Abstract
BACKGROUND
Gastrointestinal stromal tumors (GIST) are prevalent neoplasm originating from the gastrointestinal mesenchyme. Approximately 50% of GIST patients experience tumor recurrence within 5 years. Thus, there is a pressing need to accurately evaluate risk stratification preoperatively.
AIM
To assess the application of a deep learning model (DLM) combined with computed tomography features for predicting risk stratification of GISTs.
METHODS
Preoperative contrast-enhanced computed tomography (CECT) images of 551 GIST patients were retrospectively analyzed. All image features were independently analyzed by two radiologists. Quantitative parameters were statistically analyzed to identify significant predictors of high-risk malignancy. Patients were randomly assigned to the training (n = 386) and validation cohorts (n = 165). A DLM and a combined DLM were established for predicting the GIST risk stratification using convolutional neural network and subsequently evaluated in the validation cohort.
RESULTS
Among the analyzed CECT image features, tumor size, ulceration, and enlarged feeding vessels were identified as significant risk predictors (P < 0.05). In DLM, the overall area under the receiver operating characteristic curve (AUROC) was 0.88, with the accuracy (ACC) and AUROCs for each stratification being 87% and 0.96 for low-risk, 79% and 0.74 for intermediate-risk, and 84% and 0.90 for high-risk, respectively. The overall ACC and AUROC were 84% and 0.94 in the combined model. The ACC and AUROCs for each risk stratification were 92% and 0.97 for low-risk, 87% and 0.83 for intermediate-risk, and 90% and 0.96 for high-risk, respectively. Differences in AUROCs for each risk stratification between the two models were significant (P < 0.05).
CONCLUSION
A combined DLM with satisfactory performance for preoperatively predicting GIST stratifications was developed using routine computed tomography data, demonstrating superiority compared to DLM.
Keywords: Gastrointestinal stromal tumors; Deep learning; Risk stratification; Tomography, X-ray computed; Prognosis
Core Tip: The deep learning model (DLM) was validated to accurately predict the risk classification of gastrointestinal stromal tumors. The combined DLM outperformed DLM in predicting risk stratification. The combined model has potential to guide and facilitate clinical decision-making.
INTRODUCTION
As is well documented, gastrointestinal stromal tumors (GIST) are prevalent tumors originating from the gastrointestinal mesenchyme. Approximately 50% of GIST patients experience tumor recurrence within 5 years, and surgical intervention alone is inadequate for achieving an optimal prognosis[1,2]. Earlier studies established that 85% of GISTs are associated with receptor tyrosine kinase (c-kit) mutations, and 3%–5% are linked to platelet derived growth factor receptor alpha gene mutation[1,3-5]. The mutation type determines response to imatinib[6-8]. Considering the lack of effective conventional chemotherapy drugs, and the activity of Imatinib, adjuvant Imatinib may be a potential treatment option for patients with GIST[1,5,9]. A study assessing the efficacy of long-term imatinib treatment in advanced GIST patients documented a median overall survival exceeding four years[10]. Moreover, considering the recurrence and metastasis rate of intermediate- and high-risk GISTs after surgery, targeted drug therapy can improve the prognosis of GISTs[11,12]. Therefore, there is an urgent need to accurately evaluate risk stratification prior to surgery to obtain valuable information for evaluating the necessity of surgery and adjuvant treatment.
At present, the risk stratification of GISTs is based on histologic (mitotic index) and imaging characteristics (including tumor size and site) of the lesion as outlined in the National Institutes of Health (NIH) consensus classification system[13]. However, it is challenging to determine the mitotic index without histological examination. Indeed, the malignancy risk of most tumors is confirmed histologically after surgery. Although, endoscopic biopsy has also been widely used preoperatively, its utility may be limited if the tumor sample contains large areas of necrosis or hemorrhage, yielding inconclusive results[14-16].
Recently, the deep learning model (DLM), composed of multi-types of self-learning units, has emerged as a promising technique for analyzing medical imaging data[17-20]. Notably, DLM has demonstrated efficacy in clinical applications such as the assessment of differentiation grades in meningioma and renal cell carcinoma, as well as in predicting the molecular subtypes and grades of glioma[17-19,21]. Overall, deep learning transforms medical images into high-dimensional mineable data, offering rapid insights with high repeatability and providing a novel approach for GIST risk assessment[22-24]. This study aimed to establish and validate DLMs for predicting preoperative GIST risk stratification based on routine post-contrast computed tomography (CT) and clinical data.
MATERIALS AND METHODS
Characteristics of patients
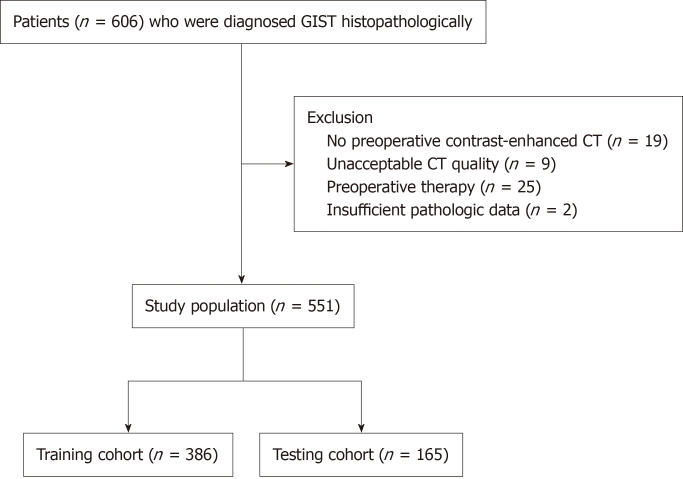
This study was approved by our institutional ethics committee. All the patients signed the informed consent form before examinations. From January 2012 to December 2022, 606 patients with GISTs were initially enrolled in this retrospective study. A total of 55 patients were subsequently excluded for the following reasons: (1) Lack of preoperative contrast-enhanced CT images (n = 19); (2) Suboptimal CT image quality (n = 9); (3) Preoperative therapy or experienced metastasis to other sites (n = 25); and (4) Absence of required pathologic data (n = 2). Finally, a total of 551 patients were included in this study (256 men and 295 women; mean age 60.3 ± 9.8 years). The study population flow chart is illustrated in Figure 1.
Figure 1.
Study population flowchart. CT: Computed tomography; GIST: Gastrointestinal stromal tumors.
All patients underwent complete surgical resection. GIST risk classification was based on National Comprehensive Cancer Network (NCCN) guidelines[15]. According to risk categories, patients in this study were classified into the high-risk (high risk), intermediate-risk (intermediate risk), and low-risk (very low and low risk) groups.
CT image acquisition
All patients underwent abdominal contrast-enhanced CT examination covering the whole tumor. After a non-contrast CT scan (Scanner: Philips Iqon, GE Healthcare Discovery CT750 HD or SIEMENS 64-MDCT) with a thickness of 1.0-1.5 mm, three-phase contrast-enhanced scans were performed, with 90 to 100 mL iodine contrast medium (Ultravist 370, Bayer Schering Pharma, Germany) intravenously injected at a rate of 2.5 to 3.0 mL/s.
Clinical and CT image feature analysis
All CT images were independently analyzed by two radiologists with 3 and 13 years of experience in abdominal radiology. In cases of disagreement, the radiologist with 13 years of experience reviewed the images to reach a consensus. Clinical information, image features and pathologic characteristics, including gender, age, tumor location, growth pattern (exophytic, endoluminal, mixed), tumor size (measured as the maximum diameter of the largest tumor section), tumor morphology (round or oval shape was considered regular, and lobulated or other irregular shapes were categorized as irregular), necrosis, ulceration, internal hemorrhagic foci, calcification, lymph node status, presence of enlarged feeding vessels, tumor boundary (clear or blurred), the pattern and degree of tumor enhancement during the venous phase and the range of tumor enhancement across the three phases, were derived from CT images and medical records. For CT value measurements of each tumor, regions of interest (ROIs) were delineated to cover tumor parenchyma while avoiding areas with evidence of cystic, necrotic or hemorrhagic changes at the level of the largest solid tumor regions and their adjacent upper and lower levels during the plain phase, arterial phase, venous phase and delay phase, respectively. The ROIs for CT value measurements were consistently sized using the copy and paste function across the different phases of images. Next, the average of three measurements was calculated. According to the difference between the venous phase and plain CT, the enhancement degree was defined as mild (CT value difference ≤ 20 HU), moderate (CT value difference between 20 HU and 40 HU), and obvious enhancement (CT value difference > 40 Hu). According to differences between the CT values of the venous and arterial phases, the enhancement pattern was defined as continuous (CT value difference ≥ 0) and attenuation (CT value difference < 0). The enhancement tumor range was calculated during the arterial phase (ER1 = arterial phase CT value-precontrast CT value), venous phase (ER2 = venous phase CT value- precontrast phase CT value), and delay phase (ER3 = delay phase CT value - precontrast scan CT value).
Image segmentation
All the CT images were exported in Joint Photographic Experts Group format. Then, two radiologists with extensive experience in abdominal imaging diagnosis (3 years and 13 years, respectively) participated in the segmentation of the entire tumor. One radiologist manually delineated the ROIs of the entire tumor layer by layer on venous phase CT images. The segmented images were subsequently confirmed by the other radiologist. Both radiologists were blinded to the pathological reports for risk stratification. Based on recommendations from previous literature[21], ImageJ (NIH, Bethesda, MD) was employed to apply an adaptive contrast filter to images. Besides, CT- segmented images were randomly selected from 20 patients, and the Dice similarity coefficient (DSC) was used to evaluate the inter-reader consistency in image segmentation. Detailed information of image preprocessing can be found in the Supplementary material 1.
The DLM construction
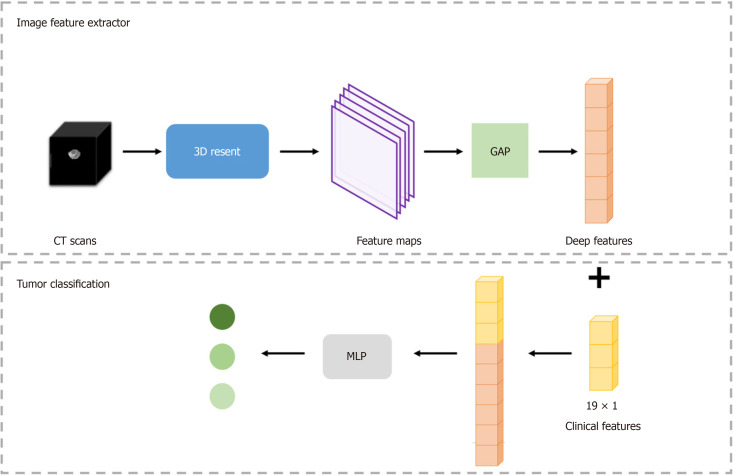
The DLM was constructed in two steps: Tumor features and tumor classification were initially extracted from CT images to generate the DLM, followed by the establishment of the combined model for tumor classification by integrating subject clinical-imaging features after statistical analysis. Figure 2 displays the detailed framework of this process. In the current study, a stratified random split was utilized at the patient level to randomly divide all patients into a training cohort and a validation cohort in a 7:3 ratio.
Figure 2.
Overall gastrointestinal stromal tumor risk stratification framework. CT: Computed tomography; MLP: Multilayer perceptron; GAP: Global average pooling.
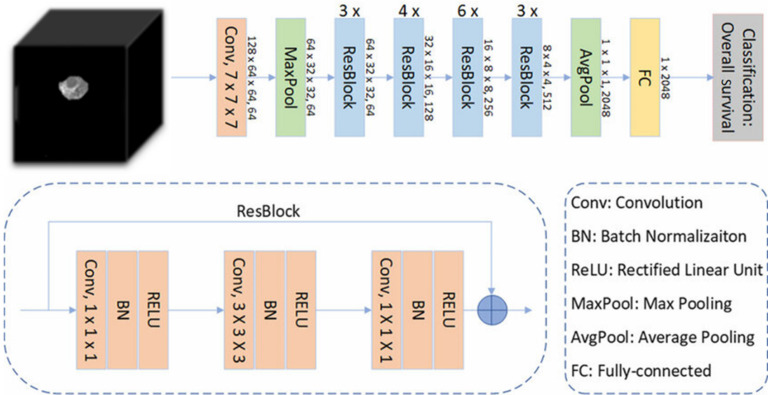
The 3D residual network (ResNet) was used to train our image dataset and establish the DLM. The 3D-ResNet is a three-dimensional convolutional neural network model based on the ResNet architecture. It is an extension of ResNet in two-dimensional image classification tasks used to process three-dimensional volume data. Besides, it accepts 3D volumetric data as input, positioning it as a powerful model for learning volume datasets. Furthermore, it can be adjusted according to the task complexity and dataset characteristics with variable depth. It can enhance the network depth by increasing the number of stacked layers of residual blocks, thereby optimizing the model's expressive ability. During the training phase, 3D-Resnet was used to extract and learn deep tumor features related to GIST risk stratification from each patient's CT images.
Multilayer perceptron (MLP) was employed for tumor risk stratification by combining imaging features and clinical data. It is a universal function approximator based on feedforward artificial neural networks that can learn and represent nonlinear relationships through multiple fully connected hidden layers and an output layer, making it suitable for various machine learning tasks. In the current model, a batch normalization (BN) layer was introduced following each linear layer in MLP to accelerate the convergence rate of the neural network, reduce the dependence of the model on the initial parameters, and improve the robustness of the model. The BN layer normalized each mini-batch data to stabilize the input of the neural network, thus improving the convergence rate and generalizability of the model. Figure 3 shows the feature extraction process. In the present study, the subject clinical-image data features of patients were concatenated with imaging features extracted by the 3D ResNet, and their feature vectors were inputted into the MLP to establish the combined DLM for tumor risk stratification.
Figure 3.
Schematic diagram of feature extraction process. BN: Batch normalization; RELU: Rectified linear unit.
Training details
In this study, the network architecture was implemented using the Pytorch framework and trained on NVIDIA GPU to accelerate training speed. Additionally, the transfer learning method was adopted, leveraging pre-training weights to improve model performance. Detailed information of model training process can be found in the Supplementary materials 2.
Statistical analysis
Statistical analysis was performed using the software SPSS version 22.0 (SPSS Inc., Chicago, IL, United States) and MedCalc version 22.002 (MedCalc Software Ltd, Ostend, Belgium). A P value of < 0.05 was considered statistically significant.
The interobserver agreement of measurements between the two radiologists was evaluated using the interclass correlation coefficient (ICC). The χ2 test, independent two-sample t-test, one-way analysis of variance and Bonferroni tests were used to evaluate the significance of correlations between various clinical- imaging features and pathological GIST risk classifications from surgical resection specimens. Ordinary logistic regression was performed to identify significant predictive factors for relapse[25,26].
To assess the performance of DLM and combined DLM, five different indicators, namely area under the receiver operating characteristic curve (AUROC), F1 score (F1), accuracy (ACC), sensitivity (SEN), and specificity (SPE), were used. AUROC was calculated along with its 95%CI.
RESULTS
Clinical-imaging characteristics
ICC analysis showed a good concordance of measurements between the two radiologists (tumor size, ICC = 0.985; CT value in the plain phase, ICC = 0.812; CT value in the arterial phase, ICC = 0.906; CT value in the venous phase, ICC = 0.921; CT value in the delay phase, ICC = 0.848). Among the analyzed CECT image features, tumor size, tumor morphology, tumor location, growth pattern, necrosis, ulceration, calcification, lymph node status, presence of enlarged feeding vessels, tumor enhancement pattern during the venous phase, and the range of tumor enhancement across the three phases were found to be significantly associated with GIST risk categories (P < 0.05). The distribution of these features in the risk categories and the results of the χ2 test are listed in Table 1. Meanwhile, ordinary logistic regression analysis identified tumor size, ulceration, and the presence of enlarged feeding vessels as statistically significant predictors (P < 0.05, Table 2).
Table 1.
Distribution of the analyzed clinical-imaging features across pathologic risk categories, mean ± SD
|
CECT features
|
|
Pathologic risk categories
|
P value
|
||
|
High (n = 213)
|
Moderated (n = 143)
|
Low (n = 195)
|
|||
| Gender | Male | 107 | 60 | 89 | 0.296 |
| Female | 106 | 83 | 106 | ||
| Location | Gastric | 139 | 136 | 171 | 0.000 |
| Non-gastric | 74 | 7 | 24 | ||
| Morphology | Regular | 54 | 94 | 170 | 0.000 |
| Irregular | 159 | 49 | 25 | ||
| Growth pattern | Endoluminal | 35 | 54 | 93 | 0.000 |
| Mixed | 51 | 22 | 28 | ||
| Exophytic | 127 | 67 | 74 | ||
| Degree of contrast enhancement in the venous phase | Mild (≤ 20 HU) | 30 | 20 | 20 | 0.424 |
| Moderate (20-40 HU) | 101 | 74 | 90 | ||
| Obvious (≥ 40 HU) | 82 | 49 | 85 | ||
| Contrast enhancement pattern during the venous phase | Continuous | 198 | 142 | 193 | 0.000 |
| Attenuation | 15 | 1 | 2 | ||
| Calcification | Present | 22 | 16 | 21 | 0.967 |
| Absent | 191 | 127 | 174 | ||
| Necrosis | Present | 155 | 65 | 33 | 0.000 |
| Absent | 58 | 78 | 162 | ||
| Ulceration | Present | 53 | 23 | 8 | 0.000 |
| Absent | 160 | 120 | 187 | ||
| Enlarged feeding vessels | Present | 183 | 60 | 14 | 0.000 |
| Absent | 30 | 83 | 181 | ||
| Lymph nodes | Present | 19 | 1 | 1 | 0.000 |
| Absent | 194 | 142 | 194 | ||
| Age | 59.44 ± 10.41 | 61.39 ± 9.83 | 60.37 ± 9.13 | 0.183 | |
| Size | 9.03 ± 4.42 | 4.90 ± 1.89 | 2.77 ± 1.20 | 0.000 | |
| Range of tumor enhancement during the arterial phase | 18.83 ± 17.81 | 14.24 ± 11.53 | 18.45 ± 17.47 | 0.004 | |
| Range of tumor enhancement during the venous phase | 41.59 ± 25.73 | 38.97 ± 18.96 | 45.22 ± 25.70 | 0.038 | |
| Range of tumor enhancement during the delay phase | 42.73 ± 18.89 | 43.72 ± 17.97 | 47.97 ± 20.27 | 0.016 | |
CECT: Contrast-enhanced computed tomography.
Table 2.
Logistic regression analysis of risk classification based on clinical-imaging feature
| P value |
95%CI
|
||
|
Lower bound
|
Upper bound
|
||
| Size | 0.000 | -0.763 | -0.473 |
| Range of tumor enhancement during the arterial phase |
0.131 |
-0.035 |
0.005 |
| Range of tumor enhancement during the venous phase |
0.220 |
-0.007 |
0.032 |
| Range of tumor enhancement during the delay phase |
0.858 |
-0.021 |
0.017 |
| Morphology | 0.602 | -0.387 | 0.608 |
| Location | 0.074 | -0.063 | 1.386 |
| Ulceration | 0.004 | -1.622 | -0.300 |
| Enlarged feeding vessels | 0.000 | -2.134 | -1.094 |
| Growth pattern | 0.224 | -0.833 | 0.328 |
| Contrast enhancement during the venous phase |
0.428 |
-2.266 |
1.384 |
| Necrosis | 0.236 | -0.195 | 0,793 |
| Lymph nodes | 0.890 | -1.934 | 1.678 |
Diagnostic performance of the DLM
All patients were randomly associated into two independent cohorts, namely a training cohort (386 patients: 176 males, mean age, 60.2 ± 9.8 years; 210 females, mean age, 59.9 ± 10.2 years) and a validation cohort (165 patients: 80 males, mean age, 59.8 ± 9.9 years; 85 females, mean age, 60.0 ± 10.1 years). There were 136 (35.2%) cases of low-risk GISTs, 101 (26.2%) cases of intermediate-risk GISTs, and 149 (38.6%) cases of high-risk GISTs in the training cohort. In contrast, the validation cohort comprised 59 (35.8%) cases of low-risk GISTs, 42 (25.5%) cases of intermediate-risk GISTs, and 64 (38.8%) cases of high-risk GISTs.
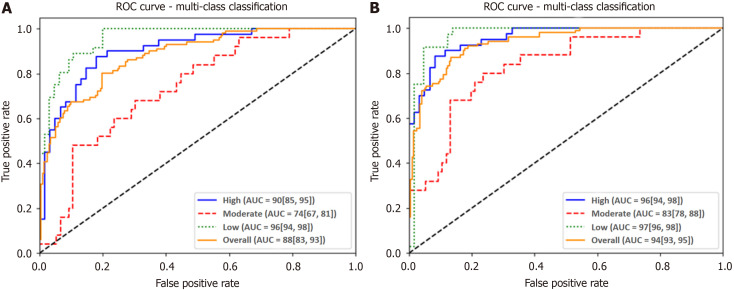
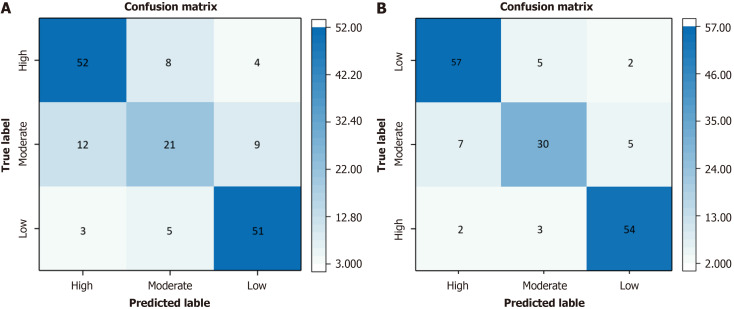
The DSC value showed a good concordance of image segmentation between the two radiologists (DSC = 99.96%). The results for the different algorithms are detailed in Table 3. The model constructed using 3D-ResNet with 34 Layers demonstrated the optimal performance, with an ACC of 75%, a SEN of 72%, a SPE of 87%, and a F1 score of 72%. The overall AUROC for DLM was 0.88 (0.83, 0.93). The ROCs are depicted in Figure 4A. In DLM, the ACC and AUROCs for each stratification were 87% (144/165) and 0.96 (0.94, 0.98) for low-risk GISTs, 79% (131/165) and 0.74 (0.67, 0.81) for intermediate-risk GISTs, and 84% (138/165) and 0.90 (0.85, 0.95) for high-risk GISTs, respectively. The results for the validation cohorts were visualized as confusion matrices to compare the GIST risk stratification predicted by DLM against the pathological risk stratification (Figure 5A).
Table 3.
Different algorithms for predicting gastrointestinal stromal tumor risk classification
|
Different method
|
Accuracy (%)
|
Sensitivity (%)
|
Specificity (%)
|
F1 score (%)
|
| 3DCNN | 60 | 51 | 68 | 52 |
| 3DResnet_50 | 66 | 58 | 71 | 59 |
| 3DResnet_18 | 71 | 66 | 76 | 67 |
| 3DResnet_34 | 75 | 72 | 87 | 72 |
| Combined model (3DResnet + MLP) |
84 | 83 | 92 | 83 |
3DCNN: Three-dimensional convolutional neural network; 3DResnet: Three-dimensional residual network; MLP: Multilayer perceptron.
Figure 4.
Receiver operating characteristic curves for the deep learning model and combined model. A: Receiver operating characteristic curve (ROC) for the deep learning model in the validation cohort; B: ROC curve for the combined model in the validation cohort. ROC: Receiver operating characteristic; AUC: Area under the curve.
Figure 5.
Confusion matrix for the deep learning model and combined model for gastrointestinal stromal tumors risk stratification. A: Confusion matrix for the deep learning model; B: Confusion matrix for the combined model.
The combined DLM achieved satisfactory performance in assessing GIST risk stratification. The overall ACC and AUROC were 84% (139/165) and 0.94 (0.93, 0.95) for the combined model. The ROCs are delineated in Figure 4B. The ACC and AUROCs for each tumor risk stratification were 92% (152/165) and 0.97 (0.96, 0.98) for low-risk GISTs, 87% (143/165) and 0.83 (0.78, 0.88) for intermediate-risk GISTs, and 90% (148/165) and 0.96 (0.94, 0.98) for high-risk GISTs, respectively. The results for the validation cohorts were visualized as confusion matrices to compare the GIST risk stratification predicted by the combined model against pathological risk stratification (Figure 5B).
The ACC, SEN, SPE, F1 score and AUROCs for each tumor risk stratification across different models are summarized in Table 4. Importantly, differences in AUROCs between DLM and the combined model were significant (P < 0.001).
Table 4.
Accuracy, sensitivity, specificity, F1 score and areas under the receiver operating characteristic curves for each tumor risk stratification, n (%)/95%CI
|
|
Accuracy (n = 165)
|
Sensitivity
|
Specificity
|
F1 score (%)
|
AUROC
|
|
| DLM | High | 138 (84); (78-90) | 81 (52/64); (76-86) | 85 (86/101); (78-91) | 79 | 0.90 (85-95) |
| Moderate | 131 (79); (71-87) | 50 (21/42); (25-74) | 89 (110/123); (82-96) | 55 | 0.74 (67-81) | |
| Low | 144 (87); (81-93) | 86 (51/59); (81-91) | 88 (93/106); (83-94) | 83 | 0.96 (94-98) | |
| Overall | 75 | 72 | 87 | 72 | 0.88 (83-93) | |
| Combined model | High | 148 (90); (86-94) | 88 (56/64); (83-93) | 91 (92/101); (83-98) | 87 | 0.96 (94-98) |
| Moderate | 143 (87); (83-92) | 69 (29/42); (66-71) | 93 (114/123); (86-98) | 72 | 0.83 (78-88) | |
| Low | 152 (92); (85-96) | 92 (54/59); (89-93) | 92 (98/106); (86-97) | 89 | 0.97 (96-98) | |
| Overall | 84 | 83 | 92 | 83 | 0.94 (93-95) | |
DLM: Deep learning model; AUROC: Areas under the receiver operating characteristic curve.
DISCUSSION
In this retrospective research, a DLM and a combined DLM were constructed. Notably, the latter (AUROC = 0.94) outperformed the former (AUROC = 0.88) in assessing GIST grading.
According to the modified NIH criteria and NCCN guidelines, the need of adjuvant treatment for GIST patients and the duration of treatment are associated with the risk stratification of GISTs[8,15,27,28]. Combining adjuvant treatment such as Imatinib before and after surgery may extend the recurrence free survival and overall survival of intermediate and high-risk GIST patients[1,5,29]. Therefore, an accurate preoperative categorization of risk classification, especially in high-risk GISTs, can provide valuable information for evaluating the necessity of surgical resection and adjuvant treatments[30-32]. In this study, two models were developed to predict preoperative GIST risk stratification: DLM and combined DLM. To the best of our knowledge, studies that combined clinical-imaging features and convolutional neural network models to establish a combined model for predicting GIST risk stratification and distinguishing between different categorizations of risk classification (high-risk, intermediate-risk and low-risk GISTs) are scarce.
Analysis of clinical-imaging features revealed that tumor size, morphology, location, growth pattern, the presence of necrosis, ulceration, calcification, lymph nodes, and enlarged feeding vessels, as well as the tumor enhancement pattern during the venous phase and the range of tumor enhancement, were significantly associated with pathologic GIST risk categories. Logistic regression analysis subsequently identified tumor size, the presence of ulcers, and enlarged feeding vessels as predictors of pathologic risk categories, consistent with the results of previous studies[25,26,33]. Zhou et al[34] reported that tumors with large sizes (> 10 cm) and enlarged feeding vessels were more likely to be a high -risk GISTs[34]. Moreover, mucosal destruction promotes the formation of ulcers due to the influence of gastric acid[35]. The NCCN guideline recommend patients with GISTs larger than 2 cm to undergo surgical resection[28], while according to Nishida’s[36] report, small GIST tumors may also be invasive and linked to a poor prognosis. Therefore, evaluating the risk stratification of GISTs exclusively based on tumor size could be insufficient. Other imaging features were also subjectively assessed and heavily relied on the experience of observers. While the degree of contrast enhancement is typically considered a characteristic of tumor biological activity, it showed no significant association with pathologic risk stratification as a predictive factor in our study, in line with the results of previous articles[26,33].
The results of our study unveiled that the DLM could accurately predict GIST risk classifications, with an AUROC of 0.88 in the validation cohort. However, the performance of the combined DLM was relatively higher (AUROC = 0.94), attributable to the combination of DLM with clinical-imaging features increasing the ability to assess the GIST risk classification. Overall, our study offers a novel method for optimizing the preoperative assessment of GIST risk stratification based on CT images, moving beyond dependence on postoperative specimens. Zhou et al[34] documented that the AUROC of the multinomial logistic regression model with subjective CT image features for GIST risk stratification was 0.806. At the same time, Wang et al[22] divided patients with GISTs into the high malignant potential group (intermediate risk and high risk) and the low malignancy potential group (very low risk and low risk) and described that the area under the curve (AUC) of the combined model (clinical features and the radiomics) was significantly higher in the validation group (0.913 vs 0.792, P = 0.019) compared to the clinical model. Importantly, Kang et al[37] concluded that the DLM (AUROCs; testing, 0.89; external validation, 0.85) outperformed the radiomics model in terms of GIST risk classification. In this study, the DLM and the combined DLM had an AUROC of 0.88 and 0.94 for distinguishing between the three types of GIST risk classifications (high-risk, intermediate-risk and low-risk) in the validation cohort. Indeed, the combined model outperformed DLM, with ACCs and AUROCs of 92% and 0.97 for low-risk GISTs, 87% and 0.83 for intermediate-risk GISTs, and 90% and 0.96 for high-risk GISTs, respectively. Notably, the AUROCs for different risk stratifications in the combined model were significantly superior to those of the DLM. These results collectively indicated that the combined DLM had superior predictive capabilities, especially for low- and high-risk GISTs.
However, the results for the intermediate-risk class were relatively unsatisfactory, with an accuracy of 87% and an AUROC of 0.83. This may be ascribed to imbalanced sample sizes. Specifically, there were only 143 cases of intermediate-risk GISTs in this study, which was lower than those of non-intermediate-risk GISTs (408 cases). This may result in relatively fewer features being extracted from intermediate-risk GISTs compared to machine learning algorithms, thereby introducing model bias. Nevertheless, compared to DLM (AUC = 0.74), the combined model (AUC = 0.83) demonstrated advantages in predicting intermediate-risk GISTs.
Of note, deep learning is a subfield of artificial intelligence that performs tasks by analyzing relationships between existing data points[38-40]. In recent years, image analysis based on deep learning algorithm has been increasingly applied to tumor diagnosis, grading, staging, prediction, and treatment evaluation. Zhu et al[41] concluded that the DLM outperformed in assessing the risk of screening-detected breast cancer. Similarly, Doppalapudi et al[42] pointed out that the accuracy of lung cancer classification predicted by DLM was 71.18%, while that of traditional machine learning models was merely 61.12%, indicating that DLM displayed superior performance for predicting lung cancer subtypes. A study investigating glioma showed that DLM achieved high performance in predicting molecular subtypes and grades, with an isocitrate dehydrogenase-AUC of 0.90, an 1p/19q co-deletion AUC of 0.85, and a grade AUC of 0.81 (grade II/III/IV)[17]. Wang et al[43] developed the convolutional neural network models with varying layers, achieving AUROCs above 0.8 for differentiating high-risk gastric GISTs from intermediate-risk and very low/low-risk gastric GISTs in the validation dataset. In the present study, the DLM based on the 3D-ResNet method increased the network depth by increasing the number of stacked layers of residual blocks, thereby improving the model's expressive ability. In addition, the clinical data of patients were concatenated with the imaging features extracted by the 3D- ResNet and then incorporated their feature vectors into the MLP for risk classification. As anticipated, the results uncovered that the DLM based on the 3D-ResNet method combined with clinical-imaging features could accurately predict GIST risk classifications.
Nevertheless, this study has several limitations that cannot be overlooked. Firstly, this was a retrospective study based on a limited sample size, resulting in an imbalance in the data for risk stratification. Therefore, an ideal DLM should be constructed with a larger training set containing datasets from multiple-centers to balance the data for different risk stratifications. Further prospective studies with external validation cohorts are warranted to validate our results. Secondly, the DLM developed in our study required manual segmentation of tumors on CT images remains a semi-automatic model. Thirdly, radiomics features were used for the risk stratification of GISTs in previous studies[22,32,39,44,45]. Therefore, future studies can compare the performance of radiomics models with DLM.
CONCLUSION
In summary, a high-performance combined DLM for preoperative prediction of the GIST risk stratification was developed and validated in this study. Noteworthily, this model has the potential to guide and facilitate clinical decision-making for GIST patients.
ACKNOWLEDGEMENTS
We are grateful to Yan-Bei Liu and Cong-Cong Yuan for their supports. We would also like to thank Yan-Bei Liu for his scientific advice and intellectual discussions.
Footnotes
Institutional review board statement: The study was reviewed and approved by Tianjin Medical University Cancer Institute and Hospital (EK20240015).
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade A
Scientific Significance: Grade B
P-Reviewer: Zheng S S-Editor: Liu H L-Editor: A P-Editor: Xu ZH
Contributor Information
Yi Li, Department of Radiology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin’s Clinical Research Center for Cancer, State Key Laboratory of Druggability Evaluation and Systematic Translational Medicine, Tianjin Key Laboratory of Digestive Cancer, Tianjin 300060, China.
Yan-Bei Liu, School of Life Sciences, Tiangong University, Tianjin 300387, China.
Xu-Bin Li, Department of Radiology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin’s Clinical Research Center for Cancer, State Key Laboratory of Druggability Evaluation and Systematic Translational Medicine, Tianjin Key Laboratory of Digestive Cancer, Tianjin 300060, China.
Xiao-Nan Cui, Department of Radiology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin’s Clinical Research Center for Cancer, State Key Laboratory of Druggability Evaluation and Systematic Translational Medicine, Tianjin Key Laboratory of Digestive Cancer, Tianjin 300060, China.
Dong-Hua Meng, Department of Radiology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin’s Clinical Research Center for Cancer, State Key Laboratory of Druggability Evaluation and Systematic Translational Medicine, Tianjin Key Laboratory of Digestive Cancer, Tianjin 300060, China.
Cong-Cong Yuan, Department of Radiology, Tianjin First Central Hospital, Tianjin 300190, China.
Zhao-Xiang Ye, Department of Radiology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin’s Clinical Research Center for Cancer, State Key Laboratory of Druggability Evaluation and Systematic Translational Medicine, Tianjin Key Laboratory of Digestive Cancer, Tianjin 300060, China. yezhaoxiang@163.com.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author at yezhaoxiang@163.com.
References
- 1.Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S, McCarter MD, Polikoff JA, Tan BR, Owzar K American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Chen Z, Liu W, Wang X, Ma S, Jin F, Wang X. Development of a Malignancy Potential Binary Prediction Model Based on Deep Learning for the Mitotic Count of Local Primary Gastrointestinal Stromal Tumors. Korean J Radiol. 2021;22:344–353. doi: 10.3348/kjr.2019.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Call J. Mutational Testing in Gastrointestinal Stromal Tumor. Curr Cancer Drug Targets. 2019;19:688–697. doi: 10.2174/1568009619666190326123945. [DOI] [PubMed] [Google Scholar]
- 5.Wozniak A, Gebreyohannes YK, Debiec-Rychter M, Schöffski P. New targets and therapies for gastrointestinal stromal tumors. Expert Rev Anticancer Ther. 2017;17:1117–1129. doi: 10.1080/14737140.2017.1400386. [DOI] [PubMed] [Google Scholar]
- 6.Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST) Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247–1253. doi: 10.1200/JCO.2009.24.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patrikidou A, Domont J, Chabaud S, Ray-Coquard I, Coindre JM, Bui-Nguyen B, Adenis A, Rios M, Bertucci F, Duffaud F, Chevreau C, Cupissol D, Pérol D, Emile JF, Blay JY, Le Cesne A French Sarcoma Group. Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French Sarcoma Group. Eur J Cancer. 2016;52:173–180. doi: 10.1016/j.ejca.2015.10.069. [DOI] [PubMed] [Google Scholar]
- 8.Tirumani SH, Baheti AD, Tirumani H, O'Neill A, Jagannathan JP. Update on Gastrointestinal Stromal Tumors for Radiologists. Korean J Radiol. 2017;18:84–93. doi: 10.3348/kjr.2017.18.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 10.Li GZ, Raut CP. Targeted therapy and personalized medicine in gastrointestinal stromal tumors: drug resistance, mechanisms, and treatment strategies. Onco Targets Ther. 2019;12:5123–5133. doi: 10.2147/OTT.S180763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SH, Lee HJ, Kim MC, Yook JH, Sohn TS, Hyung WJ, Ryu SW, Kurokawa Y, Kim YW, Han SU, Kim HH, Park DJ, Kim W, Lee SI, Cho H, Cho GS, Kim JJ, Kim KH, Yoo MW, Yang HK. Early experience of laparoscopic resection and comparison with open surgery for gastric gastrointestinal stromal tumor: a multicenter retrospective study. Sci Rep. 2022;12:2290. doi: 10.1038/s41598-022-05044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi R, Toyokawa T, Yoshii M, Tamura T, Tanaka H, Lee S, Muguruma K, Yashiro M, Ohira M. A Giant Gastric Gastrointestinal Stromal Tumor Successfully Resected Following Neoadjuvant Treatment With Imatinib Mesylate. Anticancer Res. 2020;40:1147–1152. doi: 10.21873/anticanres.14056. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81–89. doi: 10.1177/106689690201000201. [DOI] [PubMed] [Google Scholar]
- 14.Eckardt AJ, Adler A, Gomes EM, Jenssen C, Siebert C, Gottschalk U, Koch M, Röcken C, Rösch T. Endosonographic large-bore biopsy of gastric subepithelial tumors: a prospective multicenter study. Eur J Gastroenterol Hepatol. 2012;24:1135–1144. doi: 10.1097/MEG.0b013e328356eae2. [DOI] [PubMed] [Google Scholar]
- 15.Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1–41; quiz S42. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park CH, Kim GH, Lee BE, Song GA, Park DY, Choi KU, Kim DH, Jeon TY. Two staging systems for gastrointestinal stromal tumors in the stomach: which is better? BMC Gastroenterol. 2017;17:141. doi: 10.1186/s12876-017-0705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Voort SR, Incekara F, Wijnenga MMJ, Kapsas G, Gahrmann R, Schouten JW, Nandoe Tewarie R, Lycklama GJ, De Witt Hamer PC, Eijgelaar RS, French PJ, Dubbink HJ, Vincent AJPE, Niessen WJ, van den Bent MJ, Smits M, Klein S. Combined molecular subtyping, grading, and segmentation of glioma using multi-task deep learning. Neuro Oncol. 2023;25:279–289. doi: 10.1093/neuonc/noac166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Chang M, Wang R, Xi IL, Chang K, Huang RY, Vallières M, Habibollahi P, Dagli MS, Palmer M, Zhang PJ, Silva AC, Yang L, Soulen MC, Zhang Z, Bai HX, Stavropoulos SW. Deep Learning Based on MRI for Differentiation of Low- and High-Grade in Low-Stage Renal Cell Carcinoma. J Magn Reson Imaging. 2020;52:1542–1549. doi: 10.1002/jmri.27153. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Man C, Gong L, Dong D, Yu X, Wang S, Fang M, Wang S, Fang X, Chen X, Tian J. A deep learning radiomics model for preoperative grading in meningioma. Eur J Radiol. 2019;116:128–134. doi: 10.1016/j.ejrad.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Yang M, Wang S, Li X, Sun Y. Emerging role of deep learning-based artificial intelligence in tumor pathology. Cancer Commun (Lond) 2020;40:154–166. doi: 10.1002/cac2.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banzato T, Causin F, Della Puppa A, Cester G, Mazzai L, Zotti A. Accuracy of deep learning to differentiate the histopathological grading of meningiomas on MR images: A preliminary study. J Magn Reson Imaging. 2019;50:1152–1159. doi: 10.1002/jmri.26723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang P, Yan J, Qiu H, Huang J, Yang Z, Shi Q, Yan C. A radiomics-clinical combined nomogram-based on non-enhanced CT for discriminating the risk stratification in GISTs. J Cancer Res Clin Oncol. 2023;149:12993–13003. doi: 10.1007/s00432-023-05170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vernuccio F, Cannella R, Comelli A, Salvaggio G, Lagalla R, Midiri M. [Radiomics and artificial intelligence: new frontiers in medicine.] Recenti Prog Med. 2020;111:130–135. doi: 10.1701/3315.32853. [DOI] [PubMed] [Google Scholar]
- 24.Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, Thrun S. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115–118. doi: 10.1038/nature21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang TH, Hwang JI, Yang MS, Hung SW, Chan SW, Wang J, Tyan YS. Gastrointestinal stromal tumors: computed tomographic features and prediction of malignant risk from computed tomographic imaging. J Chin Med Assoc. 2007;70:367–373. doi: 10.1016/S1726-4901(08)70022-4. [DOI] [PubMed] [Google Scholar]
- 26.Grazzini G, Guerri S, Cozzi D, Danti G, Gasperoni S, Pradella S, Miele V. Gastrointestinal stromal tumors: relationship between preoperative CT features and pathologic risk stratification. Tumori. 2021;107:556–563. doi: 10.1177/0300891621996447. [DOI] [PubMed] [Google Scholar]
- 27.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973–983. doi: 10.1016/S0140-6736(13)60106-3. [DOI] [PubMed] [Google Scholar]
- 28.von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Conrad EU 3rd, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JM 3rd, Koon H, Mayerson J, McCarter M, McGarry SV, Meyer C, O'Donnell RJ, Pappo AS, Paz IB, Petersen IA, Pfeifer JD, Riedel RF, Schuetze S, Schupak KD, Schwartz HS, Tap WD, Wayne JD, Bergman MA, Scavone J. Soft Tissue Sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:758–786. doi: 10.6004/jnccn.2016.0078. [DOI] [PubMed] [Google Scholar]
- 29.Wang FH, Zheng HL, Li JT, Li P, Zheng CH, Chen QY, Huang CM, Xie JW. Prediction of recurrence-free survival and adjuvant therapy benefit in patients with gastrointestinal stromal tumors based on radiomics features. Radiol Med. 2022;127:1085–1097. doi: 10.1007/s11547-022-01549-7. [DOI] [PubMed] [Google Scholar]
- 30.Lin JX, Chen QF, Zheng CH, Li P, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Huang CM. Is 3-years duration of adjuvant imatinib mesylate treatment sufficient for patients with high-risk gastrointestinal stromal tumor? A study based on long-term follow-up. J Cancer Res Clin Oncol. 2017;143:727–734. doi: 10.1007/s00432-016-2334-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Pan X, Liu R, Zheng H, Chen L, Guan W, Wang H, Sun Y, Tang L, Guan Y, Ge Y, He J, Zhou Z. Texture analysis of CT images in predicting malignancy risk of gastrointestinal stromal tumours. Clin Radiol. 2018;73:266–274. doi: 10.1016/j.crad.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Choi IY, Yeom SK, Cha J, Cha SH, Lee SH, Chung HH, Lee CM, Choi J. Feasibility of using computed tomography texture analysis parameters as imaging biomarkers for predicting risk grade of gastrointestinal stromal tumors: comparison with visual inspection. Abdom Radiol (NY) 2019;44:2346–2356. doi: 10.1007/s00261-019-01995-4. [DOI] [PubMed] [Google Scholar]
- 33.Iannicelli E, Carbonetti F, Federici GF, Martini I, Caterino S, Pilozzi E, Panzuto F, Briani C, David V. Evaluation of the Relationships Between Computed Tomography Features, Pathological Findings, and Prognostic Risk Assessment in Gastrointestinal Stromal Tumors. J Comput Assist Tomogr. 2017;41:271–278. doi: 10.1097/RCT.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 34.Zhou C, Duan X, Zhang X, Hu H, Wang D, Shen J. Predictive features of CT for risk stratifications in patients with primary gastrointestinal stromal tumour. Eur Radiol. 2016;26:3086–3093. doi: 10.1007/s00330-015-4172-7. [DOI] [PubMed] [Google Scholar]
- 35.Meng X, Liu J, Kang J, Wang M, Guan Z, Tian D, Chen X. Lamivudine protects mice from gastric ulcer by activating PGK1 to suppress ferroptosis. Biochem Pharmacol. 2024;227:116440. doi: 10.1016/j.bcp.2024.116440. [DOI] [PubMed] [Google Scholar]
- 36.Nishida T, Goto O, Raut CP, Yahagi N. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer. 2016;122:3110–3118. doi: 10.1002/cncr.30239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang B, Yuan X, Wang H, Qin S, Song X, Yu X, Zhang S, Sun C, Zhou Q, Wei Y, Shi F, Yang S, Wang X. Preoperative CT-Based Deep Learning Model for Predicting Risk Stratification in Patients With Gastrointestinal Stromal Tumors. Front Oncol. 2021;11:750875. doi: 10.3389/fonc.2021.750875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 39.Ning Z, Luo J, Li Y, Han S, Feng Q, Xu Y, Chen W, Chen T, Zhang Y. Pattern Classification for Gastrointestinal Stromal Tumors by Integration of Radiomics and Deep Convolutional Features. IEEE J Biomed Health Inform. 2019;23:1181–1191. doi: 10.1109/JBHI.2018.2841992. [DOI] [PubMed] [Google Scholar]
- 40.Tran KA, Kondrashova O, Bradley A, Williams ED, Pearson JV, Waddell N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021;13:152. doi: 10.1186/s13073-021-00968-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu X, Wolfgruber TK, Leong L, Jensen M, Scott C, Winham S, Sadowski P, Vachon C, Kerlikowske K, Shepherd JA. Deep Learning Predicts Interval and Screening-detected Cancer from Screening Mammograms: A Case-Case-Control Study in 6369 Women. Radiology. 2021;301:550–558. doi: 10.1148/radiol.2021203758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doppalapudi S, Qiu RG, Badr Y. Lung cancer survival period prediction and understanding: Deep learning approaches. Int J Med Inform. 2021;148:104371. doi: 10.1016/j.ijmedinf.2020.104371. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Shao M, Hu H, Xiao W, Cheng G, Yang G, Ji H, Yu S, Wan J, Xie Z, Xu M. Convolutional neural network applied to preoperative venous-phase CT images predicts risk category in patients with gastric gastrointestinal stromal tumors. BMC Cancer. 2024;24:280. doi: 10.1186/s12885-024-11962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen T, Ning Z, Xu L, Feng X, Han S, Roth HR, Xiong W, Zhao X, Hu Y, Liu H, Yu J, Zhang Y, Li Y, Xu Y, Mori K, Li G. Radiomics nomogram for predicting the malignant potential of gastrointestinal stromal tumours preoperatively. Eur Radiol. 2019;29:1074–1082. doi: 10.1007/s00330-018-5629-2. [DOI] [PubMed] [Google Scholar]
- 45.Ren C, Wang S, Zhang S. Development and validation of a nomogram based on CT images and 3D texture analysis for preoperative prediction of the malignant potential in gastrointestinal stromal tumors. Cancer Imaging. 2020;20:5. doi: 10.1186/s40644-019-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author at yezhaoxiang@163.com.