Abstract
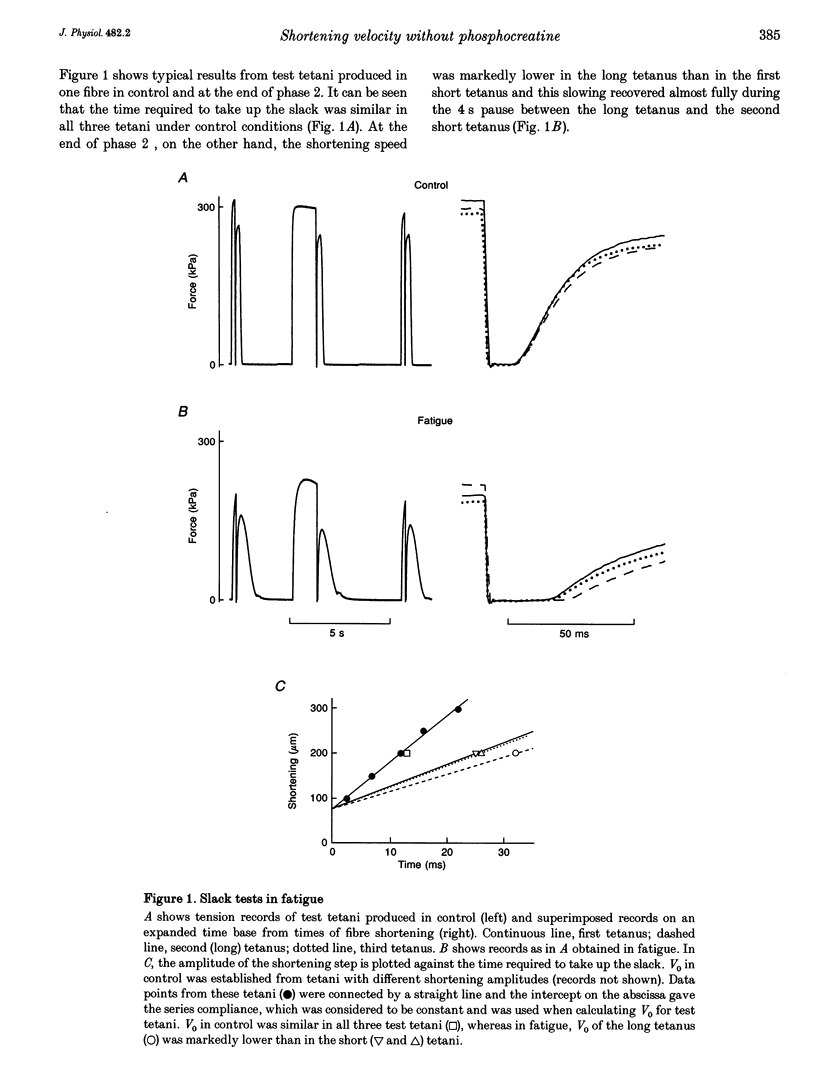
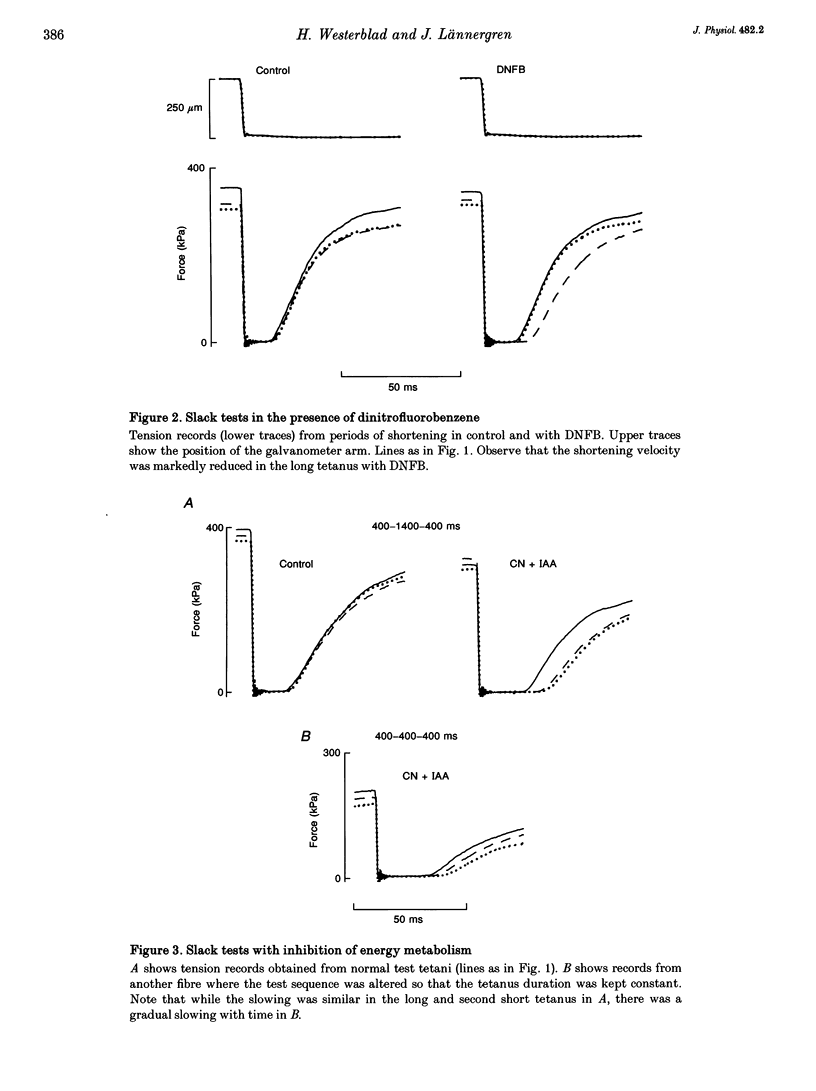
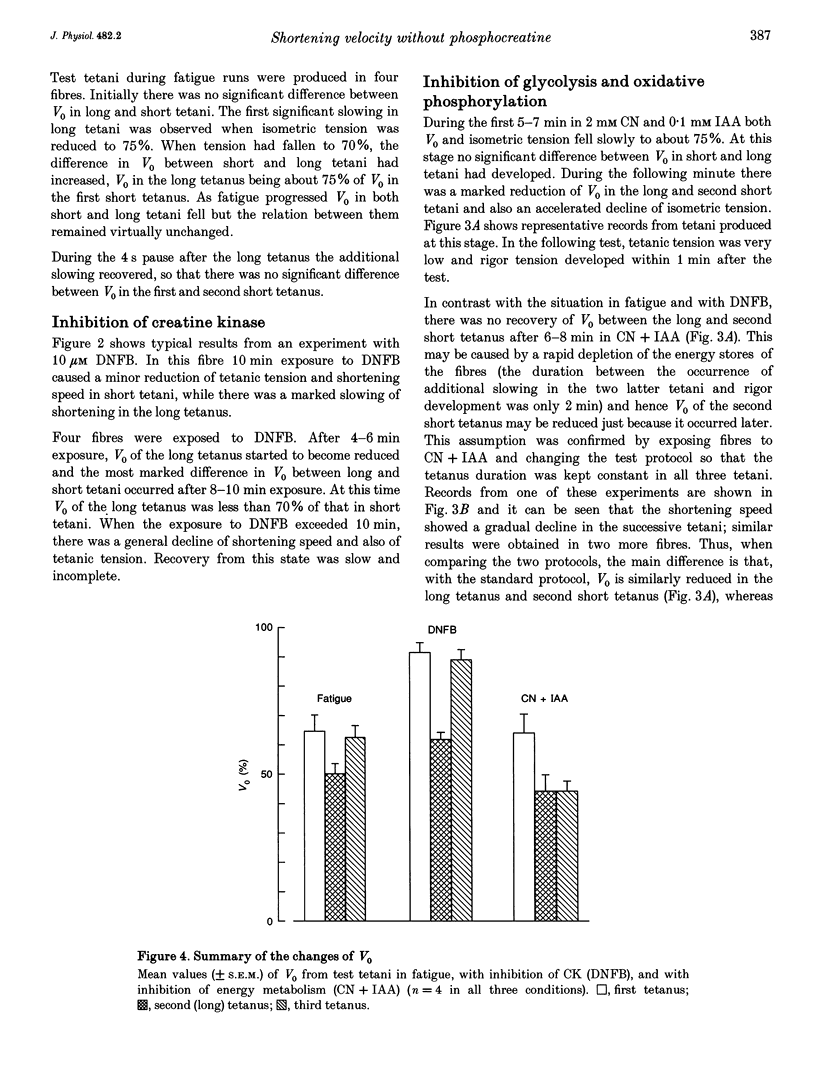
1. ADP inhibits the maximum shortening velocity (V0) in skeletal muscle. [ADP] may increase considerably during contractions and reduce V0 in the absence of energy buffering by phosphocreatine (PCr). We have tested this hypothesis by comparing V0 in long and short tetani produced in situations where PCr buffering is absent. 2. Single, intact muscle fibres were dissected from toe muscles of Xenopus and stimulated by current pulses at 20 degrees C. The test sequence consisted of a 400 ms tetanus, followed after 3 s by a 1400 ms tetanus and after an additional 4 s by a 400 ms tetanus. V0 was measured with slack tests at 200 and 1200 ms, respectively. 3. The PCr system was inactivated in three ways: (i) fatiguing fibres with repeated short tetani; (ii) inhibition of the creatine kinase (CK) reaction with dinitrofluorobenzene; and (iii) inhibition of energy metabolism with iodoacetic acid and cyanide. 4. Under control conditions V0 was similar in all three test tetani. With inactive PCr buffering V0 was about 30% lower in the long tetanus. This slowing recovered fully in the second short tetanus in fatigue and with CK inhibition. 5. Calculations suggest that [ADP] can reach very high levels (about 3 mM) during prolonged contractions in the absence of PCr buffering.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARLSON F. D., SIGER A. The creatine phosphoryltransfer reaction in iodoacetate-poisoned muscle. J Gen Physiol. 1959 Nov;43:301–313. doi: 10.1085/jgp.43.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K., Luciani G. B., Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988 Jan;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A. Intracellular pH and buffer power of type 1 and 2 fibres from skeletal muscle of Xenopus laevis. Pflugers Arch. 1987 Apr;408(4):386–389. doi: 10.1007/BF00581133. [DOI] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978 Aug 31;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga G., Lännergren J., Stienen G. J. Stable maintenance heat rate and contractile properties of different single muscle fibres from Xenopus laevis at 20 degrees C. J Physiol. 1987 Dec;393:399–412. doi: 10.1113/jphysiol.1987.sp016829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts R. H. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994 Jan;74(1):49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Nosek T. M. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989 May;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Sahlin K. Role of oxygen in regulation of glycolysis and lactate production in human skeletal muscle. Exerc Sport Sci Rev. 1990;18:1–28. [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. Force and membrane potential during and after fatiguing, continuous high-frequency stimulation of single Xenopus muscle fibres. Acta Physiol Scand. 1986 Nov;128(3):359–368. doi: 10.1111/j.1748-1716.1986.tb07989.x. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Sweeney H. L., Kushmerick M. J. A simple analysis of the "phosphocreatine shuttle". Am J Physiol. 1984 May;246(5 Pt 1):C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Moss R. L. Effects on shortening velocity of rabbit skeletal muscle due to variations in the level of thin-filament activation. J Physiol. 1986 Aug;377:487–505. doi: 10.1113/jphysiol.1986.sp016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesser A. S., Van der Laarse W. J., Elzinga G. ATP formation and ATP hydrolysis during fatiguing, intermittent stimulation of different types of single muscle fibres from Xenopus laevis. J Muscle Res Cell Motil. 1993 Dec;14(6):608–618. doi: 10.1007/BF00141558. [DOI] [PubMed] [Google Scholar]
- Nassar-Gentina V., Passonneau J. V., Rapoport S. I. Fatigue and metabolism of frog muscle fibers during stimulation and in response to caffeine. Am J Physiol. 1981 Sep;241(3):C160–C166. doi: 10.1152/ajpcell.1981.241.3.C160. [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R. Addition of phosphate to active muscle fibers probes actomyosin states within the powerstroke. Pflugers Arch. 1989 May;414(1):73–81. doi: 10.1007/BF00585629. [DOI] [PubMed] [Google Scholar]
- Tombes R. M., Brokaw C. J., Shapiro B. M. Creatine kinase-dependent energy transport in sea urchin spermatozoa. Flagellar wave attenuation and theoretical analysis of high energy phosphate diffusion. Biophys J. 1987 Jul;52(1):75–86. doi: 10.1016/S0006-3495(87)83190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Lee J. A., Lamb A. G., Bolsover S. R., Allen D. G. Spatial gradients of intracellular calcium in skeletal muscle during fatigue. Pflugers Arch. 1990 Mar;415(6):734–740. doi: 10.1007/BF02584013. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. Changes of the force-velocity relation, isometric tension and relaxation rate during fatigue in intact, single fibres of Xenopus skeletal muscle. J Muscle Res Cell Motil. 1994 Jun;15(3):287–298. doi: 10.1007/BF00123481. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. Force and membrane potential during and after fatiguing, intermittent tetanic stimulation of single Xenopus muscle fibres. Acta Physiol Scand. 1986 Nov;128(3):369–378. doi: 10.1111/j.1748-1716.1986.tb07990.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. The relation between force and intracellular pH in fatigued, single Xenopus muscle fibres. Acta Physiol Scand. 1988 May;133(1):83–89. doi: 10.1111/j.1748-1716.1988.tb08383.x. [DOI] [PubMed] [Google Scholar]


