Abstract
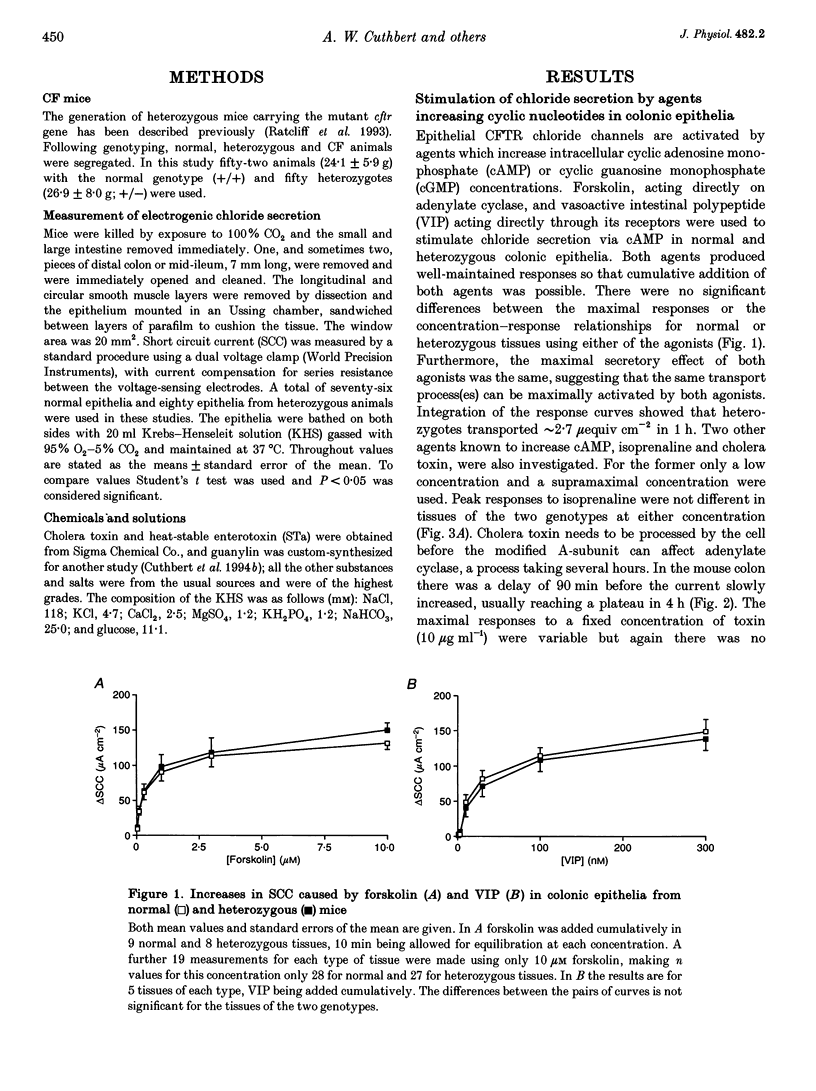
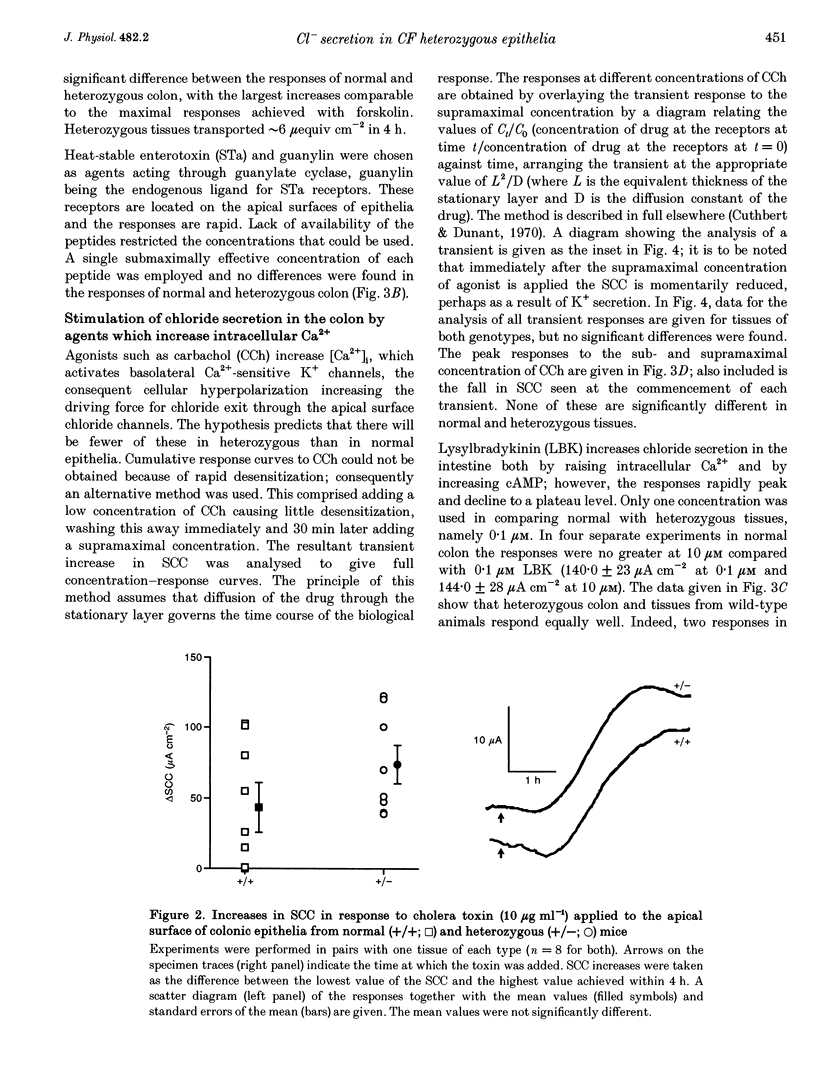
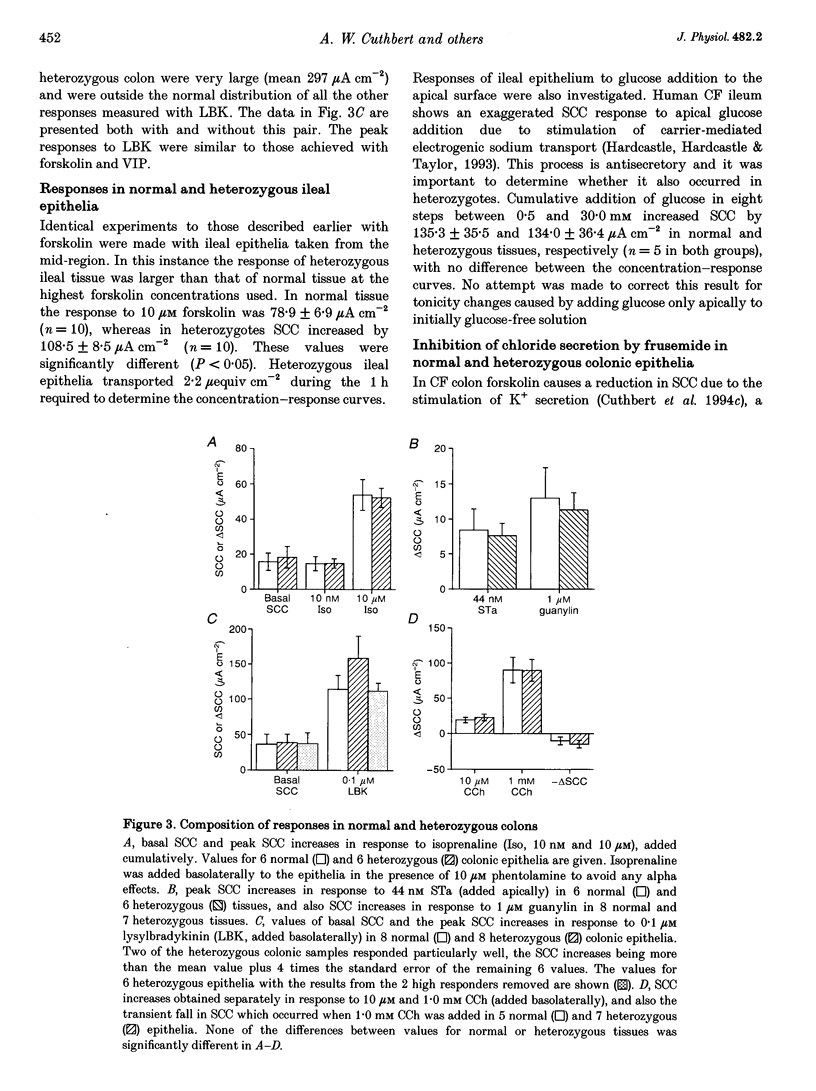
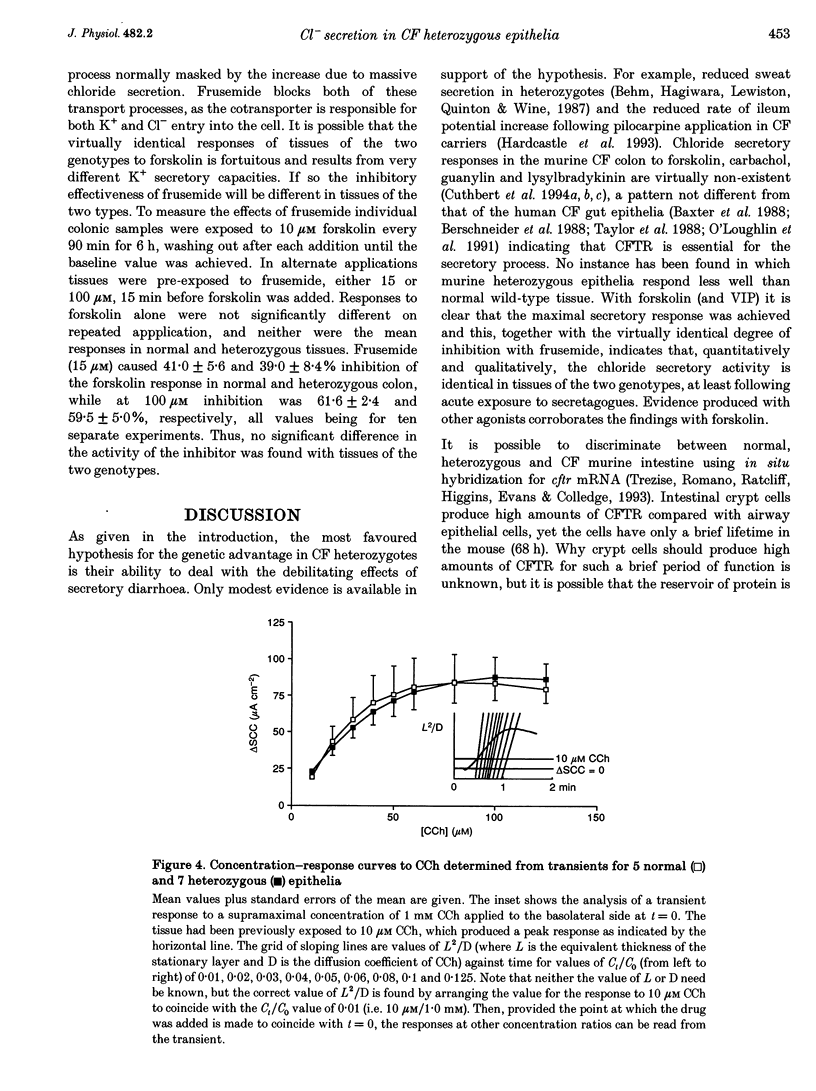
1. The delta F508 mutation of the cystic fibrosis (CF) gene is of high frequency in man (1 in 25) and in homozygotes causes cystic fibrosis. It is suggested that cystic fibrosis heterozygotes withstand secretory diarrhoea better than normal individuals and so are genetically advantaged. This hypothesis has been examined by measuring electrogenic chloride secretion in gut epithelia of normal and heterozygous CF mice. 2. Chloride secretory responses of normal and heterozygous colonic epithelia to forskolin, vasoactive intestinal polypeptide (VIP), isoprenaline, cholera toxin, heat-stable enterotoxin (STa), guanylin, carbachol and lysylbradykinin were examined. No significant differences in responses of tissues of the two genotypes were found. 3. Responses of normal and heterozygous ileal epithelia to forskolin and glucose were investigated. Heterozygous tissues responded as well as normal tissues. 4. Frusemide (furosemide) caused virtually identical inhibition of the chloride secretory responses to forskolin in colonic epithelia of both genotypes. 5. No evidence to support the genetic advantage hypothesis in ileal or colonic epithelia of the null CF mouse has been found, at least for acute responses. If the hypothesis is true then either (a) other non-cystic fibrosis transmembrane conductance regulator (non-CFTR) transport processes are involved, (b) prolonged exposure to secretagogues is required, or (c) delta F508 CFTR is responsible for the protective effect.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter P. S., Goldhill J., Hardcastle J., Hardcastle P. T., Taylor C. J. Accounting for cystic fibrosis. Nature. 1988 Sep 15;335(6187):211–211. doi: 10.1038/335211a0. [DOI] [PubMed] [Google Scholar]
- Behm J. K., Hagiwara G., Lewiston N. J., Quinton P. M., Wine J. J. Hyposecretion of beta-adrenergically induced sweating in cystic fibrosis heterozygotes. Pediatr Res. 1987 Sep;22(3):271–276. doi: 10.1203/00006450-198709000-00007. [DOI] [PubMed] [Google Scholar]
- Berschneider H. M., Knowles M. R., Azizkhan R. G., Boucher R. C., Tobey N. A., Orlando R. C., Powell D. W. Altered intestinal chloride transport in cystic fibrosis. FASEB J. 1988 Jul;2(10):2625–2629. doi: 10.1096/fasebj.2.10.2838365. [DOI] [PubMed] [Google Scholar]
- Chao A. C., de Sauvage F. J., Dong Y. J., Wagner J. A., Goeddel D. V., Gardner P. Activation of intestinal CFTR Cl- channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J. 1994 Mar 1;13(5):1065–1072. doi: 10.1002/j.1460-2075.1994.tb06355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992 May 8;256(5058):774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Dunant Y. Diffusion of drugs through stationary water layers as the rate limiting process in their action at membrane receptors. Br J Pharmacol. 1970 Nov;40(3):508–521. doi: 10.1111/j.1476-5381.1970.tb10632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Evans M. J., Colledge W. H., MacVinish L. J., Ratcliff R. Kinin-stimulated chloride secretion in mouse colon requires the participation of CFTR chloride channels. Braz J Med Biol Res. 1994 Aug;27(8):1905–1910. [PubMed] [Google Scholar]
- Cuthbert A. W., Hickman M. E., MacVinish L. J., Evans M. J., Colledge W. H., Ratcliff R., Seale P. W., Humphrey P. P. Chloride secretion in response to guanylin in colonic epithelial from normal and transgenic cystic fibrosis mice. Br J Pharmacol. 1994 May;112(1):31–36. doi: 10.1111/j.1476-5381.1994.tb13024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., MacVinish L. J., Hickman M. E., Ratcliff R., Colledge W. H., Evans M. J. Ion-transporting activity in the murine colonic epithelium of normal animals and animals with cystic fibrosis. Pflugers Arch. 1994 Oct;428(5-6):508–515. doi: 10.1007/BF00374572. [DOI] [PubMed] [Google Scholar]
- Field M., Semrad C. E. Toxigenic diarrheas, congenital diarrheas, and cystic fibrosis: disorders of intestinal ion transport. Annu Rev Physiol. 1993;55:631–655. doi: 10.1146/annurev.ph.55.030193.003215. [DOI] [PubMed] [Google Scholar]
- Gabriel S. E., Brigman K. N., Koller B. H., Boucher R. C., Stutts M. J. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science. 1994 Oct 7;266(5182):107–109. doi: 10.1126/science.7524148. [DOI] [PubMed] [Google Scholar]
- Guggino S. E. Gates of Janus: cystic fibrosis and diarrhea. Trends Microbiol. 1994 Mar;2(3):91–94. doi: 10.1016/0966-842x(94)90541-x. [DOI] [PubMed] [Google Scholar]
- Hansson G. C. Cystic fibrosis and chloride-secreting diarrhoea. Nature. 1988 Jun 23;333(6175):711–711. doi: 10.1038/333711c0. [DOI] [PubMed] [Google Scholar]
- O'Loughlin E. V., Hunt D. M., Gaskin K. J., Stiel D., Bruzuszcak I. M., Martin H. C., Bambach C., Smith R. Abnormal epithelial transport in cystic fibrosis jejunum. Am J Physiol. 1991 May;260(5 Pt 1):G758–G763. doi: 10.1152/ajpgi.1991.260.5.G758. [DOI] [PubMed] [Google Scholar]
- Quinton P. M. Human genetics. What is good about cystic fibrosis? Curr Biol. 1994 Aug 1;4(8):742–743. doi: 10.1016/s0960-9822(00)00166-4. [DOI] [PubMed] [Google Scholar]
- Ratcliff R., Evans M. J., Cuthbert A. W., MacVinish L. J., Foster D., Anderson J. R., Colledge W. H. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat Genet. 1993 May;4(1):35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- Taylor C. J., Baxter P. S., Hardcastle J., Hardcastle P. T. Failure to induce secretion in jejunal biopsies from children with cystic fibrosis. Gut. 1988 Jul;29(7):957–962. doi: 10.1136/gut.29.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]


