Abstract
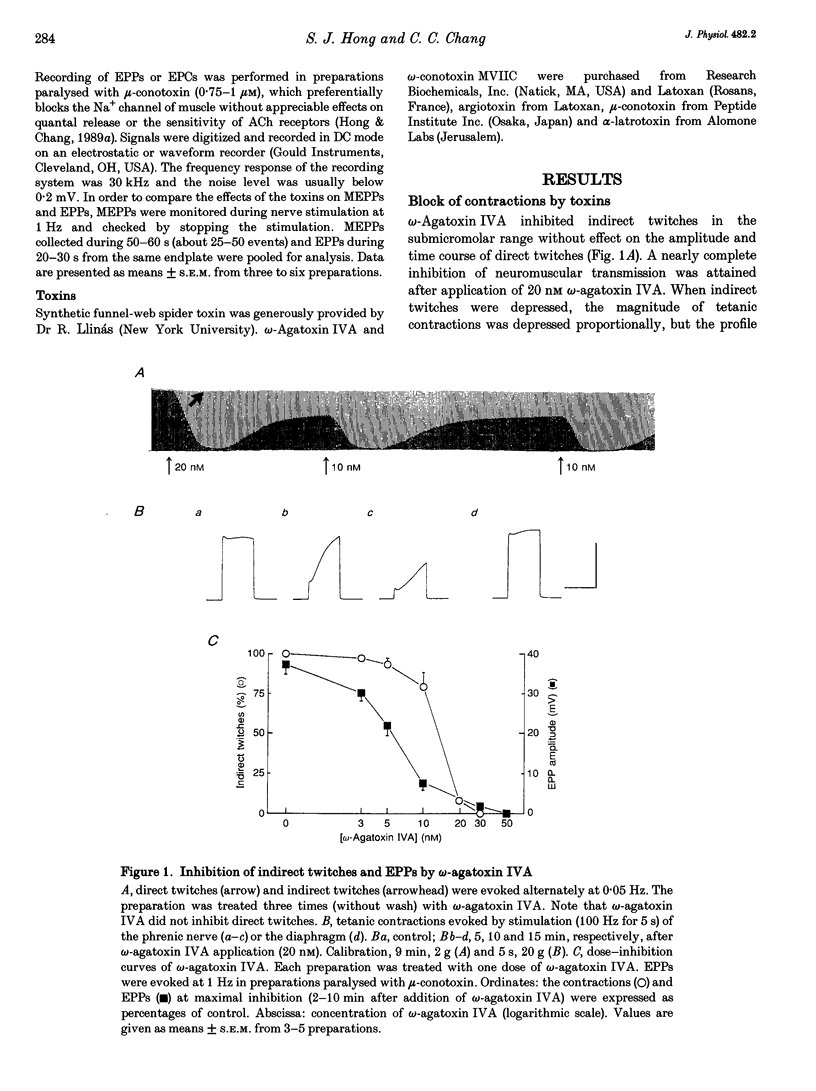
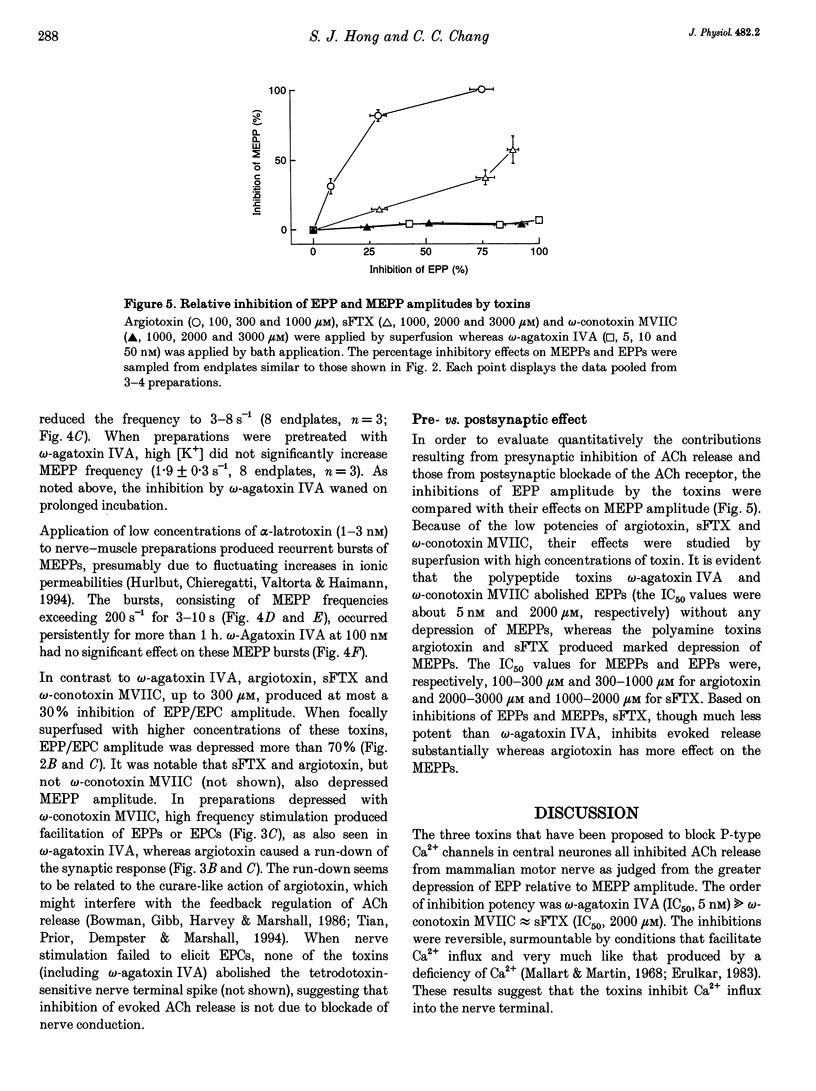
1. The effects were studied of the central neurone P-type Ca2+ channel blockers, omega-agatoxin IVA, omega-conotoxin MVIIC (polypeptide toxins) and synthetic funnel-web spider polyamine toxin on acetylcholine release from mouse motor nerve. 2. omega-Agatoxin IVA decreased the quantal content of endplate potentials and blocked synaptic transmission in the nanomolar range in a reversible manner, whereas the other toxins depressed transmission in the hundred micromolar range. 3. The polyamine toxin, but not the polypeptide toxins, decreased the amplitude of the miniature endplate potential. The increase in the frequency of miniature endplate potentials evoked by high [K+], but not that evoked by alpha-latrotoxin, was effectively antagonized by omega-agatoxin IVA. 4. In the presence of omega-agatoxin IVA, high frequency nerve stimulation produced facilitation of endplate currents and tetanic contractions. 5. The results suggest that, under physiological conditions, the Ca2+ necessary for nerve action potential-evoked acetylcholine release is translocated via a subtype of the P-type Ca2+ channel sensitive to omega-agatoxin IVA.
Full text
PDF
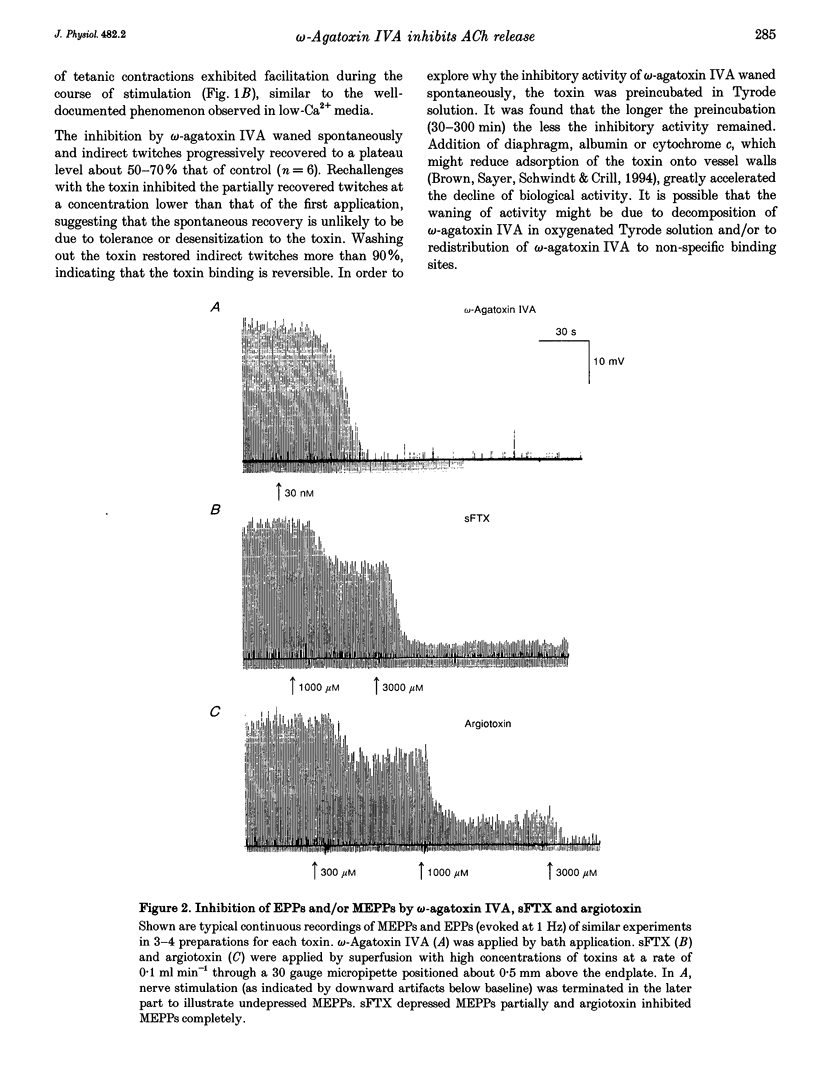


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchison W. D. Dihydropyridine-sensitive and -insensitive components of acetylcholine release from rat motor nerve terminals. J Pharmacol Exp Ther. 1989 Nov;251(2):672–678. [PubMed] [Google Scholar]
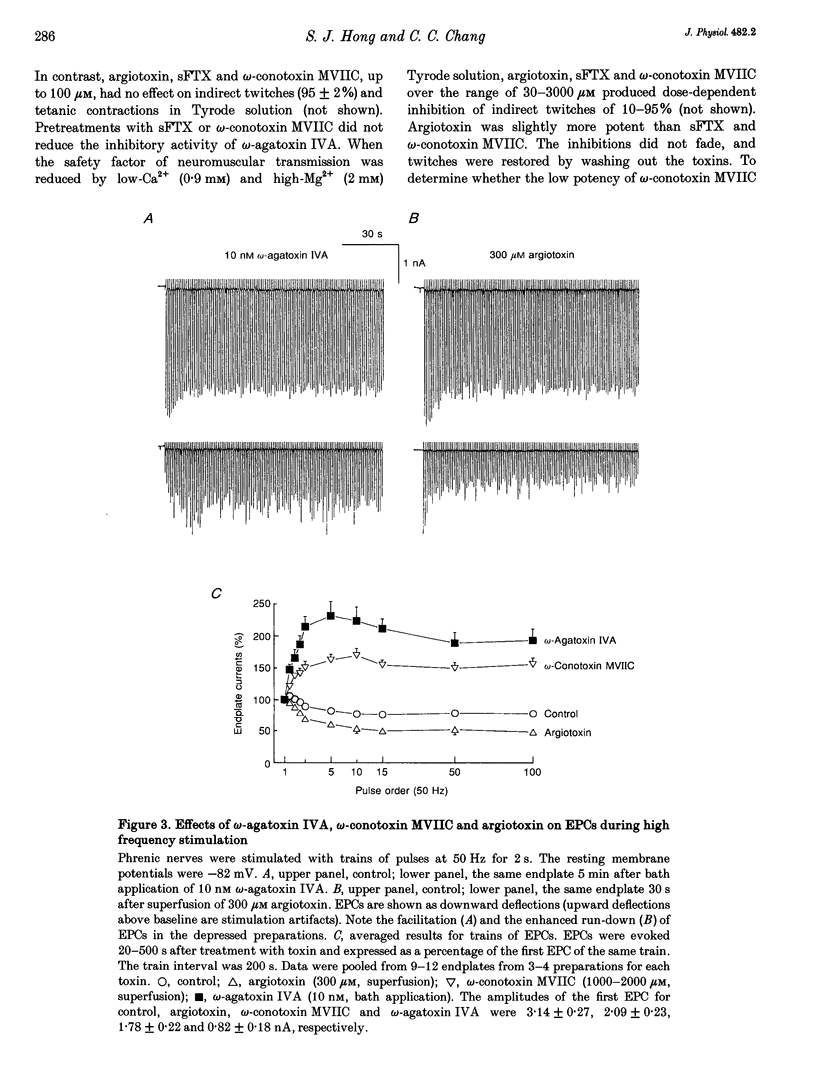
- Brown A. M., Sayer R. J., Schwindt P. C., Crill W. E. P-type calcium channels in rat neocortical neurones. J Physiol. 1994 Mar 1;475(2):197–205. doi: 10.1113/jphysiol.1994.sp020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinor P. T., Zhang J. F., Randall A. D., Zhou M., Schwarz T. L., Tsien R. W., Horne W. A. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993 Jun 3;363(6428):455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- Erulkar S. D. The modulation of neurotransmitter release at synaptic junctions. Rev Physiol Biochem Pharmacol. 1983;98:63–175. doi: 10.1007/BFb0033867. [DOI] [PubMed] [Google Scholar]
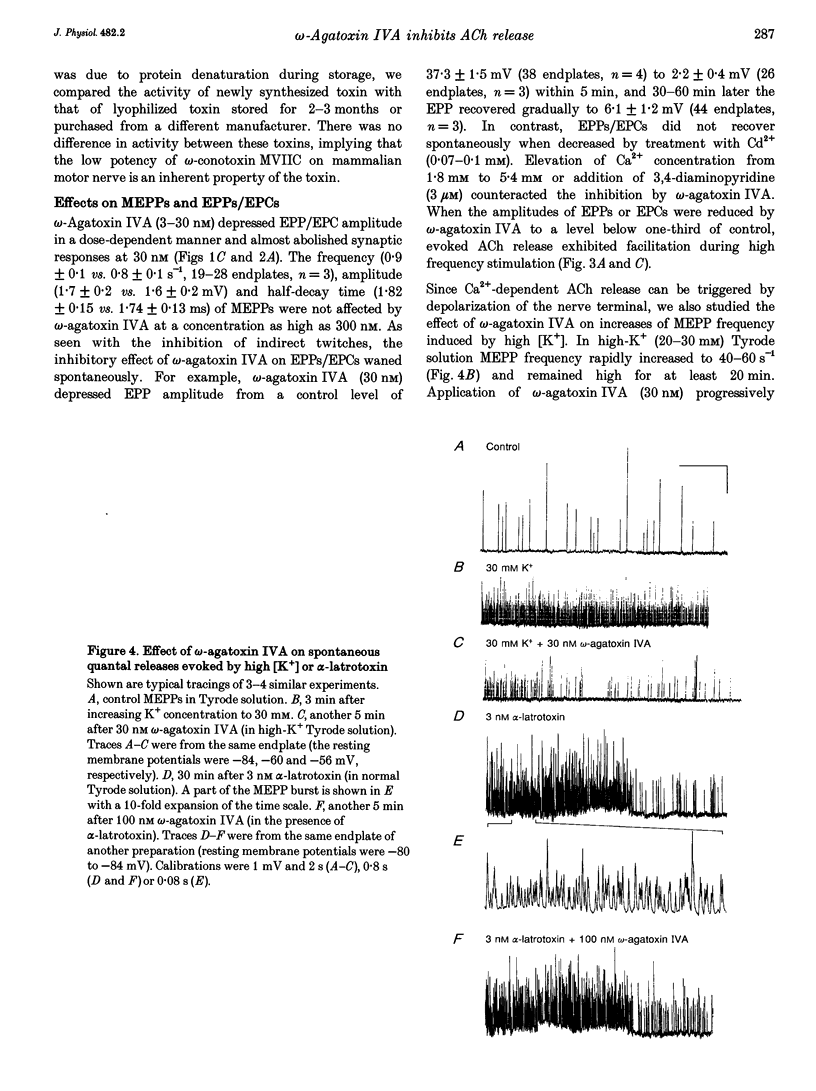
- Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- Hillyard D. R., Monje V. D., Mintz I. M., Bean B. P., Nadasdi L., Ramachandran J., Miljanich G., Azimi-Zoonooz A., McIntosh J. M., Cruz L. J. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992 Jul;9(1):69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- Hong S. J., Chang C. C. Antagonism by tubocurarine and verapamil of the regenerative acetylcholine release from mouse motor nerve. Eur J Pharmacol. 1989 Mar 14;162(1):11–17. doi: 10.1016/0014-2999(89)90598-0. [DOI] [PubMed] [Google Scholar]
- Hong S. J., Chang C. C. Use of geographutoxin II (mu-conotoxin) for the study of neuromuscular transmission in mouse. Br J Pharmacol. 1989 Jul;97(3):934–940. doi: 10.1111/j.1476-5381.1989.tb12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. J., Tsuji K., Chang C. C. Inhibition by neosurugatoxin and omega-conotoxin of acetylcholine release and muscle and neuronal nicotinic receptors in mouse neuromuscular junction. Neuroscience. 1992;48(3):727–735. doi: 10.1016/0306-4522(92)90416-y. [DOI] [PubMed] [Google Scholar]
- Hurlbut W. P., Chieregatti E., Valtorta F., Haimann C. Alpha-latrotoxin channels in neuroblastoma cells. J Membr Biol. 1994 Feb;138(1):91–102. doi: 10.1007/BF00211072. [DOI] [PubMed] [Google Scholar]
- Kerr L. M., Yoshikami D. A venom peptide with a novel presynaptic blocking action. Nature. 1984 Mar 15;308(5956):282–284. doi: 10.1038/308282a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Hillman D. E., Cherksey B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992 Sep;15(9):351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke J. I., Dunlap K., Turner T. J. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993 Nov;11(5):895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. The relation between quantum content and facilitation at the neuromuscular junction of the frog. J Physiol. 1968 Jun;196(3):593–604. doi: 10.1113/jphysiol.1968.sp008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Priestley T., Woodruff G. N., Kemp J. A. Antagonism of responses to excitatory amino acids on rat cortical neurones by the spider toxin, argiotoxin636. Br J Pharmacol. 1989 Aug;97(4):1315–1323. doi: 10.1111/j.1476-5381.1989.tb12594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti D. A., Uchitel O. D. Transmitter release and presynaptic Ca2+ currents blocked by the spider toxin omega-Aga-IVA. Neuroreport. 1993 Dec 13;5(3):333–336. doi: 10.1097/00001756-199312000-00039. [DOI] [PubMed] [Google Scholar]
- Regehr W. G., Mintz I. M. Participation of multiple calcium channel types in transmission at single climbing fiber to Purkinje cell synapses. Neuron. 1994 Mar;12(3):605–613. doi: 10.1016/0896-6273(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Rosenthal L., Meldolesi J. Alpha-latrotoxin and related toxins. Pharmacol Ther. 1989;42(1):115–134. doi: 10.1016/0163-7258(89)90024-7. [DOI] [PubMed] [Google Scholar]
- Sano K., Enomoto K., Maeno T. Effects of synthetic omega-conotoxin, a new type Ca2+ antagonist, on frog and mouse neuromuscular transmission. Eur J Pharmacol. 1987 Sep 11;141(2):235–241. doi: 10.1016/0014-2999(87)90268-8. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Sutton K. G., Dolphin A. C. Interactions of polyamines with neuronal ion channels. Trends Neurosci. 1993 Apr;16(4):153–160. doi: 10.1016/0166-2236(93)90124-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993 Nov 11;366(6451):156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Tian L., Prior C., Dempster J., Marshall I. G. Nicotinic antagonist-produced frequency-dependent changes in acetylcholine release from rat motor nerve terminals. J Physiol. 1994 May 1;476(3):517–529. doi: 10.1113/jphysiol.1994.sp020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T. J., Adams M. E., Dunlap K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9518–9522. doi: 10.1073/pnas.90.20.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchitel O. D., Protti D. A., Sanchez V., Cherksey B. D., Sugimori M., Llinás R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. B., Randall A., Tsien R. W. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994 Apr 1;264(5155):107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]



