Abstract
BACKGROUND
Esophageal cancer (EC) often occurs in the elderly, with approximately 33% of patients aged ≥ 75 years at the time of diagnosis.
AIM
To evaluate the prognostic factors for radiotherapy (RT) in elderly patients with unresectable EC.
METHODS
We retrospectively analyzed the clinical characteristics, toxic reactions, and survival information of EC patients aged ≥ 75 years who underwent intensity-modulated RT at Lu’an Hospital of Anhui Medical University between January 2016 and September 2023. Kaplan-Meier analysis was used to draw the overall survival (OS) curves, and Cox regression analysis was employed to evaluate the influence of various clinical factors on the prognosis.
RESULTS
A total of 139 patients were enrolled. The median follow-up time was 52.0 months. The median OS was 20.0 months. The 1-year, 2-year, 3-year, and 5-year OS rates were 69.8%, 38.7%, 28.2%, and 17.5%, respectively. Univariate analysis showed that age, radiation dose, and chemotherapy had no significant impact on prognosis. Multivariate analysis indicated that clinical stage [III-IVa vs I-II, hazard ratio (HR) = 2.421, 95% confidence interval (CI): 1.242-4.718, P = 0.009; IVb vs I-II, HR = 4.222, 95%CI: 1.888-9.438, P < 0.001), Charlson comorbidity index (CCI) (0 vs ≥ 1, HR = 1.539, 95%CI: 1.015-2.332, P = 0.042), and nutritional risk screening 2002 (NRS2002) (< 3 vs ≥ 3, HR = 2.491, 95%CI: 1.601-3.875, P < 0.001) were independent prognostic factors for OS.
CONCLUSION
Our results suggest that CCI and NRS2002 were independent prognostic factors of OS for unresectable elderly EC patients undergoing RT. For elderly patients with EC, full attention should be given to biological age-related indicators, such as comorbidities and nutrition, when formulating treatment protocols. These factors should be considered in future clinical practice.
Keywords: Elderly patient, Esophageal cancer, Radiotherapy, Prognosis, Comorbidity, Nutritional risk
Core Tip: Esophageal cancer (EC) often occurs in the elderly, with approximately 33% of patients aged ≥ 75 years at the time of diagnosis. Since patients aged 75 years and above are often excluded from many clinical trials of EC, there is a lack of agreement regarding the prognosis and treatment of this population. We retrospectively analyzed the clinical characteristics, toxic reactions, and survival information of elderly patients with EC aged ≥ 75 years who received intensity-modulated radiotherapy. Our analysis aimed to evaluate the prognostic factors affecting overall survival. We found that the Charlson comorbidity index and nutritional risk screening 2002 were independent prognostic factors for overall survival. Our results suggest that when formulating treatment plans for elderly patients with EC, full attention should be given to age-related biological indicators such as comorbidities and nutrition.
INTRODUCTION
Esophageal cancer (EC), a common malignant tumor of the digestive tract, poses a significant threat to human health. The Global Cancer Observatory’s 2022 online platform, Cancer Today, 2022 (GLOBOCAN 2022), reported that the global incidence and mortality rates of EC are approximately 2.6% and 4.6%, respectively, ranking 11th and 7th, respectively among all malignant tumors[1]. With the extension of life expectancy, the aging of the population, and the improvement of diagnosis methods, the proportion of elderly EC patients has gradually increased, with 33% of patients being over 75 years old at the time of diagnosis[2,3]. Elderly patients often have degraded physiological functions, multiple comorbidities, and poor treatment tolerance, necessitating particularly cautious treatment approaches for this group.
Endoscopic resection and surgery (preferred for early-stage patients) and neoadjuvant chemoradiotherapy followed by surgery (trimodality therapy) are common treatment modalities for EC[4-6]. While advancements in survival have been noted, age significantly impacts the treatment of EC. Lester et al[7] indicated that older patients with trimodality therapy encountered increased postoperative cardiopulmonary toxicity and mortality, with cardiotoxicity exhibiting a linear correlation with age and a 61% rise in relative risk for every 10-year increase in age. Given the elevated postoperative complications, heightened in-hospital mortality, and reduced overall survival (OS) among elderly patients, non-surgical treatment is typically advised[8].
For patients with locally advanced EC who refuse surgery and are medically considered inoperable or have unresectable tumors, the efficacy of radical chemoradiotherapy (CRT) has been confirmed in numerous randomized clinical trials, whether using conventional two-dimensional radiotherapy (RT) or three-dimensional intensity-modulated RT (IMRT) technology. The earliest data were obtained from the Radiation Therapy Oncology Group (RTOG) 8501 and RTOG9405 studies[9-12]. For patients with metastatic EC, systemic therapy is effective, and RT to the primary tumor can relieve patients’ dysphagia and pain and improve patients' survival[13,14]. However, the clinical trials that back these treatments excluded individuals aged over 75, with older patients showing lower tolerance to CRT and experiencing a higher incidence of side effects compared to their younger counterparts[15]. Therefore, these clinical data are underrepresented in elderly patients. There are limited data on the efficacy and safety of RT in EC patients aged 75 years and older, and there is no consensus on prognosis and treatment in this population.
Studies have revealed that elderly patients with EC often receive inadequate treatment due to concerns about side effects, comorbidities, and poor outcome, even when the disease is at a curable stage. Notably, the decision to forgo treatment was attributed to physicians in 46% of cases and to patients in 46% of cases[16]. However, the actual age of the patient does not truly reflect their biological aging level. Studies have shown that carefully selected elderly patients can tolerate the treatment and have a survival benefit[17]. More attention should be given to physiological age in medicine, which includes factors such as comorbidities, nutrition, and physical condition.
Based on the clinical information of elderly patients with unresectable EC who received IMRT at Lu’an Hospital of Anhui Medical University, this study explored prognostic factors associated with aging and provided a reference for individualized diagnosis and treatment of elderly EC patients.
MATERIALS AND METHODS
Patients
Retrospective analysis was conducted on elderly (≥ 75 years) EC patients who received IMRT with or without chemotherapy at Lu’an Hospital of Anhui Medical University from January 2016 to September 2023. All patients were pathologically diagnosed with esophageal squamous cell carcinoma (ESCC) or adenocarcinoma, had no prior history of thoracic RT, and had not undergone previous EC surgery. They were receiving RT for the first time and had complete follow-up data. Exclusion criteria included patients with severe cardiopulmonary dysfunction or other critical diseases affecting important systems. Clinical stages were classified according to the eighth edition of the American Joint Committee on Cancer (AJCC) staging system. This study was conducted in accordance with the ethical guidelines set forth in the World Medical Association Declaration of Helsinki and received approval from the Ethics Committee of Lu’an Hospital, Anhui Medical University.
Treatment
All patients received IMRT. Each patient was immobilized in the supine position, and a spiral computed tomography (CT) scan was performed with a slice thickness of 0.5 cm, covering the range from the lower edge of the mandible to the lower edge of the liver. The gross tumor volume (GTV) was defined as any visible primary tumor and included metastatic lymph nodes, determined by comprehensive esophagogram, esophagoscopy, contrast-enhanced thoracic CT or positron emission tomography. The clinical target volume (CTV) was defined as the GTV plus 3 cm cranial-caudal and 0.5 cm radial margin. For lymph nodes, the CTV was generated by extending the nodal GTV by 0.5 cm. The planning target volume (PTV) was generated by additional 0.5 cm radial margin for CTV. The prescription dose to 95% of PTV ranged from 44 Gray (Gy) to 66 Gy in 22-33 fractions. The use of chemotherapy and the choice of regimen were determined by the clinician based on the patient’s specific circumstances.
Follow-up and treatment evaluation
All patients were followed up every 3 months during the first 2 years and every 6 months thereafter. Follow-up assessments included blood tests, esophagogram, esophagoscopy, and CT scans of the neck, chest, and abdomen. Follow-ups were conducted via telephone and outpatient visits, with a final deadline of January 23, 2024. Treatment-related toxicities were evaluated according to the RTOG radiation injury grading criteria.
Statistical analysis
Continuous variables were expressed as means ± SD or medians depending on whether they were normally distributed. The median follow-up time was estimated using the reverse Kaplan-Meier method. The Kaplan-Meier function was utilized to draw the OS curves, and the log-rank test was employed to compare OS among different groups. Univariate and multivariate Cox proportional hazards regression models were used to evaluate the relationship between various clinical variables and prognosis. Potential prognostic factors with P < 0.05 in univariate analysis were included in the multivariate analysis. The hazard ratio (HR) and the corresponding 95% confidence interval (CI) were used to predict the effect of each variable on OS. The OS time was defined as the period from the date of diagnosis until death from any cause. Receiver operating characteristic (ROC) curves were drawn to verify the accuracy of the Charlson comorbidity index (CCI), nutritional risk screening 2002 (NRS2002) and clinical stage for survival prediction. The OS and ROC curves were calculated using statistical product and service solutions 24.0, while the remaining statistical analyses were performed using R software (version 4.3.0, http://www.r-project.org/).
RESULTS
Patients’ characteristics
During the study period, a total of 158 elderly (≥ 75 years) EC patients were treated with IMRT. After exclusions, 139 cases remained for analysis. The exclusions were due to the following reasons: 4 cases had concurrent malignant tumors, 1 case had small cell pathology, 8 cases had incomplete clinical data, and 6 cases were lost to follow-up. The median age was 79 years (range from 75 years to 92 years). According to the eighth edition of the AJCC staging system, 93 patients (66.9%) were distributed in stages III to IVa. Chemotherapy was administered to 103 patients (74.1%) at different phases of treatment, including 84 patients (60.4%) who received concurrent chemotherapy. 129 patients (92%) completed the planned RT, with a median radiation dose of 60 Gy (range from 20 to 66 Gy). The clinical characteristics are summarized in Table 1.
Table 1.
Demographics and clinical characteristics of patients, n (%)
|
Variables
|
Number of patients
|
| Age | |
| ≥ 75 and < 80 | 70 (50.4) |
| ≥ 80 | 69 (49.6) |
| Sex | |
| Female | 39 (28.1) |
| Male | 100 (72.9) |
| Marital status | |
| Married | 133 (95.7) |
| Unmarried | 6 (4.3) |
| Smoke | |
| No | 84 (60.4) |
| Yes | 55 (39.6) |
| Drink | |
| No | 102 (73.4) |
| Yes | 37 (26.6) |
| C stage | |
| I-II | 24 (17.3) |
| III-IVa | 93 (66.9) |
| IVb | 22 (15.8) |
| T stage | |
| T1-T2 | 22 (15.8) |
| T3-T4 | 117 (84.2) |
| N stage | |
| Negative | 22 (15.8) |
| Positive | 117 (84.2) |
| M stage | |
| M0 | 117 (84.2) |
| M1 | 22 (15.8) |
| Tumor length | |
| < 6 | 90 (64.7) |
| ≥ 6 | 49 (35.3) |
| Tumor location | |
| Upper thoracic | 34 (24.5) |
| Middle thoracic | 62 (44.6) |
| Lower thoracic | 43 (30.9) |
| ECOG | |
| 0-1 | 133 (95.7) |
| 2 | 6 (4.3) |
| CCI | |
| 0 | 62 (44.6) |
| ≥ 1 | 77 (55.4) |
| NRS2002 | |
| < 3 | 66 (47.5) |
| ≥ 3 | 73 (52.5) |
| BMI | |
| < 18.5 | 32 (23.0) |
| ≥ 18.5 | 107 (77.0) |
| Weight loss | |
| < 10% | 128 (92.1) |
| ≥ 10% | 11 (7.9) |
| Radiation dose | |
| < 60 | 57 (41.0) |
| ≥ 60 | 82 (59.0) |
| Chemotherapy | |
| No | 36 (25.9) |
| Yes | 103 (74.1) |
BMI: Body mass index; ECOG: Eastern Cooperative Oncology Group; CCI: Charlson comorbidity index; NRS2002: Nutritional risk screening 2002.
Survival and prognostic analysis
The median follow-up time was 52.0 months. The median OS was 20.0 months. The 1-year, 2-year, 3-year, and 5-year OS rates were 69.8%, 38.7%, 28.2%, and 17.5%, respectively.
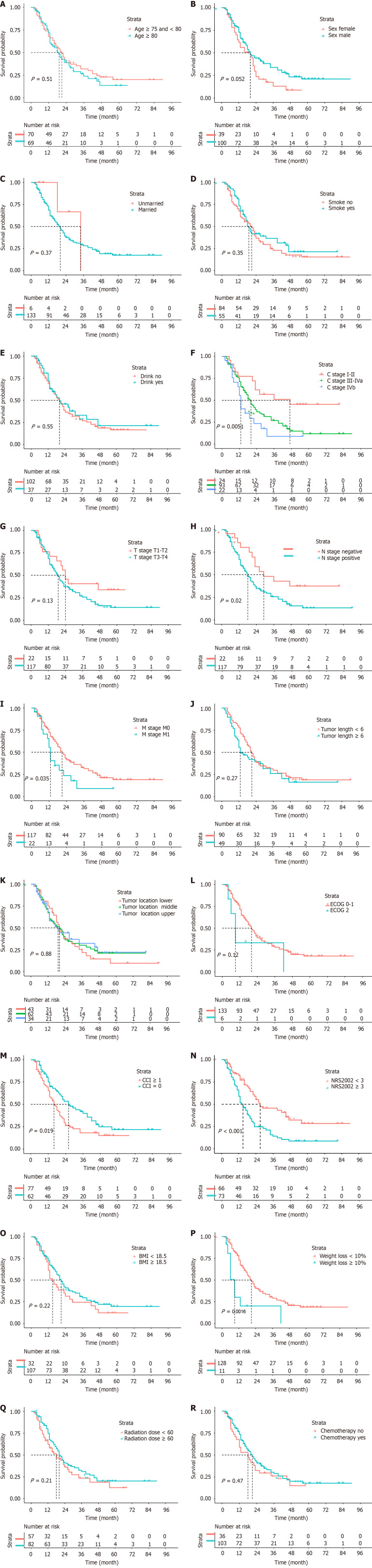
Univariate analysis showed that patients with a CCI score of ≥ 1 had worse OS compared to those with a score of 0 (HR = 1.620; 95%CI: 1.080-2.429; P = 0.019). Patients with an NRS2002 score of < 3 before RT had better OS than those with a score of ≥ 3 (HR = 2.215; 95%CI: 1.466-3.348; P < 0.001). Patients with weight loss (WL) of < 10% before RT had better OS compared to those with WL of ≥ 10% (HR = 2.947; 95%CI: 1.473-5.898; P = 0.002). Other statistically significant prognostic factors included clinical stage, lymph node metastasis, and distant metastasis. Conversely, factors such as age, radiation dose, and chemotherapy did not have a significant impact on prognosis. The survival curves of elderly patients with EC in different groups are depicted in Figure 1.
Figure 1.
The survival curves of elderly patients with esophageal cancer in different groups. A: Age; B: Sex; C: Marital status; D: Smoke; E: Drink; F: C stage; G: T stage; H: N stage; I: M stage; J: Tumor length; K: Tumor location; L: Eastern Cooperative Oncology Group; M: Charlson comorbidity index; N: Nutritional risk screening 2002; O: Body mass index; P: Weight loss; Q: Radiation dose; R: Chemotherapy. BMI: Body mass index; ECOG: Eastern Cooperative Oncology Group; CCI: Charlson comorbidity index; NRS2002: Nutritional risk screening 2002.
The subsequent multivariate analysis was conducted based on clinical stage, CCI, NRS2002, and WL. The results indicated that the clinical stage (III-IVa vs I-II, HR = 2.421, 95%CI: 1.242-4.718, P = 0.009; IVb vs I-II, HR = 4.222, 95%CI: 1.888-9.438, P < 0.001), CCI (HR = 1.539; 95%CI: 1.015-2.332; P = 0.042) and NRS2002 (HR = 2.491; 95%CI: 1.601-3.875; P < 0.001) were independent prognostic factors for elderly patients with EC. The results of both univariate and multivariate analyses are presented in Table 2.
Table 2.
Univariate and multivariate Cox analysis of the determinants of overall survival of patients
|
Variables
|
Univariate analysis
|
Multivariate analysis
|
||||||
|
HR
|
Lower 95%CI
|
Upper 95%CI
|
P value
|
HR
|
Lower 95%CI
|
Upper 95%CI
|
P value
|
|
| Age | ||||||||
| ≥ 75 and < 80 | 1 | |||||||
| ≥ 80 | 1.147 | 0.769 | 1.710 | 0.502 | ||||
| Sex | ||||||||
| Female | 1 | |||||||
| Male | 0.656 | 0.427 | 1.009 | 0.055 | ||||
| Marital status | ||||||||
| Married | 1 | |||||||
| Unmarried | 0.538 | 0.132 | 2.188 | 0.387 | ||||
| Smoke | ||||||||
| No | 1 | |||||||
| Yes | 0.821 | 0.544 | 1.239 | 0.348 | ||||
| Drink | ||||||||
| No | 1 | |||||||
| Yes | 0.869 | 0.545 | 1.387 | 0.557 | ||||
| C stage | ||||||||
| I-II | 1 | 1 | ||||||
| III-IVa | 2.261 | 1.189 | 4.298 | 0.013 | 2.421 | 1.242 | 4.718 | 0.009 |
| IVb | 3.444 | 1.593 | 7.446 | 0.002 | 4.222 | 1.888 | 9.438 | < 0.001 |
| T stage | ||||||||
| T1-T2 | 1 | |||||||
| T3-T4 | 1.575 | 0.876 | 2.831 | 0.129 | ||||
| N stage | ||||||||
| Negative | 1 | |||||||
| Positive | 2.030 | 1.104 | 3.732 | 0.023 | ||||
| M stage | ||||||||
| M0 | 1 | |||||||
| M1 | 1.761 | 1.038 | 2.986 | 0.036 | ||||
| Tumor length | ||||||||
| < 6 | 1 | |||||||
| ≥ 6 | 1.261 | 0.836 | 1.904 | 0.269 | ||||
| Tumor location | ||||||||
| Upper thoracic | 1 | |||||||
| Middle thoracic | 1.054 | 0.632 | 1.759 | 0.840 | ||||
| Lower thoracic | 1.146 | 0.665 | 1.976 | 0.623 | ||||
| ECOG | ||||||||
| 0-1 | 1 | |||||||
| 2 | 2.023 | 0.820 | 4.995 | 0.126 | ||||
| CCI | ||||||||
| 0 | 1 | 1 | ||||||
| ≥ 1 | 1.620 | 1.080 | 2.429 | 0.020 | 1.539 | 1.015 | 2.332 | 0.042 |
| NRS2002 | ||||||||
| < 3 | 1 | 1 | ||||||
| ≥ 3 | 2.215 | 1.466 | 3.348 | < 0.001 | 2.491 | 1.601 | 3.875 | < 0.001 |
| BMI | ||||||||
| < 18.5 | 1 | |||||||
| ≥ 18.5 | 0.756 | 0.482 | 1.183 | 0.221 | ||||
| Weight loss | ||||||||
| < 10% | 1 | 1 | ||||||
| ≥ 10% | 2.947 | 1.473 | 5.898 | 0.002 | 1.798 | 0.870 | 3.715 | 0.113 |
| Radiation dose | ||||||||
| < 60 | 1 | |||||||
| ≥ 60 | 0.771 | 0.511 | 1.164 | 0.217 | ||||
| Chemotherapy | ||||||||
| No | 1 | |||||||
| Yes | 0.847 | 0.543 | 1.320 | 0.463 | ||||
HR: Hazard ratio; CI: Confidence interval; BMI: Body mass index; ECOG: Eastern Cooperative Oncology Group; CCI: Charlson comorbidity index; NRS2002: Nutritional risk screening 2002.
ROC curve for survival prediction
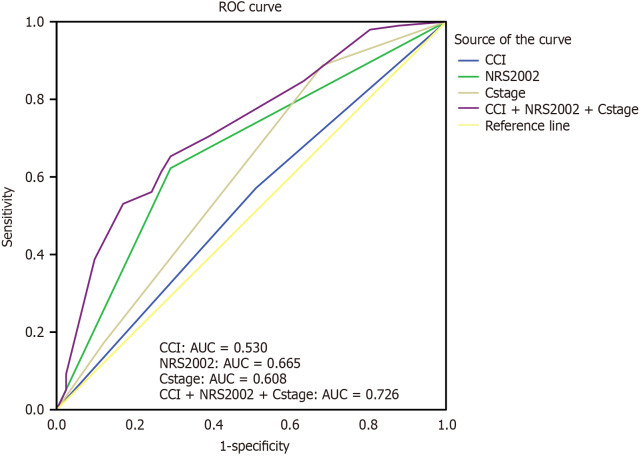
ROC curves for OS were plotted. As shown in Figure 2, the area under the curve (AUC) for NRS2002 was 0.665, the AUC for CCI was 0.530, and the AUC for clinical stage was 0.608. When all three indicators were combined for prediction, the AUC increased to 0.726 (95%CI: 0.635-0.817), demonstrating superior predictive accuracy compared to any single indicator alone.
Figure 2.
Receiver operating characteristic curves of Charlson comorbidity index, nutritional risk screening 2002, and clinical stage for predicting odds ratio in elderly patients with esophageal cancer after radiotherapy. ROC: Receiver operating characteristic; AUC: Area under curve; CCI: Charlson comorbidity index; NRS2002: Nutritional risk screening 2002.
Toxicity
Toxicity primarily occurred in grade 1 or 2, with a low incidence of grade 3 and higher adverse reactions. Table 3 demonstrated treatment-related acute toxic reactions, including leukopenia, anemia, thrombocytopenia, esophagitis, pneumonitis, and dermatitis. Specifically, the incidence of grade 3 hematological toxicity was 8.6%, grade 3 esophagitis was 5.5%, and grade 3 pneumonitis was 1.4%. Additionally, one patient experienced grade 4 hematological toxicity, while no grade 4 or higher non-hematological toxicity was observed.
Table 3.
Treatment-related acute toxicities in patients, n (%)
|
Toxicity
|
Grade 1
|
Grade 2
|
Grade 3
|
Grade 4
|
| Leukocytopenia | 31 (22.3) | 43 (30.9) | 6 (4.3) | 1 (0.7) |
| Anemia | 29 (20.8) | 21 (15.1) | 8 (5.7) | 1 (0.7) |
| Thrombopenia | 3 (2.1) | 2 (1.4) | 2 (1.4) | 1 (0.7) |
| Esophagitis | 19 (13.6) | 40 (28.7) | 7 (5.5) | 0 (0.0) |
| Pneumonitis | 1 (0.7) | 2 (1.4) | 2 (1.4) | 0 (0.0) |
| Dermatitis | 2 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
DISCUSSION
In this study, we conducted a retrospective analysis of the clinical characteristics, toxic reactions, and survival information of elderly EC patients aged ≥ 75 years who underwent IMRT and aimed to evaluate the prognostic factors for OS. Our analysis revealed that CCI and NRS2002 are independent prognostic factors for OS. These findings underscore the significance of comorbidities and nutrition in influencing the prognosis of elderly EC patients. Therefore, when formulating treatment protocols for elderly EC patients, it is crucial to consider aging-related prognostic factors, specifically comorbidities and nutrition, as indicated by CCI and NRS2002.
With the advancement of medical technology and the improvement of medical security levels, human life expectancy continues to increase, leading to a growing proportion of elderly individuals in the population. China, which accounts for about one-fifth of the world’s population, sees more than half of the global EC cases. Elderly EC patients represent a heterogeneous group that warrants significant attention. Although there are limited avenues of treatment for elderly EC patients, any treatment modality can provide substantial survival benefits compared to best supportive care[18]. Currently, many clinical studies on elderly EC have focused on patients over 70 years of age. A multi-center retrospective study from China revealed that the survival time of patients over 75 years is significantly lower than that of patients under 75 years. Therefore, the optimal treatment strategy for elderly EC patients over 75 years is still under exploration[19-21].
Due to demographic shifts, the number of elderly patients with EC is increasing, and many patients have unhealthy lifestyle habits such as smoking and alcohol consumption, leading to a higher prevalence of comorbidities among this group. Observational studies have consistently indicated that cancer patients with comorbidities experience lower survival rates compared to those without comorbidities[22-24]. The most commonly used model for quantifying comorbidities is the CCI. Currently, several studies have investigated the relationship between comorbidities and prognosis in patients with different stages of EC. For instance, Ishido et al[25] found that a CCI ≥ 2 was a prognostic factor for endoscopic treatment in elderly patients with superficial EC. Similarly, Yamashita et al[26] reported that a high CCI was associated with poor prognosis in stage II and above staged EC. Bernardi et al[27] highlighted that age alone could not directly indicate a patient’s ability to tolerate treatment, instead, comorbidities played a central role in the decision-making process, with the routine use of CCI aiding in prognostic risk stratification. While the value of CCI has been extensively studied in surgery, it has been less explored in RT for elderly EC. Our results align with these findings, showing that more than half of elderly EC patients had comorbidities, with CCI ≥ 1 accounting for 55.4% (77/139). Univariate and multivariate analyses indicated that the OS of the “CCI = 0” group was better than that of the “CCI ≥ 1” group. Therefore, our study suggests that comorbidities could affect the survival of elderly EC patients receiving RT.
Some studies have reported that approximately 60% to 85% of EC patients suffer from malnutrition, ranking first among malignant tumors[28]. Malnutrition can cause various harms to patients undergoing RT for EC, such as increasing positioning errors, side effects, and reducing efficacy. It can even lead to treatment interruptions, prolonged hospitalization, increased medical expenses, and decreased survival rates. Studies have indicated that malnutrition is a prognostic factor in elderly EC patients receiving RT[21,29]. Currently, there are many nutritional assessment tools for the prognosis assessment of EC, such as the controlling nutritional status (CONUT), the prognostic nutritional index (PNI), the patient-generated subjective global assessment, and the NRS2002[30-33]. Compared with the invasive and complex inconveniences of the first three assessment methods, the NRS2002 screening tool is non-invasive, inexpensive, time-saving, and easy to use[34]. Noh et al[35] analyzed 274 patients undergoing surgery for ESCC, using PNI, NRS2002, and CONUT to assess preoperative nutritional status. The results indicated that during a median follow-up of 55 months, a high NRS2002 was associated with poor OS and a high incidence of postoperative complications. Thus, NRS2002 was considered the most appropriate scoring system for assessing patients’ nutritional status. Song et al[36] retrospectively investigated 202 patients with locally advanced unresectable EC who received CRT to explore their prognostic factors. The results revealed that NRS ≥ 3 points was a poor prognostic factor. Similarly, the study by Wang et al[33] demonstrated that the baseline NRS2002 score serves as a simple and effective biomarker for predicting the long-term prognosis of patients with EC undergoing CRT. Consistent with previous studies, our study showed that NRS2002 was an independent prognostic factor for OS in elderly EC patients receiving RT. Therefore, pretreatment nutritional assessment and correction of malnutrition may improve survival outcomes in elderly EC patients undergoing RT.
Currently, CRT with 5-fluorouracil and cisplatin is the standard care for non-surgical EC treatment[9]. However, due to the serious toxic and side effects of double-drug concurrent chemoradiation (CCRT), most elderly EC patients often cannot tolerate it and thus cannot complete standard CCRT treatment. Ji et al[37] enrolled 298 elderly EC patients aged 70 and above to investigate the efficacy and toxicities of CCRT with S-1. The results indicated that the survival outcomes were superior in the CCRT group compared to the RT group alone, and the toxicities were manageable, suggesting that elderly patients could benefit from a treatment regimen involving combined RT and single-agent chemotherapy. Regarding EC patients 75 years and older, there are no large-scale prospective randomized clinical trials comparing the efficacy of CCRT with RT alone. Some retrospective studies have revealed that CCRT has no statistically significant survival benefit[21,38]. The findings of our study are consistent with these reports. Therefore, caution should be exercised when using CCRT in elderly EC patients aged 75 years and older, and omitting or using milder chemotherapy regimens may be more appropriate.
To further analyze the prognostic value of CCI, NRS2002, and clinical stage in elderly patients with unresectable EC, we plotted ROC curves. The combined prediction of these three factors for the survival of elderly EC patients yielded an AUC of 0.726, which outperformed the predictive ability of any individual factor. This finding suggests that treatment strategies for elderly EC patients should be tailored based on factors such as disease stage, nutritional status, and comorbidity. Individualized treatment plans should be carefully designed for elderly patients through meticulous selection, aiming to provide the best possible treatment while ensuring safety.
In the study of Yin et al[19], the median OS of elderly EC patients who received RT, including patients with metastatic lesions, was 20.68 months. The 1-year, 2-year, 3-year, and 5-year OS rates were 62.6%, 41.8%, 11.1%, and 0%, respectively. Our study showed that the median OS of elderly EC patients receiving RT was 20.0 months, and the 1-year, 2-year, 3-year, and 5-year OS rates were 69.8%, 38.7%, 28.2%, and 17.5%, respectively. Since their study enrolled patients over 70 years, and ours included patients over 75 years, it showed that we had a greater advantage in survival of elderly EC patients. In the study of Suzuki et al[38], the median OS of older patients aged ≥ 75 years with localized EC who received RT was 30.0 months, and the 2-year OS rate was 53%. Zhou et al[21] retrospectively analyzed 149 patients over the age of 75 with localized EC and reported that the 2-year OS rate was 51.6%. The survival time of patients in our study was slightly worse than that of the above two studies, which may be due to the inclusion of 15.8% of patients with metastatic EC. Therefore, RT is an effective treatment for patients with unresectable EC over the age of 75, and advanced age alone should not be a reason to exclude patients from RT.
There were some limitations to this study. Firstly, it was a single-center retrospective study with a relatively small sample size, introducing selection bias. Secondly, variables such as smoking, drinking, and comorbidity were all self-reported by elderly patients, who were elderly, leading to recall bias. Finally, some potential prognostic factors, such as elective nodal irradiation and involved-field irradiation, were not included in the study. Therefore, prospective research is still needed to provide a basis for the treatment of elderly EC patients.
CONCLUSION
This study identified prognostic factors for RT in elderly patients with unresectable EC. “CCI ≥ 1” and “NRS2002 ≥ 3” were independent prognostic factors associated with worse OS. Increasing the dose of RT and combining chemotherapy did not significantly improve survival. For elderly patients with EC, the focus should be on biological age rather than physiologic age. Future clinical practice should consider indicators associated with aging, such as comorbidities and nutritional status.
Footnotes
Institutional review board statement: This study was approved by the Ethics Committee of Lu’an Hospital of Anhui Medical University, No. 2023 LLKS035.
Informed consent statement: In this study, informed consent was obtained from the patients and their families.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Beeraka NM S-Editor: Fan M L-Editor: A P-Editor: Wang WB
Contributor Information
Li-Li Hu, Department of Cancer Center, Lu’an Hospital of Anhui Medical University, Lu’an 237002, Anhui Province, China.
Feng Rong, Department of Cancer Center, Lu’an Hospital of Anhui Medical University, Lu’an 237002, Anhui Province, China.
Lei Liu, College of Health and Elderly Care, Anhui Vocational College of City Management, Hefei 230012, Anhui Province, China.
Ling Zhang, Department of Cancer Center, Lu’an Hospital of Anhui Medical University, Lu’an 237002, Anhui Province, China.
Lei-Lei Zhang, Department of Cancer Center, Lu’an Hospital of Anhui Medical University, Lu’an 237002, Anhui Province, China.
Qun Yang, Department of Cancer Center, Lu’an Hospital of Anhui Medical University, Lu’an 237002, Anhui Province, China.
Zhao-Long Xia, Department of Cancer Center, Lu’an Hospital of Anhui Medical University, Lu’an 237002, Anhui Province, China.
Hui Wang, Department of Oncology, The Second Affiliated Hospital of Anhui Medical University, Hefei 230601, Anhui Province, China. 1223556508@qq.com.
Data sharing statement
The datasets generated and/or analyzed in the current study can be obtained from the corresponding author upon a reasonable request.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–2509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 3.van Blankenstein M, Looman CW, Siersema PD, Kuipers EJ, Coebergh JW. Trends in the incidence of adenocarcinoma of the oesophagus and cardia in the Netherlands 1989-2003. Br J Cancer. 2007;96:1767–1771. doi: 10.1038/sj.bjc.6603798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu W, Li P, Wen W, Jian Y. Comparison of Long-Term Survival Between cT1N0 Stage Esophageal Cancer Patients Receiving Endoscopic Dissection and Esophagectomy: A Meta-Analysis. Front Surg. 2022;9:917689. doi: 10.3389/fsurg.2022.917689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y, Chen Z, Yang H, Wang J, Pang Q, Zheng X, Yang H, Li T, Lordick F, D'Journo XB, Cerfolio RJ, Korst RJ, Novoa NM, Swanson SJ, Brunelli A, Ismail M, Fernando HC, Zhang X, Li Q, Wang G, Chen B, Mao T, Kong M, Guo X, Lin T, Liu M, Fu J AME Thoracic Surgery Collaborative Group. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol. 2018;36:2796–2803. doi: 10.1200/JCO.2018.79.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lester SC, Lin SH, Chuong M, Bhooshan N, Liao Z, Arnett AL, James SE, Evans JD, Spears GM, Komaki R, Haddock MG, Mehta MP, Hallemeier CL, Merrell KW. A Multi-institutional Analysis of Trimodality Therapy for Esophageal Cancer in Elderly Patients. Int J Radiat Oncol Biol Phys. 2017;98:820–828. doi: 10.1016/j.ijrobp.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Markar SR, Karthikesalingam A, Thrumurthy S, Ho A, Muallem G, Low DE. Systematic review and pooled analysis assessing the association between elderly age and outcome following surgical resection of esophageal malignancy. Dis Esophagus. 2013;26:250–262. doi: 10.1111/j.1442-2050.2012.01353.x. [DOI] [PubMed] [Google Scholar]
- 9.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, Asbell SO, Graham MV, Leichman LL. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 10.Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 11.Hulshof MCCM, Geijsen ED, Rozema T, Oppedijk V, Buijsen J, Neelis KJ, Nuyttens JJME, van der Sangen MJC, Jeene PM, Reinders JG, van Berge Henegouwen MI, Thano A, van Hooft JE, van Laarhoven HWM, van der Gaast A. Randomized Study on Dose Escalation in Definitive Chemoradiation for Patients With Locally Advanced Esophageal Cancer (ARTDECO Study) J Clin Oncol. 2021;39:2816–2824. doi: 10.1200/JCO.20.03697. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Dong B, Zhu W, Li J, Huang R, Sun Z, Yang X, Liu L, He H, Liao Z, Guan N, Kong Y, Wang W, Chen J, He H, Qiu G, Zeng M, Pu J, Hu W, Bao Y, Liu Z, Ma J, Jiang H, Du X, Hu J, Zhuang T, Cai J, Huang J, Tao H, Liu Y, Liang X, Zhou J, Tao G, Zheng X, Chen M. A Phase III Multicenter Randomized Clinical Trial of 60 Gy versus 50 Gy Radiation Dose in Concurrent Chemoradiotherapy for Inoperable Esophageal Squamous Cell Carcinoma. Clin Cancer Res. 2022;28:1792–1799. doi: 10.1158/1078-0432.CCR-21-3843. [DOI] [PubMed] [Google Scholar]
- 13.Li LQ, Fu QG, Zhao WD, Wang YD, Meng WW, Su TS. Chemoradiotherapy Versus Chemotherapy Alone for Advanced Esophageal Squamous Cell Carcinoma: The Role of Definitive Radiotherapy for Primary Tumor in the Metastatic Setting. Front Oncol. 2022;12:824206. doi: 10.3389/fonc.2022.824206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guttmann DM, Mitra N, Bekelman J, Metz JM, Plastaras J, Feng W, Swisher-McClure S. Improved Overall Survival with Aggressive Primary Tumor Radiotherapy for Patients with Metastatic Esophageal Cancer. J Thorac Oncol. 2017;12:1131–1142. doi: 10.1016/j.jtho.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi S, Ohtsu A, Doi T, Kojima T, Minashi K, Mera K, Yano T, Tahara M, Muto M, Nihei K. A retrospective study of definitive chemoradiotherapy for elderly patients with esophageal cancer. Am J Clin Oncol. 2007;30:607–611. doi: 10.1097/COC.0b013e3180ca7c84. [DOI] [PubMed] [Google Scholar]
- 16.Qu X, Biagi J, Banashkevich A, Mercer CD, Tremblay L, Mahmud A. Management and outcomes of localized esophageal and gastroesophageal junction cancer in older patients. Curr Oncol. 2015;22:e435–e442. doi: 10.3747/co.22.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bracken-Clarke D, Farooq AR, Horgan AM. Management of Locally Advanced and Metastatic Esophageal Cancer in the Older Population. Curr Oncol Rep. 2018;20:99. doi: 10.1007/s11912-018-0745-3. [DOI] [PubMed] [Google Scholar]
- 18.Mantziari S, Teixeira Farinha H, Bouygues V, Vignal JC, Deswysen Y, Demartines N, Schäfer M, Piessen G. Esophageal Cancer in Elderly Patients, Current Treatment Options and Outcomes; A Systematic Review and Pooled Analysis. Cancers (Basel) 2021;13 doi: 10.3390/cancers13092104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin H, E M, Zhang H, Wang C. The outcomes of radiotherapy and factors that predict overall survival in elderly patients with esophageal squamous cell carcinoma. Clin Transl Oncol. 2017;19:742–749. doi: 10.1007/s12094-016-1603-0. [DOI] [PubMed] [Google Scholar]
- 20.Li R, He Y, Sun X, Wang N, Zhang M, Wei K, Li H, Dong P, Du L, Chen W. The long-term survival of esophageal cancer in elderly patients: A multi-center, retrospective study from China. Cancer Med. 2023;12:4852–4863. doi: 10.1002/cam4.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou YC, Chen LL, Xu HB, Sun Q, Zhang Q, Cai HF, Jiang H. Aging-related prognosis analysis of definitive radiotherapy for very elderly esophageal cancer. Cancer Med. 2018;7:1837–1844. doi: 10.1002/cam4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Søgaard M, Thomsen RW, Bossen KS, Sørensen HT, Nørgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5:3–29. doi: 10.2147/CLEP.S47150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morishima T, Matsumoto Y, Koeda N, Shimada H, Maruhama T, Matsuki D, Nakata K, Ito Y, Tabuchi T, Miyashiro I. Impact of Comorbidities on Survival in Gastric, Colorectal, and Lung Cancer Patients. J Epidemiol. 2019;29:110–115. doi: 10.2188/jea.JE20170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jørgensen TL, Hallas J, Friis S, Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106:1353–1360. doi: 10.1038/bjc.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishido K, Tanabe S, Katada C, Kubota Y, Furue Y, Wada T, Watanabe A, Koizumi W. Usefulness of endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma in elderly patients: a single-center retrospective cohort study. Jpn J Clin Oncol. 2021;51:895–904. doi: 10.1093/jjco/hyab030. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita K, Watanabe M, Mine S, Fukudome I, Okamura A, Yuda M, Hayami M, Imamura Y. The impact of the Charlson comorbidity index on the prognosis of esophageal cancer patients who underwent esophagectomy with curative intent. Surg Today. 2018;48:632–639. doi: 10.1007/s00595-018-1630-2. [DOI] [PubMed] [Google Scholar]
- 27.Bernardi D, Asti E, Aiolfi A, Bonitta G, Luporini A, Bonavina L. Outcome of Trimodal Therapy in Elderly Patients with Esophageal Cancer: Prognostic Value of the Charlson Comorbidity Index. Anticancer Res. 2018;38:1815–1820. doi: 10.21873/anticanres.12420. [DOI] [PubMed] [Google Scholar]
- 28.Chen MF, Hsieh CC, Chen PT, Lu MS. Role of Nutritional Status in the Treatment Outcome for Esophageal Squamous Cell Carcinoma. Nutrients. 2021;13 doi: 10.3390/nu13092997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Wang L, Fang M, Li J, Song T, Zhan W, Xu H. Prognostic Value of the Geriatric Nutritional Risk Index in Patients Exceeding 70 Years Old with Esophageal Squamous Cell Carcinoma. Nutr Cancer. 2020;72:620–626. doi: 10.1080/01635581.2019.1650189. [DOI] [PubMed] [Google Scholar]
- 30.Takagi K, Buettner S, Ijzermans JNM, Wijnhoven BPL. Systematic Review on the Controlling Nutritional Status (CONUT) Score in Patients Undergoing Esophagectomy for Esophageal Cancer. Anticancer Res. 2020;40:5343–5349. doi: 10.21873/anticanres.14541. [DOI] [PubMed] [Google Scholar]
- 31.Qi Q, Song Q, Cheng Y, Wang N. Prognostic Significance of Preoperative Prognostic Nutritional Index for Overall Survival and Postoperative Complications in Esophageal Cancer Patients. Cancer Manag Res. 2021;13:8585–8597. doi: 10.2147/CMAR.S333190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen SB, Liu DT, Chen YP. The Impact of Preoperative Nutritional Status on the Survival of Patients With Esophageal Squamous Cell Carcinoma. Front Surg. 2021;8:752792. doi: 10.3389/fsurg.2021.752792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Yu B, Ye Y, Shen J, Ding N, Tang H, Xu Y, Song L, Zhu Z, Chen Y, Xie S, Chen M. Predictive Value of Nutritional Risk Screening 2002 and Prognostic Nutritional Index for Esophageal Cancer Patients Undergoing Definitive Radiochemotherapy. Nutr Cancer. 2018;70:879–885. doi: 10.1080/01635581.2018.1470656. [DOI] [PubMed] [Google Scholar]
- 34.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–336. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 35.Noh JH, Na HK, Kim YH, Song HJ, Kim HR, Choi KD, Lee GH, Jung HY. Influence of Preoperative Nutritional Status on Patients Who Undergo Upfront Surgery for Esophageal Squamous Cell Carcinoma. Nutr Cancer. 2022;74:2910–2919. doi: 10.1080/01635581.2022.2042573. [DOI] [PubMed] [Google Scholar]
- 36.Song T, Wan Q, Yu W, Li J, Lu S, Xie C, Wang H, Fang M. Pretreatment nutritional risk scores and performance status are prognostic factors in esophageal cancer patients treated with definitive chemoradiotherapy. Oncotarget. 2017;8:98974–98984. doi: 10.18632/oncotarget.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji Y, Du X, Zhu W, Yang Y, Ma J, Zhang L, Li J, Tao H, Xia J, Yang H, Huang J, Bao Y, Du D, Liu D, Wang X, Li C, Yang X, Zeng M, Liu Z, Zheng W, Pu J, Chen J, Hu W, Li P, Wang J, Xu Y, Zheng X, Chen J, Wang W, Tao G, Cai J, Zhao J, Zhu J, Jiang M, Yan Y, Xu G, Bu S, Song B, Xie K, Huang S, Zheng Y, Sheng L, Lai X, Chen Y, Cheng L, Hu X, Ji W, Fang M, Kong Y, Yu X, Li H, Li R, Shi L, Shen W, Zhu C, Lv J, Huang R, He H, Chen M. Efficacy of Concurrent Chemoradiotherapy With S-1 vs Radiotherapy Alone for Older Patients With Esophageal Cancer: A Multicenter Randomized Phase 3 Clinical Trial. JAMA Oncol. 2021;7:1459–1466. doi: 10.1001/jamaoncol.2021.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki G, Yamazaki H, Aibe N, Masui K, Kimoto T, Shimizu D, Nishimura T, Nakashima A, Machida K, Kawabata K, Ota Y, Fujiwara H, Ishikawa T, Yamada K. Definitive Radiotherapy for Older Patients Aged ≥75 Years With Localized Esophageal Cancer. In Vivo. 2019;33:925–932. doi: 10.21873/invivo.11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed in the current study can be obtained from the corresponding author upon a reasonable request.