Abstract
Ferroptosis is a nonapoptotic form of cell death characterized by iron‐dependent lipid peroxidation in membrane phospholipids. Since its identification in 2012, extensive research has unveiled its involvement in the pathophysiology of numerous diseases, including cancers, neurodegenerative disorders, organ injuries, infectious diseases, autoimmune conditions, metabolic disorders, and skin diseases. Oxidizable lipids, overload iron, and compromised antioxidant systems are known as critical prerequisites for driving overwhelming lipid peroxidation, ultimately leading to plasma membrane rupture and ferroptotic cell death. However, the precise regulatory networks governing ferroptosis and ferroptosis‐targeted therapy in these diseases remain largely undefined, hindering the development of pharmacological agonists and antagonists. In this review, we first elucidate core mechanisms of ferroptosis and summarize its epigenetic modifications (e.g., histone modifications, DNA methylation, noncoding RNAs, and N6‐methyladenosine modification) and nonepigenetic modifications (e.g., genetic mutations, transcriptional regulation, and posttranslational modifications). We then discuss the association between ferroptosis and disease pathogenesis and explore therapeutic approaches for targeting ferroptosis. We also introduce potential clinical monitoring strategies for ferroptosis. Finally, we put forward several unresolved issues in which progress is needed to better understand ferroptosis. We hope this review will offer promise for the clinical application of ferroptosis‐targeted therapies in the context of human health and disease.
Keywords: epigenetics, ferroptosis, human disease, lipid peroxidation
The core molecules driving the induction and execution of ferroptosis represent promising therapeutic targets for treating ferroptosis‐related diseases.
1. INTRODUCTION
Regulated cell death (RCD) refers to a controllable and intervenable form of cell death, playing a fundamental role in maintaining homeostasis and biological processes. 1 Ferroptosis, first described in 2012 by the Stockwell laboratory during a screen for agents selectively lethal to RAS‐mutant cancer cells, represents a unique, nonapoptotic form of RCD.2 Unlike apoptosis, autophagy, and necroptosis, ferroptosis is driven by iron‐dependent overwhelming lipid peroxidation of polyunsaturated fatty acids (PUFAs) in membrane phospholipids, underscoring the pivotal role of lipid metabolism in its initiation and execution. 3 The oxidizable lipids in the form of PUFA‐containing phospholipids (PUFA‐PLs) provide the substrates for ferroptosis execution. 4 , 5 Under conditions of iron overload and compromised antioxidant defenses, these lipids undergo uncontrolled peroxidation, leading to plasma membrane permeabilization, rupture, and eventual cell death. 4 , 5
The regulation of ferroptosis is complex and involves both epigenetic modulators and nonepigenetic factors such as histone modifications, DNA methylation, noncoding RNAs (ncRNAs) regulation, N6‐methyladenosine (m6A) modification, genetic mutations, transcriptional regulators, and posttranslational modifications (PTMs). 5 , 6 , 7 , 8 These elements directly or indirectly control the governing mechanisms of ferroptosis, including iron accumulation, lipid metabolism, and antioxidant responses. Dysregulation of the ferroptotic network has been increasingly implicated in the pathogenesis of various diseases, such as cancers, neurodegenerative disorders, organ injuries, infectious diseases, autoimmune conditions, metabolic disorders, and skin diseases. 9 , 10 A detailed understanding of ferroptosis and its role in disease pathophysiology paves the way for innovative therapeutic strategies.
To date, several ferroptosis‐targeted therapies have been developed, focusing on modulating the key drivers of ferroptosis and offering potential new treatment options. This review overviews the core mechanisms of ferroptosis, focusing on its prerequisites and execution, and the intricate regulatory network of ferroptosis modulated by epigenetic and nonepigenetic factors was also illustrated. Additionally, we discuss the emerging role of ferroptosis in human disease and summarize the primary pharmacological strategies aimed at modulating this process. Last, we highlight potential clinical monitoring tools for ferroptosis, providing a foundation for future clinical applications.
2. CORE MECHANISMS OF FERROPTOSIS
Plasma membrane rupture represents the final phase in various forms of RCDs. In contrast to other RCDs that necessitate specific pore‐forming proteins for their execution, the occurrence of ferroptosis relies on distinct lipid‐centric mechanisms to disrupt plasma membrane integrity. 11 , 12 The peroxidation of PUFA‐PLs is a key event in ferroptosis, with oxidizable lipids, overloaded iron, and impaired antioxidant systems serving as critical prerequisites that drive this overwhelming peroxidation, ultimately resulting in plasma membrane rupture and ferroptotic cell death (Figure 1).
FIGURE 1.
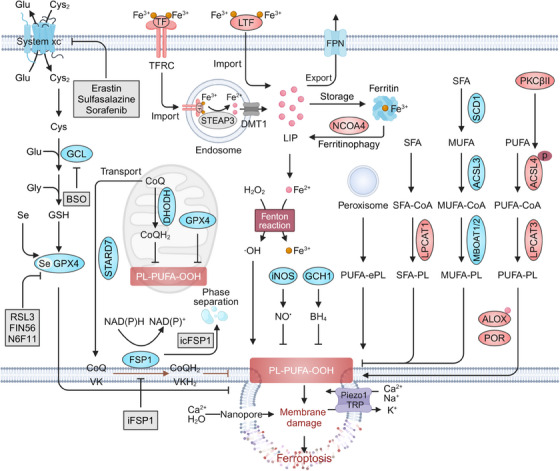
Core mechanisms of ferroptosis. Oxidizable lipids in the cellular membrane, particularly PUFA‐PLs mediated by ACSL4 and LPCAT3, are preferential substrates of iron‐dependent nonenzymatic and enzymatic lipid peroxidation. When GPX4‐dependent or ‐independent antioxidant systems (e.g., FSP1, DHODH, GCH1, iNOS, 7‐DHC) are compromised, cellular defense against lipid peroxidation diminishes, allowing uncontrolled lipid peroxidation. The lethal accumulation of lipid peroxidation overwhelms antioxidant defenses and membrane repair capacity, activating mechanosensitive cation channels, disrupting ion homeostasis, and ultimately leading to membrane rupture and ferroptotic cell death. ACSL4, acyl‐CoA synthetase long‐chain family member 4; ALOX, arachidonate lipoxygenase; BH4, tetrahydrobiopterin; BSO, buthionine sulfoximine; CoQ, coenzyme Q; Cys, cysteine; Cys2, cystine; DHODH, dihydroorotate dehydrogenase; DMT1, divalent metal transporter 1; FPN, ferroportin; FSP1, ferroptosis suppressor protein 1; GCH1, GTP cyclohydrolase‐1; GCL, glutamate–cysteine ligase; Glu, glutamate; GPX4, glutathione peroxidase 4; GSH, glutathione; H₂O₂, hydrogen peroxide; LIP, labile iron pool; iNOS, inducible nitric oxide synthase; LPCAT3, lysophosphatidylcholine acyltransferase 3; MBOAT, membrane bound O‐acyltransferase; MUFA, monounsaturated fatty acid; LTF, lactotransferrin; NCOA4, nuclear receptor coactivator 4; PKCβII, protein kinase C; POR, cytochrome P450 oxidoreductase; PUFA, polyunsaturated fatty acid; SCD1, stearoyl‐CoA desaturase; Se, selenium; SFA, saturated fatty acid; STARD7, StAR‐related lipid transfer domain‐containing 7; STEAP3, six‐transmembrane epithelial antigens of the prostate 3; TF, transferrin; TFRC, transferrin receptor; TRP, transient receptor potential; VK, vitamin K.
2.1. Ferroptosis prerequisites
2.1.1. Oxidizable lipids
The preferential substrates in the cellular membrane, particularly PUFA‐PLs, are essential for lipid oxidation and ferroptosis onset. This is because PUFA tails within PLs contain more than one double bond and bis‐allylic moieties, which are highly vulnerable to oxidative damage and converted to PL hydroperoxides (PL‐PUFA‐OOHs). 13 The incorporation of PUFA into PLs requires the action of lipid metabolism enzymes, specifically acyl‐CoA synthetase long‐chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3). 14 ACSL4 catalyzes the conversion of PUFA into PUFA‐CoAs, which can be integrated into PLs by LPCAT3. 15 The activity of ACSL4 can be amplified by protein kinase C (PKCβII) through phosphorylation at Thr328 and dimerization, thus facilitating ferroptosis. 16 Interestingly, recent research by Brent R. Stockwell's team has demonstrated that exogenous supplementation with PUFAs stimulates the biosynthesis of PLs with two PUFA tails (diacyl‐PUFA PLs; PL‐PUFA2s), 17 rather than those with a single PUFA tail (PL‐PUFA1s), which were previously considered as the primary substrates for lipid peroxidation. The formation of PL‐PUFA2s facilitates mitochondrial reactive oxygen species (ROS) production and lipid peroxidation, thus inducing ferroptosis in multiple cancer cell lines. 17 In addition to PUFA‐PLs, polyunsaturated ether phospholipids (PUFA‐ePLs), synthesized by peroxisomes, may also serve as substrates for lipid peroxidation and ferroptosis induction in certain cell lines. 18 , 19 Moreover, Ca2+‐independent phospholipase A2β (iPLA2β) can cleave oxidized PUFA residues from PLs to detoxicate lipid peroxidation and ferroptosis mediated by TP53 and ROS stress. 20
In contrast, monounsaturated fatty acids (MUFAs) are protective against ferroptosis by competitively inhibiting the biosynthesis of PL‐PUFA. 17 Biologically, endogenous MUFAs are converted by saturated fatty acids (SFAs) through stearoyl‐CoA desaturase, and ACSL3 catalyzes MUFAs into their corresponding MUFA‐CoAs. 21 , 22 Subsequently, members of membrane bound O‐acyltransferase (MBOAT) family, including MBOAT1 and MBOAT2, mediate the incorporation of MUFA‐CoA into PLs, thereby competitively reducing the levels of PLs with PUFA tails and suppressing ferroptosis. 23 Exogenous supplementation with MUFAs, such as oleic acid, can hamper the interaction between PUFA and PL‐PUFA1s to reduce PL‐PUFA2s content, particularly when co‐treated with PUFAs like docosahexaenoic acid. 17 This process contributes to the ferroptosis‐inhibitory role of MUFAs. In addition to MUFAs, the enzyme LPCAT1‐mediated incorporation of both endogenous and exogenous SFAs into PLs counteracts the levels of PUFA‐PLs in the membrane, thereby conferring resistance to ferroptosis. 24 In summary, the interplay between various lipids and associated lipid‐modifying enzymes is critical for regulating lipid peroxide production and determining vulnerability to ferroptosis.
2.1.2. Overloaded iron
In line with its name, iron is a primary driving force behind ferroptosis through both nonenzymatic and enzymatic processes. In biological systems, iron mainly varies between ferrous (Fe2+) and ferric (Fe3+) redox states. 25 Notably, the reaction between labile Fe2+ and hydrogen peroxide (H₂O₂) results in the formation of Fe3+ and hydroxyl radicals (OH•), a highly mobile and toxic form of ROS, and this process is commonly known as the Fenton reaction. 26 Hydroxyl radicals produced by Fenton reaction can attack PUFA‐PLs to initiate and propagate nonenzymatic lipid peroxidation and ferroptosis. 27 Moreover, iron and its derivatives serve as catalytic centers for various enzymes, such as arachidonate lipoxygenases (ALOXs) and cytochrome P450 (CYP450) oxidoreductase (POR), which are involved in the generation of lipid hydroperoxides. 27 Hence, manipulating labile iron levels can regulate cellular sensitivity to ferroptosis by a series of iron metabolism processes, including iron import, storage, utilization, and export.
Under physiological conditions, Fe3+‐bound transferrin (TF) is recognized and internalized via the membrane protein transferrin receptor (TFRC)‐mediated endocytosis. 28 Subsequently, Fe3+ can be reduced to Fe2+ by six‐transmembrane epithelial antigens of the prostate 3 within endosomes, and transported to the cytoplasm via divalent metal transporter 1 (DMT1/SLC11A2). 28 The released ferrous iron contributes to the formation of labile iron pool (LIP), which initiates both nonenzymatic and enzymatic lipid peroxidation. Interfering with this endocytosis process by genetic inhibition of TFRC indeed decreases Fe2+ levels and relieves ferroptosis. 29 Similarly, lactotransferrin is also implicated in the iron import and functions as a ferroptosis‐promoting factor. 30 The excess Fe2+ can bind to ferritin for iron storage. 31 Ferritin consists of ferritin heavy chain 1 (FTH1) and ferritin light chain (FTL), with only FTH1 possessing ferroxidase activity, which converts Fe2+ into nontoxic Fe3+ for storage. 32 Consequently, inhibition of ferritin expression, especially FTH1, increases labile iron levels and promotes ferroptosis. 33 , 34 In response to intracellular iron demand, ferritin can be selectively degraded by nuclear receptor coactivator 4 (NCOA4)‐mediated ferritinophagy to increase iron availability, which dictates ferroptosis sensitivity. 35 , 36 , 37 Additionally, excess Fe2+ can be exported by ferroportin (FPN/SLC40A1) in the cellular membrane. Therefore, it is not surprising that suppression of FPN expression can lead to increased cellular iron abundance and induce a proferroptotic state. 38 , 39 Together, the regulation of iron content through various factors controls cellular vulnerability to ferroptosis.
2.1.3. Compromised antioxidant systems
Ferroptosis is antagonized by two main antioxidant systems, and impairment of these antioxidant mechanisms can induce or sensitize cells to ferroptosis. The first system involves glutathione (GSH) peroxidase 4 (GPX4), the only known enzyme that directly reduces membrane PL peroxides to alcohols to terminate lipid peroxidation. 40 Notably, GPX4 is a selenoprotein, and selenium is essential for its expression and activity. 41 Supplementation with selenium enhances both the transcription and protein synthesis of GPX4. 42 , 43 , 44 In contrast, treatment with statins disrupts the translation of selenoprotein, particularly GPX4, leading to increased levels of cellular lipid peroxidation and heightened susceptibility to ferroptosis. 45 GPX4 detoxifies PL peroxides dependent on its active site, namely selenocysteine. Small molecule inhibitors, such as RSL3, can react with the selenocysteine of GPX4, resulting in direct inactivation of GPX4 and potent induction of ferroptosis. 46 Genetic ablation of GPX4 or enhanced degradation of GPX4 by pharmacological compounds (e.g., N6F11 and FIN56) also triggers an increase in lipid peroxides and consequent ferroptotic death. 47 , 48 , 49 Furthermore, GPX4 exists in three isoforms: mitochondrial, cytosolic, and nuclear GPX4. 4 Although mitochondrial GPX4 may contribute to inhibiting mitochondrial lipid peroxidation and ferroptosis, cytosolic GPX4 is generally regarded as the most crucial isoform for preventing ferroptosis. 50 Increasing evidence suggests that cationic residues in cytosolic GPX4 enable electrostatic interactions with the plasma membrane surface, catalyzing the reduction of PL‐PUFA‐OOHs via a charge‐driven substrate recognition mechanism, despite the absence of plasma membrane‐targeting signals in cytosolic GPX4. 51 GPX4 utilizes GSH as a cofactor to detoxify PL hydroperoxide. 40 , 47 GSH is synthesized from glycine, cysteine and glutamate via the enzyme glutamate–cysteine ligase (GCL), with cysteine serving as the rate‐limiting factor. 52 Cells mainly import cystine (the oxidized form of cysteine) via the system xc−, a cystine‐glutamate antiporter composed of solute carrier family 7 member 11 (SLC7A11) and solute carrier family 3 member 2 (SLC3A2). 53 Once imported, cystine is immediately reduced to cysteine through an NADPH‐consuming reaction. 54 Consequently, blocking system xc− activity with pharmacological agents including erastin, sulfasalazine (SAS), and sorafenib could result in impaired cystine uptake, GSH depletion, indirect inactivation of GPX4, and ultimate ferroptosis induction. 2 , 55 , 56 Similarly, culturing cells in cystine‐starved medium triggers the rapid loss of GSH and GPX4 inactivation, thus inducing ferroptosis. 57 Direct inhibition of GSH synthesis using the GCL inhibitor buthionine sulfoximine (BSO) also inactivates GPX4 and triggers ferroptosis in certain cell lines. 40 Together, system xc−‐mediated cystine import, GSH synthesis, and GPX4 activity constitute a robust ferroptosis protection system, and intervention in this pathway can lead to ferroptotic cell death.
Although the system xc−–GSH–GPX4 system is the center of ferroptosis surveillance, GPX4‐independent mechanisms have been identified to protect against ferroptosis. Other systems are controlled by enzymes including ferroptosis suppressor protein 1 (FSP1), 58 , 59 dihydroorotate dehydrogenase (DHODH), 50 GTP cyclohydrolase‐1 (GCH1), 60 and inducible nitric oxide synthase (iNOS). 61 These enzymes generate metabolites with lipophilic radical‐trapping antioxidant (RTA) properties, thereby effectively interrupting PL peroxidation cascades. Thus, interfering with these antioxidant systems can promote the accumulation of lipid peroxides and ferroptosis. FSP1 is a NAD(P)H‐ubiquinone reductase that can reduce coenzyme Q (CoQ) and vitamin K to their corresponding hydroquinone (CoQH2 and VKH2), which function as potent RTAs and prevent lipid peroxidation. 58 , 59 , 62 Notably, the plasma‐membrane localization of FSP1, mediated by the N‐terminal myristoylation, is essential for its ferroptosis‐suppressing activity. 58 Dissociation of FSP1 from the membrane and its phase separation induced by the compound icFSP1, or direct inhibition of FSP1 enzyme activity via iFSP1, could significantly enhance ferroptosis. 59 , 63 In addition, DHODH, a mitochondrial enzyme involved in pyrimidine biosynthesis, suppresses mitochondrial lipid peroxidation by reducing CoQ to CoQH2, which coordinates with mitochondrial GPX4 to prevent ferroptosis within the mitochondria. 50 Inhibition or inactivation of DHODH has been linked to the promotion of ferroptosis in cancer cells characterized by low mitochondrial GPX4 expression. Furthermore, CoQ biosynthesis relies on StAR‐related lipid transfer domain‐containing 7 (STARD7) within mitochondria. 64 The transport of CoQ from mitochondria to the cytoplasm and plasm membrane requires the presence of cytosolic STARD7. 64 Both processes are essential for CoQ‐dependent antioxidant defense against ferroptosis. 64 Moreover, GCH1 is implicated in the biosynthesis of the antioxidant tetrahydrobiopterin (BH4), which selectively protects PL‐PUFA2s from oxidative damage, thus safeguarding cells from ferroptosis. 60 Inhibition of GCH1 expression or the regeneration of BH4 favors a proferroptotic state in cancer cells. 65 , 66 In addition to CoQH2, VKH2, and BH4, nitric oxide (NO•) derived from iNOS acts as another GPX4‐independent ferroptosis resistance factor, probably through its interaction with 15‐lipoxygenase (15‐LOX) and lipid radicals generated by 15‐LOX. 61 M2 macrophages express lower levels of iNOS and NO• compared with M1 macrophages, rendering them more vulnerable to ferroptosis. 61 Metabolites involved in cholesterol biosynthesis, such as 7‐dehydrocholesterol (7‐DHC), also display ferroptosis‐modulating activity. 67 , 68 , 69 By reducing lipid peroxyl radicals, 7‐DHC neutralizes PL peroxidation in both the plasma membrane and mitochondria, thus alleviating ferroptosis. 67 , 68 Inhibition of 7‐DHC synthesis through the deletion of the upstream enzyme sterol‐C5‐desaturase significantly increases cellular sensitivity to ferroptosis. 67 , 68 Collectively, these endogenous RTAs and associated metabolic enzymes form a complex network for scavenging deleterious lipid hydroperoxides. Inhibition of RTA biosynthesis by genetic or pharmacological approaches sensitizes cells to ferroptosis across diverse contexts.
2.2. Ferroptosis execution
In most forms of RCD, the terminal events typically involve permeabilization and plasma membrane rupture. 70 Ferroptosis is primarily executed through mechanisms centered on lipid peroxidation, which regulates plasma membrane integrity. Lipid peroxidation occurs in three phases including initiation, propagation, and termination. 26 The initiation of PL peroxidation involves both nonenzymatic and enzymatic mechanisms, as described above. Once PL peroxidation is initiated and not promptly neutralized, an auto‐amplifying lipid peroxidation chain reaction will occur. During this propagation process, the phospholipid radical (PL•) reacts with molecular oxygen, leading to formation of the phospholipid peroxyl radical (PLOO•). This peroxyl radical subsequently interacts with a PUFA within the PL, generating a lipid peroxide (PLOOH) and another new PL•, which can initiate another radical chain reaction. This autooxidation process can be terminated by GPX4 and GPX4‐independent antioxidant systems. Upon proferroptotic stimuli, the accumulation of lipid peroxides seems to occur in a sequential manner across different subcellular locations. Recent studies show that lipid peroxidation first accumulates in the endoplasmic reticulum membrane, followed by further cumulation in the plasma membrane, both of which are crucial for the initiation of ferroptotic cell death. 71 , 72
The accumulation of lipid peroxides in the plasma membrane could increase plasma membrane tension, which subsequently activates mechanosensitive cation channels, including Piezo1 and transient receptor potential. 12 The opening of these channels causes an influx of Ca2+ and Na+, alongside an efflux of K+. Meanwhile, the inactivation of Na+/K+‐ATPase cooperatively potentiates the imbalance in ion fluxes. 12 The loss of ion homeostasis and subsequent osmotic changes across the membrane lead to cell rounding and plasma membrane breakdown. 12 Blocking this osmotic process with high molecular weight polyethyleneglycol, an osmoprotectant, significantly delays cell swelling, plasma membrane damage, and ferroptotic cell death. 73 , 74 The formation of nanoscale pores in the membrane has been observed during ferroptosis. 73 , 74 The opening of nanopores facilitates Ca2+ and water influx, leading to osmotic swelling, plasma membrane breakdown, and ultimately ferroptotic cell death. 73 , 74 However, it remains unclear whether pore‐forming proteins, such as gasdermin family proteins—known for mediating membrane rupture during pyroptosis—contribute to pore formation and permeabilization in ferroptotic cells. Furthermore, Ca2+ influx during ferroptosis could activate the endosomal sorting complexes required for transport (ESCRT)‐III complex. 73 , 75 ESCRT‐III typically mediates plasma membrane repair in response to necroptosis or pyroptosis. 76 , 77 Genetic inhibition of ESCRT‐III has been shown to increase sensitivity to ferroptosis, suggesting its membrane repair role in ferroptosis. These findings indicate the existence of a complex interplay within cells between plasma membrane damage and repair, and ferroptosis appears to execute when the damage is overwhelming.
3. EPIGENETIC AND NONEPIGENETIC REGULATION IN FERROPTOSIS
Ferroptosis response is governed by a complex network involving both epigenetic modifications (e.g., histone modifications, DNA methylation, ncRNAs, and m6A modification) and nonepigenetic modifications (e.g., genetic mutations, transcriptional regulation, and PTMs) (Figures 2 and 3). These modifications can influence its sensitivity or trigger ferroptosis by dynamically regulating the expression and activity of key ferroptosis‐related molecules, providing potential personalized targets for the development of ferroptosis‐based therapies.
FIGURE 2.
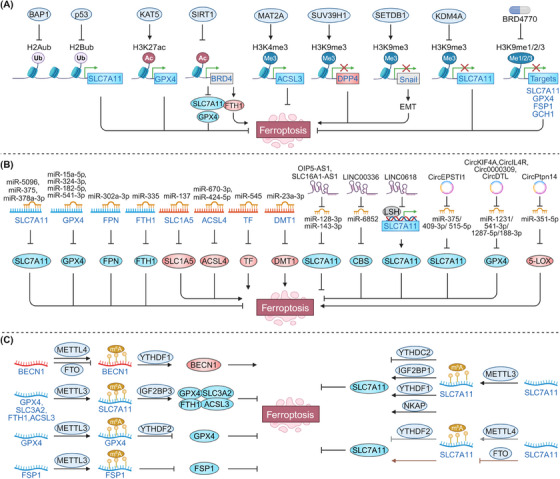
Epigenetic regulation in ferroptosis. (A) Posttranslational modifications of histones, such as acylation, methylation, and ubiquitination, regulate DNA accessibility and the expression of ferroptosis‐related genes, thereby modulating the cellular ferroptosis response. (B) miRNAs regulate ferroptosis by inhibiting mRNA translation or promoting mRNA degradation, while lncRNAs and circRNAs function as competing endogenous RNAs (ceRNAs), sponging miRNAs to modulate the expression of ferroptosis‐related genes such as SLC7A11 and GPX4. (C) m6A modifications regulate ferroptosis by altering the mRNA stability of key genes such as SLC7A11, GPX4, and FSP1 through the coordinated actions of methyltransferases, demethylases, and reader proteins. ACSL4, acyl‐CoA synthetase long‐chain family member 4; BAP1, BRCA1‐associated deubiquitinase 1; BECN1, beclin 1; CBS, cystathionine beta‐synthase; DMT1, divalent metal transporter 1; DPP4, dipeptidyl peptidase 4; FPN, ferroportin; FSP1, ferroptosis suppressor protein 1; FTH1, ferritin heavy chain 1; FTO, FTO alpha‐ketoglutarate dependent dioxygenase; GCH1, GTP cyclohydrolase‐1; GPX4, glutathione peroxidase 4; H2Aub, ubiquitination of histones H2A; H2Bub, ubiquitination of histones H2B; IGF2BP3, insulin‐like growth factor 2 mRNA binding protein 3; KAT5, lysine acetyltransferase 5; LSH, lymphoid‐specific helicase; NKAP, NF‐κB activating protein; m6A, N6‐methyladenosine; MAT2A, methionine adenosyltransferase 2A; METTL4, methyltransferase‐like 4; SIRT1, sirtuin 1; SLC1A5, solute carrier family 1 member 5; SLC3A2, solute carrier family 3 member 2; SLC7A11, solute carrier family 7 member 11; Snail, snail family transcriptional repressor 1; TF, transferrin; YTHDF1, YTH N6‐methyladenosine RNA binding protein F1.
FIGURE 3.
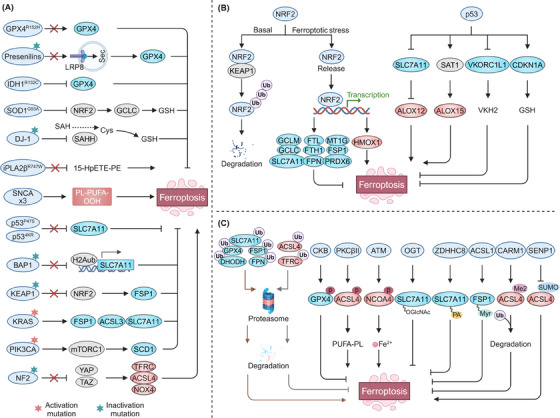
Nonepigenetic regulation in ferroptosis. (A) Genetic mutations in neurodegenerative diseases and cancers are key modulators of pathways influencing ferroptosis susceptibility as shown. (B) NRF2 transcriptionally regulates genes involved in GSH and GPX4 biosynthesis, iron metabolism, NADPH production, and FSP1, thereby modulating cellular susceptibility to ferroptosis. TP53 transcriptionally inhibits SLC7A11 and VKORC1L1 and upregulates SAT1, sensitizing cells to ferroptosis. However, under cystine deprivation, TP53 suppresses ferroptosis by promoting CDKN1A expression. (C) Core ferroptosis‐regulating proteins, including SLC7A11, GPX4, ACSL4, FSP1, and DHODH, can undergo multiple PTMs, such as ubiquitination, phosphorylation, acetylation, O‐GlcNAcylation, S‐palmitoylation, N‐myristoylation, methylation, and SUMOylation, thereby influencing ferroptosis sensitivity. ACSL4, acyl‐CoA synthetase long‐chain family member 4; ALOX, arachidonate lipoxygenase; BAP1, BRCA1‐associated deubiquitinase 1; CARM1, coactivator‐associated arginine methyltransferase 1; DHODH, dihydroorotate dehydrogenase; EGFR, epidermal growth factor receptor; FPN, ferroportin; FSP1, ferroptosis suppressor protein 1; FTH1, ferritin heavy chain 1; GCH1, GTP cyclohydrolase‐1; GPX4, glutathione peroxidase 4; GSH, glutathione; HMOX1, heme oxygenase 1; IDH1, isocitrate dehydrogenase 1; iPLA2β, phospholipase A2β; KEAP1, kelch‐like ECH‐associated protein 1; NF2, neurofibromin 2; NRF2, nuclear factor erythroid 2‐related factor 2; OGT, O‐linked N‐acetylglucosamine (GlcNAc) transferase; SAHH, s‐adenosylhomocysteine hydrolase; SAT1, spermidine/spermine N1‐acetyltransferase 1; SCD1, stearoyl‐CoA desaturase; SENP1, SUMO‐specific peptidase 1; SOD1, superoxide dismutase 1; TF, transferrin; TFRC, transferrin receptor; VKORC1L1, vitamin K epoxide reductase complex subunit 1‐like 1. GCLC, glutamate–cysteine ligase catalytic subunit; SNCA, synuclein α; PIK3CA, phosphatidylinositol‐4,5‐bisphosphate 3‐kinase catalytic subunit α; YAP, Yes1‐associated transcriptional regulator; TAZ, transcriptional coactivator with PDZ‐binding motif; NOX4, NADPH oxidase 4; CDKN1A, cyclin dependent kinase inhibitor 1A; CKB, creatine kinase B; ATM, ATM serine/threonine kinase; ZDHHC8, zinc finger DHHC‐type containing 8.
3.1. Epigenetic regulation in ferroptosis
3.1.1. Histone modifications
Histones are the fundamental protein components of chromatin. Histone octamers, consisting of two copies each of four histones (H2A, H2B, H3, and H4), are wrapped by DNA forming the basic repeating unit of chromatin known as the nucleosome. Various modifications, including acylation, methylation, and ubiquitination, have been identified on the amino termini or tails of histones, which regulate DNA accessibility and the expression of ferroptosis‐related genes (Figure 2A).
All histone core proteins are subject to ubiquitination, with H2A and H2B being the most frequently modified. SLC7A11 can be epigenetically activated through the ubiquitination of histones H2A (H2Aub) and H2B (H2Bub). 78 , 79 , 80 , 81 Tumor suppressors such as BRCA1 associated with deubiquitinase 1 (BAP1) and TP53 reduce the occupancy of H2Aub and H2Bub at the SLC7A11 promoter in a de‐ubiquitination‐dependent manner, suppressing its expression and inducing ferroptosis. 78 , 79 Recent studies have also shown that the histone H2A deubiquitinase MYSM1 is essential for hematopoietic stem cells function by protecting against ferroptosis. Mechanistically, MYSM1 deficiency reduces the translation rate of ferroptosis‐protective genes, thereby increasing the vulnerability of hematopoietic stem cells to ferroptosis and impairing their function. 82
Histone acetylation typically serves as a positive regulatory modification, enhancing gene expression by reducing histone‐DNA interactions and loosening chromatin structure through the neutralization of histone's positive charge. 83 Inhibition of lysine acetyltransferase 5 (KAT5), which reduces H3K27ac abundance at the GPX4 promoter, downregulates GPX4 and promotes ferroptosis in breast cancer cells. 84 NAD+‐dependent histone deacetylases like SIRT1 and SIRT3 also induce ferroptosis by epigenetically inhibiting epithelial–mesenchymal transition in cancer cells. 85 , 86 Acetylation recognition by bromodomain‐containing (BRD) proteins, such as BRD4, further modulates ferroptosis sensitivity. The BRD4 inhibitor has been shown to elevate H3K4me3 and H3K27ac levels upstream of BRD4 by either inhibiting the histone methyltransferase G9a or enhancing histone deacetylase SIRT1 activity. This, in turn, disrupts BRD4's ability to recognize acetylation sites on histones at GPX4 and SLC7A11 genes, resulting in their downregulation and the subsequent induction of ferroptosis. 87 Thus, BRD4 inhibitors could be used alone or in combination with immunotherapy to kill several BRD4‐proficient tumors by inducing ferroptosis. 87 , 88 , 89 Furthermore, transcription factors like HIC1 and HNF4A compete with histone acetyltransferase KAT2B, impacting the transcriptional regulation of pro‐ and antiferroptosis genes and ferroptosis sensitivity. 90
Histone methylation also plays a significant role, with different sites and levels of methylation conferring distinct functions. H3K4me3 and H3K9me3 are among the most studied methylation markers in ferroptosis. H3K4me3 typically promotes transcription, while H3K9me3 represses it. 91 Methionine adenosyltransferase 2A (MAT2A) increases H3K4me3 occupancy at the ACSL3 promoter, enhancing ACSL3 expression and ferroptosis resistance. 92 Histone methyltransferase SUV39H1 catalyzes H3K9me3 on the DPP4 promoter, thereby repressing its expression and DPP4–NOX1 complex formation, ultimately inhibiting lipid peroxidation and ferroptosis in clear cell renal carcinoma. The histone methyltransferase SETDB1 has been shown to promote ferroptosis by enhancing E‐cadherin expression through increasing H3K9me3 levels on the Snail promoter. 93 In contrast, lysine demethylases KDM4A reduce H3K9me3 occupancy at the SLC7A11 promoter, upregulating SLC7A11 and resisting ferroptosis. 81 Additionally, BRD4770 has been reported to activate the expression of key ferroptosis‐regulatory genes, including FSP1, SLC7A11, GPX4, and GCH1, by inhibiting H3K9me1/2/3 modifications, thus conferring resistance to ferroptosis in vascular smooth muscle cells. 94
3.1.2. DNA methylation
DNA methylation involves the addition of a methyl group to cytosine residues within DNA, a process mediated by DNA methyltransferases. 95 This modification is typically associated with gene silencing. 95 Key ferroptosis defense genes, such as GPX4 and FSP1, can be silenced by DNA methylation, enhancing ferroptosis susceptibility. 96 , 97 For instance, glycine increases levels of the DNA methylation donor SAM, thereby promoting GPX4 methylation and inducing ferroptosis in rheumatoid arthritis. 96 Hypermethylation of the FSP1 promoter underpins the dependence of acute lymphoblastic leukemia on the GSH system, increasing its vulnerability to ferroptosis, 97 while DNA methylation silences lipid metabolism genes like ELOVL fatty acid elongase 5 and fatty acid desaturase 1, conferring resistance to ferroptosis in gastric cancers. 98
3.1.3. ncRNAs regulation
ncRNAs, accounting for over 90% of human genome‐derived RNAs, play critical roles in biological processes and disease. 99 ncRNAs are classified into various categories primarily according to their size, each playing a crucial role in regulating ferroptosis (Figure 2B). Among these, microRNAs (miRNAs) regulate ferroptosis by inhibiting messenger RNAs (mRNAs) translation or promoting mRNA degradation. 100 Specific miRNAs, such as miR‐5096, miR‐375, and miR‐378a‐3p, induce ferroptosis through the downregulation of SLC7A11. 101 , 102 , 103 Similarly, miRNAs including miR‐15a‐5p, miR‐324‐3p, miR‐182‐5p, and miR‐541‐3p facilitate ferroptosis by suppressing GPX4. 102 , 104 , 105 , 106 Conversely, miR‐137 inhibits ferroptosis by downregulating the glutamine transporter SLC1A5, 107 while miR‐670‐3p and miR‐424‐5p reduce ferroptosis by targeting the lipid metabolism gene ACSL4. 108 , 109 Moreover, miRNAs act as versatile modulators of ferroptosis by influencing various iron metabolism processes. For example, miR‐302a‐3p and miR‐335 inhibit FPN expression and enhance FTH1 degradation, thereby increasing cellular iron levels and promoting ferroptosis. 110 , 111 In contrast, miR‐545 and miR‐23a‐3p target TF and DMT1 to prevent iron accumulation, thus suppressing ferroptosis. 112 , 113
LncRNAs also regulate ferroptosis, often functioning as competing endogenous RNAs (ceRNAs). 99 By competitively binding to miRNAs, lncRNAs modulate the availability of miRNAs to interact with their target mRNAs, thereby influencing the expression of key genes involved in ferroptosis. For example, lncRNA OIP5‐AS1 and lncRNA SLC16A1‐AS1 upregulate SLC7A11 and inhibit ferroptosis by targeting miR‐128‐3p and miR‐143‐3p. 114 , 115 LncRNA LINC00336 promotes resistance to ferroptosis by upregulating cystathionine beta‐synthase and activating the transsulfuration pathway through competing with miR‐6852. 116 Additionally, lncRNAs interact with proteins to regulate gene expression and ferroptosis susceptibility. 117 , 118 , 119 For example, nuclear lncRNA LINC0618 interacts with lymphoid‐specific helicase, reducing its binding to the SLC7A11 promoter, thereby inhibiting SLC7A11 transcription and promoting ferroptosis. 120 Similarly, cytosolic lncRNA LINC00472/P53RRA binds to G3BP stress granule assembly factor 1 (G3BP1), displacing TP53 and retaining it in the nucleus, which in turn promotes ferroptosis by affecting the transcription of multiple metabolic genes. 121
Notably, circular RNAs (circRNAs), a subclass of lncRNAs generated through back‐splicing of pre‐mRNA, also act as ceRNAs in regulating ferroptosis sensitivity. CircEPSTI1 promotes SLC7A11 expression and inhibits ferroptosis by sponging several miRNAs, including miR‐375, miR‐409‐3p, and miR‐515‐5p. 122 CircKIF4A, circIL4R, circDTL, and circ0000309 protect against ferroptosis by enhancing GPX4 expression via competitive binding to miR‐1231, miR‐541‐3p, miR‐1287‐5p, and miR‐188‐3p, respectively. 105 , 123 , 124 , 125 Conversely, circPtpn14 promotes ferroptosis by targeting miR‐351‐5p, which has been reported to inhibit the expression of 5‐LOX. 126
3.1.4. m6A modification
m6A is the most prevalent internal modification in eukaryotic mRNA and refers to a methylation that takes place at the N6 site of adenosine. 127 This process is reversible, with methyltransferases (writers) adding the modification and demethylases (erasers) removing it, while reader proteins recognize the modified mRNA. 127 m6A modifications regulate ferroptosis by altering the mRNA stability of ferroptosis‐related genes (Figure 2C). Elevated levels of m6A modification have been observed during ferroptosis in hepatic stellate cells, attributed to increased expression of methyltransferase‐like 4 (METTL4) and decreased expression of the demethylase FTO. 128 Inhibiting this elevated m6A modification can suppress the stability of BECN1 mRNA mediated by the m6A reader protein YTH m6A RNA binding protein F1 (YTHDF1), thereby defending against ferroptosis. 128 Consistently, demethylase FTO reduces SLC7A11 expression through m6A demethylation, sensitizing cells to ferroptosis. 129 Methyltransferases METTL14 and METTL3 promote ferroptosis by accelerating the degradation of SLC7A11 mRNA through a YTHDF2 and YTHDC2‐dependent mechanism, respectively. 130 , 131 Interestingly, METTL3 can also stabilize and increase SLC7A11 mRNA levels, protecting against ferroptosis by facilitating the recognition of the m6A‐modified SLC7A11 motif by YTHDF1 and IGF2 mRNA‐binding protein 1. 132 , 133 Additionally, another m6A reader protein, NF‐κB activating protein, enhances the splicing of SLC7A11 mRNA in a METTL3‐dependent manner, further inhibiting ferroptosis. 134 Several antiferroptotic genes, including GPX4, ACSL3, FTH1, and SLC3A2, are stabilized by METTL3‐mediated m6A methylation via IGF2BP3 recognition, contributing to desensitization to ferroptosis. 135 METTL3 has also been implicated in promoting the methylation of FSP1 and GPX4 mRNAs, which inhibits their expression and increases vulnerability to ferroptosis. 136 , 137 , 138
3.2. Nonepigenetic regulation in ferroptosis
3.2.1. Genetic mutations
Ample evidence underscores the critical role of genetic mutations in the regulation of ferroptosis, laying the groundwork for understanding the involvement of ferroptosis in disease pathophysiology and identifying populations suitable for ferroptosis‐targeted therapies. 139 , 140 , 141 Currently, research on the role of genetic mutations in ferroptosis regulation focuses on neurodegenerative diseases and cancers, which are closely associated with genetic mutations (Figure 3A). 139 , 140 , 141 , 142
Neurodegenerative disease‐causing mutations in proteins play an important role in modulating pathways that affect ferroptosis susceptibility, supporting the notion that ferroptosis serves as a mechanism in the development of neurodegenerative diseases. For example, the R152H missense mutation in GPX4 leads to partially reduced enzymatic activity and impaired ferroptosis resistance, which could be linked to the pathological phenotypes observed in spondylometaphyseal dysplasia patients carrying this mutation. 143 Familial Alzheimer's disease and amyotrophic lateral sclerosis (ALS)‐associated mutations in presenilins and superoxide dismutase 1 confer ferroptosis vulnerability by inhibiting GPX4 expression through limiting LRP8‐mediated selenium uptake and impairing the NRF2 pathway and GSH synthesis, respectively. 144 , 145 Furthermore, Parkinson's disease‐related loss‐of‐function mutations in DJ‐1 (E64D, M26I, A104T, L166P) and iPLA2β (R747W) increase susceptibility to ferroptosis by suppressing s‐adenosylhomocysteine hydrolase‐mediated cysteine generation for GSH production and inhibiting the hydrolysis of 15‐HpETE from phosphatidylethanolamine (PE). 146 , 147 In addition, α‐synuclein triplication‐mediated increases in α‐synuclein levels confer vulnerability of neurons to ferroptosis by affecting ether‐linked phospholipid synthesis, contributing to familial Parkinson's disease pathology. 148 , 149
Moreover, loss‐of‐function mutations in tumor suppressor genes often confer resistance to ferroptosis, promoting tumorigenesis. For instance, acetylation‐deficient p53 mutants (p53 4KR: K117R, K161R, K162R, K98R) and the African‐specific S47 polymorphism (p53 P47S) contribute to ferroptosis resistance by impairing p53's ability to downregulate SLC7A11. 150 , 151 Similarly, loss‐of‐function mutations in BAP1 and kelch‐like ECH‐associated protein 1 (KEAP1) have also been reported to lose their ability to inhibit SLC7A11, thereby facilitating tumor growth. 78 , 152 , 153 KEAP1 mutations have also been shown to upregulate FSP1 expression, leading to ferroptosis resistance in non‐small cell lung cancer cells. 154 The activation of oncogenes can also confer resistance to ferroptosis. For example, oncogenic mutations in KRAS promote ferroptosis resistance by upregulating SLC7A11, ACSL3, and FSP1, while mutations in PIK3CA enhance mechanistic target of rapamycin kinase signaling, which also contributes to ferroptosis resistance. 155 , 156 , 157 , 158 Notably, in certain cases, mutations in tumour suppressors and oncogenes can confer vulnerability to ferroptosis in cancers. It was reported that inactivation of neurofibromin 2 renders cancer cells susceptible to ferroptosis through inhibiting YAP/TAZ signaling, whereas activation of epidermal growth factor receptor (EGFR) and isocitrate dehydrogenase 1 promote ferroptosis by inhibiting SLC7A11 and GPX4, respectively. 159 , 160 , 161
3.2.2. Transcriptional regulation
Transcriptional master regulators are critical in coordinating pathways that govern ferroptosis sensitivity. Numerous transcription factors, such as TP53, 162 , 163 , 164 NRF2, 154 , 157 , 165 , 166 , 167 NFE2L1, 159 , 168 , 169 YAP1/TAZ, 159 ATF3, 170 HIF2α, 171 ZEB1, 45 STAT1, 172 PPARα, 173 and MYCN, 174 , 175 , 176 can shape the ferroptosis threshold in cells by directly or indirectly modulating ferroptosis vulnerability‐governed genes or metabolites levels. Notably, the role of these transcription factors is context specific, as some transcription factors play significant roles in ferroptosis regulation in certain cell types but not in others. 4 In this section, we focus on the complex roles of transcription factors NRF2 and TP53 in ferroptosis regulation (Figure 3B).
NRF2 is a major transcriptional activator of antioxidant defense mechanisms. Under basal conditions, NRF2 is bound by KEAP1 and undergoes proteasomal degradation, but during ferroptosis, it is released and translocated to the nucleus to promote the expression of target genes. 34 Many of the genes involved in antiferroptotic pathway are targets of NRF2, such as genes involved in GSH biosynthesis (e.g., SLC7A11, GCLC, and GCLM), GPX4 synthesis (peroxiredoxin 6), iron regulation (e.g., FTH1/FTL and SLC40A1, and metallothionein 1G), and NADPH production (e.g., G6PD, PGD), as well as the key antiferroptosis factor FSP1. 153 , 154 , 177 , 178 NRF2 can positively regulate the transcription of these genes to resist ferroptosis. Notably, the role of NRF2 in resisting ferroptosis seems to be context‐dependent. In cells with high ferrous ion levels, it promotes ferroptosis by upregulating heme oxygenase 1 (HMOX1) expression. 179
TP53, another crucial regulator, exhibits dual roles in ferroptosis regulation. As mentioned above, TP53 can transcriptionally and epigenetically repress SLC7A11 expression, sensitizing ferroptosis through an ALOX12‐dependent lipid peroxidation response. 79 , 162 , 180 , 181 Moreover, TP53 sensitizes ferroptosis by promoting polyamine catabolism through the upregulation of spermidine/spermine N1‐acetyltransferase 1 (SAT1) and inhibiting vitamin K synthesis via downregulation of vitamin K epoxide reductase complex subunit 1‐like 1. 163 , 182 However, TP53 can suppress ferroptosis under conditions of cystine deprivation by promoting the expression of CDKN1A, thereby conserving intracellular GSH. 164
3.2.3. PTMs
PTMs of ferroptosis‐related proteins are crucial in modulating ferroptosis susceptibility by influencing protein structure, activity, localization, and function (Figure 3C). 8
Among these modifications, ubiquitination plays a pivotal role in protein degradation and stability via the proteasome system, directly impacting the levels of key proteins involved in ferroptosis. 183 , 184 Core ferroptosis proteins, such as SLC7A11, 185 GPX4, 186 ACSL4, 187 FSP1, 188 DHODH, 189 and iron metabolism‐related proteins including TFRC and SLC40A1, 190 , 191 , 192 can be directly labeled by ubiquitin to undergo ubiquitination‐proteasomal degradation, thereby affecting ferroptosis response. Moreover, linear ubiquitination, mediated by the HOIL‐interacting protein, has been shown to stabilize GPX4, thereby conferring protection against ferroptosis. 193 Interestingly, ubiquitination's role extends beyond protein degradation to influencing protein localization, which further regulates ferroptosis. For instance, a recent study demonstrated that the E3 ubiquitin ligase TRIM21 mediates K63‐linked ubiquitination of FSP1 at Lys322 and Lys366, promoting its translocation to the plasma membrane, thus mitigating ferroptosis. 194
Phosphorylation is another emerging mechanism regulating ferroptosis. Three key ferroptosis‐related proteins, GPX4, ACSL4, and NCOA4, are subject to phosphorylation, which modulates their ferroptotic function. Specifically, creatine kinase B‐induced phosphorylation of GPX4 at Ser104 inhibits ferroptosis by preventing its interaction with HSC70, thereby reducing autophagic degradation. 195 Similarly, phosphorylation of ACSL4 at Thr328 by PKCBII enhances its dimerization and activity, driving ferroptotic cell death. 16 Furthermore, the serine/threonine kinase ATM‐mediated phosphorylation of NCOA4 at Ser550 plays a crucial role in ferritinophagy and the ferroptosis induction. 37
Acetylation, O‐GlcNAcylation, S‐palmitoylation, N‐myristoylation, methylation, and small ubiquitin‐like modifier (SUMO)ylation also contribute to ferroptosis regulation. For example, inhibition of acetylation in ALOX12 reduces ferroptosis susceptibility. 196 O‐GlcNAc transferase‐mediated O‐GlcNAcylation and zinc finger DHHC‐type palmitoyltransferase 8 (ZDHHC8)‐mediated S‐palmitoylation of SLC7A11, 197 , 198 as well as ACSL1‐induced N‐myristoylation of FSP1, 58 , 199 enhance ferroptosis resistance. Conversely, coactivator‐associated arginine methyltransferase 1‐mediated methylation of ACSL4 reduces ferroptosis susceptibility, while inhibition of ACSL4 SUMOylation by SUMO‐specific peptidase 1 promotes ferroptosis. 200 , 201
Together, these findings underscore the multifaceted role of PTMs in the regulation of ferroptosis. The specific effects of PTMs on ferroptosis are likely determined by the substrate involved and the particular type and site of modification. A deeper understanding of the mechanisms through which PTMs regulate ferroptosis will provide insights for developing targeted therapies for ferroptosis‐related diseases.
4. THE ROLE OF FERROPTOSIS IN DISEASES
In recent years, ferroptosis has been recognized as a physiological process vital for maintaining homeostasis, particularly in tumor suppression. Dysregulated ferroptosis is implicated in the pathogenesis of various diseases, including cancer, neurodegenerative diseases, organ injury, infectious diseases, autoimmune diseases, metabolic diseases, and skin diseases (Figure 4). Impaired system xc−–GPX4 pathways, iron overload and elevated oxidizable lipids contents are common key ferroptotic mechanisms mediating these diseases. Deciphering the specific cellular and molecular mechanisms triggering ferroptosis across different diseases will facilitate the development of disease‐specific ferroptosis‐targeted therapeutic approaches.
FIGURE 4.
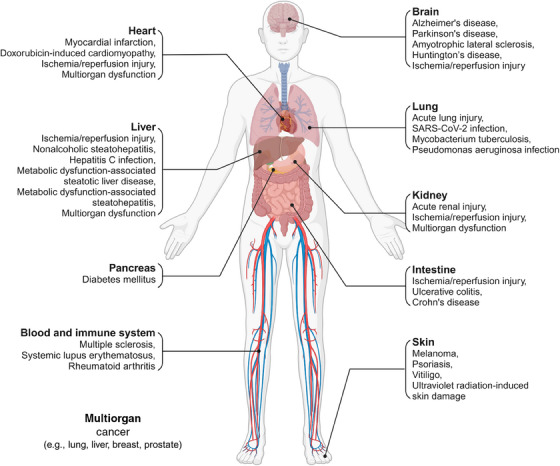
Role of ferroptosis in various diseases across different organs and tissues. Ferroptosis serves as an intrinsic tumor‐suppressive mechanism, with its evasion supporting tumorigenesis and progression. Additionally, ferroptosis activation is implicated in the pathogenesis of multiple neurodegenerative diseases, organ injuries, metabolic dysfunction‐associated steatotic liver disease, and dermatological conditions such as psoriasis, vitiligo, and UV‐induced skin damage. Notably, due to its complex interaction with the immune system, ferroptosis may exert dual effects, particularly in immune and infectious diseases.
4.1. Cancer
The initial identification of ferroptosis in RAS‐mutant cancer cells through a cytotoxicity screening of compounds established a direct connection between ferroptosis and cancer pathophysiology. 2 Since then, a growing body of evidence has elucidated the critical role of ferroptosis in tumor biology and its therapeutic potential. 202 , 203 Ferroptosis functions as an intrinsic defense mechanism against tumorigenesis and tumor progression. 162 The inability to induce ferroptosis may promote tumor development. For instance, the retention of tumor‐suppressive function in p533KR mutant, which possesses ferroptosis‐promoting capabilities while lacking traditional abilities to promote cell‐cycle arrest, apoptosis, and senescence, alongside the loss of tumor‐suppressive function in p534KR mutant, which lacks ferroptosis regulatory activity, support the above viewpoint. 151 , 162 Besides, the reports of several tumor suppressor proteins function as ferroptosis promoters also provide evidence for this. 202 , 204
To counteract this intrinsic tumor suppressive mechanism, tumors have developed multiple mechanisms to evade ferroptosis, which supports their growth and metastasis. 5 , 202 These include inhibition of PUFA‐PLs synthesis and peroxidation, restriction of labile iron availability, and upregulation of cellular defense systems such as SLC7A11, GPX4, and FSP1, all of which enable cancer cells to bypass ferroptosis and continue proliferating. 5 Of particular interest is the protective role of lymph fluid in metastasizing melanoma cells, where elevated levels of oleic acid and reduced free iron create an environment that shields these cells from ferroptosis, facilitating their metastasis. 205
Despite these evasive mechanisms, certain cancer cells exhibit susceptibility to ferroptosis due to oncogene addiction and metabolic reprogramming, which provides a potential vulnerability for overcoming both intrinsic and acquired resistance to therapy. 5 , 45 , 160 , 206 , 207 For example, EGFR‐mutant non‐small cell lung cancer cells, as well as de‐differentiated and persistent cancer cells are vulnerable to ferroptosis due to their dependence on cystine and metabolic rewiring or the acquisition of a mesenchymal state. 45 , 160 , 206 Inducing ferroptosis has been functionally validated as an effective approach to suppress tumor growth across multiple cancer models in preclinical studies. 14 , 34 , 57 , 208
Additionally, ferroptosis not only directly modulates the fate of tumor cells but also plays a significant role in the tumor microenvironment to influence tumor development. Ferroptosis exhibits dual effects in antitumor immunity by directly modulating the fate and function of immune cells and indirectly inducing the release of multiple signals from ferroptotic cancer cells (e.g., DAMPs, MHC class I molecules, cytokines, and PTGS2). 202 The complex interplay between ferroptosis and the tumor microenvironment highlights the significance of therapeutic time window for ferroptosis‐targeted therapy in cancer. On one hand, owing to the immunostimulatory effects of ferroptosis and its involvement in immunotherapy, ferroptosis induction appears to be a promising strategy for enhancing antitumor immunity and offers synergistic effects when combined with immunotherapy to kill well‐established tumors. 172 , 209 , 210 , 211 On the other hand, ferroptosis inhibition also could impede tumorigenesis, particularly in early‐stage tumors, due to the immune‐suppressive effect of ferroptosis in polymorphonuclear myeloid‐derived suppressor cells. 212 , 213 Thus, ferroptosis‐targeted therapy in cancer must consider the intricate nature of ferroptosis in the tumor microenvironment.
4.2. Neurodegenerative diseases
Neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease, ALS, and Huntington's disease, are characterized by progressive neuronal death and neurological dysfunction. 214 Although the exact pathogenesis of neurodegenerative diseases remains unclear, a common pathophysiological hallmark, including iron accumulation and lipid peroxidation within affected regions, suggests that ferroptosis, an iron‐dependent form of cell death, plays a significant role in neuronal degeneration in these disorders. 27 , 140 , 215 , 216 , 217 , 218 Furthermore, glutamate excitotoxicity, a key contributor to neurodegenerative diseases, may also involve ferroptosis by inhibiting system xc− and triggering STING‐dependent autophagic degradation of GPX4. 2 , 219 , 220 Several pathogenic genes and proteins implicated in neurodegenerative diseases, such as DJ‐1 and PLA2G6 in Parkinson's disease and β‐amyloid and tau in Alzheimer's disease, have been associated with ferroptosis. 146 , 147 , 221 , 222
Experimental models have demonstrated that conditional genetic deletion of GPX4, a key ferroptosis regulator, leads to neurodegenerative phenotypes, including cognitive impairment and motor neuron death. 145 , 223 Ferroptosis inhibitors, lipophilic antioxidants, and iron chelators have shown promise in ameliorating neurodegeneration in ALS, Alzheimer's disease, and Parkinson's disease. 177 , 223 , 224 , 225 Indeed, several approved drugs for neurodegenerative diseases, such as idebenone for Alzheimer's disease, and edaravone for ALS, have been proven to alleviate neurodegeneration by inhibiting ferroptosis. 226 , 227 , 228 Additionally, Copper(II)‐diacetylbis(N4‐methylthiosemicarbazone) (CuATSM), a novel drug currently undergoing clinical trials for patients with neurodegenerative diseases, has also been reported to possess antiferroptotic properties. 229 All of these findings suggest that ferroptosis is a contributing factor in neurodegenerative diseases, and its inhibition may serve as a promising therapeutic strategy for these conditions.
4.3. Organ injury
Ferroptosis has been identified as a key driver of tissue injury across multiple organs, with varying susceptibility across different organs and cell types. 7 Transgenic studies in mice have shown that proximal renal tubule cells are particularly sensitive to ferroptosis outside of the brain, as evidenced by the spontaneous development of acute renal failure in tamoxifen‐induced Gpx4 knockout mice. 47 Ischemia/reperfusion‐induced organ damage, which underlies multiple devastating diseases, including myocardial infarction 230 and stroke, 42 , 231 as well as injuries in other organs such as the liver, 47 kidney, 232 lung, 233 and intestine, 234 has been partially identified as a consequence of ferroptotic cell death. Inhibition of ferroptosis has consistently alleviated ischemia/reperfusion‐induced damage in various preclinical models. Furthermore, suppressing ferroptosis presents a promising therapeutic approach for improving outcomes in solid organ transplantation, where ischemia/reperfusion injury is an inevitable complication during implantation. 235 , 236
Beyond ischemia/reperfusion injury, ferroptosis has also been observed in other modes of organ injury, including doxorubicin (DOX)‐induced cardiomyopathy, 230 rhabdomyolysis‐induced kidney injury, 237 and environmental pollutants‐associated liver and lung injuries. 238 , 239 In critically ill patients, features of ferroptosis, including elevated malondialdehyde (MDA) and catalytic iron levels, have been observed in cases of multiorgan dysfunction, suggesting that ferroptosis inhibition may offer therapeutic benefits in these contexts. 240
4.4. Infectious diseases
Ferroptosis has been reported to be activated during several infectious diseases and is associated with the burden of infections. Pseudomonas aeruginosa triggers ferroptosis in bronchial epithelial cells by secreting ALOX15, which catalyzes the oxidation of host arachidonic acid‐phosphatidylethanolamine (AA‐PE) into 15‐hydroperoxy‐AA‐PE (15‐HOO‐AA‐PE). 241 Elevated levels of oxidized AA‐PE correlate with worse clinical outcomes, likely due to ALOX15‐mediated ferroptotic activity promoting persistent biofilm formation and compromising the bronchial epithelial barrier function. 241 Similarly, Mycobacterium tuberculosis induces ferroptosis in macrophages, and ferroptosis inhibitors significantly reduce bacterial burden in infected mice. 242 Supporting this, zebrafish Hmox1a protects against Mycobacterium marinum infection by limiting iron availability and reducing susceptibility to ferroptosis. 243 In contrast, ferroptosis has been shown to inhibit hepatitis C virus (HCV) replication by altering the conformation of the HCV replicase complex through lipid peroxidation, with FADS2‐dependent fatty acid desaturation playing a key role. 244 Additionally, ferroptotic characteristics, such as lipid alterations and upregulation of TFRC, have been observed in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐infected hamsters, suggesting a potential link between SARS‐CoV‐2 infection and ferroptosis. 245 However, the precise causal relationship between ferroptosis and these infections, as well as its role in subsequent inflammatory responses, requires further investigation.
4.5. Autoimmune diseases
Ferroptosis has been implicated in autoimmune diseases, such as multiple sclerosis, systemic lupus erythematosus (SLE), inflammatory bowel disease, and rheumatoid arthritis. Recent research identified STING‐dependent ferroptosis in neurons as a critical regulator in inflammation‐induced neurodegeneration, including multiple sclerosis. 220 Moreover, ferroptotic neurons have been shown to enhance T‐cell activation by modulating T‐cell receptor signaling, thereby accelerating the progression of experimental autoimmune encephalitis, the murine model of multiple sclerosis. 246 These findings suggest that ferroptosis plays a detrimental role in the development of multiple sclerosis. Similarly, in SLE, autoantibody‐ and interferon‐alpha‐mediated suppression of GPX4 induces ferroptosis in neutrophils, contributing to the immunopathogenesis of the disease. Inhibiting neutrophil ferroptosis has been shown to significantly reduce lupus severity in mice. 247 Ferroptosis is also associated with the progression of SLE‐related conditions, such as lupus nephritis, 248 likely due to the susceptibility of human proximal tubular cells to ferroptotic triggers present in lupus serum. Further research is needed to identify endogenous triggers of ferroptosis in SLE, which may illuminate the disease's pathological processes and provide novel therapeutic targets. In inflammatory bowel disease, ferroptosis is notably elevated in intestinal epithelial cells in both ulcerative colitis and Crohn's disease, accompanied by reduced GPX4 activity. 249 , 250 Additionally, Gpx4‐deficient mice fed with PUFAs develop enteritis resembling Crohn's disease, underscoring the role of ferroptosis in inflammatory bowel disease pathogenesis. 250 In the context of rheumatoid arthritis, enhanced ferroptosis in chondrocytes and anti‐inflammatory macrophages correlates with disease progression and severity, while promoting ferroptosis in synovial fibroblasts has been shown to alleviate inflammation and improve symptoms. 251 , 252 , 253 Therefore, targeting cell‐specific ferroptosis offers promising therapeutic strategies for autoimmune diseases.
4.6. Metabolic disease
Ferroptosis, as a consequence of dysregulated metabolism, has increasingly been linked to metabolic disorders, such as metabolic dysfunction‐associated steatotic liver disease (MASLD) and diabetes mellitus. 254 In the liver, ferroptotic stress contributes to the onset and progression of MASLD. 255 Initially, hepatic iron overload and lipid peroxidation induce ferroptosis, leading to the formation of lipid droplets and simple hepatic steatosis, characterized by lipid accumulation. 255 Over time, excessive lipid buildup disrupts hepatic lipid metabolism, exacerbating ferroptosis‐mediated hepatocyte damage and triggering inflammatory responses. This can drive the progression from simple hepatic steatosis to metabolic dysfunction‐associated steatohepatitis (MASH), a more advanced stage of MASLD. 255 , 256 , 257 In addition to hepatocyte ferroptosis, a recent study found that NCF1‐mediated ferroptosis in Kupffer cells worsens MASH progression. 258 Inhibition of ferroptosis has been shown to significantly alleviate MASH and its progression toward fibrosis and hepatocellular carcinoma. 258 , 259 , 260 , 261 , 262
In diabetes, ferroptosis‐associated pathways are activated in response to hyperglycemia. 263 , 264 Activated ferroptosis contributes to pancreatic β‐cell dysfunction and the development of various diabetic complications. 56 , 263 , 264 , 265 , 266 However, a recent study revealed that the deficiency of CXCL16 in islet‐resident macrophages leads to excessive exposure to oxidized low‐density lipoprotein, which promotes ferroptosis in pathogenic CD8+ T cells, thereby inhibiting the progression of diabetes. 267 These findings underscore the complexity of ferroptosis in metabolic diseases. Further research is required to elucidate the interactions between ferroptosis and tissue‐resident cells to better understand its role in metabolic diseases pathogenesis.
4.7. Skin diseases
Ferroptosis is also a key contributor to pathogenesis of various skin diseases. 9 In psoriasis, ferroptosis triggers a cascade of inflammatory responses through lipid peroxidation in keratinocytes, contributing to the initiation and progression of psoriatic lesions. 9 Studies have shown that ferroptosis inhibitors, such as ferrostatin‐1, can effectively alleviate inflammatory symptoms in psoriasis. 268 Similarly, in vitiligo, ferroptosis is a key factor. The elevated iron content in melanocytes and impaired antioxidant defenses make these cells highly susceptible to ferroptosis, leading to skin depigmentation. 269 Ferroptosis also exacerbates skin damage induced by ultraviolet (UV) radiation. UV‐exposed skin exhibits abnormal iron metabolism and increased lipid peroxidation, worsening skin damage. The application of ferroptosis inhibitors has been shown to effectively mitigate UV‐induced skin damage. 270 In conclusion, ferroptosis plays a central role in the pathogenesis of various skin diseases. Drugs that modulate ferroptosis offer significant therapeutic potential, presenting promising avenues for treatment.
5. THERAPEUTIC APPROACHES TARGETING FERROPTOSIS
The role of ferroptosis in disease pathogenesis, both through its activation and inhibition, highlights lipid metabolism, iron homeostasis, and redox systems as key pathways for therapeutic intervention. Modulating these pathways offers a promising strategy for the treatment of ferroptosis‐related diseases. This section primarily summarizes clinical drugs that exhibit therapeutic potential by targeting key components of ferroptosis, providing a foundation for future clinical applications (Table 1).
TABLE 1.
List of clinical trials associated with ferroptosis‐targeted drugs.
| Agents | Mechanism and/or target | Effects | Experimental model | Outcomes | Indication | Trial phase and identifier number | References |
|---|---|---|---|---|---|---|---|
| Targeting lipid metabolism pathway | |||||||
| Rosiglitazone | ACSL4 inhibitor | Inhibiting ferroptosis | Renal Gpx4−/− mice; I/R injury mice 14 | Reduces mortality associated with acute kidney injury; prevents I/R intestinal injury | Solid tumor malignancies | Phase II NCT04114136 | 14 , 234 |
| Prostate cancer | Phase III NCT00182052 | ||||||
| Ulcerative colitis | Phase II NCT00065065 | ||||||
| HIV infection | Phase II NCT00367744 | ||||||
| Sarcoma | Phase II NCT00004180 | ||||||
| Alzheimer's disease | Phase I NCT00688207 | ||||||
| MASH | Phase II NCT00492700 | ||||||
| Kidney transplant | Phase II NCT00309309 | ||||||
| Baicalein | ALOX12/15 inhibitor; ACSL4 inhibitor | Inhibiting ferroptosis | Cisplatin and folic acid‐induced AKI in mice; CPT‐11‐induced delayed diarrhea in rat; heart I/R injury in rat | Alleviates renal inflammatory responses and AKI; alleviates colonic pathological injury and decreased inflammatory factor; improves myocardial I/R challenge‐induced ST segment elevation, coronary flow, left ventricular systolic pressure, infarct area, and pathological changes | Influenza | Phase II NCT03830684 | 271 , 272 , 273 |
| Zileuton | ALOX5 selective inhibitor | Inhibiting ferroptosis | HT22 cells | Protects HT22 neuronal cells from glutamate oxidative toxicity in a ferroptosis‐dependent mechanism. | Chronic myelogenous leukemia | Phase I NCT02047149; Phase I NCT01130688 | 274 |
| Sickle cell disease | Phase I NCT01136941 | ||||||
| Head and neck cancer; lung cancer | Phase II NCT00056004; Phase II NCT00070486 | ||||||
| Tobacco use disorder | Phase II NCT02348203; Phase II NCT01021215 | ||||||
| Acne vulgaris | Phase II NCT00098358 | ||||||
| NDGA | Pan‐LOX inhibitor | Inhibiting ferroptosis | Acute lymphoblastic leukemia cells | Blocks RSL3‐induced lipid peroxidation and cell death | Prostate cancer | Phase II NCT00678015 | 275 |
| High grade glioma | Phase I NCT02575794 | ||||||
| Brain and central nervous System tumors | Phase I NCT00404248 | ||||||
| Targeting iron homeostasis | |||||||
| DFO | Iron chelator | Inhibiting ferroptosis | MCD‐induced MASH in mice; hepatic I/R injury in mice; aged (15–18 months) C57 mice intraperitoneally injected with LPS; diabetic MCAO rat; MCAO rat | Attenuates the Severity of MASH; attenuates hepatic I/R injury and lipid peroxidation; improves inflammation and sickness behavior in aged mice treated with LPS; prevents vasoregression and microglia activation while improving AQP4 polarity as well as blood‐brain barrier permeability; reduce the area of cerebral infarction, improve the pathological structure of cerebral ischemia rats | Cardiomyopathy Hypotension, acute renal failure Ischemic stroke AKI Aneurysmal subarachnoid hemorrhage | Phase IV NCT00800761 | 236 , 266 , 276 , 278 |
| Phase II NCT0087088 | |||||||
| Phase II NCT00777140 | |||||||
| Phase II NCT04633889 | |||||||
| Phase II NCT04566991 | |||||||
| DFP | Iron chelator | Inhibiting ferroptosis | DSS‐induced ulcerative colitis in mice | Relieves the inflammation and impaired colon, and increase body weight | Acute myocardial infarction type 1 | Phase I NCT05604131 | 279 |
| Neurodegeneration | Phase II NCT00907283 | ||||||
| Stroke | Phase II NCT05111821 | ||||||
| DFX | Iron chelator | Inhibiting ferroptosis | Myocardial I/R injury in mice | Reduces myocardial injury and infarct size | Myelodysplasia | Phase II NCT03387475 | 280 |
| Sickle cell disease | Phase II NCT05392101 | ||||||
| DXZ | Iron chelator | Inhibiting ferroptosis | DOX‐ and I/R‐induced cardiomyopathy in mice | Prevents DOX‐induced cardiomyopathy and reduces the severity of cardiac I/R Injury. | During congenital heart surgery | Phase II NCT04997291 | 230 |
| Acute myeloid leukemia | Phase II NCT03589729 | ||||||
| Targeting redox systems | |||||||
| SAS | SLC7A11 inhibitor | Promoting ferroptosis | H22‐luc hepatoma ascites mice | Reduces tumor burden | Glioma; glioblastoma; recurrent glioblastoma | Phase I NCT04205357 | 281 |
| Breast cancer; chronic pain due to malignancy | Phase II NCT03847311 | ||||||
| Sorafenib | SLC7A11 inhibitor | Promoting ferroptosis | MGC803 xenografts; HT‐1080 xenografts; 786‐O xenografts | Suppresses tumor growth | Hepatocellular carcinoma | Phase IV NCT01203787 | 282 , 283 , 284 |
| Acute myeloid leukemia | Phase III NCT01371981 | ||||||
| Neuroblastoma | Phase II NCT02559778 | ||||||
| Carcinoma, non‐small‐cell lung | Phase III NCT00449033 | ||||||
| BSO | Inhibition of GCL; GSH‐depleting | Promoting ferroptosis | BJ‐derived cell; HT‐1080 cells; | Induces selective lethality in BJ‐derived tumorigenic cells expressing oncogenic HRAS; inhibits HT‐1080 cells viability | Neuroblastoma | Phase I NCT00005835, Phase I NCT00002730 | 40 , 172 |
| Cisplatin | GSH‐depleting | Promoting ferroptosis | MKN‐45 xenografts; LLC xenografts | Suppresses tumor growth | NSCLC | Phase III NCT01656551 | 285 , 286 |
| Bladder cancer | Phase III NCT04574960 | ||||||
| Cervical cancer | Phase III NCT01561586 | ||||||
| Cancer of pancreas | Phase II NCT03649321 | ||||||
| NAC | GSH synthesis regulator | Inhibiting ferroptosis | ICH in mice; polycystic ovary syndrome model in rats; diabetic nephropathy model in beagle; intermittent hypoxia‐induced myocardial injury in mice | Improves functional recovery at least 7 days following ICH in mice; attenuates gravid uterine and placental ferroptosis in a PCOS‐like rat model with fetal loss; ameliorates kidney injury in diabetic nephropathy; alleviates intermittent hypoxia‐related myocardial injury | Progressive MS | Phase II NCT05122559 | 287 , 288 , 289 , 290 |
| Neurofibromatosis 1 | Phase II NCT04481048 | ||||||
| Skin disorder | Early phase 1 NCT05287724 | ||||||
| Mitochondrial disease | Phase I NCT05241262 | ||||||
| Diabetic neuropathies | Phase IV NCT04766450 | ||||||
| Vascular cognitive impairment no dementia | Phase II NCT03306979 | ||||||
| SLE | Phase II NCT00775476 | ||||||
| Mifepristone | Promoting GSH synthesis | Inhibiting ferroptosis | Acetaminophen induced liver injury in mice | Protects against APAP‐induced acute liver injury, evidenced by decreased ALT, AST level and histological recovery | Meningioma | Phase III NCT03015701 | 291 |
| Breast cancer | Phase III NCT05016349 | ||||||
| NSCLC | Phase II NCT02642939 | ||||||
| Prostate cancer | Phase II NCT00140478 | ||||||
| Hepatitis C virus infection | Phase II NCT00255177 | ||||||
| Endocrine disease; diabetes | Phase II NCT01419535 | ||||||
| WA | Alkylation of GPX4 | Promoting ferroptosis | IMR‐32 xenografts | Suppresses the growth and relapse rate of neuroblastoma xenografts | Recurrent ovarian cancer | Phase I NCT05610735 | 292 |
| Gemcitabine | Inhibiting GPX4 | Promoting ferroptosis | A549 cells | Inhibits A549 cells proliferation | Pancreatic cancer | Phase II NCT06015659 | 293 |
| Biliary tract cancer | Phase II NCT05357196 | ||||||
| Adult solid tumor | Phase I NCT05147272 | ||||||
| SeMet | GPX4 activators | Inhibiting ferroptosis | DOX‐induced acute cardiotoxicity in mice | Protects mice from DOX‐induced cardiotoxicity | Liver disease | Phase IV NCT01650181 | 294 |
| Precancerous/nonmalignant condition; prostate cancer | Phase III NCT00030901 | ||||||
| Lung cancer | Phase II NCT00526890 | ||||||
| Colorectal cancer | Phase II NCT00625183 | ||||||
| ccRCC | Phase I NCT05363631 | ||||||
| Brequinar | DHODH inhibitor | Promoting ferroptosis | NCI‐H226 xenografts and TC494 lung cancer PDXs | Suppresses the tumor growth of GPX4low xenografts | SARS‐CoV‐2 infection | Phase II NCT04575038 | 50 |
| Acute myeloid leukemia | Phase II NCT03760666 | ||||||
| Leflunomide | DHODH inhibitor | Promoting ferroptosis | SB‐driven hepatocarcinogenesis in mice | Constrains tumor mass and number, and achieves a much healthier liver and prolonged survival time | SARS‐CoV‐2 infection | Phase III NCT05007678 | 189 |
| Neuroendocrine tumors | Phase II NCT06540937 | ||||||
| Brain and central nervous system tumors | Phase II NCT00003775 | ||||||
| Idiopathic pulmonary Hemosiderosis | Phase II NCT05937191 | ||||||
| Advanced pancreatic adenocarcinoma | Phase I NCT06454383 | ||||||
| Menaquinone‐4 | RTA | Inhibiting ferroptosis | Hepatocyte‐specific Gpx4−/− mice; I/R injury in mice | Protects against related pathologic changes in liver; Protects against liver or kidney I/R injury in mice | Diabetes | Phase IV NCT00960973 | 62 |
| Osteoporosis | Phase IV NCT00548509 | ||||||
| Hepatocellular carcinoma | Phase III NCT00165633 | ||||||
| Promethazine | RTA | Inhibiting ferroptosis | Cisplatin‐induced AKI and LPS/galactosamine‐induced liver injury in mice | Ameliorates AKI and increases the survival rate in mice; improves LPS/GalN‐induced acute liver injury | Pruritus | Phase IV NCT04805073 | 295 |
| Diabetic gastroparesis | Phase II NCT02130622 | ||||||
| Edaravone | RTA | Inhibiting ferroptosis | CSDS depression model in mice; permanent MCAO mice | Ameliorates depressive and anxiety‐like behaviors; alleviates cerebral ischemic injury in the mice with permanent MCAO | Acute ischemic stroke | Phase III NCT02430350 | 296 , 297 |
| Nasopharyngeal carcinoma, brain necrosis | Phase II NCT01865201 | ||||||
| Myocardial infarction | Phase IV NCT00265239 | ||||||
| Cerebral infarction | Phase IV NCT00200356 | ||||||
| ALS | Phase III NCT00415519 | ||||||
| CuATSM | RTA | Inhibiting ferroptosis | Mouse embryonic fibroblasts and hippocampal cells | Rescues embryonic fibroblasts and hippocampal cells from ferroptosis | ALS | Phase III NCT04082832 | 298 |
Abbreviations: ACSL4, acyl‐CoA synthetase long‐chain family member 4; AKI, acute kidney injury; ALOX12/15, arachidonate lipoxygenases 12/15; ALS, amyotrophic lateral sclerosis; ALT, alanine aminotransferase; APAP, acetaminophen; AST, aspartate aminotransferase; BSO, buthionine sulfoximine; ccRCC, clear cell renal cell carcinoma; CSDS, chronic social defeat stress; DFO, deferoxamine; DFP, deferiprone; DFX, deferasirox; DHODH, dihydroorotate dehydrogenase; DOX, doxorubicin; DSS, dextran sulfate sodium; DXZ, dexrazoxane; GCL, glutamate–cysteine ligase; Gpx4, glutathione peroxidase 4; GSH, glutathione; I/R, ischemia/reperfusion; ICH, intracranial hemorrhage; LLC, Lewis lung carcinoma; LPS, lipopolysaccharide; MASH, metabolic dysfunction‐associated steatohepatitis; MCAO, middle cerebral artery occlusion; MCD, methionine/choline‐deficient diet; MS, multiple sclerosis; NAC, N‐acetylcysteine; NCT, national clinical trial; NDGA, nordihydroguaiaretic acid; NSCLC, non‐small cell lung cancer; PCOS, polycystic ovary syndrome; PDXs, patient‐derived xenografts; RTA, radical‐trapping antioxidant; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; SAS, sulfasalazine; SB, sleeping beauty; SeMet, selenomethionine; SLC7A11, solute carrier family 7 member 11; SLE, systemic lupus erythematosus; WA, withaferin A.
5.1. Targeting lipid metabolic pathway
Since the incorporation of PUFA‐PLs into cell membranes is a prerequisite for ferroptosis, lipid metabolic pathways that modulate membrane lipid composition present promising therapeutic targets. 299 , 300 For example, thiazolidinediones, such as rosiglitazone, reduce mortality associated with acute kidney injury and prevent ischemia/reperfusion intestinal injury by selectively inhibiting ACSL4, an enzyme that facilitates the incorporation of PUFA‐PLs into membranes. 14 , 234 Similarly, baicalin, a natural flavonoid glycoside, has shown myocardial protection against ischemia/reperfusion by suppressing ACSL4‐mediated ferroptosis. 273 However, clinical drugs targeting other key molecules involved in membrane lipid composition, such as LPCAT3, ACSL3, and MBOAT1/2, have yet to be identified. Interestingly, exogenous lipid supplementation, particularly a PUFA‐rich diet, may serve as an adjuvant therapy for ferroptosis‐related diseases, as it has been shown to delay tumor growth in colon cancer by enhancing acidosis‐driven ferroptosis. This effect is augmented by ferroptosis inducers such as SAS or erastin, and blocked by the inhibitor ferrostatin‐1. 301
Additionally, peroxidation of PUFA‐PLs is a critical event in ferroptosis. ALOX enzymes, which catalyze PUFA oxidation, are important regulators of ferroptosis. 302 The United States Food and Drug Administration (US FDA)‐approved drug Zileuton, an ALOX5 inhibitor, has shown neuroprotective effects by preventing ALOX5‐induced glutamate excitotoxicity and ferroptosis. 274 Selective ALOX12/15 inhibitor baicalin has been found to effectively mitigate cisplatin‐induced acute kidney injury and CPT‐11‐induced gastrointestinal dysfunction by suppressing ALOX12/15‐dependent ferroptosis. 271 , 272 Pan‐LOX inhibitor nordihydroguaiaretic acid has also shown promise in inhibiting ferroptosis in acute lymphoblastic leukemia. 275 However, its therapeutic effects and applications in improving ferroptosis‐induced diseases remain unclear, warranting further study.
5.2. Targeting iron homeostasis
Excess iron is a major driver of ferroptosis, and iron chelation therapies using agents like deferoxamine, deferiprone, and deferasirox can effectively alleviate diseases associated with iron overload, including neurodegeneration, organ injury, and MASH, by inhibiting ferroptosis. 236 , 266 , 276 , 277 , 278 , 279 , 280 , 303 , 304 Dexrazoxane (DXZ), the only US FDA‐approved iron chelator for reducing DOX‐induced cardiotoxicity, also exhibits therapeutic effects related to ferroptosis by chelating mitochondrial iron. 230 However, iron chelation poses risks of adverse effects such as anemia and renal toxicity, limiting its broader clinical application. 305 , 306 , 307
Regulating iron homeostasis‐related molecules offers another potential therapeutic strategy for ferroptosis‐involved diseases. For example, baicalin, previously recognized for its antiferroptotic effects through the inhibition of ALOX12/15 and ACSL4, has also been shown to induce ferroptosis in bladder cancer cells by downregulating FTH1, inhibiting tumor growth. 308 Clinical trials involving iron export regulators, such as the FPN inhibitor vamifeport and hepcidin antagonists PRS‐080, NOX‐H94, and LY2787106, which reduce intracellular iron levels by alleviating hepcidin‐mediated FPN suppression, have been reported. However, their potential to modulate ferroptosis in the management of ferroptosis‐related diseases has yet to be fully elucidated. 309
5.3. Targeting redox systems
The maintenance of normal biological functions relies on the coordinated action of redox systems to preserve oxidative‐reductive homeostasis within the body. Dysregulation of key redox pathways, such as the system xc−–GSH–GPX4, FSP1–CoQ10–NAD(P)H, and DHODH systems, often leads to the onset of ferroptosis‐related diseases. Investigating clinically available drugs that target these redox systems is crucial for advancing the clinical translation of ferroptosis‐based therapies.
The system xc−‐GSH‐GPX4 pathway is a key guardian against ferroptosis. Small molecule inhibitors like erastin and RSL3 have been identified as research tools for targeting this pathway, while US FDA‐approved drugs such as antirheumatic SAS, antitumor kinase inhibitor sorafenib, and the muscle relaxant lanperisone have been shown to inhibit tumor growth by inducing ferroptosis through the inhibition of system xc−. 34 , 55 , 281 , 282 , 283 , 284 , 310 , 311 , 312 , 313 , 314 , 315 However, sorafenib's inability to induce ferroptosis in certain cancer cell lines highlights its limited clinical applicability for ferroptosis‐mediated tumor inhibition. 316
In the context of GSH synthesis, as previously mentioned, BSO can induce ferroptosis in tumor cells by inhibiting GCL and depleting GSH. 40 , 172 , 317 Despite its relative safety, BSO's clinical benefits for cancer patients remain limited. 318 Identifying sensitive patient populations and exploring combination strategies may expand its clinical applications. 317 , 319 , 320 , 321 Cisplatin, a widely used antitumor drug, also induces ferroptosis by depleting GSH, adding another anticancer mechanism beyond apoptosis. 322 Notably, cisplatin resistance is associated with ferroptosis resistance, and combining cisplatin with ferroptosis inducers may enhance efficacy in overcoming this resistance. 55 , 285 , 286 , 323 However, cisplatin‐induced ferroptosis is also implicated in chemotherapy‐related side effects, such as ovarian damage and acute kidney injury. 295 , 324 The antioxidant N‐acetylcysteine (NAC) has been shown to mitigate these side effects by inhibiting ferroptosis through modulation of cysteine metabolism. 287 , 288 , 289 , 290 , 324 , 325 This protective effect of NAC has also been observed in alleviating ferroptosis‐related acetaminophen (APAP) hepatotoxicity and neurodegeneration. 326 , 327 Furthermore, mifepristone has been shown to reduce APAP‐induced hepatotoxicity by promoting GSH synthesis. 291
Inhibiting GPX4 directly triggers ferroptosis, with compounds like RSL3, ML162, and FIN56 showing preclinical potential. However, due to poor pharmacokinetics, their clinical applications remain limited. Natural compounds such as withaferin A and gemcitabine have shown tumor‐inhibiting effects by targeting GPX4, offering potential therapeutic avenues. 292 , 293 , 313 , 328 However, the direct inhibition of GPX4 requires caution, as GPX4 deficiency can cause severe side effects, such as acute kidney failure and embryonic lethality. Conversely, GPX4 activators, including selenium compounds like selenomethionine and selenocysteine‐containing peptides like Tat SelPep, have demonstrated the ability to alleviate DOX‐induced cardiomyopathy and promote stroke recovery by suppressing GPX4‐dependent ferroptosis. 42 , 294 The identification of other GPX4 activators with potent ferroptosis‐suppressing capabilities, such as 1d4 and natural compounds like curculigoside and puerarin, further expands the therapeutic potential of GPX4‐targeted treatments. 329 , 330 , 331
The FSP1–CoQ10–NAD(P)H and DHODH systems function as parallel defense mechanisms against ferroptosis, making them promising therapeutic targets. Small molecules like iFSP1, 59 NPD4928, 332 and FSEN1 333 selectively inhibit FSP1, sensitizing cancer cells to ferroptosis and providing a strategy to enhance therapies resistant to ferroptosis. In contrast, diphenylbutene derivative compounds 3f can inhibit ferroptosis by increasing FSP1 protein levels, offering protection against ischemic stroke. 334 DHODH, which compensates for the loss of GPX4, also holds therapeutic potential for tumors with low GPX4 expression. Several clinical trials involving DHODH inhibitors, such as brequinar, leflunomide, and teriflunomide, have been reported. 50 , 189 , 335 Combining DHODH inhibitors with AMPK activators, SAS, or oxaliplatin has shown synergistic tumor inhibition through ferroptosis induction. 50 , 189 , 336
Free radicals play a crucial role in lipid peroxidation, driving ferroptosis through the formation and propagation of lipid radicals. RTAs can block these processes, with lipophilic RTAs such as ferrostatin‐1 and liproxstatin‐1 being potent specific ferroptosis inhibitors. 2 Compared with ferrostatin‐1, liproxstatin‐1 demonstrates superior pharmacokinetics and efficacy in treating various ferroptosis‐related diseases in vivo, such as hepatic ischemia/reperfusion injury and acute renal failure. 47 However, liproxstatin‐1's inhibition of CYP450 limits its clinical utility, as this inhibition may slow down drug metabolism and clearance, thus increasing the risk of adverse effects. 47 Recent study suggests that sulfane sulfur species, particularly hydropersulfides (RSSH), may act as endogenous radical scavengers and could offer novel approaches to ferroptosis treatment. 337 , 338 Furthermore, vitamin K derivative hydroquinone, reduced by FSP1, exhibits RTA functionality and significant antiferroptotic effects. 62 Additionally, diarylamine derivatives, such as phenothiazine, a key pharmaceutical core structure, have been identified as effective RTA inhibitors. Among them, the derivative drug promethazine demonstrates a stronger ability to protect kidney function compared with ferrostatin‐1, highlighting the potential therapeutic applications of diarylamine derivatives in treating ferroptosis‐related diseases. 295 , 339 The clinically approved RTA edaravone, used to treat cerebral ischemic injury and ALS, likely exerts its neuroprotective effects through ferroptosis suppression. 297 , 340 , 341 CuATSM, a clinical candidate for treating Parkinson's disease and ALS, also suppresses ferroptosis via its RTA activity. 298 These discoveries underscore the importance of RTAs in ferroptosis‐related therapies, offering new opportunities for clinical translation.
6. POTENTIAL CLINICAL MONITORING APPROACHES FOR FERROPTOSIS
Given the involvement of ferroptosis in disease pathogenesis and therapy, identifying effective clinical monitoring approaches is crucial. Several biomarkers of ferroptosis have been described (Table 2), including mitochondrial alterations (e.g., shrinkage, increased membrane density, and reduced cristae), elevated ferrous iron levels and iron metabolism, increased lipid peroxidation along with its byproducts (e.g., lipid ROS, MDA, and 4‐hydroxynonenal [4‐HNE]), and changes in ferroptosis‐related genes (e.g., CHAC1, PTGS2, SLC7A11, and ACSL4). 342 , 343 , 344 , 345 While elevated ferrous iron levels and lipid peroxidation are key biomarkers for ferroptosis, their transient nature and low expression levels pose challenges for direct detection in clinical settings. 342 , 344 , 345 Biomarkers such as 4‐HNE, MDA, hyperoxidized peroxiredoxin 3, and TFRC show relative potential for clinical detection of ferroptosis due to their stability and detectability in tissue sections. 202 However, these molecules indicate the ferroptotic response in an indirect and static manner. More potential clinical monitoring approaches for detecting ferroptosis directly and dynamically should be developed.
TABLE 2.
Summary of ferroptosis biomarkers.
| Name | Property | Alteration | Detection technique | Samples | Description | References |
|---|---|---|---|---|---|---|
| Lipid ROS | Oxidation product | Up | BODIPY 581/591 C11 probes | Cells | Detecting ROS formed from hydroperoxides, but not to hydroperoxides themselves | 2 , 346 |
| 4‐HNE | Oxidation product | Up | Antibody staining | Cells; ex vivo tissue | Aldehyde secondary products of lipid peroxidation | 347 , 348 |
| MDA | Oxidation product | Up | TBARSs assay; antibody staining | Cells; ex vivo tissue | Aldehyde secondary products of lipid peroxidation | 349 , 350 |
| TFRC | Protein | Increased plasma localization | 3F3‐FMA antibody and additional anti‐TFRC antibodies | Cells; ex vivo tissue | Selectively staining ferroptotic cells in tissue sections | 351 |
| PRDX3 | Protein | Hyperoxidized and translocated from mitochondria to plasma membranes | Antibody staining | Cells; ex vivo tissue | Identifying ferroptotic cells in tissue sections | 352 |
| PTGS2 | mRNA | Up | qRT‐PCR assay | Cells; ex vivo tissue | Upregulated in specific contexts | 40 , 347 |
| CHAC1 | mRNA | Up | qRT‐PCR assay | Cells; ex vivo tissue | Primarily upregulated by system xc− inhibitors, but not by other ferroptosis inducers | 56 |
| SLC7A11 | Protein | Up | Antibody assay | Cells; ex vivo tissue | Upregulated in specific contexts | 311 |
| ACSL4 | Protein | Up | Antibody assay | Cells; ex vivo tissue | Upregulated in specific contexts | 353 |
Abbreviations: 4‐HNE, 4‐hydroxynonenal; ACSL4, acyl‐CoA synthetase long chain family member; CHAC1, ChaC glutathione‐specific gamma‐glutamylcyclotransferase 1; MDA, malondialdehyde; PRDX3, peroxiredoxin 3; PTGS2, prostaglandin‐endoperoxide synthase 2; ROS, reactive oxygen species; SLC7A11, solute carrier family 7 member 11; TBARSs, thiobarbituric acid reactive substances; TFRC, transferrin receptor; qRT‐PCR, quantitative real‐time PCR.
Imaging techniques are gaining attention for their ability to detect ferroptosis both spatially and temporally. Fluorescence‐ and probe‐based methods are regarded as key approaches in ferroptosis imaging. Three such techniques, BODIPY 581/591 C11, photochemical activation of membrane lipid peroxidation, and hydrogen peroxide fluorescent probes, have been developed to capture and visualize ferroptotic processes. 346 , 354 , 355 , 356 Among these, BODIPY 581/591 C11 has become a primary method for identifying ferroptosis by specifically detecting lipid ROS production. However, these techniques are largely restricted to tissue sections and cell cultures, limiting their use for dynamic, in vivo detection of ferroptotic activity in clinical settings. Similarly, mass spectrometry remains limited to the detection of lipid hydroperoxide levels in ex vivo tissue samples during ferroptosis. 61 Notably, this technique offers detailed insights into lipid molecular mass, elemental composition, and chemical structure throughout the ferroptotic process, facilitating the identification of potential biomarkers for ferroptosis. 72 , 357 Moreover, positron emission tomography (PET) imaging with tracers like 18F‐TRX, 18F‐FSPG, and 68Ga‐NOTATf enables real‐time, noninvasive monitoring of ferroptosis in vivo, by measuring the intracellular LIP, system xc− activity, and TF uptake, respectively. 358 , 359 , 360 , 361 , 362 Notably, ongoing clinical trials using 18F‐FSPG for PET imaging in tumor patients represent a significant step forward in detecting system xc− activity, potentially revolutionizing the clinical monitoring of ferroptosis. 361 , 362
Another emerging technique for noninvasive in vivo imaging of ferroptosis is magnetic resonance imaging (MRI), which excels at resolving soft tissue and anatomical structures. Recently, an artemisinin‐based probe (Art‐Gd) was developed for contrast‐enhanced MRI to detect ferroptosis, leveraging the radical formation reaction between labile Fe2⁺ and artemisinin. This reaction allows Art‐Gd to form complexes that enhance tissue retention and improve longitudinal relaxation time (T1) contrast, enabling real‐time MRI detection of ferroptosis in vivo. 363 However, MRI's limitations in molecular‐scale imaging pose challenges, as there is a scarcity of contrast agents capable of detecting other ferroptosis‐related molecules such as lipid peroxides, GSH, and iron transporters. Further research is needed to overcome these limitations and enable the detection of multiple ferroptosis biomarkers, providing a more accurate and comprehensive assessment of this process.
7. OPPORTUNITIES AND CHALLENGES
Numerous preclinical studies suggest that targeting ferroptosis offers promising therapeutic opportunities for related conditions. However, our understanding of ferroptosis remains insufficient, with several unresolved issues potentially hindering its clinical translation.
Although overwhelming lipid oxidation and subsequent plasma membrane rupture are recognized as central events in ferroptosis, the intricate cellular biology underlying this process, including intracellular lipid transport between organelles, the propagation of lipid peroxidation reactions within cells, the degradation of specific lipid species during peroxidation, and the role of fragmented oxidative products in membrane rupture—remains to be fully elucidated. 4 Additionally, much of the current understanding of ferroptosis stems from studies in cultured cancer cells. More research is needed to explore ferroptosis in cell lines from other diseases and normal tissues, as these may involve distinct mechanisms. 4 A comprehensive understanding of the biological activities of ferroptosis is essential for advancing research in this field.
A host of molecules and metabolic products related to lipid and iron metabolism, as well as redox systems, have been identified as regulators of ferroptosis. However, further research is required to identify and quantify endogenous triggers and inhibitors of ferroptosis across various pathophysiological states. This will enhance our understanding of ferroptosis responses in different individuals, tissues, and conditions, elucidate the causal relationships between ferroptosis and diseases, and guide the development of targeted biomarkers and therapies, as well as the evaluation of their efficacy and side effects. 364
In terms of clinical monitoring, assessing ferroptosis remains in its early stages. There is a need for more specific and less toxic detection reagents to enable precise, noninvasive real‐time in vivo monitoring of ferroptosis. Given the specific substances released from ferroptotic cells, developing detection methods based on blood, urine, and fecal samples represents a promising future direction. Additionally, identifying populations most likely to benefit from ferroptosis‐targeted therapies and achieving precise, ferroptosis‐specific treatments are key goals in ongoing ferroptosis research.
AUTHOR CONTRIBUTIONS
Guangtong Deng designed the review. Qian Zhou and Yu Meng searched for literature and wrote the manuscript. Qian Zhou and Yu Meng drew the figures and tables. Jiayuan Le, Yuming Sun, Yating Dian, Lei Yao, Yixiao Xiong, Furong Zeng, and Xiang Chen and helped edit and revise the manuscript. Guangtong Deng, Qian Zhou, Y. L., and Furong Zeng provided funding support. All authors have read and approved the article and agree with publication in this journal.
CONFLICT OF INTEREST STATEMENT
The authors have declared that no conflict of interest exists.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
We thank Biorender (https://www.biorender.com/) for the assistance for the illustration. This work was supported by the National Natural Science Foundation of China (Grant Nos. 82272849 to G. D., 82403139 to Y. L.), Huxiang Youth Talent Program (Grant Nos. 2023RC3072 to G. D., 2024RC3043 to F. Z.), Natural Science Fund for Outstanding Youths in Hunan Province (Grant Nos. 2023JJ20093 to G. D.), Postdoctoral Fellowship Program of CPSF (Grant Nos. GZB20240875 to Q. Z., GZC20242053 to Y. L.), Changsha Municipal Natural Science Foundation (Grant Nos. kq2403010 to Y. L.), and Xiangya Hospital Youth Fund (Grant Nos. 2023Q17 to Y. L.).
Zhou Q, Meng Y, Le J, et al. Ferroptosis: mechanisms and therapeutic targets. MedComm. 2024;5:e70010. 10.1002/mco2.70010
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Green DR. The coming decade of cell death research: five riddles. Cell. 2019;177(5):1094‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron‐dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie Y, Hou W, Song X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dixon SJ, Olzmann JA. The cell biology of ferroptosis. Nat Rev Mol Cell Biol. 2024;25(6):424‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22(7):381‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17(9):2054‐2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang M, Luo H, Yi X, Wei X, Jiang DS. The epigenetic regulatory mechanisms of ferroptosis and its implications for biological processes and diseases. MedComm. 2023;4(3):e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Yan D, Liu J, Tang D, Chen X. Protein modification and degradation in ferroptosis. Redox Biol. 2024;75:103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le J, Meng Y, Wang Y, et al. Molecular and therapeutic landscape of ferroptosis in skin diseases. Chin Med J (Engl). 2024;137(15):1777‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen F, Kang R, Tang D, Liu J. Ferroptosis: principles and significance in health and disease. J Hematol Oncol. 2024;17(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dondelinger Y, Priem D, Huyghe J, Delanghe T, Vandenabeele P, Bertrand MJM. NINJ1 is activated by cell swelling to regulate plasma membrane permeabilization during regulated necrosis. Cell Death Dis. 2023;14(11):755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirata Y, Cai R, Volchuk A, et al. Lipid peroxidation increases membrane tension, Piezo1 gating, and cation permeability to execute ferroptosis. Curr Biol. 2023;33(7):1282‐1294. e1285. [DOI] [PubMed] [Google Scholar]
- 13. Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA. 2016;113(34):E4966‐E4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dixon SJ, Winter GE, Musavi LS, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. 2015;10(7):1604‐1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang HL, Hu BX, Li ZL, et al. PKCbetaII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol. 2022;24(1):88‐98. [DOI] [PubMed] [Google Scholar]
- 17. Qiu B, Zandkarimi F, Bezjian CT, et al. Phospholipids with two polyunsaturated fatty acyl tails promote ferroptosis. Cell. 2024;187(5):1177‐1190. e1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zou Y, Henry WS, Ricq EL, et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. 2020;585(7826):603‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reed A, Ware T, Li H, Fernando Bazan J, Cravatt BF. TMEM164 is an acyltransferase that forms ferroptotic C20:4 ether phospholipids. Nat Chem Biol. 2023;19(3):378‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen D, Chu B, Yang X, et al. iPLA2beta‐mediated lipid detoxification controls p53‐driven ferroptosis independent of GPX4. Nat Commun. 2021;12(1):3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tesfay L, Paul BT, Konstorum A, et al. Stearoyl‐CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res. 2019;79(20):5355‐5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Magtanong L, Ko PJ, To M, et al. Exogenous monounsaturated fatty acids promote a ferroptosis‐resistant cell state. Cell Chem Biol. 2019;26(3):420‐432. e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liang D, Feng Y, Zandkarimi F, et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell. 2023;186(13):2748‐2764. e2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Z, Hu Y, Zheng H, et al. LPCAT1‐mediated membrane phospholipid remodelling promotes ferroptosis evasion and tumour growth. Nat Cell Biol. 2024;26(5):811‐824. [DOI] [PubMed] [Google Scholar]
- 25. Stoyanovsky DA, Tyurina YY, Shrivastava I, et al. Iron catalysis of lipid peroxidation in ferroptosis: regulated enzymatic or random free radical reaction? Free Radic Biol Med. 2019;133:153‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35(6):830‐849. [DOI] [PubMed] [Google Scholar]
- 27. Long H, Zhu W, Wei L, Zhao J. Iron homeostasis imbalance and ferroptosis in brain diseases. MedComm. 2023;4(4):e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson GJ, Vulpe CD. Mammalian iron transport. Cell Mol Life Sci. 2009;66(20):3241‐3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang LJ, Zhou YJ, Xiong XM, et al. Ubiquitin‐specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic Biol Med. 2021;162:339‐352. [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Liu Y, Liu J, Kang R, Tang D. NEDD4L‐mediated LTF protein degradation limits ferroptosis. Biochem Biophys Res Commun. 2020;531(4):581‐587. [DOI] [PubMed] [Google Scholar]
- 31. Bayir H, Dixon SJ, Tyurina YY, Kellum JA, Kagan VE. Ferroptotic mechanisms and therapeutic targeting of iron metabolism and lipid peroxidation in the kidney. Nat Rev Nephrol. 2023;19(5):315‐336. [DOI] [PubMed] [Google Scholar]
- 32. Shesh BP, Connor JR. A novel view of ferritin in cancer. Biochim Biophys Acta Rev Cancer. 2023;1878(4):188917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fang X, Cai Z, Wang H, et al. Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11‐mediated ferroptosis. Circ Res. 2020;127(4):486‐501. [DOI] [PubMed] [Google Scholar]
- 34. Sun X, Ou Z, Chen R, et al. Activation of the p62‐Keap1‐NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hou W, Xie Y, Song X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26(9):1021‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu H, Liu Q, Shan X, Gao W, Chen Q. ATM orchestrates ferritinophagy and ferroptosis by phosphorylating NCOA4. Autophagy. 2023;19(7):2062‐2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bao WD, Pang P, Zhou XT, et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer's disease. Cell Death Differ. 2021;28(5):1548‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oliveira T, Hermann E, Lin D, Chowanadisai W, Hull E, Montgomery M. HDAC inhibition induces EMT and alterations in cellular iron homeostasis to augment ferroptosis sensitivity in SW13 cells. Redox Biol. 2021;47:102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1‐2):317‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cardoso BR, Hare DJ, Bush AI, Roberts BR. Glutathione peroxidase 4: a new player in neurodegeneration? Mol Psychiatry. 2017;22(3):328‐335. [DOI] [PubMed] [Google Scholar]
- 42. Alim I, Caulfield JT, Chen Y, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 2019;177(5):1262‐1279. e1225. [DOI] [PubMed] [Google Scholar]
- 43. Conrad M, Proneth B. Selenium: tracing another essential element of ferroptotic cell death. Cell Chem Biol. 2020;27(4):409‐419. [DOI] [PubMed] [Google Scholar]
- 44. Ingold I, Berndt C, Schmitt S, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide‐induced ferroptosis. Cell. 2018;172(3):409‐422. e421. [DOI] [PubMed] [Google Scholar]
- 45. Viswanathan VS, Ryan MJ, Dhruv HD, et al. Dependency of a therapy‐resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547(7664):453‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Conrad M, Pratt DA. The chemical basis of ferroptosis. Nat Chem Biol. 2019;15(12):1137‐1147. [DOI] [PubMed] [Google Scholar]
- 47. Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li J, Liu J, Zhou Z, et al. Tumor‐specific GPX4 degradation enhances ferroptosis‐initiated antitumor immune response in mouse models of pancreatic cancer. Sci Transl Med. 2023;15(720):eadg3049. [DOI] [PubMed] [Google Scholar]
- 49. Shimada K, Skouta R, Kaplan A, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12(7):497‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mao C, Liu X, Zhang Y, et al. DHODH‐mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593(7860):586‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Labrecque CL, Fuglestad B. Electrostatic drivers of GPx4 interactions with membrane, lipids, and DNA. Biochemistry. 2021;60(37):2761‐2772. [DOI] [PubMed] [Google Scholar]
- 52. Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30(1‐2):42‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261(5):2256‐2263. [PubMed] [Google Scholar]
- 54. Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond). 2018;38(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roh JL, Kim EH, Jang HJ, Park JY, Shin D. Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett. 2016;381(1):96‐103. [DOI] [PubMed] [Google Scholar]
- 56. Dixon SJ, Patel DN, Welsch M, et al. Pharmacological inhibition of cystine‐glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Badgley MA, Kremer DM, Maurer HC, et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368(6486):85‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bersuker K, Hendricks JM, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Doll S, Freitas FP, Shah R, et al. FSP1 is a glutathione‐independent ferroptosis suppressor. Nature. 2019;575(7784):693‐698. [DOI] [PubMed] [Google Scholar]
- 60. Kraft VAN, Bezjian CT, Pfeiffer S, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6(1):41‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kapralov AA, Yang Q, Dar HH, et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol. 2020;16(3):278‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mishima E, Ito J, Wu Z, et al. A non‐canonical vitamin K cycle is a potent ferroptosis suppressor. Nature. 2022;608(7924):778‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nakamura T, Hipp C, Santos Dias Mourao A, et al. Phase separation of FSP1 promotes ferroptosis. Nature. 2023;619(7969):371‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Deshwal S, Onishi M, Tatsuta T, et al. Mitochondria regulate intracellular coenzyme Q transport and ferroptotic resistance via STARD7. Nat Cell Biol. 2023;25(2):246‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jiang Y, Zhao J, Li R, et al. CircLRFN5 inhibits the progression of glioblastoma via PRRX2/GCH1 mediated ferroptosis. J Exp Clin Cancer Res. 2022;41(1):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu Z, Kang R, Yang N, et al. Tetrahydrobiopterin inhibitor‐based antioxidant metabolic strategy for enhanced cancer ferroptosis‐immunotherapy. J Colloid Interface Sci. 2024;658:100‐113. [DOI] [PubMed] [Google Scholar]
- 67. Freitas FP, Alborzinia H, Dos Santos AF, et al. 7‐Dehydrocholesterol is an endogenous suppressor of ferroptosis. Nature. 2024;626(7998):401‐410. [DOI] [PubMed] [Google Scholar]
- 68. Li Y, Ran Q, Duan Q, et al. 7‐Dehydrocholesterol dictates ferroptosis sensitivity. Nature. 2024;626(7998):411‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yamada N, Karasawa T, Ito J, et al. Inhibition of 7‐dehydrocholesterol reductase prevents hepatic ferroptosis under an active state of sterol synthesis. Nat Commun. 2024;15(1):2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yuan J, Ofengeim D. A guide to cell death pathways. Nat Rev Mol Cell Biol. 2024;25(5):379‐395. [DOI] [PubMed] [Google Scholar]
- 71. von Krusenstiern AN, Robson RN, Qian N, et al. Identification of essential sites of lipid peroxidation in ferroptosis. Nat Chem Biol. 2023;19(6):719‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kagan VE, Mao G, Qu F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pedrera L, Espiritu RA, Ros U, et al. Ferroptotic pores induce Ca(2+) fluxes and ESCRT‐III activation to modulate cell death kinetics. Cell Death Differ. 2021;28(5):1644‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Riegman M, Sagie L, Galed C, et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat Cell Biol. 2020;22(9):1042‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dai E, Meng L, Kang R, Wang X, Tang D. ESCRT‐III‐dependent membrane repair blocks ferroptosis. Biochem Biophys Res Commun. 2020;522(2):415‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gong YN, Guy C, Olauson H, et al. ESCRT‐III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell. 2017;169(2):286‐300. e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ruhl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. ESCRT‐dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 2018;362(6417):956‐960. [DOI] [PubMed] [Google Scholar]
- 78. Zhang Y, Shi J, Liu X, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20(10):1181‐1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang Y, Yang L, Zhang X, et al. Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Rep. 2019;20(7):e47563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang Y, Zhao Y, Wang H, et al. Histone demethylase KDM3B protects against ferroptosis by upregulating SLC7A11. FEBS Open Bio. 2020;10(4):637‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen M, Jiang Y, Sun Y. KDM4A‐mediated histone demethylation of SLC7A11 inhibits cell ferroptosis in osteosarcoma. Biochem Biophys Res Commun. 2021;550:77‐83. [DOI] [PubMed] [Google Scholar]
- 82. Zhao J, Jia Y, Mahmut D, et al. Human hematopoietic stem cell vulnerability to ferroptosis. Cell. 2023;186(4):732‐747. e716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sabari BR, Zhang D, Allis CD, Zhao Y. Metabolic regulation of gene expression through histone acylations. Nat Rev Mol Cell Biol. 2017;18(2):90‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li H, Liu W, Zhang X, Wu F, Sun D, Wang Z. Ketamine suppresses proliferation and induces ferroptosis and apoptosis of breast cancer cells by targeting KAT5/GPX4 axis. Biochem Biophys Res Commun. 2021;585:111‐116. [DOI] [PubMed] [Google Scholar]
- 85. Lee J, You JH, Kim MS, Roh JL. Epigenetic reprogramming of epithelial‐mesenchymal transition promotes ferroptosis of head and neck cancer. Redox Biol. 2020;37:101697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu L, Li Y, Cao D, et al. SIRT3 inhibits gallbladder cancer by induction of AKT‐dependent ferroptosis and blockade of epithelial‐mesenchymal transition. Cancer Lett. 2021;510:93‐104. [DOI] [PubMed] [Google Scholar]
- 87. Sui S, Zhang J, Xu S, Wang Q, Wang P, Pang D. Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)‐JQ1 in cancer cells. Cell Death Dis. 2019;10(5):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schmitt A, Grimm M, Kreienkamp N, et al. BRD4 inhibition sensitizes diffuse large B‐cell lymphoma cells to ferroptosis. Blood. 2023;142(13):1143‐1155. [DOI] [PubMed] [Google Scholar]
- 89. Meng Y, Sun HY, He Y, et al. BET inhibitors potentiate melanoma ferroptosis and immunotherapy through AKR1C2 inhibition. Mil Med Res. 2023;10(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang X, Du L, Qiao Y, et al. Ferroptosis is governed by differential regulation of transcription in liver cancer. Redox Biol. 2019;24:101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wei X, Yi X, Zhu XH, Jiang DS. Histone methylation and vascular biology. Clin Epigenetics. 2020;12(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ma M, Kong P, Huang Y, et al. Activation of MAT2A‐ACSL3 pathway protects cells from ferroptosis in gastric cancer. Free Radic Biol Med. 2022;181:288‐299. [DOI] [PubMed] [Google Scholar]
- 93. Liu T, Xu P, Ke S, et al. Histone methyltransferase SETDB1 inhibits TGF‐beta‐induced epithelial‐mesenchymal transition in pulmonary fibrosis by regulating SNAI1 expression and the ferroptosis signaling pathway. Arch Biochem Biophys. 2022;715:109087. [DOI] [PubMed] [Google Scholar]
- 94. Chen Y, Yi X, Huo B, et al. BRD4770 functions as a novel ferroptosis inhibitor to protect against aortic dissection. Pharmacol Res. 2022;177:106122. [DOI] [PubMed] [Google Scholar]
- 95. Kalra A, Meltzer SJ. The role of DNA methylation in gastrointestinal disease: an expanded review of malignant and nonmalignant gastrointestinal diseases. Gastroenterology. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ling H, Li M, Yang C, et al. Glycine increased ferroptosis via SAM‐mediated GPX4 promoter methylation in rheumatoid arthritis. Rheumatology (Oxford). 2022;61(11):4521‐4534. [DOI] [PubMed] [Google Scholar]
- 97. Pontel LB, Bueno‐Costa A, Morellato AE, Carvalho Santos J, Roue G, Esteller M. Acute lymphoblastic leukemia necessitates GSH‐dependent ferroptosis defenses to overcome FSP1‐epigenetic silencing. Redox Biol. 2022;55:102408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lee JY, Nam M, Son HY, et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci USA. 2020;117(51):32433‐32442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Slack FJ, Chinnaiyan AM. The role of non‐coding RNAs in oncology. Cell. 2019;179(5):1033‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yadav P, Sharma P, Sundaram S, Venkatraman G, Bera AK, Karunagaran D. SLC7A11/xCT is a target of miR‐5096 and its restoration partially rescues miR‐5096‐mediated ferroptosis and anti‐tumor effects in human breast cancer cells. Cancer Lett. 2021;522:211‐224. [DOI] [PubMed] [Google Scholar]
- 102. Ding C, Ding X, Zheng J, et al. miR‐182‐5p and miR‐378a‐3p regulate ferroptosis in I/R‐induced renal injury. Cell Death Dis. 2020;11(10):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ni H, Qin H, Sun C, et al. MiR‐375 reduces the stemness of gastric cancer cells through triggering ferroptosis. Stem Cell Res Ther. 2021;12(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fan K, Huang W, Qi H, et al. The Egr‐1/miR‐15a‐5p/GPX4 axis regulates ferroptosis in acute myocardial infarction. Eur J Pharmacol. 2021;909:174403. [DOI] [PubMed] [Google Scholar]
- 105. Deng SH, Wu DM, Li L, et al. miR‐324‐3p reverses cisplatin resistance by inducing GPX4‐mediated ferroptosis in lung adenocarcinoma cell line A549. Biochem Biophys Res Commun. 2021;549:54‐60. [DOI] [PubMed] [Google Scholar]
- 106. Xu Q, Zhou L, Yang G, et al. CircIL4R facilitates the tumorigenesis and inhibits ferroptosis in hepatocellular carcinoma by regulating the miR‐541‐3p/GPX4 axis. Cell Biol Int. 2020;44(11):2344‐2356. [DOI] [PubMed] [Google Scholar]
- 107. Luo M, Wu L, Zhang K, et al. miR‐137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018;25(8):1457‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bao C, Zhang J, Xian SY, Chen F. MicroRNA‐670‐3p suppresses ferroptosis of human glioblastoma cells through targeting ACSL4. Free Radic Res. 2021;55(7):853‐864. [DOI] [PubMed] [Google Scholar]
- 109. Ma LL, Liang L, Zhou D, Wang SW. Tumor suppressor miR‐424‐5p abrogates ferroptosis in ovarian cancer through targeting ACSL4. Neoplasma. 2021;68(1):165‐173. [DOI] [PubMed] [Google Scholar]
- 110. Wei D, Ke YQ, Duan P, Zhou L, Wang CY, Cao P. MicroRNA‐302a‐3p induces ferroptosis of non‐small cell lung cancer cells via targeting ferroportin. Free Radic Res. 2021;55(7):821‐830. [DOI] [PubMed] [Google Scholar]
- 111. Li X, Si W, Li Z, et al. miR‑335 promotes ferroptosis by targeting ferritin heavy chain 1 in in vivo and in vitro models of Parkinson's disease. Int J Mol Med. 2021;47(4):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zheng S, Hu L, Song Q, et al. miR‐545 promotes colorectal cancer by inhibiting transferring in the non‐normal ferroptosis signaling. Aging (Albany NY). 2021;13(24):26137‐26147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Song Y, Wang B, Zhu X, et al. Human umbilical cord blood‐derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol. 2021;37(1):51‐64. [DOI] [PubMed] [Google Scholar]
- 114. Zhang Y, Guo S, Wang S, et al. LncRNA OIP5‐AS1 inhibits ferroptosis in prostate cancer with long‐term cadmium exposure through miR‐128‐3p/SLC7A11 signaling. Ecotoxicol Environ Saf. 2021;220:112376. [DOI] [PubMed] [Google Scholar]
- 115. Li YZ, Zhu HC, Du Y, Zhao HC, Wang L. Silencing lncRNA SLC16A1‐AS1 induced ferroptosis in renal cell carcinoma through miR‐143‐3p/SLC7A11 signaling. Technol Cancer Res Treat. 2022;21:15330338221077803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang M, Mao C, Ouyang L, et al. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2019;26(11):2329‐2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Qi W, Li Z, Xia L, et al. LncRNA GABPB1‐AS1 and GABPB1 regulate oxidative stress during erastin‐induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci Rep. 2019;9(1):16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhang JK, Zhang Z, Guo ZA, et al. The BMSC‐derived exosomal lncRNA Mir9‐3hg suppresses cardiomyocyte ferroptosis in ischemia‐reperfusion mice via the Pum2/PRDX6 axis. Nutr Metab Cardiovasc Dis. 2022;32(2):515‐527. [DOI] [PubMed] [Google Scholar]
- 119. Sui X, Hu N, Zhang Z, Wang Y, Wang P, Xiu G. ASMTL‐AS1 impedes the malignant progression of lung adenocarcinoma by regulating SAT1 to promote ferroptosis. Pathol Int. 2021;71(11):741‐751. [DOI] [PubMed] [Google Scholar]
- 120. Wang Z, Chen X, Liu N, et al. A nuclear long non‐coding RNA LINC00618 accelerates ferroptosis in a manner dependent upon apoptosis. Mol Ther. 2021;29(1):263‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mao C, Wang X, Liu Y, et al. A G3BP1‐interacting lncRNA promotes ferroptosis and apoptosis in cancer via nuclear sequestration of p53. Cancer Res. 2018;78(13):3484‐3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wu P, Li C, Ye DM, et al. Circular RNA circEPSTI1 accelerates cervical cancer progression via miR‐375/409‐3P/515‐5p‐SLC7A11 axis. Aging (Albany NY). 2021;13(3):4663‐4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shanshan W, Hongying M, Jingjing F, Yiming Y, Yu R, Rui Y. CircDTL functions as an oncogene and regulates both apoptosis and ferroptosis in non‐small cell lung cancer cells. Front Genet. 2021;12:743505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen W, Fu J, Chen Y, et al. Circular RNA circKIF4A facilitates the malignant progression and suppresses ferroptosis by sponging miR‐1231 and upregulating GPX4 in papillary thyroid cancer. Aging (Albany NY). 2021;13(12):16500‐16512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jin J, Wang Y, Zheng D, Liang M, He Q. A novel identified circular RNA, mmu_mmu_circRNA_0000309, involves in germacrone‐mediated improvement of diabetic nephropathy through regulating ferroptosis by targeting miR‐188‐3p/GPX4 signaling axis. Antioxid Redox Signal. 2022;36(10‐12):740‐759. [DOI] [PubMed] [Google Scholar]
- 126. Wu C, Du M, Yu R, et al. A novel mechanism linking ferroptosis and endoplasmic reticulum stress via the circPtpn14/miR‐351‐5p/5‐LOX signaling in melatonin‐mediated treatment of traumatic brain injury. Free Radic Biol Med. 2022;178:271‐294. [DOI] [PubMed] [Google Scholar]
- 127. He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6‐methyladenosine and its role in cancer. Mol Cancer. 2019;18(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shen M, Li Y, Wang Y, et al. N(6)‐methyladenosine modification regulates ferroptosis through autophagy signaling pathway in hepatic stellate cells. Redox Biol. 2021;47:102151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ji FH, Fu XH, Li GQ, He Q, Qiu XG. FTO prevents thyroid cancer progression by SLC7A11 m6A methylation in a ferroptosis‐dependent manner. Front Endocrinol (Lausanne). 2022;13:857765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Fan Z, Yang G, Zhang W, et al. Hypoxia blocks ferroptosis of hepatocellular carcinoma via suppression of METTL14 triggered YTHDF2‐dependent silencing of SLC7A11. J Cell Mol Med. 2021;25(21):10197‐10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ma L, Chen T, Zhang X, et al. The m(6)A reader YTHDC2 inhibits lung adenocarcinoma tumorigenesis by suppressing SLC7A11‐dependent antioxidant function. Redox Biol. 2021;38:101801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Liu L, He J, Sun G, et al. The N6‐methyladenosine modification enhances ferroptosis resistance through inhibiting SLC7A11 mRNA deadenylation in hepatoblastoma. Clin Transl Med. 2022;12(5):e778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Xu Y, Lv D, Yan C, et al. METTL3 promotes lung adenocarcinoma tumor growth and inhibits ferroptosis by stabilizing SLC7A11 m(6)A modification. Cancer Cell Int. 2022;22(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sun S, Gao T, Pang B, et al. RNA binding protein NKAP protects glioblastoma cells from ferroptosis by promoting SLC7A11 mRNA splicing in an m(6)A‐dependent manner. Cell Death Dis. 2022;13(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Xu X, Cui J, Wang H, et al. IGF2BP3 is an essential N(6)‐methyladenosine biotarget for suppressing ferroptosis in lung adenocarcinoma cells. Mater Today Bio. 2022;17:100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Song Z, Jia G, Ma P, Cang S. Exosomal miR‐4443 promotes cisplatin resistance in non‐small cell lung carcinoma by regulating FSP1 m6A modification‐mediated ferroptosis. Life Sci. 2021;276:119399. [DOI] [PubMed] [Google Scholar]
- 137. Li N, Yi X, He Y, et al. Targeting ferroptosis as a novel approach to alleviate aortic dissection. Int J Biol Sci. 2022;18(10):4118‐4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhang H, Liu J, Zhou Y, et al. Neutrophil extracellular traps mediate m(6)A modification and regulates sepsis‐associated acute lung injury by activating ferroptosis in alveolar epithelial cells. Int J Biol Sci. 2022;18(8):3337‐3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lei G, Zhuang L, Gan B. The roles of ferroptosis in cancer: tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell. 2024;42(4):513‐534. [DOI] [PubMed] [Google Scholar]
- 140. Ryan SK, Ugalde CL, Rolland AS, Skidmore J, Devos D, Hammond TR. Therapeutic inhibition of ferroptosis in neurodegenerative disease. Trends Pharmacol Sci. 2023;44(10):674‐688. [DOI] [PubMed] [Google Scholar]
- 141. Wang Y, Wu S, Li Q, Sun H, Wang H. Pharmacological inhibition of ferroptosis as a therapeutic target for neurodegenerative diseases and strokes. Adv Sci (Weinh). 2023;10(24):e2300325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Poduri A, Evrony GD, Cai X, Walsh CA. Somatic mutation, genomic variation, and neurological disease. Science. 2013;341(6141):1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Liu H, Forouhar F, Seibt T, et al. Characterization of a patient‐derived variant of GPX4 for precision therapy. Nat Chem Biol. 2022;18(1):91‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Greenough MA, Lane DJR, Balez R, et al. Selective ferroptosis vulnerability due to familial Alzheimer's disease presenilin mutations. Cell Death Differ. 2022;29(11):2123‐2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wang T, Tomas D, Perera ND, et al. Ferroptosis mediates selective motor neuron death in amyotrophic lateral sclerosis. Cell Death Differ. 2022;29(6):1187‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sun WY, Tyurin VA, Mikulska‐Ruminska K, et al. Phospholipase iPLA(2)beta averts ferroptosis by eliminating a redox lipid death signal. Nat Chem Biol. 2021;17(4):465‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Cao J, Chen X, Jiang L, et al. DJ‐1 suppresses ferroptosis through preserving the activity of S‐adenosyl homocysteine hydrolase. Nat Commun. 2020;11(1):1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Angelova PR, Choi ML, Berezhnov AV, et al. Alpha synuclein aggregation drives ferroptosis: an interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 2020;27(10):2781‐2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mahoney‐Sanchez L, Bouchaoui H, Boussaad I, et al. Alpha synuclein determines ferroptosis sensitivity in dopaminergic neurons via modulation of ether‐phospholipid membrane composition. Cell Rep. 2022;40(8):111231. [DOI] [PubMed] [Google Scholar]
- 150. Jennis M, Kung CP, Basu S, et al. An African‐specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 2016;30(8):918‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wang SJ, Li D, Ou Y, et al. Acetylation is crucial for p53‐mediated ferroptosis and tumor suppression. Cell Rep. 2016;17(2):366‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Rosell R, Jain A, Codony‐Servat J, et al. Biological insights in non‐small cell lung cancer. Cancer Biol Med. 2023;20(7):500‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Dodson M, Castro‐Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Koppula P, Lei G, Zhang Y, et al. A targetable CoQ‐FSP1 axis drives ferroptosis‐ and radiation‐resistance in KEAP1 inactive lung cancers. Nat Commun. 2022;13(1):2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Hu K, Li K, Lv J, et al. Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS‐mutant lung adenocarcinoma. J Clin Invest. 2020;130(4):1752‐1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Padanad MS, Konstantinidou G, Venkateswaran N, et al. Fatty acid oxidation mediated by Acyl‐CoA synthetase long chain 3 is required for mutant KRAS lung tumorigenesis. Cell Rep. 2016;16(6):1614‐1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Muller F, Lim JKM, Bebber CM, et al. Elevated FSP1 protects KRAS‐mutated cells from ferroptosis during tumor initiation. Cell Death Differ. 2023;30(2):442‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K‐AKT‐mTOR signaling suppresses ferroptosis via SREBP‐mediated lipogenesis. Proc Natl Acad Sci USA. 2020;117(49):31189‐31197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wu J, Minikes AM, Gao M, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2‐YAP signalling. Nature. 2019;572(7769):402‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Poursaitidis I, Wang X, Crighton T, et al. Oncogene‐Selective Sensitivity to Synchronous Cell Death following Modulation of the Amino Acid Nutrient Cystine. Cell Rep. 2017;18(11):2547‐2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wang TX, Liang JY, Zhang C, Xiong Y, Guan KL, Yuan HX. The oncometabolite 2‐hydroxyglutarate produced by mutant IDH1 sensitizes cells to ferroptosis. Cell Death Dis. 2019;10(10):755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Jiang L, Kon N, Li T, et al. Ferroptosis as a p53‐mediated activity during tumour suppression. Nature. 2015;520(7545):57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Yang X, Wang Z, Zandkarimi F, et al. Regulation of VKORC1L1 is critical for p53‐mediated tumor suppression through vitamin K metabolism. Cell Metab. 2023;35(8):1474‐1490. e1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Tarangelo A, Magtanong L, Bieging‐Rolett KT, et al. p53 suppresses metabolic stress‐induced ferroptosis in cancer cells. Cell Rep. 2018;22(3):569‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Anandhan A, Dodson M, Shakya A, et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci Adv. 2023;9(5):eade9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Takahashi N, Cho P, Selfors LM, et al. 3D culture models with CRISPR screens reveal hyperactive NRF2 as a prerequisite for spheroid formation via regulation of proliferation and ferroptosis. Mol Cell. 2020;80(5):828‐844. e826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Kuang F, Liu J, Xie Y, Tang D, Kang R. MGST1 is a redox‐sensitive repressor of ferroptosis in pancreatic cancer cells. Cell Chem Biol. 2021;28(6):765‐775. e765. [DOI] [PubMed] [Google Scholar]
- 168. Forcina GC, Pope L, Murray M, et al. Ferroptosis regulation by the NGLY1/NFE2L1 pathway. Proc Natl Acad Sci USA. 2022;119(11):e2118646119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kotschi S, Jung A, Willemsen N, et al. NFE2L1‐mediated proteasome function protects from ferroptosis. Mol Metab. 2022;57:101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Wang L, Liu Y, Du T, et al. ATF3 promotes erastin‐induced ferroptosis by suppressing system Xc(). Cell Death Differ. 2020;27(2):662‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zou Y, Palte MJ, Deik AA, et al. A GPX4‐dependent cancer cell state underlies the clear‐cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10(1):1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Wang W, Green M, Choi JE, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Venkatesh D, O'Brien NA, Zandkarimi F, et al. MDM2 and MDMX promote ferroptosis by PPARalpha‐mediated lipid remodeling. Genes Dev. 2020;34(7‐8):526‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Alborzinia H, Chen Z, Yildiz U, et al. LRP8‐mediated selenocysteine uptake is a targetable vulnerability in MYCN‐amplified neuroblastoma. EMBO Mol Med. 2023;15(8):e18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Alborzinia H, Florez AF, Kreth S, et al. MYCN mediates cysteine addiction and sensitizes neuroblastoma to ferroptosis. Nat Cancer. 2022;3(4):471‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Floros KV, Cai J, Jacob S, et al. MYCN‐amplified neuroblastoma is addicted to iron and vulnerable to inhibition of the system Xc‐/glutathione axis. Cancer Res. 2021;81(7):1896‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Mishima E, Conrad M. Nutritional and metabolic control of ferroptosis. Annu Rev Nutr. 2022;42:275‐309. [DOI] [PubMed] [Google Scholar]
- 178. Fujita H, Tanaka YK, Ogata S, et al. PRDX6 augments selenium utilization to limit iron toxicity and ferroptosis. Nat Struct Mol Biol. 2024;31(8):1277‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Wang Z, Li R, Hou N, et al. PRMT5 reduces immunotherapy efficacy in triple‐negative breast cancer by methylating KEAP1 and inhibiting ferroptosis. J Immunother Cancer. 2023;11(6):e006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Chu B, Kon N, Chen D, et al. ALOX12 is required for p53‐mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21(5):579‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Lei G, Zhang Y, Hong T, et al. Ferroptosis as a mechanism to mediate p53 function in tumor radiosensitivity. Oncogene. 2021;40(20):3533‐3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ou Y, Wang SJ, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53‐mediated ferroptotic responses. Proc Natl Acad Sci USA. 2016;113(44):E6806‐E6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Meng Y, Sun H, Li Y, et al. Targeting ferroptosis by ubiquitin system enzymes: a potential therapeutic strategy in cancer. Int J Biol Sci. 2022;18(14):5475‐5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Sun H, Meng Y, Yao L, et al. Ubiquitin‐specific protease 22 controls melanoma metastasis and vulnerability to ferroptosis through targeting SIRT1/PTEN/PI3K signaling. MedComm. 2024;5(8):e684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Aboushousha R, van der Velden J, Hamilton N, et al. Glutaredoxin attenuates glutathione levels via deglutathionylation of Otub1 and subsequent destabilization of system x(C)(). Sci Adv. 2023;9(37):eadi5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Sun X, Huang N, Li P, et al. TRIM21 ubiquitylates GPX4 and promotes ferroptosis to aggravate ischemia/reperfusion‐induced acute kidney injury. Life Sci. 2023;321:121608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Jin ZL, Gao WY, Guo F, et al. Ring finger protein 146‐mediated long‐chain fatty‐acid‐coenzyme a ligase 4 ubiquitination regulates ferroptosis‐induced neuronal damage in ischemic stroke. Neuroscience. 2023;529:148‐161. [DOI] [PubMed] [Google Scholar]
- 188. Ma Y, Huang L, Zhang Z, et al. CD36 promotes tubular ferroptosis by regulating the ubiquitination of FSP1 in acute kidney injury. Genes Dis. 2024;11(1):449‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Zhan M, Ding Y, Huang S, et al. Lysyl oxidase‐like 3 restrains mitochondrial ferroptosis to promote liver cancer chemoresistance by stabilizing dihydroorotate dehydrogenase. Nat Commun. 2023;14(1):3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Wu Y, Jiao H, Yue Y, et al. Ubiquitin ligase E3 HUWE1/MULE targets transferrin receptor for degradation and suppresses ferroptosis in acute liver injury. Cell Death Differ. 2022;29(9):1705‐1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Guo S, Chen Y, Xue X, et al. TRIB2 desensitizes ferroptosis via betaTrCP‐mediated TFRC ubiquitiantion in liver cancer cells. Cell Death Discov. 2021;7(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Tang Z, Jiang W, Mao M, Zhao J, Chen J, Cheng N. Deubiquitinase USP35 modulates ferroptosis in lung cancer via targeting ferroportin. Clin Transl Med. 2021;11(4):e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Dong K, Wei R, Jin T, et al. HOIP modulates the stability of GPx4 by linear ubiquitination. Proc Natl Acad Sci USA. 2022;119(44):e2214227119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Gong J, Liu Y, Wang W, et al. TRIM21‐promoted FSP1 plasma membrane translocation confers ferroptosis resistance in human cancers. Adv Sci (Weinh). 2023;10(29):e2302318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Wu K, Yan M, Liu T, et al. Creatine kinase B suppresses ferroptosis by phosphorylating GPX4 through a moonlighting function. Nat Cell Biol. 2023;25(5):714‐725. [DOI] [PubMed] [Google Scholar]
- 196. Wang Y, Yu R, Wu L, Yang G. Hydrogen sulfide guards myoblasts from ferroptosis by inhibiting ALOX12 acetylation. Cell Signal. 2021;78:109870. [DOI] [PubMed] [Google Scholar]
- 197. Tang J, Long G, Hu K, et al. Targeting USP8 inhibits O‐GlcNAcylation of SLC7A11 to promote ferroptosis of hepatocellular carcinoma via stabilization of OGT. Adv Sci (Weinh). 2023;10(33):e2302953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Wang Z, Wang Y, Shen N, et al. AMPKalpha1‐mediated ZDHHC8 phosphorylation promotes the palmitoylation of SLC7A11 to facilitate ferroptosis resistance in glioblastoma. Cancer Lett. 2024;584:216619. [DOI] [PubMed] [Google Scholar]
- 199. Zhang Q, Li N, Deng L, et al. ACSL1‐induced ferroptosis and platinum resistance in ovarian cancer by increasing FSP1 N‐myristylation and stability. Cell Death Discov. 2023;9(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Feng S, Rao Z, Zhang J, et al. Inhibition of CARM1‐mediated methylation of ACSL4 promotes ferroptosis in colorectal cancer. Adv Sci (Weinh). 2023;10(36):e2303484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Xu X, Mao Y, Feng Z, Dai F, Gu T, Zheng J. SENP1 inhibits ferroptosis and promotes head and neck squamous cell carcinoma by regulating ACSL4 protein stability via SUMO1. Oncol Rep. 2024;51(2):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Zhou Q, Meng Y, Li D, et al. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct Target Ther. 2024;9(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Du S, Zeng F, Sun H, et al. Prognostic and therapeutic significance of a novel ferroptosis related signature in colorectal cancer patients. Bioengineered. 2022;13(2):2498‐2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Stockwell BR. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185(14):2401‐2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Ubellacker JM, Tasdogan A, Ramesh V, et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 2020;585(7823):113‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Tsoi J, Robert L, Paraiso K, et al. Multi‐stage differentiation defines melanoma subtypes with differential vulnerability to drug‐induced iron‐dependent oxidative stress. Cancer Cell. 2018;33(5):890‐904. e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Hangauer MJ, Viswanathan VS, Ryan MJ, et al. Drug‐tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551(7679):247‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Yang WH, Ding CC, Sun T, et al. The Hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep. 2019;28(10):2501‐2508. e2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Yang F, Xiao Y, Ding JH, et al. Ferroptosis heterogeneity in triple‐negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2023;35(1):84‐100. e108. [DOI] [PubMed] [Google Scholar]
- 210. Jiang Z, Lim SO, Yan M, et al. TYRO3 induces anti‐PD‐1/PD‐L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J Clin Invest. 2021;131(8):e139434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Wang S, Zhu L, Li T, et al. Disruption of MerTK increases the efficacy of checkpoint inhibitor by enhancing ferroptosis and immune response in hepatocellular carcinoma. Cell Rep Med. 2024;5(2):101415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Du S, Zeng F, Deng G. Tumor neutrophils ferroptosis: a targetable immunosuppressive mechanism for cancer immunotherapy. Signal Transduct Target Ther. 2023;8(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Kim R, Hashimoto A, Markosyan N, et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022;612(7939):338‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Katsnelson A, De Strooper B, Zoghbi HY. Neurodegeneration: from cellular concepts to clinical applications. Sci Transl Med. 2016;8(364):364ps318. [DOI] [PubMed] [Google Scholar]
- 215. Steel K. Alzheimer's disease. N Engl J Med. 2010;362(19):1844. author reply 1844–1845. [PubMed] [Google Scholar]
- 216. Schneider SA. Neurodegenerations with brain iron accumulation. Parkinsonism Relat Disord. 2016;22(Suppl 1):S21‐S25. [DOI] [PubMed] [Google Scholar]
- 217. Sato T, Shapiro JS, Chang HC, Miller RA, Ardehali H. Aging is associated with increased brain iron through cortex‐derived hepcidin expression. Elife. 2022;11:e73456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Tian Y, Tian Y, Yuan Z, et al. Iron metabolism in aging and age‐related diseases. Int J Mol Sci. 2022;23(7):e73456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. McCormick AV, Wheeler JM, Guthrie CR, Liachko NF, Kraemer BC. Dopamine D2 receptor antagonism suppresses tau aggregation and neurotoxicity. Biol Psychiatry. 2013;73(5):464‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Woo MS, Mayer C, Binkle‐Ladisch L, et al. STING orchestrates the neuronal inflammatory stress response in multiple sclerosis. Cell. 2024;187(15):4043‐4060. e4030. [DOI] [PubMed] [Google Scholar]
- 221. Chen R, Park HA, Mnatsakanyan N, et al. Parkinson's disease protein DJ‐1 regulates ATP synthase protein components to increase neuronal process outgrowth. Cell Death Dis. 2019;10(6):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Derry PJ, Hegde ML, Jackson GR, et al. Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer's disease from a ferroptosis perspective. Prog Neurobiol. 2020;184:101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Hambright WS, Fonseca RS, Chen L, Na R, Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017;12:8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Do Van B, Gouel F, Jonneaux A, et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiol Dis. 2016;94:169‐178. [DOI] [PubMed] [Google Scholar]
- 225. Skouta R, Dixon SJ, Wang J, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136(12):4551‐4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Avci B, Gunaydin C, Guvenc T, Yavuz CK, Kuruca N, Bilge SS. Idebenone ameliorates rotenone‐induced Parkinson's disease in rats through decreasing lipid peroxidation. Neurochem Res. 2021;46(3):513‐522. [DOI] [PubMed] [Google Scholar]
- 227. Guo S, Lei Q, Guo H, Yang Q, Xue Y, Chen R. Edaravone attenuates abeta 1‐42‐induced inflammatory damage and ferroptosis in HT22 cells. Neurochem Res. 2023;48(2):570‐578. [DOI] [PubMed] [Google Scholar]
- 228. Homma T, Kobayashi S, Sato H, Fujii J. Edaravone, a free radical scavenger, protects against ferroptotic cell death in vitro. Exp Cell Res. 2019;384(1):111592. [DOI] [PubMed] [Google Scholar]
- 229. Southon A, Szostak K, Acevedo KM, et al. Cu(II) (atsm) inhibits ferroptosis: implications for treatment of neurodegenerative disease. Br J Pharmacol. 2020;177(3):656‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Fang X, Wang H, Han D, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7):2672‐2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Tuo QZ, Lei P, Jackman KA, et al. Tau‐mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry. 2017;22(11):1520‐1530. [DOI] [PubMed] [Google Scholar]
- 232. Linkermann A, Skouta R, Himmerkus N, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci USA. 2014;111(47):16836‐16841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Li Y, Cao Y, Xiao J, et al. Inhibitor of apoptosis‐stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion‐induced acute lung injury. Cell Death Differ. 2020;27(9):2635‐2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Li Y, Feng D, Wang Z, et al. Ischemia‐induced ACSL4 activation contributes to ferroptosis‐mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26(11):2284‐2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Li W, Feng G, Gauthier JM, et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J Clin Invest. 2019;129(6):2293‐2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Yamada N, Karasawa T, Wakiya T, et al. Iron overload as a risk factor for hepatic ischemia‐reperfusion injury in liver transplantation: potential role of ferroptosis. Am J Transplant. 2020;20(6):1606‐1618. [DOI] [PubMed] [Google Scholar]
- 237. Stoppe C, Averdunk L, Goetzenich A, et al. The protective role of macrophage migration inhibitory factor in acute kidney injury after cardiac surgery. Sci Transl Med. 2018;10(441):eaan4886. [DOI] [PubMed] [Google Scholar]
- 238. Mu Y, Sun J, Li Z, et al. Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere. 2022;291(Pt 2):132944. [DOI] [PubMed] [Google Scholar]
- 239. Guohua F, Tieyuan Z, Xinping M, Juan X. Melatonin protects against PM2.5‐induced lung injury by inhibiting ferroptosis of lung epithelial cells in a Nrf2‐dependent manner. Ecotoxicol Environ Saf. 2021;223:112588. [DOI] [PubMed] [Google Scholar]
- 240. Van Coillie S, Van San E, Goetschalckx I, et al. Targeting ferroptosis protects against experimental (multi)organ dysfunction and death. Nat Commun. 2022;13(1):1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Dar HH, Tyurina YY, Mikulska‐Ruminska K, et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft‐ferroptosis in bronchial epithelium. J Clin Invest. 2018;128(10):4639‐4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Amaral EP, Costa DL, Namasivayam S, et al. A major role for ferroptosis in Mycobacterium tuberculosis‐induced cell death and tissue necrosis. J Exp Med. 2019;216(3):556‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Luo K, Stocker R, Britton WJ, Kikuchi K, Oehlers SH. Haem oxygenase limits Mycobacterium marinum infection‐induced detrimental ferrostatin‐sensitive cell death in zebrafish. FEBS J. 2022;289(3):671‐681. [DOI] [PubMed] [Google Scholar]
- 244. Yamane D, Hayashi Y, Matsumoto M, et al. FADS2‐dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication. Cell Chem Biol. 2022;29(5):799‐810. e794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Bednash JS, Kagan VE, Englert JA, et al. Syrian hamsters as a model of lung injury with SARS‐CoV‐2 infection: pathologic, physiologic, and detailed molecular profiling. Transl Res. 2022;240:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Luoqian J, Yang W, Ding X, et al. Ferroptosis promotes T‐cell activation‐induced neurodegeneration in multiple sclerosis. Cell Mol Immunol. 2022;19(8):913‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Li P, Jiang M, Li K, et al. Glutathione peroxidase 4‐regulated neutrophil ferroptosis induces systemic autoimmunity. Nat Immunol. 2021;22(9):1107‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Alli AA, Desai D, Elshika A, et al. Kidney tubular epithelial cell ferroptosis links glomerular injury to tubulointerstitial pathology in lupus nephritis. Clin Immunol. 2023;248:109213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Xu M, Tao J, Yang Y, et al. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020;11(2):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Mayr L, Grabherr F, Schwarzler J, et al. Dietary lipids fuel GPX4‐restricted enteritis resembling Crohn's disease. Nat Commun. 2020;11(1):1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Wu J, Feng Z, Chen L, et al. TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen‐induced arthritis mouse models. Nat Commun. 2022;13(1):676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Liu Y, Luo X, Chen Y, et al. Heterogeneous ferroptosis susceptibility of macrophages caused by focal iron overload exacerbates rheumatoid arthritis. Redox Biol. 2024;69:103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Zhou R, Chen Y, Li S, et al. TRPM7 channel inhibition attenuates rheumatoid arthritis articular chondrocyte ferroptosis by suppression of the PKCalpha‐NOX4 axis. Redox Biol. 2022;55:102411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Ajoolabady A, Aslkhodapasandhokmabad H, Libby P, et al. Ferritinophagy and ferroptosis in the management of metabolic diseases. Trends Endocrinol Metab. 2021;32(7):444‐462. [DOI] [PubMed] [Google Scholar]
- 255. Sui Y, Geng X, Wang Z, Zhang J, Yang Y, Meng Z. Targeting the regulation of iron homeostasis as a potential therapeutic strategy for nonalcoholic fatty liver disease. Metabolism. 2024;157:155953. [DOI] [PubMed] [Google Scholar]
- 256. Tsurusaki S, Tsuchiya Y, Koumura T, et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019;10(6):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Lv T, Xiong X, Yan W, Liu M, Xu H, He Q. Mitochondrial general control of amino acid synthesis 5 like 1 promotes nonalcoholic steatohepatitis development through ferroptosis‐induced formation of neutrophil extracellular traps. Clin Transl Med. 2023;13(7):e1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Zhang J, Wang Y, Fan M, et al. Reactive oxygen species regulation by NCF1 governs ferroptosis susceptibility of Kupffer cells to MASH. Cell Metab. 2024;36(8):1745‐1763. e1746. [DOI] [PubMed] [Google Scholar]
- 259. Liu B, Yi W, Mao X, Yang L, Rao C. Enoyl coenzyme A hydratase 1 alleviates nonalcoholic steatohepatitis in mice by suppressing hepatic ferroptosis. Am J Physiol Endocrinol Metab. 2021;320(5):E925‐E937. [DOI] [PubMed] [Google Scholar]
- 260. Gong Y, Liu Z, Zhang Y, Zhang J, Zheng Y, Wu Z. AGER1 deficiency‐triggered ferroptosis drives fibrosis progression in nonalcoholic steatohepatitis with type 2 diabetes mellitus. Cell Death Discov. 2023;9(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Yu Y, Jiang L, Wang H, et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136(6):726‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. He F, Zhang P, Liu J, et al. ATF4 suppresses hepatocarcinogenesis by inducing SLC7A11 (xCT) to block stress‐related ferroptosis. J Hepatol. 2023;79(2):362‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Liu P, Zhang Z, Cai Y, Li Z, Zhou Q, Chen Q. Ferroptosis: Mechanisms and role in diabetes mellitus and its complications. Ageing Res Rev. 2024;94:102201. [DOI] [PubMed] [Google Scholar]
- 264. Li D, Jiang C, Mei G, et al. Quercetin alleviates ferroptosis of pancreatic beta cells in type 2 diabetes. Nutrients. 2020;12(10):2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Wang H, Yu X, Liu D, et al. VDR activation attenuates renal tubular epithelial cell ferroptosis by regulating Nrf2/HO‐1 signaling pathway in diabetic nephropathy. Adv Sci (Weinh). 2024;11(10):e2305563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Abdul Y, Li W, Ward R, et al. Deferoxamine treatment prevents post‐stroke vasoregression and neurovascular unit remodeling leading to improved functional outcomes in type 2 male diabetic rats: role of endothelial ferroptosis. Transl Stroke Res. 2021;12(4):615‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Srivastava N, Hu H, Peterson OJ, et al. CXCL16‐dependent scavenging of oxidized lipids by islet macrophages promotes differentiation of pathogenic CD8(+) T cells in diabetic autoimmunity. Immunity. 2024;57(7):1629‐1647. e1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Shou Y, Yang L, Yang Y, Xu J. Inhibition of keratinocyte ferroptosis suppresses psoriatic inflammation. Cell Death Dis. 2021;12(11):1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Wu X, Jin S, Yang Y, et al. Altered expression of ferroptosis markers and iron metabolism reveals a potential role of ferroptosis in vitiligo. Pigment Cell Melanoma Res. 2022;35(3):328‐341. [DOI] [PubMed] [Google Scholar]
- 270. Feng Z, Qin Y, Huo F, et al. NMN recruits GSH to enhance GPX4‐mediated ferroptosis defense in UV irradiation induced skin injury. Biochim Biophys Acta Mol Basis Dis. 2022;1868(1):166287. [DOI] [PubMed] [Google Scholar]
- 271. Guo S, Zhou L, Liu X, Gao L, Li Y, Wu Y. Baicalein alleviates cisplatin‐induced acute kidney injury by inhibiting ALOX12‐dependent ferroptosis. Phytomedicine. 2024;130:155757. [DOI] [PubMed] [Google Scholar]
- 272. Pei J, Zou Y, Zhou W, Wang Y. Baicalein, a component of banxia xiexin decoction, alleviates CPT‐11‐induced gastrointestinal dysfunction by inhibiting ALOX15‐mediated ferroptosis. Chem Biol Drug Des. 2023;102(6):1568‐1577. [DOI] [PubMed] [Google Scholar]
- 273. Fan Z, Cai L, Wang S, Wang J, Chen B. Baicalin prevents myocardial ischemia/reperfusion injury through inhibiting ACSL4 mediated ferroptosis. Front Pharmacol. 2021;12:628988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Liu Y, Wang W, Li Y, Xiao Y, Cheng J, Jia J. The 5‐lipoxygenase inhibitor zileuton confers neuroprotection against glutamate oxidative damage by inhibiting ferroptosis. Biol Pharm Bull. 2015;38(8):1234‐1239. [DOI] [PubMed] [Google Scholar]
- 275. Probst L, Dachert J, Schenk B, Fulda S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem Pharmacol. 2017;140:41‐52. [DOI] [PubMed] [Google Scholar]
- 276. Qi J, Kim JW, Zhou Z, Lim CW, Kim B. Ferroptosis affects the progression of nonalcoholic steatohepatitis via the modulation of lipid peroxidation‐mediated cell death in mice. Am J Pathol. 2020;190(1):68‐81. [DOI] [PubMed] [Google Scholar]
- 277. Fernandez‐Mendivil C, Luengo E, Trigo‐Alonso P, Garcia‐Magro N, Negredo P, Lopez MG. Protective role of microglial HO‐1 blockade in aging: Implication of iron metabolism. Redox Biol. 2021;38:101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Liu H, An N, Wang L, et al. Protective effect of Xingnaojing injection on ferroptosis after cerebral ischemia injury in MCAO rats and SH‐SY5Y cells. J Ethnopharmacol. 2023;301:115836. [DOI] [PubMed] [Google Scholar]
- 279. Chen Y, Zhang P, Chen W, Chen G. Ferroptosis mediated DSS‐induced ulcerative colitis associated with Nrf2/HO‐1 signaling pathway. Immunol Lett. 2020;225:9‐15. [DOI] [PubMed] [Google Scholar]
- 280. Ishimaru K, Ikeda M, Miyamoto HD, et al. Deferasirox targeting ferroptosis synergistically ameliorates myocardial ischemia reperfusion injury in conjunction with cyclosporine A. J Am Heart Assoc. 2024;13(1):e031219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Meng J, Yang X, Huang J, et al. Ferroptosis‐enhanced immunotherapy with an injectable dextran‐chitosan hydrogel for the treatment of malignant ascites in hepatocellular carcinoma. Adv Sci (Weinh). 2023;10(20):e2300517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Xu X, Li Y, Wu Y, et al. Increased ATF2 expression predicts poor prognosis and inhibits sorafenib‐induced ferroptosis in gastric cancer. Redox Biol. 2023;59:102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Ye LF, Chaudhary KR, Zandkarimi F, et al. Radiation‐induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem Biol. 2020;15(2):469‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Chang K, Chen Y, Zhang X, et al. DPP9 stabilizes NRF2 to suppress ferroptosis and induce sorafenib resistance in clear cell renal cell carcinoma. Cancer Res. 2023;83(23):3940‐3955. [DOI] [PubMed] [Google Scholar]
- 285. Wang Y, Zheng L, Shang W, et al. Wnt/beta‐catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ. 2022;29(11):2190‐2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Zhou Z, Zhao Y, Chen S, et al. Cisplatin promotes the efficacy of immune checkpoint inhibitor therapy by inducing ferroptosis and activating neutrophils. Front Pharmacol. 2022;13:870178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Karuppagounder SS, Alin L, Chen Y, et al. N‐acetylcysteine targets 5 lipoxygenase‐derived, toxic lipids and can synergize with prostaglandin E(2) to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Ann Neurol. 2018;84(6):854‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Hu M, Zhang Y, Ma S, et al. Suppression of uterine and placental ferroptosis by N‐acetylcysteine in a rat model of polycystic ovary syndrome. Mol Hum Reprod. 2021;27(12):gaab067. [DOI] [PubMed] [Google Scholar]
- 289. Li Q, Liao J, Chen W, et al. NAC alleviative ferroptosis in diabetic nephropathy via maintaining mitochondrial redox homeostasis through activating SIRT3‐SOD2/Gpx4 pathway. Free Radic Biol Med. 2022;187:158‐170. [DOI] [PubMed] [Google Scholar]
- 290. Huang J, Xie H, Yang Y, et al. The role of ferroptosis and endoplasmic reticulum stress in intermittent hypoxia‐induced myocardial injury. Sleep Breath. 2023;27(3):1005‐1011. [DOI] [PubMed] [Google Scholar]
- 291. Shi Y, Xu N, Liu B, et al. Mifepristone protects acetaminophen induced liver injury through NRF2/GSH/GST mediated ferroptosis suppression. Free Radic Biol Med. 2024;222:229‐243. [DOI] [PubMed] [Google Scholar]
- 292. Hassannia B, Wiernicki B, Ingold I, et al. Nano‐targeted induction of dual ferroptotic mechanisms eradicates high‐risk neuroblastoma. J Clin Invest. 2018;128(8):3341‐3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. He H, Liang L, Huang J, et al. KIF20A is associated with clinical prognosis and synergistic effect of gemcitabine combined with ferroptosis inducer in lung adenocarcinoma. Front Pharmacol. 2022;13:1007429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Huang C, Guo Y, Li T, et al. Pharmacological activation of GPX4 ameliorates doxorubicin‐induced cardiomyopathy. Redox Biol. 2024;70:103024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Mishima E, Sato E, Ito J, et al. Drugs repurposed as antiferroptosis agents suppress organ damage, including AKI, by functioning as lipid peroxyl radical scavengers. J Am Soc Nephrol. 2020;31(2):280‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Dang R, Wang M, Li X, et al. Edaravone ameliorates depressive and anxiety‐like behaviors via Sirt1/Nrf2/HO‐1/Gpx4 pathway. J Neuroinflammation. 2022;19(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Zhang Y, Zou Z, Liu S, et al. Edaravone‐loaded poly(amino acid) nanogel inhibits ferroptosis for neuroprotection in cerebral ischemia injury. Asian J Pharm Sci. 2024;19(2):100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Zilka O, Poon JF, Pratt DA. Radical‐trapping antioxidant activity of copper and nickel bis(thiosemicarbazone) complexes underlies their potency as inhibitors of ferroptotic cell death. J Am Chem Soc. 2021;143(45):19043‐19057. [DOI] [PubMed] [Google Scholar]
- 299. Li Z, Lange M, Dixon SJ, Olzmann JA. Lipid quality control and ferroptosis: from concept to mechanism. Annu Rev Biochem. 2024;93(1):499‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Zeng F, Ye L, Zhou Q, et al. Inhibiting SCD expression by IGF1R during lorlatinib therapy sensitizes melanoma to ferroptosis. Redox Biol. 2023;61:102653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Dierge E, Debock E, Guilbaud C, et al. Peroxidation of n‐3 and n‐6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis‐mediated anticancer effects. Cell Metab. 2021;33(8):1701‐1715. e1705. [DOI] [PubMed] [Google Scholar]
- 302. Angeli JPF, Shah R, Pratt DA, Conrad M. Ferroptosis inhibition: mechanisms and opportunities. Trends Pharmacol Sci. 2017;38(5):489‐498. [DOI] [PubMed] [Google Scholar]
- 303. Li W, Abdul Y, Chandran R, et al. Deferoxamine prevents poststroke memory impairment in female diabetic rats: potential links to hemorrhagic transformation and ferroptosis. Am J Physiol Heart Circ Physiol. 2023;324(2):H212‐H225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Qin X, Zhang J, Wang B, et al. Ferritinophagy is involved in the zinc oxide nanoparticles‐induced ferroptosis of vascular endothelial cells. Autophagy. 2021;17(12):4266‐4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Kontoghiorghes GJ. Ethical issues and risk/benefit assessment of iron chelation therapy: advances with deferiprone/deferoxamine combinations and concerns about the safety, efficacy and costs of deferasirox. Hemoglobin. 2008;32(1‐2):1‐15. [DOI] [PubMed] [Google Scholar]
- 306. Hoffbrand AV, Cohen A, Hershko C. Role of deferiprone in chelation therapy for transfusional iron overload. Blood. 2003;102(1):17‐24. [DOI] [PubMed] [Google Scholar]
- 307. Sheth S. Iron chelation: an update. Curr Opin Hematol. 2014;21(3):179‐185. [DOI] [PubMed] [Google Scholar]
- 308. Kong N, Chen X, Feng J, et al. Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1. Acta Pharm Sin B. 2021;11(12):4045‐4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Richard F, van Lier JJ, Roubert B, Haboubi T, Gohring UM, Durrenberger F. Oral ferroportin inhibitor VIT‐2763: First‐in‐human, phase 1 study in healthy volunteers. Am J Hematol. 2020;95(1):68‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)‐ cystine transporter: a new action for an old drug. Leukemia. 2001;15(10):1633‐1640. [DOI] [PubMed] [Google Scholar]
- 311. Lei G, Zhang Y, Koppula P, et al. The role of ferroptosis in ionizing radiation‐induced cell death and tumor suppression. Cell Res. 2020;30(2):146‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Kerkhove L, Geirnaert F, Rifi AL, et al. Repurposing sulfasalazine as a radiosensitizer in hypoxic human colorectal cancer. Cancers (Basel). 2023;15(8):2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Zhu S, Zhang Q, Sun X, et al. HSPA5 regulates ferroptotic cell death in cancer cells. Cancer Res. 2017;77(8):2064‐2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Idei U, Ohta T, Yamatani H, Seino M, Nagase S. Mechanism of cell death by combined treatment with an xCT inhibitor and paclitaxel: an alternative therapeutic strategy for patients with ovarian clear cell carcinoma. Int J Mol Sci. 2023;24(14):11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Shaw AT, Winslow MM, Magendantz M, et al. Selective killing of K‐ras mutant cancer cells by small molecule inducers of oxidative stress. Proc Natl Acad Sci USA. 2011;108(21):8773‐8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Zheng J, Sato M, Mishima E, Sato H, Proneth B, Conrad M. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell Death Dis. 2021;12(7):698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Rodman SN, Spence JM, Ronnfeldt TJ, et al. Enhancement of radiation response in breast cancer stem cells by inhibition of thioredoxin‐ and glutathione‐dependent metabolism. Radiat Res. 2016;186(4):385‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Bailey HH, Mulcahy RT, Tutsch KD, et al. Phase I clinical trial of intravenous L‐buthionine sulfoximine and melphalan: an attempt at modulation of glutathione. J Clin Oncol. 1994;12(1):194‐205. [DOI] [PubMed] [Google Scholar]
- 319. Nishizawa S, Araki H, Ishikawa Y, et al. Low tumor glutathione level as a sensitivity marker for glutamate‐cysteine ligase inhibitors. Oncol Lett. 2018;15(6):8735‐8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Rao Z, Xia Y, Jia Q, et al. Iron‐based metal‐organic framework co‐loaded with buthionine sulfoximine and oxaliplatin for enhanced cancer chemo‐ferrotherapy via sustainable glutathione elimination. J Nanobiotechnology. 2023;21(1):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Zeng L, Ding S, Cao Y, et al. A MOF‐based potent ferroptosis inducer for enhanced radiotherapy of triple negative breast cancer. ACS Nano. 2023;17(14):13195‐13210. [DOI] [PubMed] [Google Scholar]
- 322. Guo J, Xu B, Han Q, et al. Ferroptosis: a novel anti‐tumor action for cisplatin. Cancer Res Treat. 2018;50(2):445‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin‐resistant head and neck cancer cells to artesunate‐induced ferroptosis. Redox Biol. 2017;11:254‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Zhang S, Liu Q, Chang M, et al. Chemotherapy impairs ovarian function through excessive ROS‐induced ferroptosis. Cell Death Dis. 2023;14(5):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Lapenna D. Glutathione and glutathione‐dependent enzymes: from biochemistry to gerontology and successful aging. Ageing Res Rev. 2023;92:102066. [DOI] [PubMed] [Google Scholar]
- 326. Teschke R. Treatment of drug‐induced liver injury. Biomedicines. 2022;11(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Monti DA, Zabrecky G, Kremens D, et al. N‐acetyl cysteine may support dopamine neurons in Parkinson's disease: preliminary clinical and cell line data. PLoS One. 2016;11(6):e0157602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Conche C, Finkelmeier F, Pesic M, et al. Combining ferroptosis induction with MDSC blockade renders primary tumours and metastases in liver sensitive to immune checkpoint blockade. Gut. 2023;72(9):1774‐1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Li C, Deng X, Zhang W, et al. Novel allosteric activators for ferroptosis regulator glutathione peroxidase 4. J Med Chem. 2019;62(1):266‐275. [DOI] [PubMed] [Google Scholar]
- 330. Wang S, Liu W, Wang J, Bai X. Curculigoside inhibits ferroptosis in ulcerative colitis through the induction of GPX4. Life Sci. 2020;259:118356. [DOI] [PubMed] [Google Scholar]
- 331. Liu B, Zhao C, Li H, Chen X, Ding Y, Xu S. Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem Biophys Res Commun. 2018;497(1):233‐240. [DOI] [PubMed] [Google Scholar]
- 332. Yoshioka H, Kawamura T, Muroi M, et al. Identification of a small molecule that enhances ferroptosis via inhibition of ferroptosis suppressor protein 1 (FSP1). ACS Chem Biol. 2022;17(2):483‐491. [DOI] [PubMed] [Google Scholar]
- 333. Hendricks JM, Doubravsky CE, Wehri E, et al. Identification of structurally diverse FSP1 inhibitors that sensitize cancer cells to ferroptosis. Cell Chem Biol. 2023;30(9):1090‐1103. e1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Fang Y, Tan Q, Zhou H, Gu Q, Xu J. Discovery of novel diphenylbutene derivative ferroptosis inhibitors as neuroprotective agents. Eur J Med Chem. 2022;231:114151. [DOI] [PubMed] [Google Scholar]
- 335. Jiang X, Wang W, Lei L, et al. Antirheumatic drug leflunomide attenuates atherosclerosis by regulating lipid metabolism and endothelial dysfunction via DHODH/AMPK signaling pathway. Int J Biol Sci. 2024;20(10):3725‐3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Yang C, Zhao Y, Wang L, et al. De novo pyrimidine biosynthetic complexes support cancer cell proliferation and ferroptosis defence. Nat Cell Biol. 2023;25(6):836‐847. [DOI] [PubMed] [Google Scholar]
- 337. Wu Z, Khodade VS, Chauvin JR, Rodriguez D, Toscano JP, Pratt DA. Hydropersulfides inhibit lipid peroxidation and protect cells from ferroptosis. J Am Chem Soc. 2022;144(34):15825‐15837. [DOI] [PubMed] [Google Scholar]
- 338. Barayeu U, Schilling D, Eid M, et al. Hydropersulfides inhibit lipid peroxidation and ferroptosis by scavenging radicals. Nat Chem Biol. 2023;19(1):28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Shah R, Margison K, Pratt DA. The potency of diarylamine radical‐trapping antioxidants as inhibitors of ferroptosis underscores the role of autoxidation in the mechanism of cell death. ACS Chem Biol. 2017;12(10):2538‐2545. [DOI] [PubMed] [Google Scholar]
- 340. Kikuchi K, Tanaka E, Murai Y, Tancharoen S. Clinical trials in acute ischemic stroke. CNS Drugs. 2014;28(10):929‐938. [DOI] [PubMed] [Google Scholar]
- 341. Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018;62(1):20‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Cao PHA, Dominic A, Lujan FE, et al. Unlocking ferroptosis in prostate cancer ‐ the road to novel therapies and imaging markers. Nat Rev Urol. 2024;21(10):615‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Kloditz K, Fadeel B. Three cell deaths and a funeral: macrophage clearance of cells undergoing distinct modes of cell death. Cell Death Discov. 2019;5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Zou Y, Schreiber SL. Progress in understanding ferroptosis and challenges in its targeting for therapeutic benefit. Cell Chem Biol. 2020;27(4):463‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Drummen GP, van Liebergen LC, Op den Kamp JA, Post JA. C11‐BODIPY(581/591), an oxidation‐sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radic Biol Med. 2002;33(4):473‐490. [DOI] [PubMed] [Google Scholar]
- 347. Chen X, Huang J, Yu C, et al. A noncanonical function of EIF4E limits ALDH1B1 activity and increases susceptibility to ferroptosis. Nat Commun. 2022;13(1):6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Wen W, Xu Y, Qian W, et al. PUFAs add fuel to Crohn's disease‐associated AIEC‐induced enteritis by exacerbating intestinal epithelial lipid peroxidation. Gut Microbes. 2023;15(2):2265578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Xu Y, Li Y, Li J, Chen W. Ethyl carbamate triggers ferroptosis in liver through inhibiting GSH synthesis and suppressing Nrf2 activation. Redox Biol. 2022;53:102349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Park MW, Cha HW, Kim J, et al. NOX4 promotes ferroptosis of astrocytes by oxidative stress‐induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer's diseases. Redox Biol. 2021;41:101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Feng H, Schorpp K, Jin J, et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020;30(10):3411‐3423. e3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Cui S, Ghai A, Deng Y, et al. Identification of hyperoxidized PRDX3 as a ferroptosis marker reveals ferroptotic damage in chronic liver diseases. Mol Cell. 2023;83(21):3931‐3939. e3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478(3):1338‐1343. [DOI] [PubMed] [Google Scholar]
- 354. Wang F, Naowarojna N, Zou Y. Stratifying ferroptosis sensitivity in cells and mouse tissues by photochemical activation of lipid peroxidation and fluorescent imaging. STAR Protoc. 2022;3(2):101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Wang F, Graham ET, Naowarojna N, et al. PALP: A rapid imaging technique for stratifying ferroptosis sensitivity in normal and tumor tissues in situ. Cell Chem Biol. 2022;29(1):157‐170. e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Li Z. Imaging of hydrogen peroxide (H(2)O(2)) during the ferroptosis process in living cancer cells with a practical fluorescence probe. Talanta. 2020;212:120804. [DOI] [PubMed] [Google Scholar]
- 357. Weigand I, Schreiner J, Rohrig F, et al. Active steroid hormone synthesis renders adrenocortical cells highly susceptible to type II ferroptosis induction. Cell Death Dis. 2020;11(3):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Zhao N, Huang Y, Wang YH, et al. Ferronostics: measuring tumoral ferrous iron with PET to predict sensitivity to iron‐targeted cancer therapies. J Nucl Med. 2021;62(7):949‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Shibata Y, Yasui H, Higashikawa K, Kuge Y. Transferrin‐based radiolabeled probe predicts the sensitivity of human renal cancer cell lines to ferroptosis inducer erastin. Biochem Biophys Rep. 2021;26:100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Hoehne A, James ML, Alam IS, et al. [(18)F]FSPG‐PET reveals increased cystine/glutamate antiporter (xc‐) activity in a mouse model of multiple sclerosis. J Neuroinflammation. 2018;15(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Park SY, Na SJ, Kumar M, et al. Clinical evaluation of (4S)‐4‐(3‐[(18)F]fluoropropyl)‐L‐glutamate ((18)F‐FSPG) for PET/CT imaging in patients with newly diagnosed and recurrent prostate cancer. Clin Cancer Res. 2020;26(20):5380‐5387. [DOI] [PubMed] [Google Scholar]
- 362. Park SY, Mosci C, Kumar M, et al. Initial evaluation of (4S)‐4‐(3‐[(18)F]fluoropropyl)‐L‐glutamate (FSPG) PET/CT imaging in patients with head and neck cancer, colorectal cancer, or non‐Hodgkin lymphoma. EJNMMI Res. 2020;10(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Zeng F, Nijiati S, Liu Y, et al. Ferroptosis MRI for early detection of anticancer drug‐induced acute cardiac/kidney injuries. Sci Adv. 2023;9(10):eadd8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Dixon SJ, Pratt DA. Ferroptosis: A flexible constellation of related biochemical mechanisms. Mol Cell. 2023;83(7):1030‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


