Abstract
This paper summarizes several attractive surface acoustic wave (SAW) biosensors, including Love-wave sensors, dual-channel SAW sensors, langasite SAW sensors, and SAW syringe filters. SAW sensors with different piezoelectric materials and high-frequency SAW sensors used for identifying the food pathogenic bacteria Escherichia coli (E. coli) are discussed together with the examples of methods based on such sensing technology that have been effectively utilized in diagnostics and epidemiological research. This review also emphasizes some of the limitations of using these biosensors, which have prompted the increased need for more rapid, sensitive, selective, portable, power-efficient, and low-cost methods for detecting these pathogens. It is envisioned that SAW devices will have remarkable significance in the future.
This paper summarizes several attractive surface acoustic wave (SAW) biosensors, including Love-wave sensors, dual-channel SAW sensors, langasite SAW sensors, and SAW syringe filters.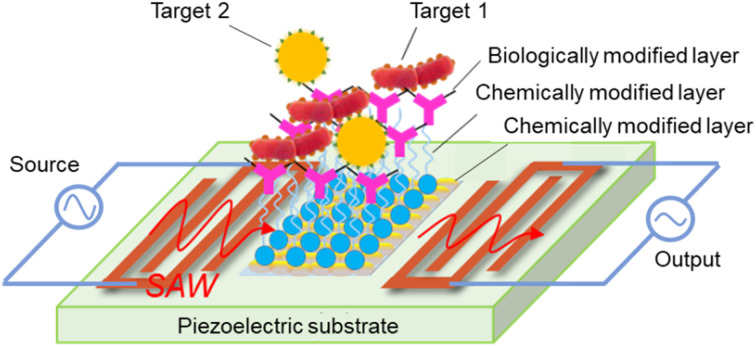
1. Introduction
Foodborne illnesses are a common and growing concern in both developed and developing nations, making food safety an increasingly essential public health issue.1,2 Foodborne pathogens (Table 1) are particularly dangerous for children, with 220 million becoming sick and 96 000 dying each year, and the yearly cost of these illnesses is US $5–6 billion.3 The danger of infection with the well-known food pathogen Escherichia coli (E. coli) has increased over time. E. coli is a Gram-negative bacterium of a rare serotype, belonging to the family Enterobacteriaceae, and is typically found residing in the intestines of healthy humans and animals.4–7 It was first isolated in 1885 from children's feces by the German bacteriologist Theodor Escherich. The bacteria measure approximately 2–6 μm in length and around 1–1.5 μm in diameter.8–10E. coli, a member of the coliform bacterial group, is predominantly responsible for the majority of urinary tract infections. ‘Coliforms’ belong to four species: E. coli (human intestines), Citrobacter (bacteremia, brain abscesses, and pneumonia), Enterobacter (urinary tract infections, meningitis, and sinusitis), and Klebsiella (gastrointestinal tracts of animals). These bacteria produce a powerful toxin known as Shiga toxin. This toxin attacks small blood vessels and can cause serious damage to intestinal cells, resulting in hemorrhagic colitis (HUS), bloody diarrhea and severe abdominal pain, stomach cramps, vomiting, dehydration, seizures, stroke, and kidney failure,11–13 and is generally found to be responsible for the majority (20%) of outbreaks globally.3,14 The presence of higher than 1 CFU E. coli O157 : H7 in 25 g of food has been considered a dangerous level.15,16 Although the majority of E. coli bacteria are not harmful, a few can cause serious food poisoning.
Common global diseases caused by bacterial infection, with the main sources of infection, key effects, annual deaths, and common detection methods.
| Pathogen | Source | Effects | Annual deaths | Detection method | Reference |
|---|---|---|---|---|---|
| E. coli O157 : H7 | Raw vegetables, undercooked beef unpasteurized milk, fruit juice | Diarrhea, stomach pain, hemorrhagic colitis, and HUS | 200 000 | Conventional methods, ELISA PCR, biosensors, etc. | 3 |
| Salmonella | Raw and undercooked egg, fruits and vegetables | Diarrhea, fever, and stomach cramps | 155 000 | RT-PCR, SPR, QCM, impedimetry, etc. | 17 |
| 18 | |||||
| Shigella | Salads and unclean water | Diarrhea, fever, and stomach cramps | 1.1 million | PCR, rep-PCR, culture methods, immunological methods etc. | 19 |
| 17 | |||||
| Clostridium botulinum | Meat, poultry, cooked dried beans and gravies | Diarrhea and stomach cramps | — | PCR, nested PCR, real-time PCR | 20 |
| Norovirus | Eating food or drinking liquids contaminated by an infected person | Vomiting, diarrhea, and stomach cramps | 200 000 | Real-time PCR | 10 |
| Staphylococcus aureus | Cooked ham, salads, and bakery products | High fever, nausea, and vomiting | 19 832 | Conventional methods, molecular biological methods, optical immunoassays | 9 |
| Bacillus cereus | Raw plant foods, such as rice, potatoes, peas, beans, and spices | Intestinal illnesses with nausea, vomiting, and diarrhea | — | Immunosensors and DNA biosensors etc. | 21 |
| Listeria monocytogenes | Raw, unpasteurized milks and cheeses, ice cream, raw or processed vegetables, fruits | Fever, muscle aches, headache | 260 | Conventional culture methods, PCR etc. | 22 |
Table 1 describes different types of foodborne pathogens and their sources, yearly mortality rate, effects on the human body, and different detection methods. The satisfactory microbiological quality for E. coli O157 is <20 CFU g−1, with the acceptable range being 20–<100 CFU g−1,3 and the time taken for the occurrence of diarrhea can range from 1 to 8 days. Primary sources of E. coli exposure include contaminated water and food, particularly raw vegetables, undercooked ground beef (hamburger), unpasteurized milk, and fruit juice.9,11,23,24 Additionally, the utilization of shared facilities facilitates E. coli transmission, thereby increasing the likelihood of an outbreak. To minimize the risk of exposure among human and animal populations, it is essential to develop sensors that can rapidly detect hazardous E. coli in food and water supplies.
As a result, several studies have been published to detect E. coli contamination in food and water, and have proposed various methods and sensors, including surface plasmon resonance biosensor,25 magnetoelastic immunosensor,14 polymerase chain reaction (PCR),26 enzyme-linked immunosorbent assay (ELISA),27 piezoelectric immunosensor,28 and quartz crystal microbalance (QCM).29 The majority of these detection techniques are based on optical, electronic, and electrochemical methods, which offer a rapid response and ease of operation.30 However, these methods are specific and sensitive, and also have limitations related to their high costs, time-consuming procedures (48–72 h), need for expensive laboratory facilities and trained personnel, and complex procedures, which obstruct the widespread use of these technologies for diagnosis.11 To overcome such limitations and replace these old procedures, the use of surface acoustic wave (SAW) devices for both sensing and actuation is proposed on a single platform to create rapid, real-time, cost-effective, and labor-efficient techniques for the detection of E. coli bacteria.
For sensor applications, the quartz crystal microbalance (QCM) is a simple and commonly utilized acoustic transducer, with minimal manufacturing costs. However, because of its low resonance frequency, it has limited sensitivity. On the other hand, surface acoustic wave (SAW) sensors are well-known and frequently employed in gas and liquid media with a variety of commercial applications. SAW sensors are more sensitive than QCM sensors (Table 2) because waves in QCM sensors must propagate through the whole piezoelectric substrate, but waves in SAW sensors only have to propagate through a guiding layer at the surface, resulting in more concentrated energy. SAW sensors also exhibit greater sensitivity compared to QCM sensors, as indicated in Table 2. SAW sensors exhibit superior sensitivity due to the higher operating frequency and concentration of acoustic wave energy at the surface. This characteristic renders SAW sensors more advantageous compared to QCM sensors. SAW sensors are capable of functioning at elevated frequencies, ranging from several hundred megahertz (MHz) to gigahertz (GHz). In contrast, QCM sensors are limited to operating within the frequency range 5–30 MHz. Owing to the aforementioned advantages, research on SAW sensors in the bio-analytical domain, particularly for applications such as E. coli detection, is experiencing rapid growth. SAW sensors are capable of detecting changes in mass or viscosity through wave velocity attenuation,29 offering high sensitivity and enhanced compatibility with complementary metal-oxide semiconductor (CMOS) technology.31 The detection limit of SAW sensors is approximately in the picograms range (10−12 g).32 Additionally, they also provide a wide array of functionalities within a compact and durable package.33
Comparison of SAW and QCM techniques.
| Technical index | SAW | QCM |
| Acoustic wave type | Surface | Bulk |
| Sensitivity | High | Low |
| Frequency | 10 MHz to 3 GHz | 5–10 MHz |
| Dielectric constant | LiNbO3 = 29, LiTaO3 = 43 | 3.8 |
| Detection range | 10−12 g | ∼1 Å to 1 μm or 10−9 g |
| Piezoelectric material | ST-cut quartz, LiNbO3, LiTaO3 | AT-cut quartz |
| Temperature range | 25 °C to 1000 °C | −190 °C to 125 °C |
| Working area | Liquids and gases | Liquids and gases |
| Cost | Low | High |
| Applications | Filters, cell monitoring and manipulation, signal processing units, sensors, and actuators | Detection of metals in vacuum, vapors, chemical analytes, biomolecules, and contaminants in the environment |
Table 2 shows that SAW sensors offer superior performance or more advantages than QCM sensors. SAW-based biosensors also offer more benefits than other types of biosensors, such as optical and mass-based biosensors. SAW technology plays an important role in many physical, chemical, electrical engineering, and biological sensing applications.34–37 SAW devices have been typically used in the Rayleigh and shear horizontal (SH) SAW propagation modes,31 wherein Rayleigh SAW is suitable for gas-sensing applications, but not well suited to liquid sensing. This is because Rayleigh waves have both a surface-normal component as well as a surface-parallel component with respect to the propagation direction and hence can couple strongly with a liquid.38 They can even work without batteries and can also operate in harsh environments.
In this review, we emphasize recent advances in the use of SAW sensors for detecting E. coli bacteria, including the Love-wave immunosensor, dual-channel SAW sensor, and SAW syringe filter. Both detection and separation/concentration techniques have advanced significantly in recent years. With a focus on distinct domains, this review examines the existing conventional approaches as well as developments in biosensor techniques for detecting E. coli bacterial infections.
2. Working principle of SAW biosensors
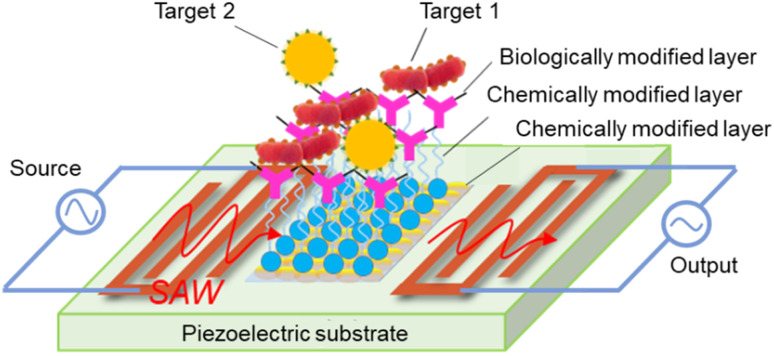
SAW was first reported by Lord Rayleigh in 1885, and updated by White and Voltmer in 1965. A SAW element is made of a piezoelectric substrate, such as quartz (SiO2), lithium niobate (LiNbO3), lithium tantalite (LiTaO3), zinc oxide (ZnO), or aluminum nitride (AlN),39–41 and interdigitated transducers (IDTs). For applications involving biological detection, it is necessary to implement chemical and biological modifications within the detection region of the SAW sensor, as shown in Fig. 1. SAWs can be generated by applying an appropriate electric field to the IDTs. Under the dynamic change of the electrical input signal caused by electromechanical coupling, the electrical component creates mechanical energy as acoustic waves in the piezoelectric substrate.
Fig. 1. Basic structure of a SAW biosensor.
In applications involving the detection of chemical and biological substances, SAW biosensors rely on mass loading, surface perturbation, chemisorption, and biological affinity to achieve a highly sensitive response to the surface of the substance under test. The signal generated by the reaction of the test sample with the biologically active substance is subsequently converted into an electrical signal output by the sensor. Biomolecules, including antibodies, enzymes, phages, and DNA probes, are coated on the surface of the SAW biosensor to capture specific target molecules, such as antigens, viruses, and DNA sequences. Upon binding the target biomolecule (bacteria or virus) to the specific recognition molecule on the biologically modified layer, the mass-loading-based effect brings about shifts in the SAW resonance frequency, phase, and other characteristics. By amplifying, receiving, transforming, and filtering these altered characteristic signals, a quantitative detection and analysis of the measured object can be achieved.
The sensitive layer of SAW biosensors is the core recognition element of microorganisms, such as bacteria and viruses, which is used to deposit biological samples, but also comes into contact with corrosive environments, such as strong acids and alkalis, during the detection process. Therefore, the sensitive layer material should have good biocompatibility and certain corrosion resistance. The deposition of an Au film, oxide film (ZnO, In2O3, GO),42–44 polymer film (PEI, PEN),45,46 composite film (ZnO–SnO2),47 and other specialty film materials on the surface of the sensitive layer for chemical modification facilitates capture of the target chemical molecules and enhances the adhesion of biomolecules. Among these materials, Au is of particular interest to researchers due to its chemical stability and its capacity to form self-assembled molecular layers through the combination of a range of biomolecules with end-modified sulfhydryl groups.48
3. Applications of SAW biosensors for E. coli detection
Over the last several decades, SAW biosensor technology has been increasingly developed for the detection of foodborne pathogenic bacteria.49 Numerous researchers have successfully developed methods to detect bacteria or microorganisms in water and food, owing to the technology's high stability, low limit of detection (LOD), low power consumption, flexible design, ease of miniaturization, and potential for integration into microfluidic devices.11,39,50,51 It has also been applied for detecting microorganisms in real time because the sensors are relatively cost-effective, due to their small size, high sensitivity11,41,45 and high electromechanical coupling coefficient. Table 3 shows a comparison of several SAW biosensors for E. coli detection. These SAW biosensors all demonstrate high electromechanical coupling performance and high sensitivity for E. coli detection.33,52–54
SAW biosensors based on different sensitive layers for the detection of E. coli.
| Type of substrate | Wave mode | Dielectric constant | TCF (ppm per °C) | Velocity (m s−1) | Detection limit or sensitivity | Reference |
|---|---|---|---|---|---|---|
| 64°YX LiNbO3 | SH-SAW | 85.2 | 80 | 4742 | 1.8 × 10−15 M | 55 |
| AIN | Love wave | 8.5 | 19 | 5700 | 6.54 × 105 CFU mL−1 | 45 |
| LiTaO3 | SH-SAW (LGS) | 470 | −33 | 4160 | 106 cells per mL | 56 |
| SiO2 | Love wave | 3.8 | — | — | 105 cells per mL | 57 |
3.1. Love-wave sensors
A Love wave is a type of horizontally polarized shear wave that is produced at the surface boundary of an elastic medium as a result of multiple total reflections. The propagation characteristics are mainly determined by the shear modulus, whereby the amplitude decays exponentially along the depth direction, and along the surface with the regularity of r−1/2. The existence of the Love wave was first predicted by the mathematician Augustus Love in 1911 in the context of seismic research.58 Physical information about the base and the waveguide layer are conveyed by the Love wave throughout its propagation process. The mechanical properties of the material, comprising the waveguide layer, including the elastic modulus and density, exhibit a discernible pattern of change in their spatial distribution.59,60 The presence of a low-velocity layer on a semi-infinite elastomer results in the accumulation of acoustic waves within the waveguide layer, due to the discrepancy in mechanical properties between the waveguide layer and the substrate. This phenomenon reduces the acoustic penetration of the substrate and consequently decelerates the propagation speed of the wave. The concentration of acoustic waves in the waveguide layer renders the device highly sensitive to alterations in surface mass loading, viscosity, and conductivity. Consequently, Love waves are frequently employed for sensing and detection in both gas-phase and liquid-phase media. Guided shear horizontal surface acoustic wave sensors are known as Love-wave sensors. Love-wave devices are excellent for real-time sensing applications. Love-mode SAW sensors, fortunately, have received a great deal of attention during the last two decades due to their high miniaturization and sensitivity, making them suitable for use in portable devices for point-of-care testing applications or for on-site measurements.61 Also, some researchers successfully applied their Love-wave methods to detect harmful pathogenic microorganisms, such as E. coli bacteria.
3.1.1. Working principle of Love-wave sensors
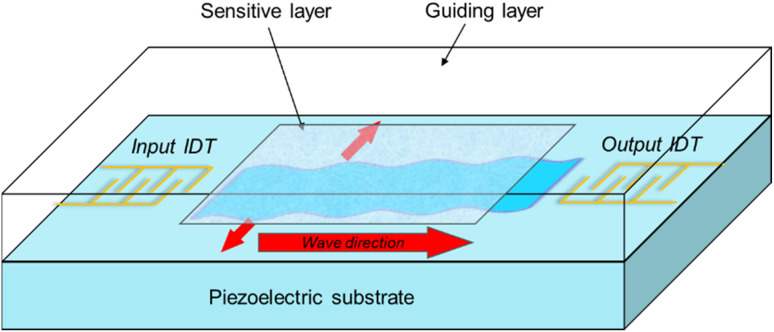
In a Love-wave sensor, a pure shear horizontal wave is created depending on the crystallographic orientation of the quartz. IDTs on the quartz substrate create and receive an elastic wave in these sensors, which are based on a piezoelectric delay line. The active sensing layer is an important part of the Love-wave sensor because any changes or disturbances on its surface induces changes in the acoustic wave velocity or amplitude, and hence a frequency shift response. Any physicochemical disturbance on the sensor surface will change the wave velocity, which may be detected with high precision by frequency in an oscillator configuration, giving the device a high measurement resolution. The basic structure of a Love-wave surface acoustic wave sensor is presented in Fig. 2.
Fig. 2. Basic structure of a Love-wave surface acoustic wave sensor.
To get Love-wave modes, the shear velocity of the guiding layer must be lower than that of the substrate. The higher the sensitivity, the bigger the difference in velocities. The sensitivity to surface mass loading for the acoustic sensor can be defined as the relative change in oscillation frequency due to mass loading on the surface,62 with the typical operation frequencies of such sensors being between 80–300 MHz.63 If the piezoelectric material is put on top of the piezoelectric substrate and over the layer with a lower shear velocity, Love waves propagate near its surface, which promote shear horizontal (SH) waves. The wave velocity and amplitude are highly influenced by changes in the medium near the surface.
3.1.2. E. coli detection using a Love-wave immunosensor
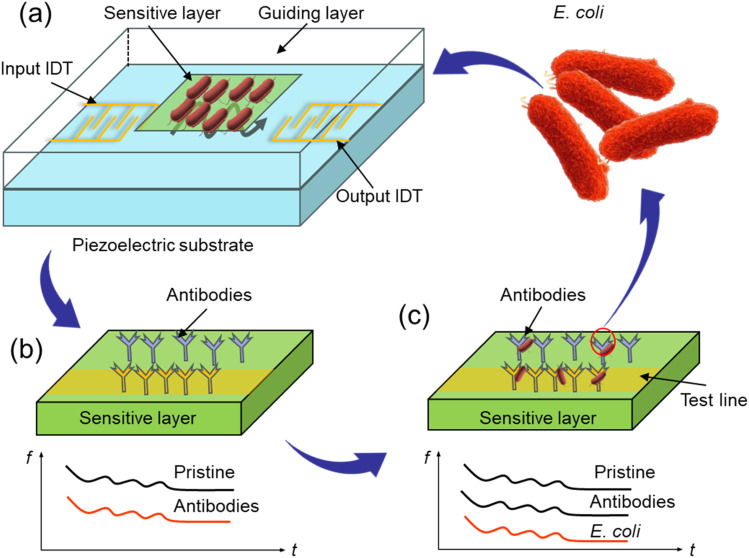
Several writers have investigated Love waves in recent years. Love-wave devices appear to be powerful devices for biodetection.64 The first Love-wave sensor for biochemical sensing was reported in 1992 by Kovacs et al.65 Then, Harding et al.66 used a Love-wave acoustic device to detect real-time antigen–antibody (Fig. 3(a)) interactions in liquid media. In 2000, Howe and Harding57 used a dual-channel Love-wave device as a biosensor to simultaneously detect Legionella and E. coli. They presented a unique methodology for coating bacteria on the sensor surface and their quantitative results were 106 cells per mL within 3 h. In 2003, Tamarin et al.67 designed a Love-wave immunosensor as a model for virus or bacteria detection in liquids. In 2007, Moll et al.68 developed an innovative method for the detection of E. coli employing an LW immunosensor. They divided their method into two steps: in the first step, goat anti-mouse antibodies (GAM) were grafted onto the sensor surface, and in the second step, E. coli bacteria were mixed with anti-E. coli antibodies and introduced onto the sensor. Later, antibody–antigen interactions were used to detect biological species, like bacteria, viruses, and toxins.69 Finally, their detection threshold was 106 bacteria per mL. More recently, the same authors70 described a multipurpose Love-wave immunosensor for the detection of bacteria, viruses, and proteins and they successfully applied this sensor to detect bacteriophages and proteins down to 4 ng mm−2. They also successfully demonstrated that E. coli bacteria could be identified up to 5.0 × 105 cells in a 500 μL chamber. Furthermore, the entire bacterial detection process could be completed in less than 1 h, exhibiting high specificity and reproducibility.
Fig. 3. Configuration of antibody binding onto the sensor surface. (a) SAW biosensors construction, (b) antibody immobilization, (c) E. coli adsorption.
In other work, a SiO2 thin film and 3-glycidoxypropyl-triethoxy silane (GPTS) were decorated on the surface of a SAW device, with GPTS (Fig. 3(b)) used to covalently bond to the SiO2 substrates by a reaction between the silanol groups of the surface and alkoxysilanes.71,72 Then the antibodies could be covalently linked to the modified surface by a reaction between the epoxy group of GPTS and the amino groups of the antibodies.73 The GPTS coupling agent (Fig. 3(c)) molecule was used to graft the antibody onto the SiO2 sensor surface, and the grafting frequency signal of the antibodies was monitored. Wave propagation was disrupted when the sensitive layer captured the species, resulting in a drop in wave propagation due to the mass addition on the sensor's surface (mass-loading effect). As a consequence, any biological species with enough total mass immobilized onto the sensor surface to alter the acoustic wave can be detected. Finally, the detection threshold was found to be 106 bacteria per mL in a 500 μL chamber at a temperature of 37 °C in under 1 h.68
The antibody could be bound to the SiO2 surface by adsorption or by a covalent link using a coupling agent. The Love-wave sensor can detect targets in the nano-molar range and has been utilized for monitoring antibody–antigen immunoreactions in aqueous solutions in real time.74 These sensors are the most recently developed devices designed for integration in “lab-on-a-chip” systems.75 However, further study is needed to improve the sensor's sensitivity before it can be used to detect individual microbes. Hopefully, SAW sensors based on Love-wave immunosensors will be widely applied in the future for a variety of applications, especially for pathogen detection.
3.2. SH-SAW biosensors with different piezoelectric materials
Shear horizontal-SAW (SH-SAW) sensors have been used in a variety of applications, including biosensors, taste sensors, and liquid- and gas-media measurements. In liquids, the SH-SAW device maintains high sensitivity and is suitable for “real-time” sensing and detection. This was the first leaky wave sensor that was only partially contained on the surface. Shear horizontal mode surface acoustic waves have a unique characteristic of complete reflection at the free edges of the substrate76 and can allow detecting complex permittivity with high sensitivity in liquids.77
In 1987, Moriizumi et al.78 described the first biosensor applications of SH-SAW transducers in a liquid medium. Deobagkar et al.79 developed a SH-SAW immunosensor for E. coli detection in water; whereby, using a 87.7 MHz oscillator, they achieved a detection range of 0.4–100 cells per L, yielding frequency changes of 1.5–5.8 kHz. As a result, the device was sensitive enough to identify E. coli at levels that are dangerous to human health. Länge et al.80 presented a new approach that involved integrating a SH-SAW biosensor in a microfluidic polymer chip. In 2008, Bisoffi et al.81 developed an SH-SAW biosensor that combined the sensitivity of 325 MHz SAW with the specificity given by antibodies for the detection of viral bio-agents. They used a LiTaO3-based SAW transducer with a SiO2 waveguide sensor platform to capture adsorb antibodies. Ten et al.11 also described an SH-SAW biosensor with a SiO2 guiding layer to detect E. coli bacteria. They employed a 22-mers DNA sequence from E. coli O157 : H7 as an amine-terminated probe ssDNA that was chemically functionalized and immobilized on the thin-film sensing region [(CHO–(CH2)3–CHO) and (APTES, NH2–(CH2)3–Si(OC2H5)3)]. Their sensor performed well with a particular oligonucleotide target, with a sensitivity of 0.6439 nm/0.1 kHz and a detection limit down to 1.8 femto-molar (1.8 × 10−15 M).
For the detection of E. coli bacteria, Lamanna et al.45 developed the first conformable SAW biosensor using polyethylene naphthalate (PEN). On a PEN substrate, they created a SAW immunosensor with a piezoelectric AlN material. They also performed detection experiments with a comparable SAW immunosensor developed on a silicon substrate to evaluate and compare the performance of their device. Their investigations determined that their PEN-based SAW biosensor had a limit of detection of 6.54 × 105 CFU mL−1, which was superior to the silicon-based SAW biosensor's limit of detection of 1.04 × 105 CFU mL−1.
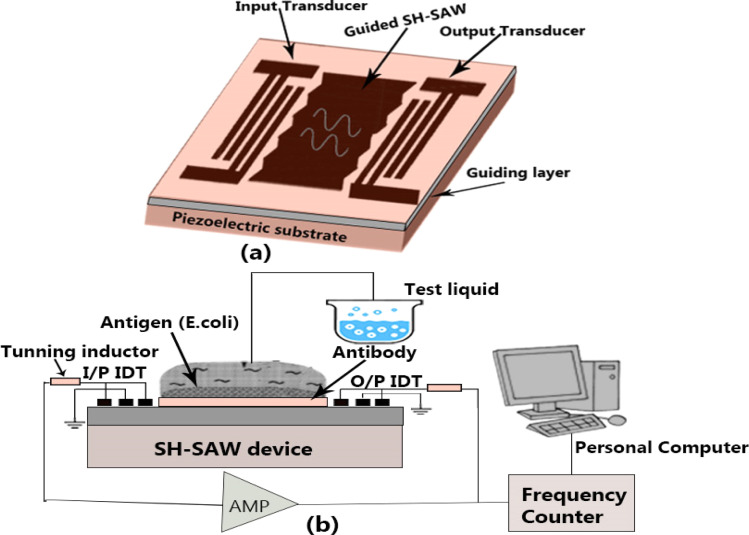
Fig. 4 illustrates a special delay line type SH-SAW biosensor. The SH-SAW is directly generated by IDT excitation and propagated in a multi-layer structure. Table 4 lists several SH-SAW mode sensors based on different piezoelectric materials. Piezoelectric materials with special crystal cutting angles, such as 36° YX LiTaO3, 64° YX LiNbO3, and ST-90° X quartz, are often used as piezoelectric substrates for the direct generation and propagation of SH-SAWs in liquid media.82 Bergaoui et al.83 reported antigen–antibody recognition using a LiTaO3 SH-SAW sensor. They showed that grafting numerous molecular layers onto the SAW sensing region allowed streptavidin–antistreptavidin identification. The antigen–antibody reactions with the sensing region of the SH-SAW biosensor device were used to evaluate its sensing qualities.
Fig. 4. Schematic representation of the SH-SAW delay-line.91.
Several types of piezoelectric materials used in SH-SAW sensors and their advantages.
| Materials | Orientation | Frequency range (MHz) | Coupling coefficient, K2 (%) | Dielectric constant | TCF [ppm per °C] | Advantage | Reference |
|---|---|---|---|---|---|---|---|
| 36° YX LiTaO3 | x-axis | 100–150 | 5–6.6 | 43 | 32 | Reduces the TCF, label-free detection, minimal energy loss, low signal loss, reproducibility | 84 and 85 |
| 64° YX LiNbO3 | x-axis | ∼100 to ∼450 | 46.4 | 85.2 | ∼80 | Low cost, low power consumption, wide application, suitable for fluid loading | 86 and 87 |
| 36° YX LiNbO3 | |||||||
| Quartz | z-axis | 134–570 | 0.1–0.2 | 3.8 | 0 | High sensitivity and temperature stability, low insertion loss | 82 and 84 |
| ZnO | c-axis | 153 | 1.5–1.7 | 8.66 | ∼−60 | Best microfluidic performance, simple operation, precision, low cost, fast response, and reduced reagent requirements | 82, 85 and 88 |
| All | 520 | 3.1–8 | 8.5 | 19 |
Acoustic wave technologies also use piezoelectric thin-film materials, including ZnO, and AlN. Among these thin-film materials, AlN thin films have been widely investigated due to their unique large wave velocity89 and higher frequency.
Before bio-molecular immobilization, piezoelectric materials create a waveguide around both transducers and reduce the attenuation. Both the particle displacement and electrical potential interact with the liquid when the surface is free and electrically active. This is an electrical interaction with the liquid that changes the velocity and/or attenuation of SH-SAW propagation and is used in liquid sensing. The SH-SAW propagating characteristics are modified by the nature and conditions of the molecules and liquid surfaces loaded on the sensing area.90 The frequency changes in response to varying amounts of E. coli O157 : H7 have been comprehensively explored with this sensor.
The main advantage of the SH-SAW sensor is that it can detect various properties of an adjacent liquid without any membrane92 with a high electromechanical coupling coefficient (K2 = 5%).93 However, it has some drawbacks; for instance, both LiNbO3 and LiTaO3 are piezoelectric materials and are dependent on the temperature, limiting the operation at elevated temperatures.94 Also, the IDTs are generally made of low-cost aluminum,80 which means that their lives in aqueous conditions are limited because of corrosion. As a result, more layers of protection are necessary.
3.3. Langasite-based SH-SAW sensors
This section describes a novel LGS SH-SAW sensor for the detection of E. coli bacteria. Langasite (Fig. 5) is a novel piezoelectric material that can be used to make surface acoustic wave devices. It offers a new opportunity for high-temperature sensors (up to 1470 °C) with a reported sensitivity of 749 Hz ng−1 m−2.95 The langasite family of crystals (LGX), including langasite (LGS), langanite (LGN), and langatate (LGT), belong to the symmetry class 32. A delay line was designed and fabricated on a (0°, 22°, 90°) with a high dielectric constant.96 Due to the delay lines, langasite SH-SAW (LGS) sensors may be used to detect bacteria. They match the requirements for sensitivity, temperature stability, compatibility with biochemically produced recognition receptors, and low attenuation in liquid environments. They also exhibit a zero-temperature coefficient of frequency (TCF).85
Fig. 5. Crystal structure of langasite.97.
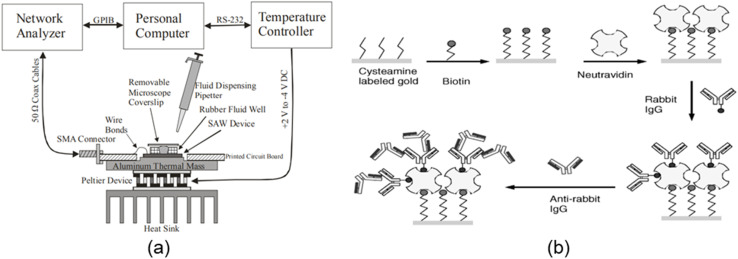
Several researchers have investigated langasite-based SAW sensors. In 2005, Pollard and Pereira Da Cunha,98 using finite thickness gratings, reported an improved pure SH-SAW transduction efficiency on LGS. Ayala et al.99 presented LGS temperature stability for SH-SAW sensor applications. No one had published research on detecting bacteria using LGS-SH-SAW sensors before Berkanpas. Berkenpas and colleagues investigated for a long time and published several articles based on LGS-SAW sensing, including for the detection of E. coli bacteria. In 2003, they effectively detected macromolecular protein assemblies using an SH-SAW transducer based on langasite (LGS) crystals.96 They reported that the device had excellent temperature stability, biocompatibility, and minimal attenuation in liquid environments. At the same time, they also suggested that it could be used to identify bacteria. In the same year, they developed an LGS SH-SAW100 sensor that could detect microbial pathogen-derived biomolecules in aqueous solutions, such as nucleic acids. They used a liquid flow-through technique to make a sensor capable of recognizing nucleic acids. Later in 2006, the same authors56 developed the LGS SH-SAW biosensor for E. coli O157 : H7 detection. They used a biotinylated polyclonal rabbit polyclonal immunoglobulin G (IgG) antibody (Fig. 6(b)) directed against E. coli to bind to a NeutrAvidinTM SAM-functionalized gold surface to derive LGS SH-SAW delay lines. In their experiment, they used two different methods: liquid-phase flow-through and dip-and-dry methods. Due to the relatively large distance of the E. coli from the sensitive LGS SH-SAW surface with the viscous decay length, the flow-through technique generated a lower (0.1–0.7) variance in the S21 response. Also, a large and detectable change in the S21 response was achieved using the dip-and-dry method (Fig. 6(a)), in which E. coli were selectively bonded and then dried onto the surface of the LGS SH-SAW delay line.
Fig. 6. Schematic structure of (a) dip-and-dry test101 and (b) immunochemical reactions and biomolecule additions on the LGS SH-SAW sensor with phase changes.102.
To evaluate the SH-SAW device's performance as a biomolecule binding sensor, a series of immunochemical reactions were generated on its surface and then measurement was made of the quantity of antigen bound onto surface-immobilized antibodies, which may bind specific antigens, to determine the concentration of target specimens. E. coli were selectively bound and then dried onto the LGS SH-SAW delay line's surface. After an initial drop, phase S21 increased slightly as the E. coli dried on the sensor surface. The dehydration of the E. coli reduced their mass, which reduced the total mass on the sensor surface, resulting in a small recovery of the phase response. Larger phase variations were observed when larger amounts of surface-bound E. coli were present. Dry E. coli attachment to the sensing zone increased the surface mass and delayed the SH-SAW, resulting in a downward phase shift. All the measurements performed well at 25 °C for this procedure (dip and dry). Finally, the detection limit was estimated at ∼106 cells per mL based on a measured <S21 noise of 0.05°. Although this sensor has numerous benefits, we hope the sensitivity of LGS SH-SAW biosensors will be improved in the future by optimizing both the bio-receptive layer and the LGS SH-SAW platform modeling and design.
3.4. SAW impedance sensor
The impedance method is one of the earliest electrical methodologies, and it is based on the measurement of changes in a medium's electrical characteristics as a result of microbial population development. The impedance-based SAW sensor is a new type of SAW device that is good for remote sensing and multi-sensor identification,103 and is capable of detecting bacteria.
This subsection introduces a novel SAW impendence sensor that can detect the density of E. coli in environmental water sources. Unique novel SAW impedance biosensors have been reported for bacteria detection based on impedance measurements of bacterial cells' electrical characteristics when they are connected with electrodes. The SAW sensor converts the impedance signal from the interface of the electrodes in the bacterial growth fluid into a frequency signal. This sensor responds to changes in the capacitance and conductance of the solution between the two electrodes. Generally, in a medium inoculated with the testing bacteria, a pair of metal electrodes are submerged. Antibodies specific to the target bacterial cells are immobilized on the electrode surface to produce an impedance biosensor for bacterial cell identification. The impedance changes in the medium produced by bacterial metabolism are monitored in real-time. Various researchers have investigated SAW-based impedance sensors, including Wang et al.,104 who for the first time used a SAW-based impedance sensor for acid phosphatase detection and micro-analysis in 1998. In 2004, Karilainen et al.105 provided a SAW biosensor for electrocardiogram (ECG) and other bio-potential monitoring. Nguyen et al.106 fabricated a SAW impedance sensor for measuring the input impedance S11 in a microfluidic approach. Yang and Bashir107 described the principles of impedance biosensors for foodborne pathogenic bacteria detection and their detection limit was ∼104 CFU mL−1 for E. coli in under 2 h. However, to the best of our knowledge, there is no reported research on the use of a SAW-based impedance sensor to detect harmful microorganisms, especially E. coli.
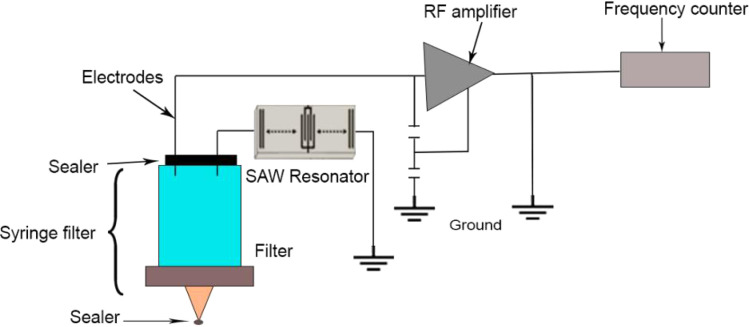
However, Chang and colleagues108 used a SAW-based impendence syringe filter (Fig. 7) to successfully detect E. coli bacteria in water sources in 2012. They modified a SAW sensor by adding a syringe filter with a Teflon cover, which allowed the CO2 gas produced by bacteria to flow upward and collect in the syringe filter's headspace. The frequency of the SAW sensor changed in response to the presence of CO2 gas around the electrode surfaces, which was created by coliform growth. The detection limit was 102–107 cells per mL. The initial density of the coliform could be determined from the growth of coliform in lauryl sulfate tryptose (LST) and by the detection of gaseous CO2. LST was utilized for the incubation of E. coli. The sensor monitored E. coli growth by frequency response curves obtained in nutritional broth (pH 7.0) at 37 °C. Larger frequency changes in CO2 gas were generated during bacterial metabolism. As the E. coli increased, the gas created covered the electrodes, producing a considerable fall in oscillator frequency and a rise in frequency as the E. coli multiplied.
Fig. 7. Schematic of a commercial syringe filter.
This sensor system also allowed for real-time bacterial growth monitoring. In comparison with conventional bacterial plating methods, the operating procedure for this SAW sensor system is quite simple, and so it may be used and implemented by both scientists and non-scientists.
3.5. Ultrahigh-frequency SAW biosensors
This section describes a high-frequency SAW sensor for detecting E. coli bacteria. This type of acoustic biosensor has the potential to offer a simple, low-cost, and reliable method for detecting bacterial loads in complex liquids.109 The sensitivity of SAW biosensors is determined by phase and frequency shifts, which are directly connected to wave types, the guiding layer size and material, and the sensing technology. SAW devices have been produced for high-frequency applications operating in the range of 100 MHz to a few GHz110 and their high acoustic wave velocity is demonstrated by piezoelectric materials (Section 3.2), which allow producing higher-frequency SAW devices. These piezoelectric materials have a linear temperature–frequency relationship, indicating that as the temperature rises, the frequency also rises. A SAW device was reported using the piezoelectric substrate LiNbO3, and it was found that as the center frequency rose to 2.55 GHz the insertion loss was reduced (13 dB).110
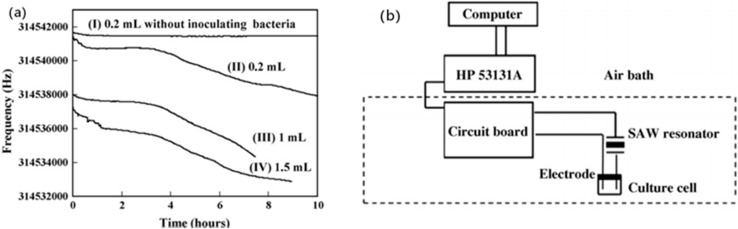
SAW-based high-frequency sensors have been researched by several authors. Thomas et al.,111 Mujahid et al.,112 Chen et al.,34 Agostini et al.,113 and Greco et al.64 utilized high-frequency SAW sensors for chemical, biological, and biochemical applications. However, the majority of these studies were focused on chemical fields instead of pathogen detection. In 2007, Chang et al.114 described (Fig. 8(b)) a new SAW (314.5 MHz) sensor for microbial count monitoring in biological cultures at ultrahigh frequencies. This sensor was used to count the number of bacteria in a biological culture. A pair of conductive electrodes were put into the transmitter's 314.5 MHz SAW stabilized oscillator. The frequency of the oscillator was changed when the electrodes were immersed in the culture solutions and the time was recorded. As the microbial metabolism changed, it produced a change in frequency (Fig. 8(a)); whereby the sensitivity increased in a parabolic manner with the resonance frequency of the device.115 Finally, their detection limit was ∼102 cells per mL in under 7 h, and the calibration curve of the detection times against the density of E. coli showed a linear correlation value (0.924). The authors also proposed that this sensor may be employed to transmit sensor signals wirelessly in dangerous areas, which would improve human safety aspects.
Fig. 8. (a) Effect of sample volume on the response of the SAW sensor. The sample was a nutrient broth (pH 7.0) inoculated with about 105 cells per mL E. coli. (b) New surface acoustic wave sensor for microbial count monitoring in biological cultures at ultrahigh frequencies.114.
Based on the signal-to-noise ratio, the SAW sensor was proven to be more sensitive than the PQC sensor.116 Even though the SAW sensor operates at an ultrahigh frequency, it demonstrated the same stability as a lower frequency PQC sensor. The SAW sensor also had higher sensitivity than the PQC sensor and a lower microbial detection threshold. If we compare it to traditional bacterial plating procedures, the process is quite simple and does not need the use of highly skilled experts.
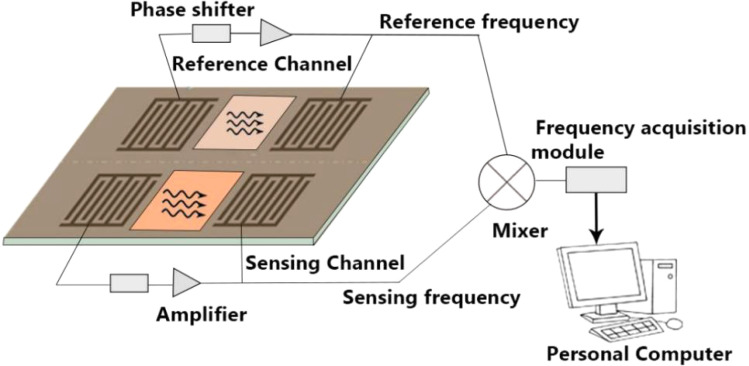
3.6. Dual-channel SAW sensors
This section describes novel dual-channel SAW sensors that can detect E. coli bacteria in water. The fundamental construction of most dual-channel SAW sensors is similar. Fig. 9 presents an illustration of a dual-channel SAW structure with two delay lines; whereby one delay line works as the reference channel, while the other works as the testing channel. The testing channel performs all the mass adsorption procedures, whereas the reference channel gives a frequency reference. Because the two channels are formed of the same material, their reactions to changes in the environment are almost the same. As a result, the new frequency difference between the two delay line oscillators, Δf, may be considered the mass adsorption response, and the velocity changes of a SAW lead to corresponding oscillation frequency changes.117 There is a basic frequency difference up to 1.3 MHz118 between the sensing channel and the reference channel when a SAW oscillator system starts to oscillate.
Fig. 9. Schematic of a dual-channel SAW sensor.
Due to them offering some advantages, SAW-based dual-channel delay lines have been investigated by some researchers in various sectors in the past few years. These include Hur et al.,117 Sakong et al.119 and Fourati et al.120 for DNA detection in liquids, Gray et al.121 for digital diagnosis of HIV, and Wang et al.118 for ultraviolet detection. In 2000, Howe and Harding57 published their research based on a dual-channel surface acoustic wave device to identify two microorganisms of Legionella and E. coli bacteria in under 3 h followed by binding to a specific antibody. They used a Love-wave device in a dual-channel set-up, where one channel worked as a sensor and the other as a reference. Their research included a series of experiments that involved coating microorganisms on the sensor surface before adding the antibody. The antibody that particularly bonded to the SAW device was connected to the concentration of the bacteria adhered to its surface. A contactless SAW biosensor works by coating a layer of SiO2 to protect the electrodes from the liquid. A digital frequency meter attached to a computer is used to measure the frequencies of both channels and the differential frequency is calculated to keep a record of the mass changes on the sensing channel. Additional research has been done to rule out the issues with using SAW devices in liquid phases. Typically, their detection limit is down to ∼106 cells per milliliter in under 3 h.
Although, this sensor has the advantage of being able to measure in real time in systems with the delay lines operating at 200 MHz (ref. 119) and reduces the electronic measurement system complexity,122 it has some limitations too. One of the biggest disadvantages is that dual-channel signals are also prone to crosstalk and the generation of noise.123 We hope this sensor will be improved. Also, the molecular interactions on the transducer's surface should be more sensitive for higher oscillation frequency SAW sensors.
4. Summary
Many technologies for detecting foodborne pathogens have been developed in recent years to address food safety and public health issues, particularly with the growing demand for fresh and minimally processed foods. Table 5 shows the different types of detection methods for E. coli and their advantages using SAW sensors. Ultrahigh-frequency SAW sensors can be used for real-time bacteria monitoring in a biological culture. An important advantage of this sensor is its lower microbial count (102 cells per mL) and the ability to perform wireless measurements in hazardous environments. This technique is quite simple when compared to traditional bacterial plating procedures, and so does not need highly skilled personnel. Dual-channel SAW biosensors, SAW syringe filters, LGS-SAW, and SiO2 nanostructure-based SAW sensors can also achieve bacteria monitoring in real time. These sensors work by grafting antigen–antibody on the surface. These methods are also simple, low cost, and can easily detect bacteria in a short time. Another type of sensor called the Love-wave sensor can be used to identify biological species as well. Also, some research has focused on unique approaches incorporating multiple antibodies that allow complete E. coli bacteria to be immobilized on the sensor surface efficiently. The sensitivity of the Love-wave sensor is substantially higher than that of other piezoelectric sensors, such as the QCM. The capacity of the Love-wave sensor means it can identify the entire E. coli bacteria with excellent repeatability and specificity. This method maintains antibody/antigen interaction specificity and can give substantial results in less than 1 h with a detection limit of 106 cells per mL. Love-wave devices are able to detect antibody concentrations as low as 1 ng mL−1.78 Among all the described methods, the Love-wave method is best for its fast detection and quick response time (<1 h).
Summary of SAW biosensors used to detect Escherichia coli O157 : H7.
| Detection range | Detection time | Temperature | pH | Waveforms | Principle | Advantages | Limitations | References | |
|---|---|---|---|---|---|---|---|---|---|
| Ultrahigh-frequency SAW | 102 cells per mL | <7 h | 30 °C | 6.5 | Rayleigh wave | Microbial metabolism affects loop capacitance inductance to change the resonant frequency | Simple, high sensitivity, does not require highly skilled technicians, low cost, and real-time bacteria monitoring | Time consuming, frequency dependent | 114 |
| Love-wave immunosensor | 106 cells per mL | <1 h | 37 °C | 7.2 | Love wave | Antibody–antigen covalent binding, mass-loading effect leading to a resonant frequency shift | Ultrahigh stability, real-time monitoring, low cost, reusable, short detection time | High IL, guiding layer effect | 68 and 124 |
| Dual-channel SAW biosensor | 105–106 cells per mL | <3 h | 25 °C | 7.4 | Love wave | Mass adsorption effect leading to a frequency difference (Δf) between the test channel and the reference channel | High sensitivity, good response, and short detection time | Influenced by temperature, pressure, competitive non-labeled methods | 57, 120 and 125 |
| LGS-SH-SAW | ∼106 cells per mL | 2–5 h | 25 °C | 7.4 | Shear horizontal wave | Antigen–antibody specific binding changes cause a phase shift | Label-free detection, reduced attenuation, high-temperature stability and flexibility, good antibody bonding | Sensitivity, detection limit | 56 and 98 |
| SAW impedance sensor | 102–107 cells per mL | 7 h | 37 °C | 7.0 | Rayleigh wave and Love wave | Changes in the impedance of the medium produced by bacterial metabolism cause changes in the frequency signal | Simple, real-time bacteria monitoring, can be used by both scientists and unprofessional | Exhibits high attenuation in liquids | 108 |
| SH-SAW biosensor | 6.54 × 105 CFU mL−1 | 2 h | 30 °C | 7.3–7.5 | Shear horizontal wave | Antibody adsorption in the waveguide layer causes a resonant frequency shift | High specificity, repeatability, reproducibility, and lower detection time | Loss of energy, orientation of the crystal, corrosion | 11, 45 and 124 |
5. Future perspective and conclusion
In this review article, we described various types of SAW-based methods for detecting E. coli bacteria and an overview of each sort of detection method is provided, as well as references to literature-based approaches. These methods are simple procedures and also easy to fabricate to detect a high amount of E. coli bacteria within a short time (<1 h). Several techniques for the fast identification of E. coli bacteria have recently been investigated and developed. However, most of them still need to be improved in terms of their sensitivity, selectivity, or accuracy before they can be used in any practical way. As a result, a reliable, accurate, fast, easy, sensitive, selective, and cost-effective detection technique is still desired.
In conclusion, fast and automated detection techniques for foodborne pathogens have a wide range of interesting applications. In the food sector and allied areas, such E. coli detection technologies would be a huge financial benefit. Even though E. coli continues to pose a serious hazard to human and animal welfare, numerous efforts are being undertaken to develop novel detection technologies. The benefits of SAW sensors are that they are extremely sensitive to changes in mass, density, and viscosity on the guiding layer and have huge potential and a significant possibility to see major advances that will enable them to become a vital player in the world of technology and point-of-care devices, especially given the ongoing desperate need for highly sensitive, selective, and cost-effective technologies for detecting microorganisms.
Though SAW devices represent a highly appealing technology for sensor applications, additional research is needed, particularly in the area of disease detection. These devices also have some challenges, including their detection limit, detection time, and specificity. Despite these challenges, SAW devices are still being investigated and exploited in sensing applications. Nanostructured SAW biosensors will be a key solution for future development to push the sensitivity and selectivity of SAW biosensors to new levels. Nanotechnology's extreme sensitivity sensing mechanism can enable nanosensing to overcome these challenges. In addition, the tendency of combining different approaches will lead to the development of new technologies or methodologies that will enhance the benefits of quick detection methods. Overall, these methods will become increasingly important in the identification and surveillance of foodborne pathogens in the future.
Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.
Author contributions
Yujia Zeng: investigation and writing. Rui Yuan: visualization. Hao Fu: conceptualization. Zhangliang Xu: supervision and writing. Drafting the article or revising it critically for important intellectual content, final approval of the version to be submitted. Song Wei: review and modification.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was support by the Natural Science Foundation of Sichuan Province (No. 2022NSFSC1996) and the National Natural Science Foundation of China (No. 52105327). The authors also want to thank the full support by Nanchong Key Laboratory of Electromagnetic Technology and Engineering (No. NCKL202005).
References
- Hua Z. Yu T. Liu D. H. Xianyu Y. L. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021;179:113076. doi: 10.1016/j.bios.2021.113076. [DOI] [PubMed] [Google Scholar]
- Saravanan A. Kumar P. S. Hemavathy R. V. Jeevanantham S. Kamalesh R. Sneha S. Yaashikaa P. R. Methods of detection of food-borne pathogens: a review. Environ. Chem. Lett. 2021;19:189–207. [Google Scholar]
- Deisingh A. Thompson M. Strategies for the detection of Escherichia coli O157 : H7 in foods. J. Appl. Microbiol. 2004;96:419–429. doi: 10.1111/j.1365-2672.2003.02170.x. [DOI] [PubMed] [Google Scholar]
- Nahar S. Jeong H. L. Kim Y. Ha A. J. W. Roy P. K. Park S. H. Ashrafudoulla M. Mizan M. F. R. Ha S. D. Inhibitory effects of Flavourzyme on biofilm formation, quorum sensing, and virulence genes of foodborne pathogens Salmonella Typhimurium and Escherichia coli. Food Res. Int. 2021;147:110461. doi: 10.1016/j.foodres.2021.110461. [DOI] [PubMed] [Google Scholar]
- Tsougeni K. Kaprou G. Loukas C. M. Papadakis G. Hamiot A. Eck M. Rabus D. Kokkoris G. Chatzandroulis S. Papadopoulos V. Dupuy B. Jobst G. Gizeli E. Tserepi A. Gogolides E. Lab-on-Chip platform and protocol for rapid foodborne pathogen detection comprising on-chip cell capture, lysis, DNA amplification and surface-acoustic-wave detection. Sens. Actuators, B. 2020;320:128345. [Google Scholar]
- Puiu M. Bala C. Microfluidics-integrated biosensing platforms as emergency tools for on -site field detection of foodborne pathogens. TrAC, Trends Anal. Chem. 2020;125:115831. [Google Scholar]
- Alahi M. E. E. Mukhopadhyay S. C. Detection methodologies for pathogen and toxins: a review. Sensors. 2017;17:1885. doi: 10.3390/s17081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. Poshtiban S. Evoy S. Recent advances in bacteriophage based biosensors for food-borne pathogen detection. Sensors. 2013;13:1763–1786. doi: 10.3390/s130201763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtis A. P. Hatfield K. Baggs J. Mu Y. See I. Epson E. Nadle J. Kainer M. A. Dumyati G. Petit S. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections-United States. Morb. Mortal. Wkly. Rep. 2019;68:214–219. doi: 10.15585/mmwr.mm6809e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopman B. A. Steele D. Kirkwood C. D. Parashar U. D. The vast and varied global burden of norovirus: Prospects for prevention and control. PLoS Med. 2016;13(4):e1001999. doi: 10.1371/journal.pmed.1001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten S. Hashim U. Gopinath S. Liu W. Foo K. Sam S. Rahman S. Voon C. Nordin A. Highly sensitive Escherichia coli shear horizontal surface acoustic wave biosensor with silicon dioxide nanostructures. Biosens. Bioelectron. 2017;93:146–154. doi: 10.1016/j.bios.2016.09.035. [DOI] [PubMed] [Google Scholar]
- Peng L. Zeng L. T. Jin H. W. Yang L. X. Xiao Y. Lan Z. Q. Yu Z. P. Ouyang S. Zhang L. R. Sun N. Discovery and antibacterial study of potential PPK1 inhibitors against uropathogenic E. coli. J. Enzyme Inhib. Med. Chem. 2020;35(1):1224–1232. doi: 10.1080/14756366.2020.1766453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X. Ye Y. L. Zhang Y. Z. Sun X. L. Current research progress of mammalian cell-based biosensors on the detection of foodborne pathogens and toxins. Crit. Rev. Food Sci. Nutr. 2021;61(22):3819–3835. doi: 10.1080/10408398.2020.1809341. [DOI] [PubMed] [Google Scholar]
- Saiz P. G. de Luis R. F. Lasheras A. Arriortua M. I. Lopes A. C. Magnetoelastic resonance sensors: principles, applications, and perspectives. ACS Sens. 2022;7(5):1248–1268. doi: 10.1021/acssensors.2c00032. [DOI] [PubMed] [Google Scholar]
- Biernbaum E. N. Kudva I. T. AB5 Enterotoxin-mediated pathogenesis: perspectives gleaned from shiga toxins. Toxins. 2022;14(1):62. doi: 10.3390/toxins14010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. M. Huang T. Y. Ye C. X. Chen L. Liang Y. Wang K. Liu J. Y. Formation and Control of the Viable but Non-culturable State of Foodborne Pathogen Escherichia coli O157:H7. Front. Microbiol. 2020;11:1202. doi: 10.3389/fmicb.2020.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detzner J. Steil D. Pohlentz G. Legros N. Humpf H. U. Mellmann A. Karch H. Müthing J. Real-time interaction analysis of Shiga toxins and membrane microdomains of primary human brain microvascular endothelial cells. Glycobiology. 2020;30(3):174–185. doi: 10.1093/glycob/cwz091. [DOI] [PubMed] [Google Scholar]
- Paniel N. Noguer T. Detection of Salmonella in food matrices, from conventional methods to recent aptamer-sensing technologies. Foods. 2019;8(9):371. doi: 10.3390/foods8090371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren B. Parish M. Schneider K. Shigella as a foodborne pathogen and current methods for detection in food. Crit. Rev. Food Sci. Nutr. 2006;46(7):551–567. doi: 10.1080/10408390500295458. [DOI] [PubMed] [Google Scholar]
- Kaneko I. Miyamoto K. Mimura K. Yumine N. Utsunomiya H. Akimoto S. McClane B. A. Detection of enterotoxigenic Clostridium perfringens in meat samples by using molecular methods. Appl. Environ. Microbiol. 2011;77(21):7526–7532. doi: 10.1128/AEM.06216-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarao N. Tran S.-L. Marin M. Vidic J. Advanced methods for detection of Bacillus cereus and its pathogenic factors. Sensors. 2020;20(9):2667. doi: 10.3390/s20092667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris M. Palomba P. Quartuccio M. Rapid detection of Listeria monocytogenes in foods, by a combination of PCR and DNA probe. Mol. Cell. Probes. 2001;15(5):275–280. doi: 10.1006/mcpr.2001.0372. [DOI] [PubMed] [Google Scholar]
- Gu Y. Y. Chen C. Y. Mao Z. M. Bachman H. Becker R. Rufo J. Wang Z. Y. Zhang P. R. Mai J. Yang S. J. Zhang J. X. Zhao S. G. Ouyang Y. S. Wong D. T. W. Sadovsky Y. Huang T. J. Acoustofluidic centrifuge for nanoparticle enrichment and separation. Sci. Adv. 2021;7(1):eabc0467. doi: 10.1126/sciadv.abc0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. Y. Belwal T. Li L. Lin X. Y. Xu Y. Q. Luo Z. S. Nanomaterial-based biosensors for sensing key foodborne pathogens: advances from recent decades. Compr. Rev. Food Sci. Food Saf. 2020;19(4):1465–1487. doi: 10.1111/1541-4337.12576. [DOI] [PubMed] [Google Scholar]
- Zhao Y. Tong R. J. Xia F. Peng Y. Current status of optical fiber biosensor based on surface plasmon resonance. Biosens. Bioelectron. 2019;142:111505. doi: 10.1016/j.bios.2019.111505. [DOI] [PubMed] [Google Scholar]
- Zhang X. M. Payne M. Kaur S. Lan R. T. Improved Genomic Identification, Clustering, and Serotyping of Shiga Toxin-Producing Escherichia coli Using Cluster/Serotype-Specific Gene Markers. Front. Cell. Infect. Microbiol. 2022;11:772574. doi: 10.3389/fcimb.2021.772574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Bu T. Cao Y. Y. Wu H. Y. Xi J. Feng Q. L. Xuan C. Y. Wang L. A versatile PdRu bimetallic nanoenzyme-integrated enzyme-linked immunosorbent assay for highly sensitive Escherichia coli O157:H7 Detection. Anal. Chem. 2023;95(24):9237–9243. doi: 10.1021/acs.analchem.3c00743. [DOI] [PubMed] [Google Scholar]
- Sharma P. Chauhan R. Pande V. Basu T. Kumar R. A. Rapid sensing of Tilletia indica-Teliospore in wheat extract by a piezoelectric label free immunosensor. Bioelectrochemistry. 2022;147:108175. doi: 10.1016/j.bioelechem.2022.108175. [DOI] [PubMed] [Google Scholar]
- Barrientos K. Rocha M. I. Jaramillo M. Vásquez N. A. High frequency (100, 150 MHz) quartz crystal microbalance (QCM) piezoelectric genosensor for the determination of the Escherichia coli O157 rfbE gene. Anal. Lett. 2022;55(17):2697–2709. [Google Scholar]
- Thakur B. Zhou G. Chang J. Pu H. Jin B. Sui X. Yuan X. Yang C.-H. Magruder M. Chen J. Rapid detection of single E. coli bacteria using a graphene-based field-effect transistor device. Biosens. Bioelectron. 2018;110:16–22. doi: 10.1016/j.bios.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Matatagui D. Bastida A. Horrillo M. C. Novel SH-SAW biosensors for ultra-fast recognition of growth factors. Biosensors. 2022;12(1):17. doi: 10.3390/bios12010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. W., Sardari S. E., Iliadis A. A., Ghodssi R.A bacterial biofilm surface acoustic wave sensor for real time biofilm growth monitoring. In Proccedings of 2010 IEEE Sensors, 2010, 1568–1571 [Google Scholar]
- Mandal D. Banerjee S. Surface acoustic wave (SAW) sensors: physics, materials, and applications. Sensors. 2022;22(3):820. doi: 10.3390/s22030820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Zhou J. Tang H. Liu Y. Shen Y. Yin X. Zheng J. Zhang H. Wu J. Shi X. Ultrahigh-frequency surface acoustic wave sensors with giant mass-loading effects on electrodes. ACS Sens. 2020;5(6):1657–1664. doi: 10.1021/acssensors.0c00259. [DOI] [PubMed] [Google Scholar]
- Xu Z. Yuan Y. J. Implementation of guiding layers of surface acoustic wave devices: a review. Biosens. Bioelectron. 2018;99:500–512. doi: 10.1016/j.bios.2017.07.060. [DOI] [PubMed] [Google Scholar]
- Länge K. Bulk and surface acoustic wave sensor arrays for multi-analyte detection: a review. Sensors. 2019;19(24):5382. doi: 10.3390/s19245382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh K. Jeoti V. Soeung S. Drieberg M. Goh M. Aslam M. Z. A comparative survey on silicon based and surface acoustic wave (SAW)-based RFID tags: potentials, challenges, and future directions. IEEE Access. 2020;8:91624–91647. [Google Scholar]
- Lee K. L. Kowach G. Li F. Voiculescu I. Liquid viscosity sensor using a surface acoustic wave device for medical applications including blood and plasma. Sensors. 2023;23(13):5911. doi: 10.3390/s23135911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Länge K. Bulk and surface acoustic wave sensor arrays for multi-analyte detection: a review. Sensors. 2019;19(24):5382. doi: 10.3390/s19245382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo B.-S. Huh J.-S. Lee D.-D. Fabrication of polymer SAW sensor array to classify chemical warfare agents. Sens. Actuators, B. 2007;121(1):47–53. [Google Scholar]
- Piro L. Lamanna L. Guido F. Balena A. Mariello M. Rizzi F. De Vittorio M. Flexible SAW microfluidic devices as wearable pH sensors based on ZnO nanoparticles. Nanomaterials. 2021;11(6):1479. doi: 10.3390/nano11061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinoiu I. Viespe C. ZnO metal oxide semiconductor in surface acoustic wave sensors: a review. Sensors. 2020;20(18):5118. doi: 10.3390/s20185118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z. Yasui T. Liu Q. Nagashima K. Takahashi T. Shimada T. Yanagida T. Baba Y. Fabrication of a robust In2O3 nanolines FET device as a biosensor platform. Micromachines. 2021;12(6):642. doi: 10.3390/mi12060642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasceno B. S. Horta I. M. de Oliveira R. S. Pereira R. M. Schatkoski V. M. Bacher G. Massi M. Thim G. P. Pereira A. L. d. J. da Silva Sobrinho A. S. Recent improvements on surface acoustic wave sensors based on graphenic nanomaterials. Mater. Sci. Semicond. Process. 2023;167:107811. [Google Scholar]
- Lamanna L. Rizzi F. Bhethanabotla V. R. De Vittorio M. Conformable surface acoustic wave biosensor for E. coli fabricated on PEN plastic film. Biosens. Bioelectron. 2020;163:112164. doi: 10.1016/j.bios.2020.112164. [DOI] [PubMed] [Google Scholar]
- Palla-Papavlu A. Voicu S. I. Dinescu M. Sensitive materials and coating technologies for surface acoustic wave sensors. Chemosensors. 2021;9(5):105. [Google Scholar]
- Arasavalli N. Alli D. R. Babu B. Ravichandra G. Mokshagni K. S. ZnO: SnO2 nanocomposite efficacy for gas sensing and microbial applications. Indian J. Biochem. Biophys. 2022;59(5):586–594. [Google Scholar]
- Wang C. Ding Y. Li M. Li H. Xu S. Li C. Qian L. Yang B. Surface acoustic wave sensor based on Au/TiO2/PEDOT with dual response to carbon dioxide and humidity. Anal. Chim. Acta. 2022;1190:339264. doi: 10.1016/j.aca.2021.339264. [DOI] [PubMed] [Google Scholar]
- Ma Z. L. Meliana C. Munawaroh H. S. H. Karaman C. Karimi-Maleh H. Low S. S. Show P. L. Recent advances in the analytical strategies of microbial biosensor for detection of pollutants. Chemosphere. 2022;306:135515. doi: 10.1016/j.chemosphere.2022.135515. [DOI] [PubMed] [Google Scholar]
- Nawaz F. Jeoti V. SAW sensor read range limitations and perspectives. Wireless Networks. 2014;20(8):2581–2587. [Google Scholar]
- Jeng M. J. Sharma M. Li Y. C. Lu Y. C. Yu C. Y. Tsai C. L. Huang S. F. Chang L. B. Lai C. S. Surface acoustic wave sensor for C-reactive protein detection. Sensors. 2020;20(22):6640. doi: 10.3390/s20226640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun H. Han X. Wang F. Nie W. C. Yang Z. D. Zhou L. T. Zhang F. P. Sun H. Han Y. M. Kong D. Q. Zhang K. L. Simulation and optimization of surface acoustic wave devices with high electromechanical coupling factor and zero temperature coupling coefficient by pattern piezoelectric layer. IEEE Sens. J. 2023;23(10):10597–10604. [Google Scholar]
- Cheng C. H. Yatsuda H. Goto M. Kondoh J. Liu S. H. Wang R. Y. L. Application of shear horizontal surface acoustic wave (SH-SAW) immunosensor in Point-of-Care diagnosis. Biosensors. 2023;13(6):605. doi: 10.3390/bios13060605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. B. Mayrhofer P. M. He X. L. Gillinger M. Ye Z. Wang X. Z. Bittner A. Schmid U. Luo J. K. High performance AlScN thin film based surface acoustic wave devices with large electromechanical coupling coefficient. Appl. Phys. Lett. 2014;105(13):133502. [Google Scholar]
- Ten S. Hashim U. Nordin A. Gopinath S. C. Liu W. Foo K. Sam S. T. Rahman S. Voon C. H. Nuzaihan M. Recognition of bacterial DNA on SAW-based biosensors. Nanobiosens. Biomol. Targeting. 2019:117–146. [Google Scholar]
- Berkenpas E. Millard P. Da Cunha M. P. Detection of Escherichia coli O157: H7 with langasite pure shear horizontal surface acoustic wave sensors. Biosens. Bioelectron. 2006;21:2255–2262. doi: 10.1016/j.bios.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Howe E. Harding G. A comparison of protocols for the optimisation of detection of bacteria using a surface acoustic wave (SAW) biosensor. Biosens. Bioelectron. 2000;15(11–12):641–649. doi: 10.1016/s0956-5663(00)00116-0. [DOI] [PubMed] [Google Scholar]
- Love A. E. H. Some Problems of Geodynamics, Cambridge University Press, Dover, 1911 [Google Scholar]
- Kiełczyński P. Szalewski M. Balcerzak A. Wieja K. Propagation of ultrasonic Love waves in nonhomogeneous elastic functionally graded materials. Ultrasonics. 2016;65:220–227. doi: 10.1016/j.ultras.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Feng X. Ke L. Gao Y. Love wave propagation in one-dimensional piezoelectric quasicrystal multilayered nanoplates with surface effects. Appl. Math. Mech. 2024;45(4):619–632. [Google Scholar]
- Šetka M. Bahos F. Chmela O. Matatagui D. Gràcia I. Drbohlavová J. Vallejos S. Cadmium telluride/polypyrrole nanocomposite based Love wave sensors highly sensitive to acetone at room temperature. Sens. Actuators, B. 2020;321:128573. [Google Scholar]
- Du J. Harding G. L. A multilayer structure for Love-mode acoustic sensors. Sens. Actuators, A. 1998;65(2–3):152–159. [Google Scholar]
- Zhang J. Y. Zhang X. J. Wei X. W. Xue Y. Y. Wan H. Wang P. Recent advances in acoustic wave biosensors for the detection of disease-related biomarkers: a review. Anal. Chim. Acta. 2021;1164:338321. doi: 10.1016/j.aca.2021.338321. [DOI] [PubMed] [Google Scholar]
- Greco G. Agostini M. Cecchini M. Ultra-high-frequency Love surface acoustic wave device for real-time sensing applications. IEEE Access. 2020;8:112507–112514. [Google Scholar]
- Kovacs G., Lubking G., Vellekoop M. and Venema A.Love waves for (bio)-chemical sensing in liquids. IEEE 1992 Ultrasonics Symposium Proceedings, 1992, pp. 281–285 [Google Scholar]
- Harding G. Du J. Dencher P. Barnett D. Howe E. Love wave acoustic immunosensor operating in liquid. Sens. Actuators, A. 1997;61(1–3):279–286. [Google Scholar]
- Tamarina O. Comeaub S. Déjousa C. Moynetc D. Rebierea D. Bezianc J. Pistréa J. Real time device for biosensing: design of a bacteriophage model using love acoustic waves. Biosens. Bioelectron. 2003;18(5–6):755–763. doi: 10.1016/s0956-5663(03)00022-8. [DOI] [PubMed] [Google Scholar]
- Moll N. Pascal E. Dinh D. H. Pillot J.-P. Bennetau B. Rebière D. Moynet D. Mas Y. Mossalayi D. Pistré J. A Love wave immunosensor for whole E. coli bacteria detection using an innovative two-step immobilisation approach. Biosens. Bioelectron. 2007;22(9–10):2145–2150. doi: 10.1016/j.bios.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Li C. Zhang J. K. Xie H. Y. Luo J. T. Fu C. Tao R. Li H. L. Fu Y. Q. Highly sensitive Love mode acoustic wave platform with SiO2 wave-guiding layer and gold nanoparticles for detection of carcinoembryonic antigens. Biosensors. 2022;12(7):536. doi: 10.3390/bios12070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll N. Pascal E. Dinh D. H. Lachaud J.-L. Vellutini L. Pillot J.-P. Rebière D. Moynet D. Pistré J. Mossalayi D. Multipurpose Love acoustic wave immunosensor for bacteria, virus or proteins detection. Irbm. 2008;29(2–3):155–161. [Google Scholar]
- Hajiabadi S. H. Bedrikovetsky P. Mahani H. Khoshsima A. Aghaei H. Kalateh-Aghamohammadi M. Habibi S. Effects of surface modified nanosilica on drilling fluid and formation damage. J. Pet. Sci. Eng. 2020;194:107559. [Google Scholar]
- Bozok S. S. Ogulata R. T. Effect of antioxidant and SiO2 combination on linen fabrics. J. Nat. Fibers. 2022;19(2):727–735. [Google Scholar]
- Kusnezow W. Jacob A. Walijew A. Diehl F. Hoheisel J. D. Antibody microarrays: an evaluation of production parameters. Proteomics. 2003;3(3):254–264. doi: 10.1002/pmic.200390038. [DOI] [PubMed] [Google Scholar]
- Puiu M. Gurban A. M. Rotariu L. Brajnicov S. Viespe C. Bala C. Enhanced sensitive Love wave surface acoustic wave sensor designed for immunoassay formats. Sensors. 2015;15(5):10511–10525. doi: 10.3390/s150510511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini M. Greco G. Cecchini M. Full-SAW microfluidics-based Lab-on-a-Chip for biosensing. IEEE Access. 2019;7:70901–70909. [Google Scholar]
- Shuvalov A. L. Kutsenko A. A. Korotyaeva M. E. Poncelet O. Love waves in a coated vertically periodic substrate. Wave Motion. 2013;50(4):809–820. [Google Scholar]
- Fu Y. Q. Pang H. F. Torun H. Tao R. McHale G. Reboud J. Tao K. Zhou J. Luo J. T. Gibson D. Luo J. K. Hu P. A. Engineering inclined orientations of piezoelectric films for integrated acoustofluidics and lab-on-a-chip operated in liquid environments. Lab Chip. 2021;21(2):254–271. doi: 10.1039/d0lc00887g. [DOI] [PubMed] [Google Scholar]
- Moriizumi T., Unno Y. and Shiokawa S.New sensor in liquid using leaky SAW. IEEE 1987 Ultrasonics Symposium, 1987, pp. 579–582 [Google Scholar]
- Deobagkar D. D. Limaye V. Sinha S. Yadava R. Acoustic wave immunosensing of Escherichia coli in water. Sens. Actuators, B. 2005;104(1):85–89. [Google Scholar]
- Länge K. Blaess G. Voigt A. Götzen R. Rapp M. Integration of a surface acoustic wave biosensor in a microfluidic polymer chip. Biosens. Bioelectron. 2006;22(2):227–232. doi: 10.1016/j.bios.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Bisoffi M. Hjelle B. Brown D. Branch D. Edwards T. Brozik S. Bondu-Hawkins V. Larson R. Detection of viral bioagents using a shear horizontal surface acoustic wave biosensor. Biosens. Bioelectron. 2008;23(9):1397–1403. doi: 10.1016/j.bios.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Richardson M. Sankaranarayanan S. K. Bhethanabotla V. R. Low insertion loss and highly sensitive SH-SAW sensors based on 36 YX LiTaO3 through the incorporation of filled microcavities. IEEE Sens. J. 2014;15(2):787–796. [Google Scholar]
- Bergaoui Y. Zerrouki C. Fourati N. Fougnion J. Abdelghani A. Antigen-antibody selective recognition using LiTaO3 SH-SAW sensors: investigations on macromolecules effects on binding kinetic constants. Eur. Phys. J.: Appl. Phys. 2011;56(1):13705. [Google Scholar]
- Caliendo C. Laidoudi F. Experimental and theoretical study of multifrequency surface acoustic wave devices in a single Si/SiO2/ZnO piezoelectric structure. Sensors. 2020;20(5):1380. doi: 10.3390/s20051380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. T. Quan A. J. Fu C. Li H. L. Shear-horizontal surface acoustic wave characteristics of a (110) ZnO/SiO2/Si multilayer structure. J. Alloys Compd. 2017;693:558–564. [Google Scholar]
- Mandal D. Banerjee S. Surface acoustic wave (SAW) sensors: physics, materials, and applications. Sensors. 2022;22(3):820. doi: 10.3390/s22030820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R. Yang Y. Link S. Gong S. A1 resonators in 128° Y-cut lithium niobate with electromechanical coupling of 46.4% J. Microelectromech. Syst. 2020;29(3):313–319. [Google Scholar]
- Du X. Y. Fu Y. Q. Tan S. C. Luo J. K. Flewitt A. J. Milne W. I. Lee D. S. Park N. M. Park J. Choi Y. J. Kim S. H. Maeng S. ZnO film thickness effect on surface acoustic wave modes and acoustic streaming. Appl. Phys. Lett. 2008;93(9):094105. [Google Scholar]
- Lamanna L. Rizzi F. Guido F. Algieri L. Marras S. Mastronardi V. M. Qualtieri A. De Vittorio M. Flexible and transparent aluminum-nitride-based surface-acoustic-wave device on polymeric polyethylene naphthalate. Adv. Electron. Mater. 2021;7(5):2100084. [Google Scholar]
- Kano K. Yatsuda H. Kondoh J. Evaluation of shear horizontal surface acoustic wave biosensors using “layer parameter” obtained from sensor responses during immunoreaction. Sensors. 2021;21(14):492. doi: 10.3390/s21144924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietrantonio F. Benetti M. Cannatà D. Verona E. Girasole M. Fosca M. Dinarelli S. Staiano M. Marzullo V. Capo A. A Shear horizontal surface acoustic wave biosensor for a rapid and specific detection of d-serine. Sens. Actuators, B. 2016;226:1–6. [Google Scholar]
- Cole M. Covington J. A. Gardner J. W. Combined electronic nose and tongue for a flavour sensing system. Sens. Actuators, B. 2011;156(2):832–839. [Google Scholar]
- Campitelli A. P. Wlodarski W. Hoummady M. Identification of natural spring water using shear horizontal SAW based sensors. Sens. Actuators, B. 1998;49(3):195–201. [Google Scholar]
- Thiele J. Da Cunha M. P. High temperature LGS SAW gas sensor. Sens. Actuators, B. 2006;113(2):816–822. [Google Scholar]
- Morris R. H., Newton M. I., Roach P., Doy N., Evans C. R., Atherton S. and McHale G.Layer guided surface acoustic wave sensors using langasite substrates. In Proceedings of the 2009 IEEE International Frequency Control Symposium Joint with the 22nd European Frequency and Time Forum, 2009, pp. 245–247 [Google Scholar]
- Berkenpas E., Bitla S., Millard P. and da Cunha M. P.LGS shear horizontal SAW devices for biosensor applications. IEEE Symposium on Ultrasonics, 2003, pp. 1404–1407 [DOI] [PubMed] [Google Scholar]
- Konstantinova A. F. Golovina T. G. Dudka A. P. Goryachuk I. O. Sokolov V. I. Measurement and calculation of the refractive indices of Langasite-family crystals Sr3NbFe3Si2O14, Ba3NbFe3Si2O14, and Ba3TaFe3Si2O14 and the relationship of optical activity with peculiarities of electron-density distribution. Crystallogr. Rep. 2022;67:951–957. [Google Scholar]
- Pollard T. da Cunha M. P. Improved pure SH SAW transduction efficiency on LGS using finite thickness gratings. IEEE Ultrason. Symp. 2005:1048–1051. [Google Scholar]
- Ayala V. C., Eisele D., Reindl L. and Josse F.Temperature stability analysis of LGS for SH-SAW sensor applications. 2010 IEEE International Frequency Control Symposium, 2010, pp. 142–145 [Google Scholar]
- Berkenpas E., da Cunha M. P., Bitla S. and Millard P.Shear horizontal SAW biosensor on langasite. InProceedings of the 2003 IEEE Sensors, 2003, 1: 661–664 [Google Scholar]
- Ten S. T. Hashim U. Gopinath S. C. B. Liu W. W. Foo K. L. Sam S. T. Rahman S. F. A. Voon C. H. Nordin A. N. Highly sensitive Escherichia coli shear horizontal surface acoustic wave biosensor with silicon dioxide nanostructures. Biosens. Bioelectron. 2017;93:146–154. doi: 10.1016/j.bios.2016.09.035. [DOI] [PubMed] [Google Scholar]
- Berkenpas E. Bitla S. Millard P. Da Cunha M. P. Pure shear horizontal SAW biosensor on langasite. IEEE Trans. Ultrason. Ferroelectrics Freq. Control. 2004;51(11):1404–1411. doi: 10.1109/tuffc.2004.1367479. [DOI] [PubMed] [Google Scholar]
- Liu Z., Fang L., Zhang C. and Dai X.Analysis of impedance-loaded SAW sensors. 2016 IEEE Sensors, 2016, pp. 1–3 [Google Scholar]
- Wang R. Cai Q. Tong D. Nie L. Yao S. Enzymatic assay of acid phosphatase and microanalysis of Cu2+ and Ag+ with a SAW-impedance sensor. Enzyme Microb. Technol. 1998;22(1):36–41. [Google Scholar]
- Karilainen A. Finnberg T. Uelzen T. Dembowski K. Muller J. Mobile patient monitoring based on impedance-loaded SAW-sensors. IEEE Trans. Ultrason. Ferroelectrics Freq. Control. 2004;51(11):1464–1469. doi: 10.1109/tuffc.2004.1367487. [DOI] [PubMed] [Google Scholar]
- Nguyen V. H. Peters O. Serve S. Schnakenberg U. Portable SAW impedance sensor using a 1-port resonator approach. Proceedings. 2017;1:361. [Google Scholar]
- Yang L. Bashir R. Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria. Biotechnol. Adv. 2008;26(2):135–150. doi: 10.1016/j.biotechadv.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Chang Y.-H. Jang H.-D. Hsu C.-L. Chang K.-S. Quantitative determination of Escherichia coli in water sources in the environment using a surface acoustic wave impedance system modified with a syringe filter. Anal. Lett. 2012;45(11):1485–1494. [Google Scholar]
- Gagliardi M. Agostini M. Lunardelli F. Lamanna L. Miranda A. Bazzichi A. Luminare A. G. Cervelli F. Gambineri F. Totaro M. Lai M. C. L. Maisetta G. Batoni G. Pistello M. Cecchini M. Surface acoustic wave-based lab-on-a-chip for the fast detection of Legionella pneumophila in water. Sens. Actuators, B. 2023;379:133299. [Google Scholar]
- Ten S., Hashim U., Sudin A., Liu W., Salleh N. and Nazwa T.Design and characteristic of CMOS fabricated acoustic waves based sensors for foodborne pathogen rapid detection. 2012 IEEE-EMBS Conference on Biomedical Engineering and Sciences, 2012. ,pp. 387–391 [Google Scholar]
- Thomas S., Rácz Z., Cole M. and Gardner J. W.High-frequency one-port colpitts SAW oscillator for chemical sensing. Proc. Of the Sixth Int. Conf. on Advances in Circuits, Electronics and Micro-electronics, 2013. pp. 13–17 [Google Scholar]
- Mujahid A. Afzal A. Dickert F. L. An overview of high frequency acoustic sensors-QCMs, SAWs and FBARs-chemical and biochemical applications. Sensors. 2019;19(20):4395. doi: 10.3390/s19204395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini M. Cecchini M. Ultra-high-frequency (UHF) surface-acoustic-wave (SAW) microfluidics and biosensors. Nanotechnology. 2021;32(31):312001. doi: 10.1088/1361-6528/abfaba. [DOI] [PubMed] [Google Scholar]
- Chang K.-S. Chang C.-K. Chen C.-Y. A surface acoustic wave sensor modified from a wireless transmitter for the monitoring of the growth of bacteria. Sens. Actuators, B. 2007;125(1):207–213. [Google Scholar]
- Singh K. J. Elmazria O. Sarry F. Nicolay P. Ghoumid K. Belgacem B. Mercier D. Bounouar J. Enhanced sensitivity of saw-based pirani vacuum pressure sensor. IEEE Sens. J. 2011;11(6):1458–1464. [Google Scholar]
- Hashwan S. S. B. Khir M. H. M. Nawi I. M. Ahmad M. R. Hanif M. Zahoor F. Al-Douri Y. Algamili A. S. Bature U. I. Alabsi S. S. Sabbea M. O. B. Junaid M. A review of piezoelectric MEMS sensors and actuators for gas detection application. Discover Nano. 2023;18(1):25. doi: 10.1186/s11671-023-03779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur Y. Han J. Seon J. Pak Y. E. Roh Y. Development of an SH-SAW sensor for the detection of DNA hybridization. Sens. Actuators, A. 2005;120(2):462–467. [Google Scholar]
- Wang W. S. Wu T. T. Chou T. H. Chen Y. Y. A ZnO nanorod-based SAW oscillator system for ultraviolet detection. Nanotechnology. 2009;20(13):135503. doi: 10.1088/0957-4484/20/13/135503. [DOI] [PubMed] [Google Scholar]
- Sakong J., Roh Y. and Roh H.3f-2 SAW sensor system with micro-fluidic channels to detect DNA molecules. 2006 IEEE Ultrasonics Symposium, 2006, pp. 548–551 [Google Scholar]
- Fourati N. Lazerges M. Vedrine C. Fougnion J.-M. Zerrouki C. Rousseau L. Lepeut P. Bonnet J. J. Pernelle C. Surface acoustic waves sensor for DNA-biosensor development. Sens. Lett. 2009;7(5):847–850. [Google Scholar]
- Gray E. R. Turbé V. Lawson V. E. Page R. H. Cook Z. C. Ferns R. B. Nastouli E. Pillay D. Yatsuda H. Athey D. Ultra-rapid, sensitive and specific digital diagnosis of HIV with a dual-channel SAW biosensor in a pilot clinical study. NPJ Digit. Med. 2018;1:35. doi: 10.1038/s41746-018-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Gaso M.-I. March-Iborra C. Montoya-Baides Á. Arnau-Vives A. Surface generated acoustic wave biosensors for the detection of pathogens: a review. Sensors. 2009;9(7):5740–5769. doi: 10.3390/s90705740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. Liu W. B. Wang B. Enhanced Frequency Stability of SAW Yarn Tension Sensor by Using the Dual Differential Channel Surface Acoustic Wave Oscillator. Sensors. 2023;23(1):464. doi: 10.3390/s23010464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zida S. I. Lin Y. D. Khung Y. L. Current trends on surface acoustic wave biosensors. Adv. Mater. Technol. 2021;6(6):2001018. [Google Scholar]
- Cheng L. N., Li H. L., Ke Y. B., He S. T. and Tong Z. Y.Experimental analysis of two methods for aptamer immobilization on love-wave aptasensors. 2013 Symposium on Piezoelectricity, Acoustic Waves, and Device Applications, 2013, pp. 1–3 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.