (Fig 3a).
Statistics.
Bar graphs represent means±SEM. Statistical analyses (t-test) were performed using Prism software (version 8.0.1, GraphPad, San Diego, CA) to evaluate the significance between the two groups. All P-values are considered significant when <0.05.
Supplementary Material
Acknowledgments
The authors thank Diane Fiolek for administrative assistance. Chunfang Guo provided expert technical assistance in the breeding and genotyping of mice. The authors thank Ingrid Bergin VDM, Pathologist and Director, In Vivo Animal Core, University of Michigan, Unit for Laboratory Animal Medicine for performing mouse necropsy. Additional thanks to the University of Michigan Transgenic Core for the production of CAG-LSL-hMMP1 transgenic mice. This work was supported by the National Institute of Health (R01AG051849 to GJF and TQ; P30AR075043 Innovation Award to GJF and TQ; and U01AG077924 to GJF, AAD, and TQ).
Glossary
- MMP1
Matrix Metalloproteinase-1
- ECM
Extracellular matrix
- Col1a2
type I collagen alpha chain 2
- TβRII
Transforming growth factor beta type II receptor
- AFM
Atomic Force Microscopy
Footnotes
Conflict of Interest
The authors state no conflict of interest.
Data availability statement
No datasets were generated or analyzed during the current study.
References
- Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc 2009;4(9):1350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseni L, Lanzafame M, Compe E, Fortugno P, Afonso-Barroso A, Peverali FA, et al. TFIIH-dependent MMP-1 overexpression in trichothiodystrophy leads to extracellular matrix alterations in patient skin. Proc Natl Acad Sci U S A 2015;112(5):1499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M, Bhatti H, Nerusu K, Bhagavathula N, Kang S, Fisher G, et al. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol 2003;78(1):43–8. [DOI] [PubMed] [Google Scholar]
- Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020;584(7822):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science 1997;276(5317):1425–8. [DOI] [PubMed] [Google Scholar]
- D’Armiento J, DiColandrea T, Dalal SS, Okada Y, Huang MT, Conney AH, et al. Collagenase expression in transgenic mouse skin causes hyperkeratosis and acanthosis and increases susceptibility to tumorigenesis. Mol Cell Biol 1995;15(10):5732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiovanni J Multistage carcinogenesis in mouse skin. Pharmacol Ther 1992;54(1):63–128. [DOI] [PubMed] [Google Scholar]
- Dufour A, Overall CM. Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol Sci 2013;34(4):233–42. [DOI] [PubMed] [Google Scholar]
- Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 2003;3(11):859–68. [DOI] [PubMed] [Google Scholar]
- Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer 2020;20(2):89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G, Wang Z, Datta S, Varani J, Kang S, Voorhees J. Pathophysiology of premature skin aging induced by ultraviolet light. New Eng J Med 1997;337:1419–29. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996;379(6563):335–9. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol 2009;174(1):101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Shao Y, He T, Qin Z, Perry D, Voorhees JJ, et al. Reduction of fibroblast size/mechanical force down-regulates TGF-beta type II receptor: implications for human skin aging. Aging Cell 2016;15(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol 2008;144(5):666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus L, Cho H, Xie X, Eliceiri GL. Additional MDA-MB-231 breast cancer cell matrix metalloproteinases promote invasiveness. J Cell Physiol 2008;216(2):480–5. [DOI] [PubMed] [Google Scholar]
- Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 2014;15(12):802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol 2014;15(12):825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonason AS, Kunala S, Price GJ, Restifo RJ, Spinelli HM, Persing JA, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci U S A 1996;93(24):14025–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010;141(1):52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011;41(2):271–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li IMH, Horwell AL, Chu G, de Crombrugghe B, Bou-Gharios G. Characterization of Mesenchymal-Fibroblast Cells Using the Col1a2 Promoter/Enhancer. Methods Mol Biol 2017;1627:139–61. [DOI] [PubMed] [Google Scholar]
- Mammoto T, Mammoto A, Ingber DE. Mechanobiology and developmental control. Annu Rev Cell Dev Biol 2013;29:27–61. [DOI] [PubMed] [Google Scholar]
- Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 2015;348(6237):880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 2007;45(9):593–605. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 2007;8(3):221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004;4(8):617–29. [DOI] [PubMed] [Google Scholar]
- Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev 2006;20(22):3161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Balimunkwe RM, Quan T. Age-related reduction of dermal fibroblast size up-regulates multiple matrix metalloproteinases as observed in aged human skin in vivo. Br J Dermatol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Voorhees JJ, Fisher GJ, Quan T. Age-associated reduction of cellular spreading/mechanical force up-regulates matrix metalloproteinase-1 expression and collagen fibril fragmentation via c-Jun/AP-1 in human dermal fibroblasts. Aging Cell 2014;13(6):1028–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Fisher G. Age-Associated Dermal Microenvironment (AADM) Promotes Human skin Connective Tissue Aging - A Mini Review. Gerontology 2014;Invited Review Article(ePub Feb 4 2015):DOI: 10.1159/000371708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Fisher GJ. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015;61(5):427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Qin Z, Robichaud P, Voorhees JJ, Fisher GJ. CCN1 contributes to skin connective tissue aging by inducing age-associated secretory phenotype in human skin dermal fibroblasts. J Cell Commun Signal 2011;5(3):201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Wang F, Shao Y, Rittie L, Xia W, Orringer JS, et al. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol 2013;133(3):658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the US Population, 2012. JAMA Dermatol 2015;151(10):1081–6. [DOI] [PubMed] [Google Scholar]
- Sonnylal S, Shi-Wen X, Leoni P, Naff K, Van Pelt CS, Nakamura H, et al. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum 2010;62(5):1523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaegen ME, Mangelberger D, Harms PW, Vozheiko TD, Weick JW, Wilbert DM, et al. Merkel cell polyomavirus small T antigen is oncogenic in transgenic mice. J Invest Dermatol 2015;135(5):1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A, Nuschke A, Yates CC. Skin tissue repair: Matrix microenvironmental influences. Matrix Biol 2016;49:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Hammerberg C, Li Y, He T, Quan T, Voorhees JJ, et al. Expression of catalytically active matrix metalloproteinase-1 in dermal fibroblasts induces collagen fragmentation and functional alterations that resemble aged human skin. Aging Cell 2013;12(4):661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Quan T, Hammerberg C, Voorhees JJ, Fisher GJ. A mouse model of skin aging: fragmentation of dermal collagen fibrils and reduced fibroblast spreading due to expression of human matrix metalloproteinase-1. J Dermatol Sci 2015;78(1):79–82. [DOI] [PubMed] [Google Scholar]
- Young D, Das N, Anowai A, Dufour A. Matrix Metalloproteases as Influencers of the Cells’ Social Media. Int J Mol Sci 2019;20(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltz C, Gullberg D. The integrin-collagen connection--a glue for tissue repair? J Cell Sci 2016;129(4):653–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.