Abstract
1. A large proportion of sympathetic preganglionic neurones contain nitric oxide synthase. The purpose of this study was to determine the effects of facilitation and inhibition of nitric oxide synthesis within the lower thoracic spinal cord (which contains the majority of renal preganglionic neurones) on renal sympathetic nerve activity (rSNA). 2. In anaesthetized rabbits, rSNA was recorded before and after intrathecal injection (50 microliters of 0.5 M solution) of either L-arginine, a precursor of nitric oxide, or N omega-nitro-L-arginine methyl ester (L-NAME), an inhibitor of nitric oxide synthase, into the lower thoracic spinal cord. Spinal cord sections were also stained for the presence of NADPH diaphorase, a marker of nitric oxide synthesizing neurones. 3. A high density of NADPH diaphorase-containing neurones was found within the intermediolateral cell column of the lower thoracic spinal cord. 4. Intrathecal injection of L-arginine and L-NAME resulted in a large increase (113 +/- 25%) and decrease (43 +/- 8%), respectively, in rSNA. In contrast, injection of the inactive isomers D-arginine and D-NAME had no significant effect on rSNA. 5. The results indicate that endogenous nitric oxide in the lower thoracic spinal cord (1) has a potent excitatory action on renal sympathetic preganglionic neurones, and (2) helps to maintain the tonic activity of renal sympathetic nerves under resting conditions.
Full text
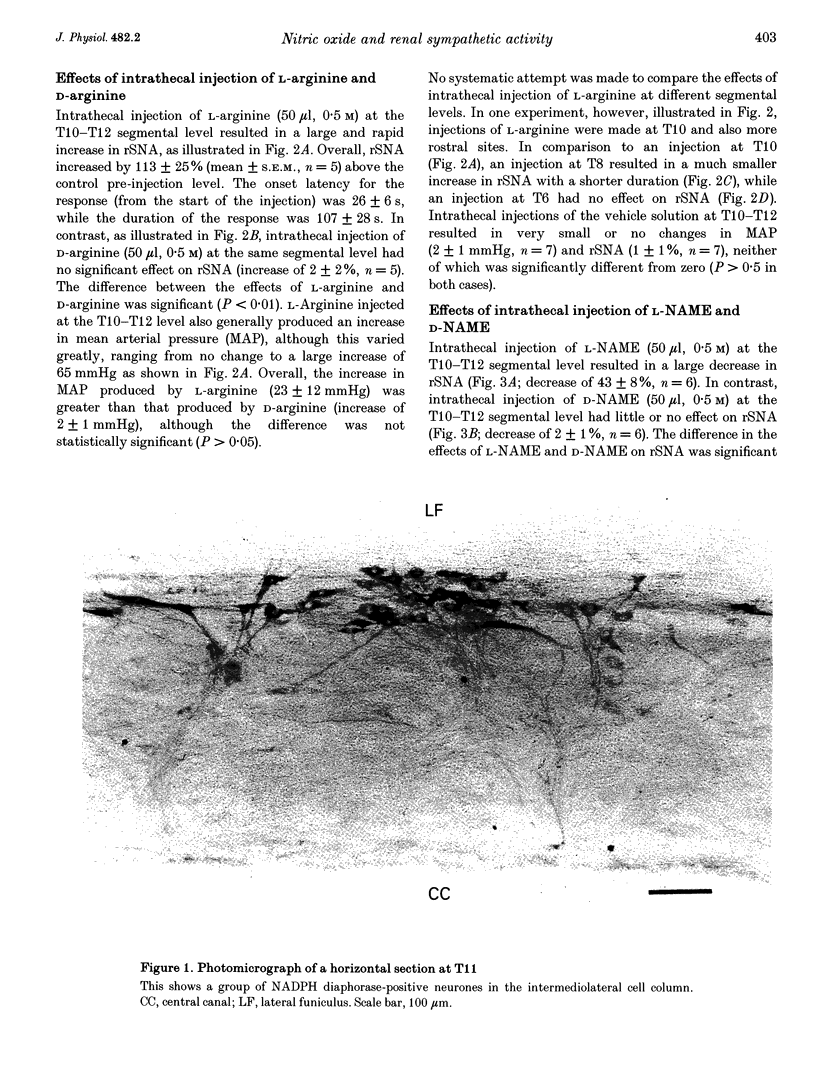
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Edwards S. L., Furness J. B., Bredt D. S., Snyder S. H. The distribution of nitric oxide synthase-containing autonomic preganglionic terminals in the rat. Brain Res. 1993 Jun 18;614(1-2):78–85. doi: 10.1016/0006-8993(93)91020-s. [DOI] [PubMed] [Google Scholar]
- Anderson C. R. NADPH diaphorase-positive neurons in the rat spinal cord include a subpopulation of autonomic preganglionic neurons. Neurosci Lett. 1992 May 25;139(2):280–284. doi: 10.1016/0304-3940(92)90571-n. [DOI] [PubMed] [Google Scholar]
- Blottner D., Baumgarten H. G. Nitric oxide synthetase (NOS)-containing sympathoadrenal cholinergic neurons of the rat IML-cell column: evidence from histochemistry, immunohistochemistry, and retrograde labeling. J Comp Neurol. 1992 Feb 1;316(1):45–55. doi: 10.1002/cne.903160105. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Glatt C. E., Hwang P. M., Fotuhi M., Dawson T. M., Snyder S. H. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991 Oct;7(4):615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Dampney R. A. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994 Apr;74(2):323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- DiBona G. F. Neural control of renal function in health and disease. Clin Auton Res. 1994 Apr;4(1-2):69–74. doi: 10.1007/BF01828841. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Dun S. L., Wu S. Y., Förstermann U., Schmidt H. H., Tseng L. F. Nitric oxide synthase immunoreactivity in the rat, mouse, cat and squirrel monkey spinal cord. Neuroscience. 1993 Jun;54(4):845–857. doi: 10.1016/0306-4522(93)90579-5. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Harada S., Tokunaga S., Momohara M., Masaki H., Tagawa T., Imaizumi T., Takeshita A. Inhibition of nitric oxide formation in the nucleus tractus solitarius increases renal sympathetic nerve activity in rabbits. Circ Res. 1993 Mar;72(3):511–516. doi: 10.1161/01.res.72.3.511. [DOI] [PubMed] [Google Scholar]
- Hayes K., Weaver L. C. Selective control of sympathetic pathways to the kidney, spleen and intestine by the ventrolateral medulla in rats. J Physiol. 1990 Sep;428:371–385. doi: 10.1113/jphysiol.1990.sp018217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Faris P. L., Hartman B. K., Xu X. Localization of NADPH diaphorase in neurons of the rostral ventral medulla: possible role of nitric oxide in central autonomic regulation and oxygen chemoreception. Brain Res. 1993 Feb 12;603(1):173–179. doi: 10.1016/0006-8993(93)91318-m. [DOI] [PubMed] [Google Scholar]
- Li Y. W., Ding Z. Q., Wesselingh S. L., Blessing W. W. Renal and adrenal sympathetic preganglionic neurons in rabbit spinal cord: tracing with herpes simplex virus. Brain Res. 1992 Feb 21;573(1):147–152. doi: 10.1016/0006-8993(92)90124-r. [DOI] [PubMed] [Google Scholar]
- Lin Y. Q., Bennett M. R. Nitric oxide modulation of quantal secretion in chick ciliary ganglia. J Physiol. 1994 Dec 1;481(Pt 2):385–394. doi: 10.1113/jphysiol.1994.sp020447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka H., Nishida H., Nomura G., Van Vliet B. N., Toshima H. Hypertension induced by nitric oxide synthesis inhibition is renal nerve dependent. Hypertension. 1994 Jun;23(6 Pt 2):971–975. doi: 10.1161/01.hyp.23.6.971. [DOI] [PubMed] [Google Scholar]
- Meckler R. L., Weaver L. C. Splenic, renal, and cardiac nerves have unequal dependence upon tonic supraspinal inputs. Brain Res. 1985 Jul 8;338(1):123–135. doi: 10.1016/0006-8993(85)90254-9. [DOI] [PubMed] [Google Scholar]
- Poree L. R., Schramm L. P. Role of cervical neurons in propriospinal inhibition of thoracic dorsal horn neurons. Brain Res. 1992 Dec 25;599(2):302–308. doi: 10.1016/0006-8993(92)90405-x. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott T. R., Bennett M. R. The effect of nitric oxide on the efficacy of synaptic transmission through the chick ciliary ganglion. Br J Pharmacol. 1993 Oct;110(2):627–632. doi: 10.1111/j.1476-5381.1993.tb13857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi H., Sakuma I., Yoshioka M., Kobayashi T., Yasuda H., Kitabatake A., Saito H., Gross S. S., Levi R. A central nervous system action of nitric oxide in blood pressure regulation. J Pharmacol Exp Ther. 1992 Jul;262(1):343–347. [PubMed] [Google Scholar]