Abstract
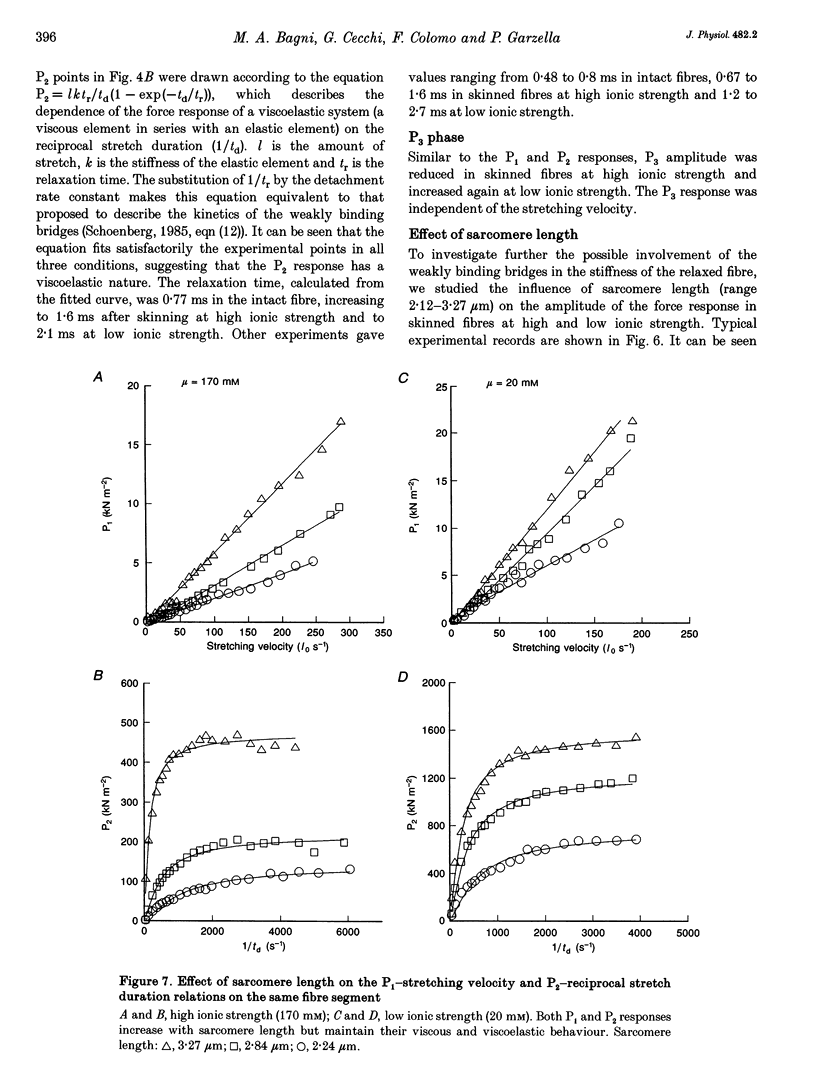
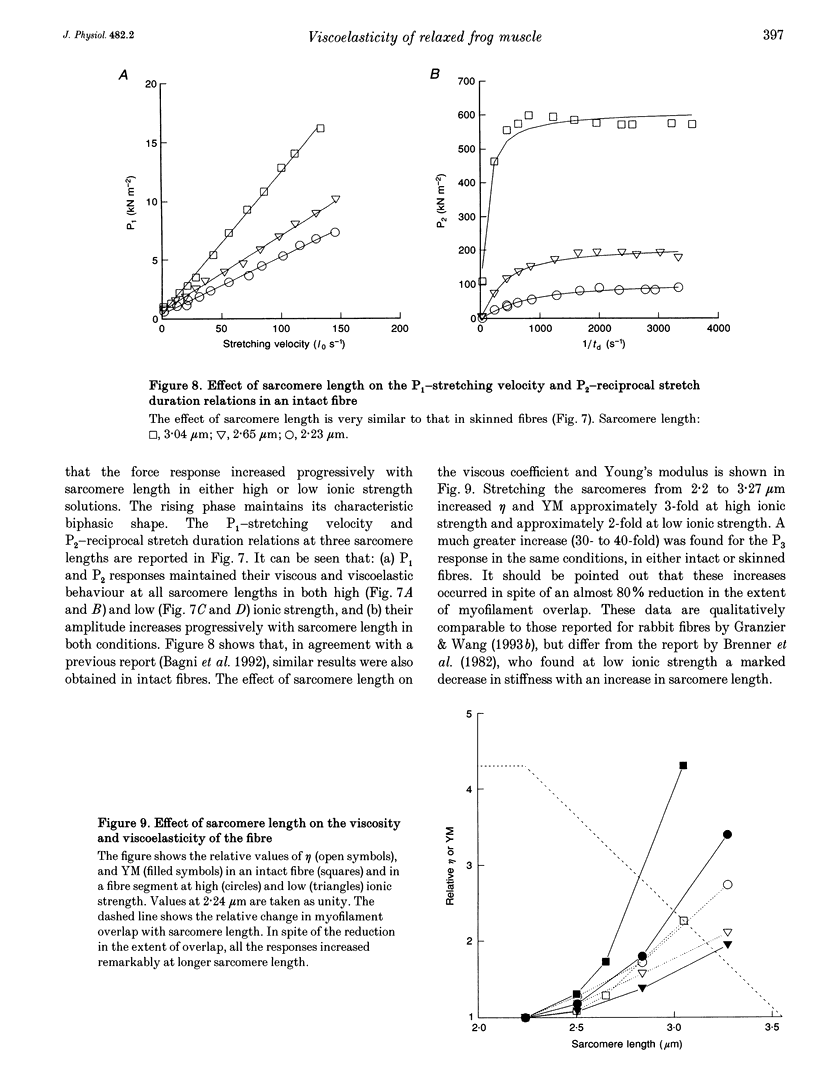
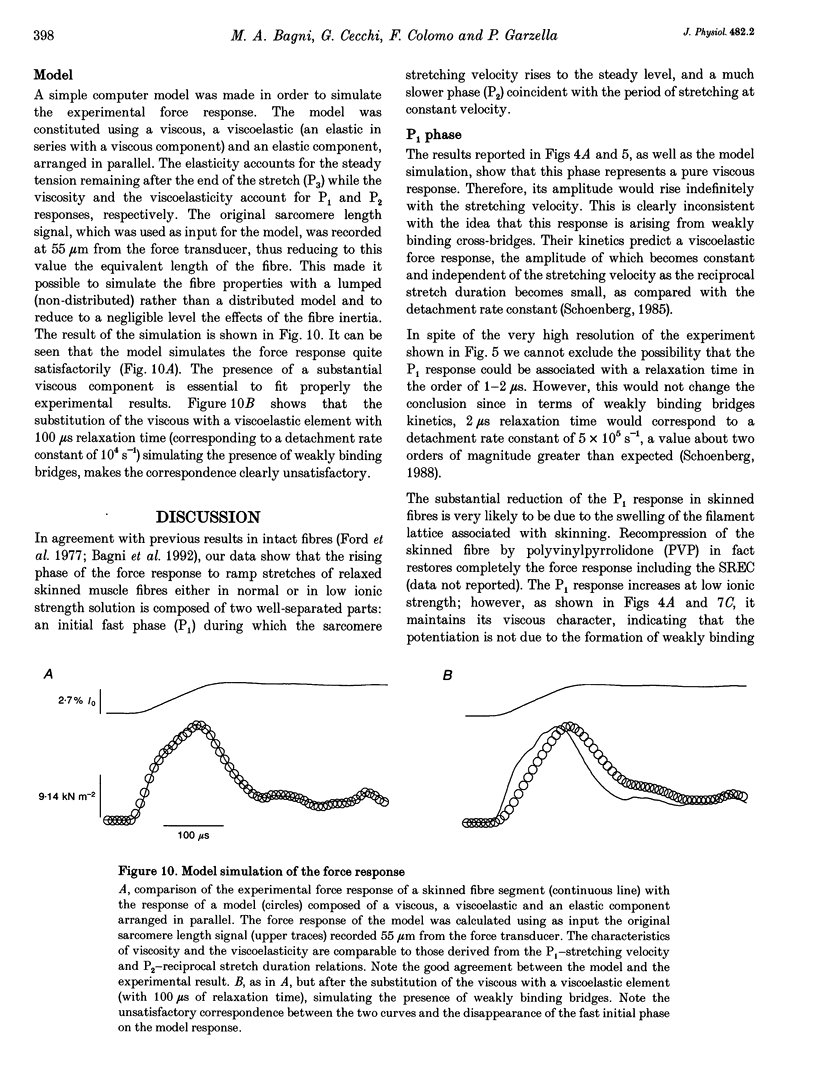
1. Passive force responses to ramp stretches at various velocities were measured in intact and skinned single muscle fibres isolated from the lumbricalis muscle of the frog. Force was measured using a fast capacitance transducer and sarcomere length was measured using a laser light diffraction technique at a point very close to the fixed end so as to avoid effects of fibre inertia. Experiments were performed at 15 degrees C with sarcomere length between 2.13 and 3.27 microns under high (170 mM) and low (20 mM) ionic strength. 2. The analysis shows that the force response is the sum of at least three components: (i) elastic (force proportional to the amount of stretch), (ii) viscous (force proportional to rate of stretch), and (iii) viscoelastic (resembling the response of a pure viscous element in series with an elastic element). 3. The amplitude of all these components increased progressively with sarcomere length in the whole range measured. 4. A further component, attributable to the short-range elasticity (SREC), was present in the force response of the intact fibres. 5. The amplitude of the force response decreased substantially upon skinning at high ionic strength but increased again at low ionic strength. The SREC was completely abolished by skinning. 6. None of the components of the force response was found to have the properties expected from the previously postulated 'weakly binding bridges'.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagni M. A., Cecchi G., Colomo F., Garzella P. Are weakly binding bridges present in resting intact muscle fibers? Biophys J. 1992 Nov;63(5):1412–1415. doi: 10.1016/S0006-3495(92)81718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Podolsky R. J. X-ray diffraction evidence for cross-bridge formation in relaxed muscle fibers at various ionic strengths. Biophys J. 1984 Sep;46(3):299–306. doi: 10.1016/S0006-3495(84)84026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Chock P. B., Eisenberg E. Mechanism of action of troponin . tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J Biol Chem. 1981 Jan 25;256(2):575–578. [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H. L., Wang K. Interplay between passive tension and strong and weak binding cross-bridges in insect indirect flight muscle. A functional dissection by gelsolin-mediated thin filament removal. J Gen Physiol. 1993 Feb;101(2):235–270. doi: 10.1085/jgp.101.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H. L., Wang K. Passive tension and stiffness of vertebrate skeletal and insect flight muscles: the contribution of weak cross-bridges and elastic filaments. Biophys J. 1993 Nov;65(5):2141–2159. doi: 10.1016/S0006-3495(93)81262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M. Characterization of the myosin adenosine triphosphate (M.ATP) crossbridge in rabbit and frog skeletal muscle fibers. Biophys J. 1988 Jul;54(1):135–148. doi: 10.1016/S0006-3495(88)82938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M. Equilibrium muscle cross-bridge behavior. Theoretical considerations. Biophys J. 1985 Sep;48(3):467–475. doi: 10.1016/S0006-3495(85)83802-9. [DOI] [PMC free article] [PubMed] [Google Scholar]



