Abstract
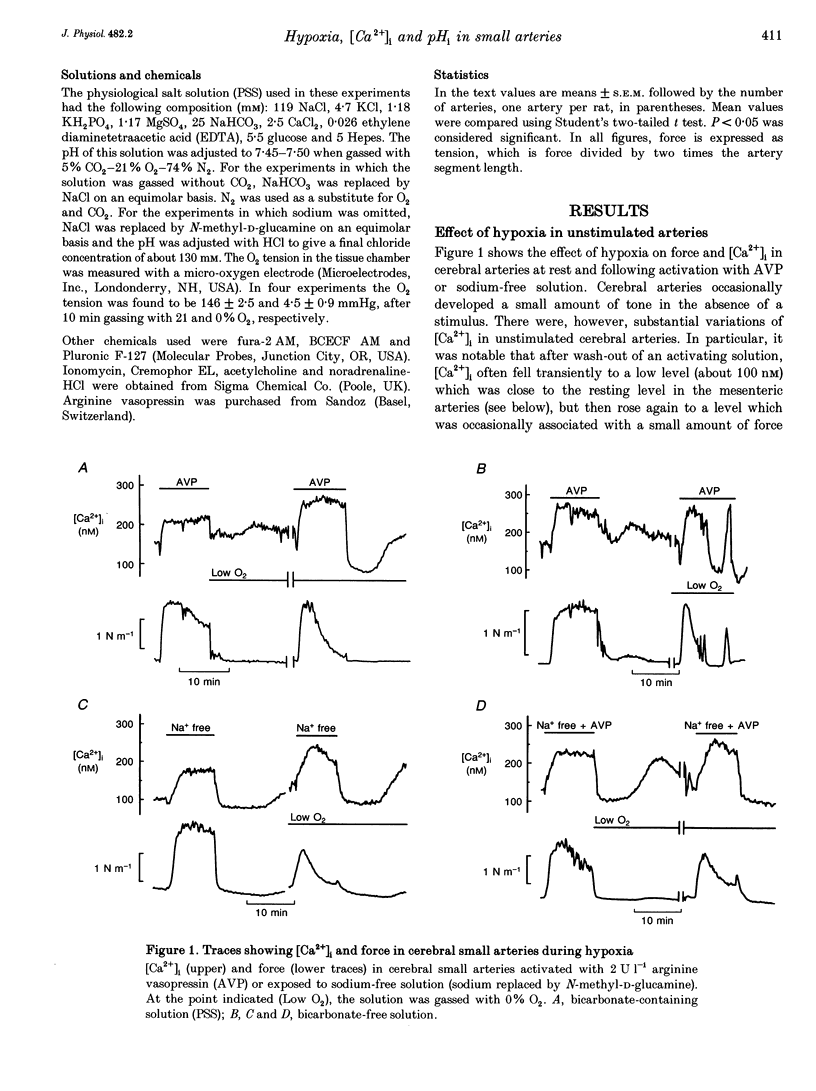
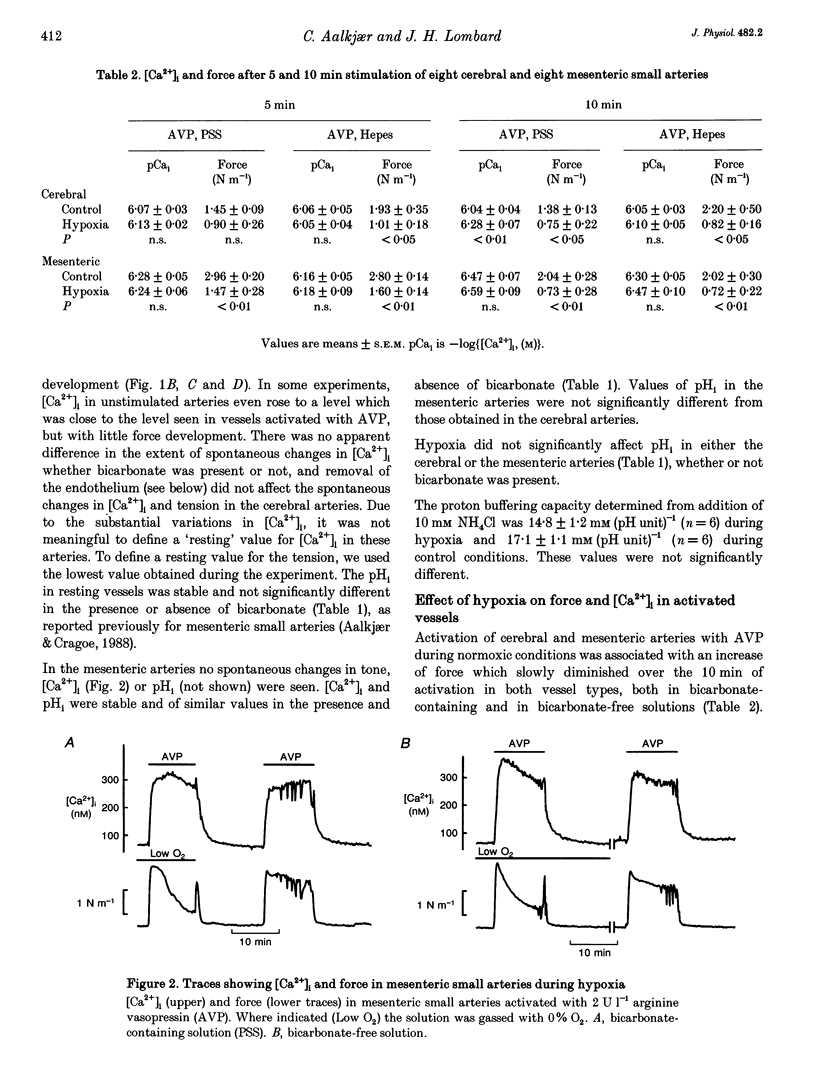
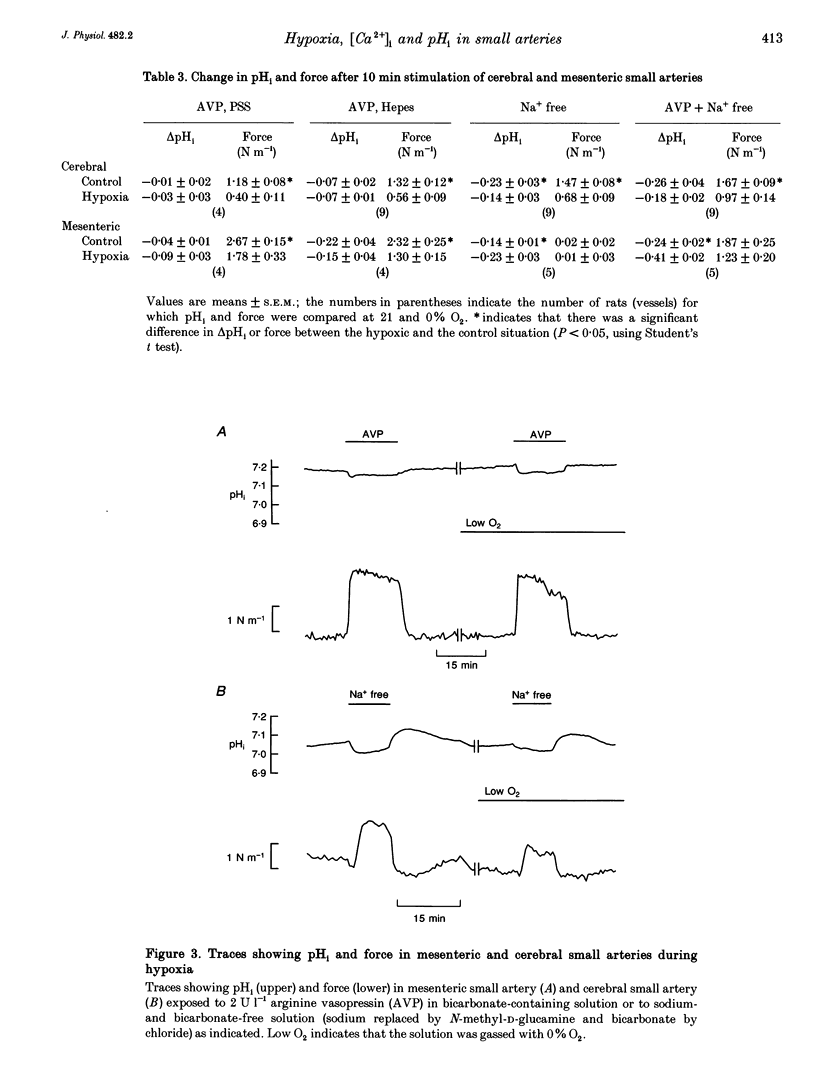
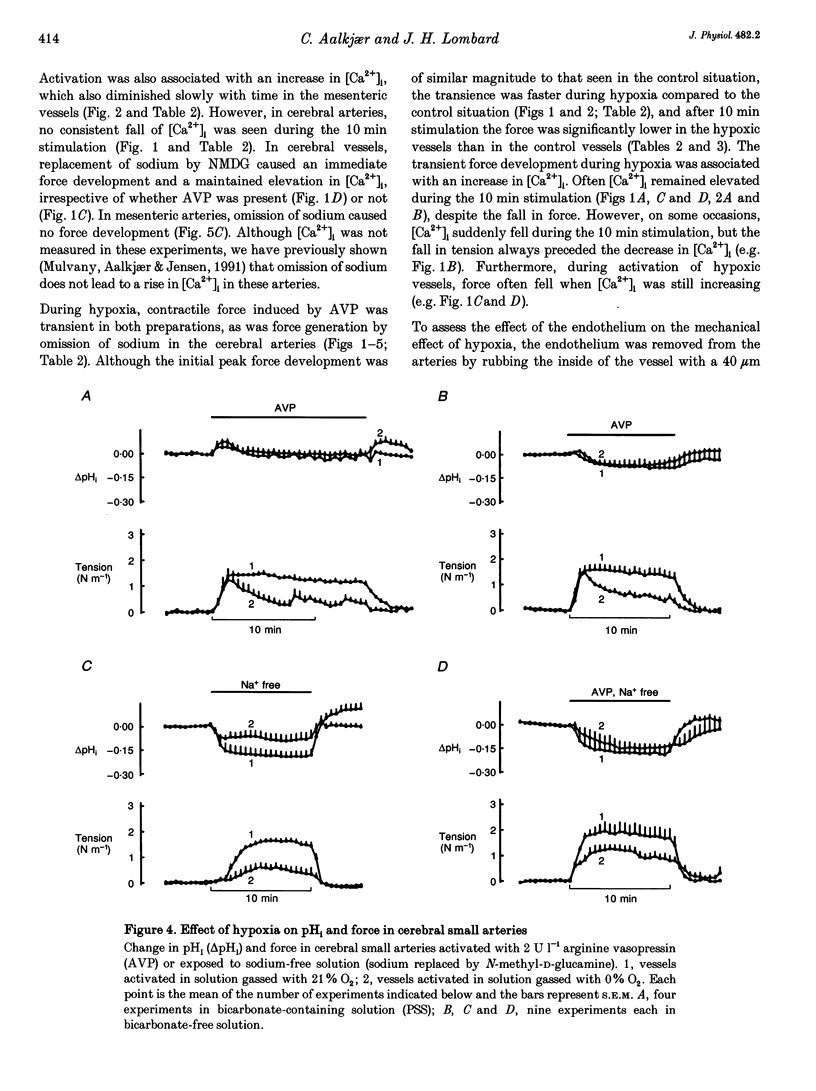
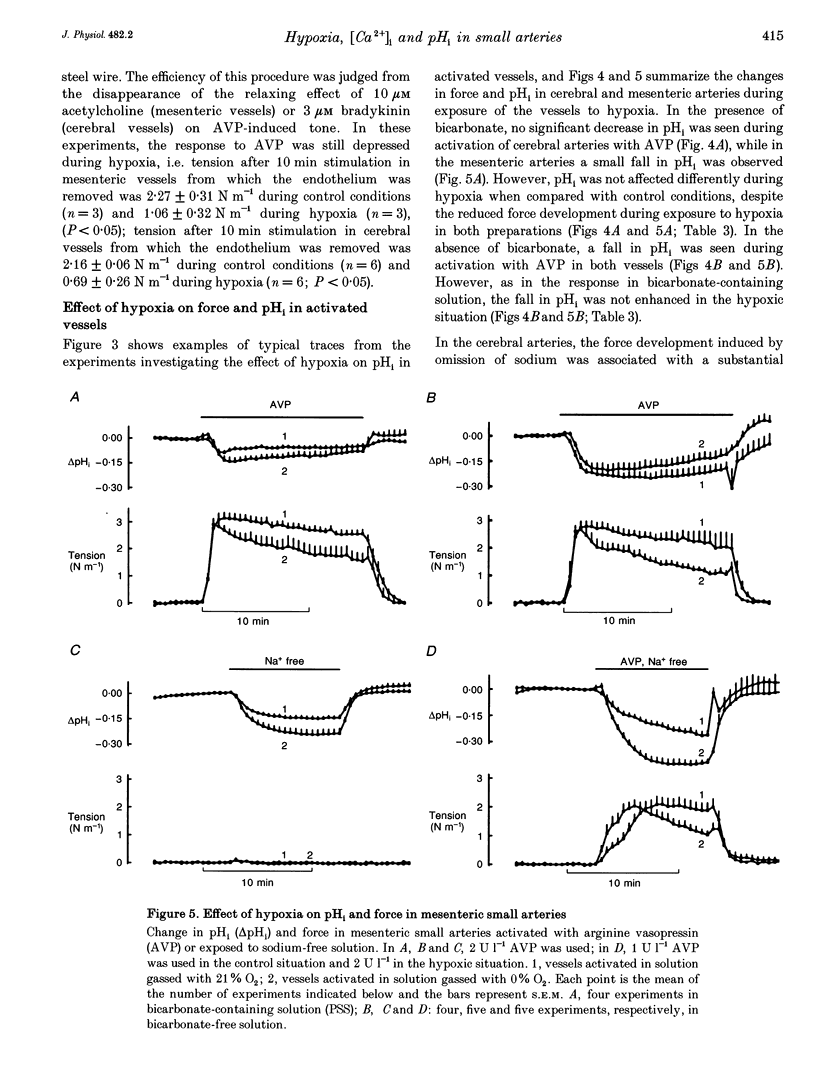
1. The effect of severe hypoxia on force, intracellular Ca2+ concentration ([Ca2+]i) and pHi was studied in isolated small arteries from rat brain and rat mesenterium. The arteries were mounted for isometric force recording while [Ca2+]i was measured with fura-2 or pHi was measured with bis-carboxyethylcarboxyfluorescein (BCECF). 2. Hypoxia reduced the force development in response to arginine vasopressin (AVP) while [Ca2+]i was unchanged or only slightly reduced. Inhibition of acid extrusion by omission of sodium caused no force development in mesenteric arteries, but the fall in pHi was enhanced during hypoxia. In cerebral arteries, hypoxia reduced the force development associated with omission of sodium, and the fall in pHi was less than during normoxic conditions. When acid extrusion was intact, pHi was not affected by hypoxia and the changes in pHi during activation with AVP were similar during hypoxia and in the control situation. 3. Although a decrease in smooth muscle [Ca2+]i may be partly responsible for the reduced force development during hypoxia, [Ca2+]i-independent mechanism(s) may play an even more important role. Furthermore, although hypoxia and force development are associated with enhanced acid production, acid extrusion maintains pHi near the control level and it is unlikely that a decrease in smooth muscle pHi plays any role in the reduced force development during hypoxia.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalkjaer C., Cragoe E. J., Jr Intracellular pH regulation in resting and contracting segments of rat mesenteric resistance vessels. J Physiol. 1988 Aug;402:391–410. doi: 10.1113/jphysiol.1988.sp017211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aalkjaer C., Hughes A. Chloride and bicarbonate transport in rat resistance arteries. J Physiol. 1991 May;436:57–73. doi: 10.1113/jphysiol.1991.sp018539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aalkjaer C., Mulvany M. J. Effect of changes in intracellular pH on the contractility of rat resistance vessels. Prog Biochem Pharmacol. 1988;23:150–158. [PubMed] [Google Scholar]
- Aalkjaer C., Mulvany M. J. Steady-state effects of arginine vasopressin on force and pHi of isolated mesenteric resistance arteries from rats. Am J Physiol. 1991 Dec;261(6 Pt 1):C1010–C1017. doi: 10.1152/ajpcell.1991.261.6.C1010. [DOI] [PubMed] [Google Scholar]
- Berk B. C., Aronow M. S., Brock T. A., Cragoe E., Jr, Gimbrone M. A., Jr, Alexander R. W. Angiotensin II-stimulated Na+/H+ exchange in cultured vascular smooth muscle cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1987 Apr 15;262(11):5057–5064. [PubMed] [Google Scholar]
- CARRIER O., Jr, WALKER J. R., GUYTON A. C. ROLE OF OXYGEN IN AUTOREGULATION OF BLOOD FLOW IN ISOLATED VESSELS. Am J Physiol. 1964 May;206:951–954. doi: 10.1152/ajplegacy.1964.206.5.951. [DOI] [PubMed] [Google Scholar]
- Carr P., Graves J. E., Poston L. Carbon dioxide induced vasorelaxation in rat mesenteric small arteries precontracted with noradrenaline is endothelium dependent and mediated by nitric oxide. Pflugers Arch. 1993 May;423(3-4):343–345. doi: 10.1007/BF00374415. [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Baron C., Papadopoulos M. T. Phosphoinositide metabolism and metabolism-contraction coupling in rabbit aorta. Am J Physiol. 1988 Dec;255(6 Pt 2):H1476–H1483. doi: 10.1152/ajpheart.1988.255.6.H1476. [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Grubb B., Aronson R. D. Effect of cyanide on oxygen tension-dependent mechanical tension in rabbit aorta. Circ Res. 1979 Mar;44(3):368–378. doi: 10.1161/01.res.44.3.368. [DOI] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Ekmehag B. L. Electrical and mechanical responses to inhibition of cell respiration in vascular smooth muscle of the rat portal vein. Acta Physiol Scand. 1989 Sep;137(1):41–51. doi: 10.1111/j.1748-1716.1989.tb08719.x. [DOI] [PubMed] [Google Scholar]
- Ekmehag B. L., Hellstrand P. Contractile and metabolic characteristics of creatine-depleted vascular smooth muscle of the rat portal vein. Acta Physiol Scand. 1988 Aug;133(4):525–533. doi: 10.1111/j.1748-1716.1988.tb08437.x. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D., Bonnet P., Greene A. S., England S. K., Rusch N. J., Lombard J. H., Harder D. R. Hypoxia increases the activity of Ca(2+)-sensitive K+ channels in cat cerebral arterial muscle cell membranes. Pflugers Arch. 1994 Oct;428(5-6):621–630. doi: 10.1007/BF00374586. [DOI] [PubMed] [Google Scholar]
- Hellstrand P., Johansson B., Norberg K. Mechanical, electrical, and biochemical effects of hypoxia and substrate removal on spontaneously active vascular smooth muscle. Acta Physiol Scand. 1977 May;100(1):69–83. doi: 10.1111/j.1748-1716.1977.tb05923.x. [DOI] [PubMed] [Google Scholar]
- Izzard A. S., MacIver D. H., Cragoe E. J., Heagerty A. M. Intracellular pH in rat resistance arteries during the development of experimental hypertension. Clin Sci (Lond) 1991 Jul;81(1):65–72. doi: 10.1042/cs0810065. [DOI] [PubMed] [Google Scholar]
- Jensen P. E., Hughes A., Boonen H. C., Aalkjaer C. Force, membrane potential, and [Ca2+]i during activation of rat mesenteric small arteries with norepinephrine, potassium, aluminum fluoride, and phorbol ester. Effects of changes in pHi. Circ Res. 1993 Aug;73(2):314–324. doi: 10.1161/01.res.73.2.314. [DOI] [PubMed] [Google Scholar]
- Jensen P. E., Mulvany M. J., Aalkjaer C. Endogenous and exogenous agonist-induced changes in the coupling between [Ca2+]i and force in rat resistance arteries. Pflugers Arch. 1992 Apr;420(5-6):536–543. doi: 10.1007/BF00374630. [DOI] [PubMed] [Google Scholar]
- Jensen P. E., Mulvany M. J., Aalkjaer C., Nilsson H., Yamaguchi H. Free cytosolic Ca2+ measured with Ca(2+)-selective electrodes and fura 2 in rat mesenteric resistance arteries. Am J Physiol. 1993 Aug;265(2 Pt 2):H741–H746. doi: 10.1152/ajpheart.1993.265.2.H741. [DOI] [PubMed] [Google Scholar]
- Kahn A. M., Bishara M., Cragoe E. J., Jr, Allen J. C., Seidel C. L., Navran S. S., O'Neil R. G., McCarty N. A., Shelat H. Effects of serotonin on intracellular pH and contraction in vascular smooth muscle. Circ Res. 1992 Dec;71(6):1294–1304. doi: 10.1161/01.res.71.6.1294. [DOI] [PubMed] [Google Scholar]
- Korner P. I., Angus J. A. Structural determinants of vascular resistance properties in hypertension. Haemodynamic and model analysis. J Vasc Res. 1992 Jul-Aug;29(4):293–312. doi: 10.1159/000158945. [DOI] [PubMed] [Google Scholar]
- Lombard J. H., Smeda J., Madden J. A., Harder D. R. Effect of reduced oxygen availability upon myogenic depolarization and contraction of cat middle cerebral artery. Circ Res. 1986 Apr;58(4):565–569. doi: 10.1161/01.res.58.4.565. [DOI] [PubMed] [Google Scholar]
- Lövgren B., Hellstrand P. Functional role of aerobic glycolysis in rat portal vein. Acta Physiol Scand. 1987 Feb;129(2):211–219. doi: 10.1111/j.1748-1716.1987.tb08061.x. [DOI] [PubMed] [Google Scholar]
- Lövgren B., Hellstrand P. Graded effects of oxygen and respiratory inhibitors on cell metabolism and spontaneous contractions in smooth muscle of the rat portal vein. Acta Physiol Scand. 1985 Apr;123(4):485–495. doi: 10.1111/j.1748-1716.1985.tb07614.x. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Aalkjaer C., Jensen P. E. Sodium-calcium exchange in vascular smooth muscle. Ann N Y Acad Sci. 1991;639:498–504. doi: 10.1111/j.1749-6632.1991.tb17343.x. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Namm D. H., Zucker J. L. Biochemical alterations caused by hypoxia in the isolated rabbit aorta. Correlation with changes in arterial contractility. Circ Res. 1973 Apr;32(4):464–470. doi: 10.1161/01.res.32.4.464. [DOI] [PubMed] [Google Scholar]
- Pearce W. J., Ashwal S., Long D. M., Cuevas J. Hypoxia inhibits calcium influx in rabbit basilar and carotid arteries. Am J Physiol. 1992 Jan;262(1 Pt 2):H106–H113. doi: 10.1152/ajpheart.1992.262.1.H106. [DOI] [PubMed] [Google Scholar]
- Rodman D. M., Hasunuma K., Peach J. L., McMurtry I. F. Inhibitor of ATP-sensitive K+ channel alters neither hypoxic contraction nor relaxation of rat aorta. Blood Vessels. 1990;27(6):365–368. doi: 10.1159/000158830. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Spurway N. C., Wray S. A phosphorus nuclear magnetic resonance study of metabolites and intracellular pH in rabbit vascular smooth muscle. J Physiol. 1987 Dec;393:57–71. doi: 10.1113/jphysiol.1987.sp016810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Wadsworth R. M. Vasoconstrictor and vasodilator effects of hypoxia. Trends Pharmacol Sci. 1994 Feb;15(2):47–53. doi: 10.1016/0165-6147(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Wray S. Smooth muscle intracellular pH: measurement, regulation, and function. Am J Physiol. 1988 Feb;254(2 Pt 1):C213–C225. doi: 10.1152/ajpcell.1988.254.2.C213. [DOI] [PubMed] [Google Scholar]
- Wray S. The effects of metabolic inhibition on uterine metabolism and intracellular pH in the rat. J Physiol. 1990 Apr;423:411–423. doi: 10.1113/jphysiol.1990.sp018030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Beckerath N., Cyrys S., Dischner A., Daut J. Hypoxic vasodilatation in isolated, perfused guinea-pig heart: an analysis of the underlying mechanisms. J Physiol. 1991 Oct;442:297–319. doi: 10.1113/jphysiol.1991.sp018794. [DOI] [PMC free article] [PubMed] [Google Scholar]



