Abstract
Gelatin is used in a broad range of tissue engineering applications due to its bioactivity, mild processing conditions, and ease of modification which has increased interest in its use as a growth factor delivery vehicle. Traditional methods to control growth factor sequestration and delivery relied on controlling hydrogel mesh size via chemical crosslinking with corollary changes to the physical properties of the hydrogel. To decouple growth factor release from scaffold properties, affinity sequestration modalities were developed to preserve bioactivity of the growth factor through interactions with the modified gelatin. This review will provide a summary of these mechanisms, highlight current gelatin growth factor delivery systems, and address the future perspective of gelatin matrices for growth factor delivery in tissue engineering.
Keywords: Gelatin, Growth Factor, Controlled Release, Sequestration, Affinity
Polymeric Matrices for Growth Factor Delivery
The core aim of tissue engineering is to guide cellular processes to enhance tissue regeneration and restore function. One of the strategies used to instruct cellular responses during tissue repair is the delivery of growth factors that promote cell migration, proliferation, and differentiation [1]. The ability of growth factors to direct cellular behavior is dependent on the concentration as well as the spatial dispersion. Bolus delivery of growth factors display limited efficacy and adverse side effects such as ectopic growth and carcinogenic effects. Researchers attempt to address these limitations by developing materials to provide localized delivery and controlled release [2].
Advances in polymeric material design over the last 25 years have enabled the development of tunable platforms for growth factor delivery [3–5]. Synthetic polymers (e.g. poly(lactide-co-glycolide) (PLGA), polylactide (PLA), polyglycolide (PGA), polycaprolactone (PCL)) offer several advantages including ease of manufacture, tunable degradation, and established use in small molecule delivery [6]. However, the harsh processing conditions required for fabrication of synthetic polymers, such as high temperatures or organic solvents, can denature growth factors leading to a loss in bioactivity [7]. To circumvent this loss of bioactivity due to processing, growth factors can be loaded into the matrix after fabrication. Post-fabrication loading can restrict the loading capacity to adsorption to the surface or absorption in the water-swollen polymer matrix [2]. In addition, degradation of synthetic polymers can result in an inflammatory response due to toxic by-products or changes to the local pH [6]. As an alternative, natural polymers and their derivatives, such as collagen, gelatin, chitosan, and alginate, are often processed in aqueous solvents. These mild processing conditions allow for in-line loading of the growth factors with a corollary increase in loading capacity over synthetic matrices. As biological materials, degradation byproducts are cytocompatible and readily cleared from the body [8, 9]. One of the more common natural polymers used for growth factor delivery is gelatin due to its versatile fabrication processing, ease of modification, and its electrostatic properties that confer growth factor affinity [10, 11]. There has been an increase in the development of gelatin delivery systems that provide tunable delivery of growth factors to support bioactivity retention [12–14]. However, sequestration and release is primarily governed by an increase in the crosslink density resulting in structural changes to the gelatin matrix [15]. The focus of this review is to provide a summary of alternative mechanisms to enhance growth factor sequestration in gelatin matrices, current gelatin growth factor matrices in practice, and future perspectives of gelatin matrices in tissue engineering.
Affinity Sequestration to Control Growth Factor Release
The efficacy of growth factor therapy for tissue engineering applications is highly dependent on retaining the bioactivity during fabrication and application. Gelatin matrices offer advantages over synthetic polymeric carriers due to its mild fabrication conditions (e.g. aqueous solution processing) and high growth factor loading during fabrication [16, 17]. Standard processing of collagen to generate gelatin also increases its solubility and provides ease of fabrication as compared to collagen delivery vehicles. The selected hydrolytic treatment (acidic or basic) determines the isoelectric point (IEP) of gelatin matrices, the pH at which the charge on the gelatin is zero [18–20]. Acidic pre-treatment results in positively-charged gelatin (type-A gelatin) with an IEP between pH 8–9. Alkaline pre-treatment hydrolyzes amide residues to carboxyl residues leading to negatively-charged gelatin (type-B gelatin) with an IEP between pH 4.8–5.4 [21]. The net charge of gelatin enables electrostatic interactions with oppositely charged growth factors which inherently sequesters growth factors. However, rapid dissolution of gelatin during implantation requires gelatin matrices to be crosslinked into hydrogels (Box 1) [22]. The crosslink density determines the mesh size of the hydrogel, which is a primary consideration in the sequestration and release of growth factors in gelatin matrices (Figure 1). Growth factors that are smaller than the effective mesh size diffuse out rapidly and are at risk for proteolytic degradation; whereas, growth factors that are larger than the effective mesh size are sequestered and protected. As such, modulation of the mesh size by changing the gel crosslink density provides a mechanism to tune the release profile. Crosslink density also affects a number of gel physical properties including swelling, mechanical properties, and degradation rate [15]. Growth factor conjugation has been investigated as an alternative to sequester growth factors irrespective of the hydrogel mesh size. As mentioned in Box 1, gelatin contains several chemical groups that enable covalent crosslinking within gelatin or to adjacent gelatin molecules. These chemical reactions can covalently bind growth factors to gelatin to enhance sequestration [23–25]. Release of conjugated growth factors will be delayed until cleavage of gelatin matrices and/or linkers permit diffusion (Figure 2) [26]. Among these conjugation modalities are bi-functional crosslinkers such as diisocyanates or susuccinimidyl valerate which facilitate covalent bonding with the available free amines on gelatin and growth factors [26, 27]. However, it is possible for side reactions to occur such as a single bi-functional crosslinker binding two growth factors or multiple bi-functional crosslinkers binding a single growth factor due to the ratio of amines present of growth factors [27]. The latter could affect the hydrogel mesh size by behaving as an additional crosslink point with the gelatin. Alternatively, a two-step process of functionalization of the gelatin and growth factor independently following by a conjugation step provides additional control over the reaction. One of the most common examples of this process is the use of methacrylated gelatin and acrylated or methacrylated growth factors that undergo free radical polymerization in the presence of a photo-initiator and UV irradiation [28]. Although conjugation modalities have been successful at immbolizing growth factor for sustained sequestration, the poor control of conjugation sites on growth factors, typically non-specific amino groups, puts these techniques at a high potential for bioactivity loss [29]. To address this limitation, affinity sequestration (see Glossary) has been explored as a means to sequester the growth factor for sustained release without loss of bioactivity and minimal effect of the gelatin physical properties. Common affinity sequestration approaches will be described in detail with a focus on the relationships with physiochemical properties and gelatin matrix design, Figure 3, Key Figure.
Box 1. Overview of covalent crosslinking modalities for gelatin matrices.
The crosslinking modality and degree of crosslinking can strongly impact the resulting physical properties of the gelatin matrix [70, 72]. Reagent-based crosslinking modalities enable homogenous crosslinking and are categorized as either non-zero length or zero-length based on assisted bonding or direct bonding, respectively [91, 92]. Non-zero length crosslinking reagents (e.g. aldehydes, isocyanates, and polyepoxides) react with free amine residues and/or carboxylic acid residues to form intramolecular and intermolecular (see Glossary) crosslinks within a gelatin solution. Zero-length crosslinking agents (e.g. acyl azides and carbodiimides) facilitate the direct reactions between carboxylic acid residues and amine residues on the same gelatin molecule or adjacent gelatin molecules without intermediate molecules in the network [93]. However, these chemical crosslinking modalities can have residual unreacted reagents that could compromise the biocompatibility of the gelatin matrices. Less toxic, covalent crosslinking modalities include natural enzymes such as genipin, which is a natural reagent derived from the gardenia fruit. It facilitates crosslinking in a two-step process that first reacts with amine residues of gelatin followed by reaction with esters of genipin with amine residues of gelatin [22]. Photo-polymerization is another common method of covalently crosslinking gelatin. This method requires functionalization of gelatin with a primer (e.g. acrylamide, methacrylamide) in order to undergo photo-polymerization in the presence of free radicals [94]. Use of these modalities offer versatile methods for controlling the mechanical and physical properties of gelatin matrices.
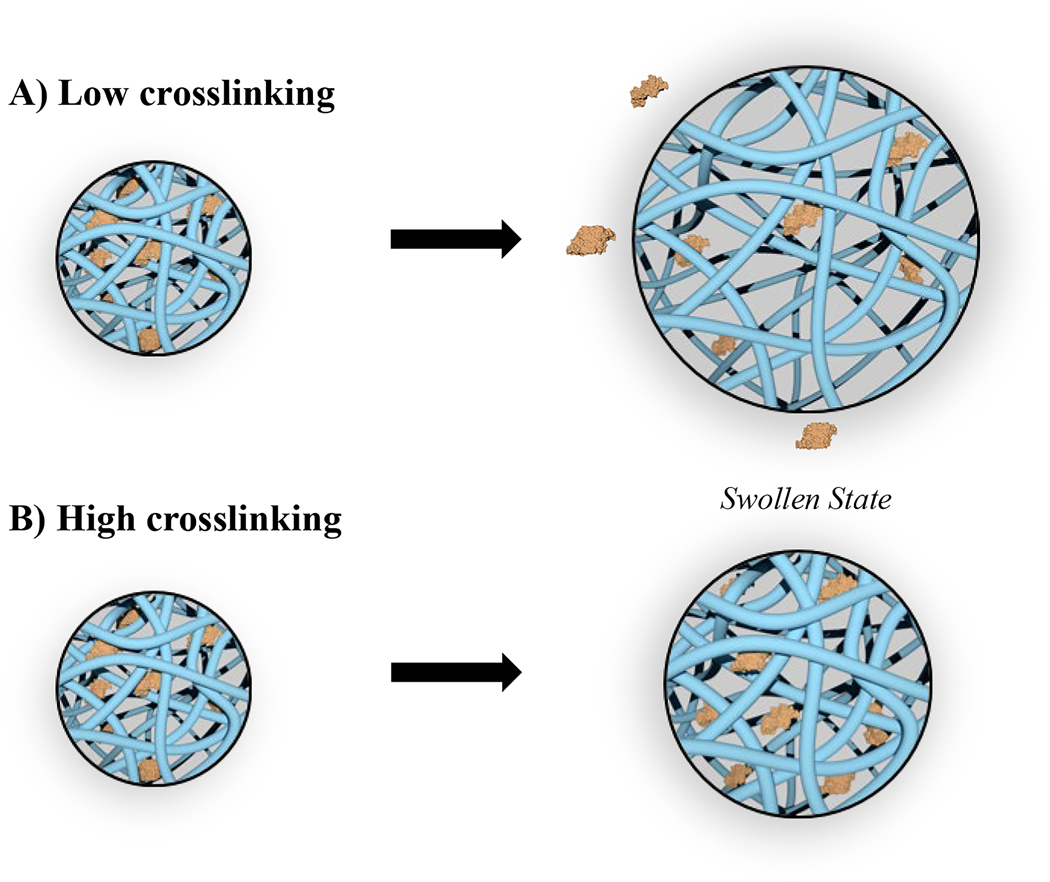
Figure 1.
The degree of crosslinking affects the hydrogel mesh size that governs growth factor release from gelatin matrices. A) Low crosslinking results in rapid swelling and diffusion. B) High crosslinking results in reduced swelling and sustained diffusion.
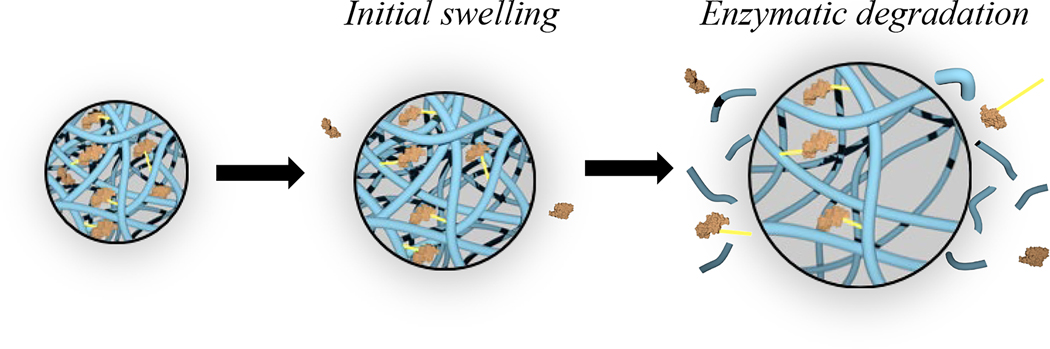
Figure 2.
Effect of conjugation on growth factor sequestration in gelatin matrices. Growth factor-conjugated gelatin matrix displays burst release due to initial swelling that releases non-conjugated growth factors followed by sustained growth factor release after proteolytic chain scission.
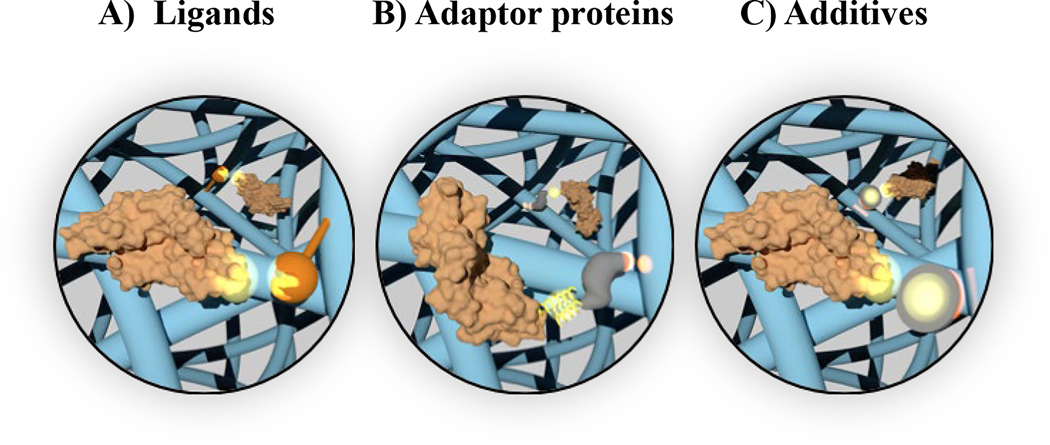
Figure 3, Key Figure. Overview of the physiochemical properties governing growth factor diffusion from gelatin matrices.
These properties include growth factor affinity to A) ligands, B) adaptor proteins, and C) nanomaterial additives incorporated into gelatin matrices.
The established interactions of growth factors and the extracellular matrix (ECM) is a rich field to draw design inspiration for sequestering growth factors. As such, ECM-derived ligands are one of the most common moieties used to sequester growth factors in gelatin matrices. These non-covalent bonds do not impair the stability or bioactivity of growth factors [29–31]. Among these target ECM ligands is heparin, a negatively-charged glycosaminoglycan that has binding domains for several growth factors. Heparin binds several growth factors via electrostatic interactions between amino acid residues and carboxyl groups. It has commonly been incorporated into gelatin through functionalization by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride/N-hydroxysulfosuccinimide (EDC/NHS). EDC/NHS reactions facilitate crosslinking between carboxyl groups on heparin and amino acid residues on gelatin, but intramolecular or intermolecular crosslinking of gelatin is also possible with this technique [31, 32]. This can be avoided by first activating the carboxyl groups on heparin with EDC prior to its incorporation into gelatin [33]. The heparin-modified gelatin matrix can then be used to sequester growth factor with high affinity and without chemically modifying the growth factor. An adapter protein is another type of ligand-based moiety that is composed of a coiled peptide and a collagen-binding domain (CBD) (see Glossary) derived from fibronectin. CBDs derived from fibronectin have high affinity towards collagen and gelatin and facilitates ready modification of gelatin matrices [30]. The coiled peptide tethered to the CBD enables electrostatic binding with a complementary coiled peptide tethered to a growth factor of interest. This technology has proven to be highly adaptable and can be modified to increase the binding strength to gelatin by altering the source of CBD [30]. Furthermore, high-throughput screening of DNA/RNA libraries using systematic evolution of ligands by exponential enrichment (SELEX) technique enables selection of peptide and oligonucleotide aptamers (see Glossary) with high binding affinity by electrostatic interactions [34, 35]. Aptamers can be incorporated into gelatin matrices by standard bioconjugation methods [34]. As an alternative to chemical modification of the gelatin matrix, nanomaterial additives with growth factor affinity have been explored to generate gelatin nanocomposite delivery systems. These additives can be readily mixed into gelatin precursor solutions and provide a high surface area to facilitate growth factor adsorption. Common nanomaterials used to sequester growth factors in gelatin matrices are nanodiamonds, carbon-based nanoparticles with truncated octahedral structures, and nanoclays. Functional groups on the surface of nanodiamonds determine interfacial interactions with growth factors. These interaction include electrostatic, hydrogen bonds, dipole-dipole, and hydrophobic adsorption and vary depending on the processing technique used during the synthesis of the nanodiamonds [36]. Surface modification of the nanodiamonds through carboxylation or hydroxylation can also be used to provide covalent conjugation to gelatin prior to crosslinking of gelatin for greater stability [36, 37]. As compared to other widely used carbon-based nanomaterials such as graphene oxide and carbon nanotubes, nanodiamonds have greater biocompatibility [36]. Two-dimensional nanoclays are another type of nanomaterial with superior biocompatibility. Nanoclays are discs composed of an octahedral sheet of magnesium oxide inserted between two parallel tetrahedral sheets of silica which results in negatively-charged surfaces and a positively-charged edge. Sodium ions adsorbed to the surface of nanoclays during manufacturing foster ionic interactions with neighboring nanoclays in dry environments. However, nanoclays dissociate in ionic aqueous solutions due to favorable interactions between the sodium ions and hydroxide molecules or other ions. Dissociation allows for rearrangement and greater access to the charged surfaces by proteins [38]. Similar to nanodiamonds, nanoclays are generally incorporated into gelatin solutions prior to crosslinking [38, 39].
There are several applications in tissue engineering that have used these affinity sequestration approaches to achieve growth factor delivery ranging from 5 days to 25 days including cardiovascular repair [39], angiogenesis [34, 40, 41], bone healing [31], and wound healing [30, 42]. For example, adaptor proteins with coiled-CBDs specific for gelatin were incorporated into EDC/NHS-crosslinked gelatin. Epidermal growth factor (EGF) (see Glossary) tethered with a complementary coil was added to the gelatin to allow for non-covalent binding. This non-covalent binding resulted in sequestration of EGF for over four days [30]. Given this relatively moderate time frame, this matrix could be employed in wound healing to initiate cell proliferation for tissue regeneration. Alternatively, the strong binding affinity of heparin to vascular endothelial growth factor (VEGF) (see Glossary) has been used in a gelatin composite wrap that was crosslinked with EDC/NHS. This matrix was able to sustained release of VEGF over three weeks rendering it useful to direct capillary formation, homogenization, and maturation of blood vessels that typically occurs during the first three weeks of angiogenesis [40]. In addition to using these modalities for moderate sequestration, they can be selected for high specificity to increase the time that growth factors are preserved in gelatin matrices. This is especially true for aptamers as demonstrated in a study that selected the acrydite oligonucleotide using SELEX due to its high bind specificity for VEGF [34]. Another advantage of these affinity sequestration is that they can be used for concentrated localization of growth factors to a particular area in the matrix. For example, a single nanodiamond can bind multiple growth factors based on its high surface area that allows for increased adsorption [36]. Furthermore, spatial concentration of nanodiamonds in gelatin matrices provides another mean to sequester growth factors by regulating the diffusion path length as later discussed. Despite the potential to sequester growth factors with minimal impact on the bioactivity, careful consideration must be given to the transient and reversible interactions that govern sequestration when sustained preservation is desired. Another consideration is that freely encapsulated nanomaterials can potentially bind the gelatin matrix due to their high surface area resulting in changes to the physical properties [41].
Gelatin Matrices in Tissue Engineering
An accompanying aspect that affects growth factor release kinetics is the fabrication technique, which determines the diffusion path length through the gelatin matrix [43]. Advancements in fabrication of gelatin-based matrices have provided several opportunities to create systems for the controlled delivery of growth factors. The geometry and size of gelatin matrices can be altered through various fabrication techniques to control growth factor sequestration. These strategies are primarily focused on changing the surface-area-to-volume ratio and the diffusion path length of the embedded growth factors [44, 45]. As a general consideration, an increase in surface area will decrease the diffusion path length with a corollary increase in the release kinetics. These considerations can be applied to the design of the gelatin delivery system regardless of the resulting geometry (e.g. microparticles, gels, fibrous meshes). The following section will describe fabrication techniques that control growth factor sequestration for growth factor delivery in a variety of tissue engineering applications.
Gelatin microparticles
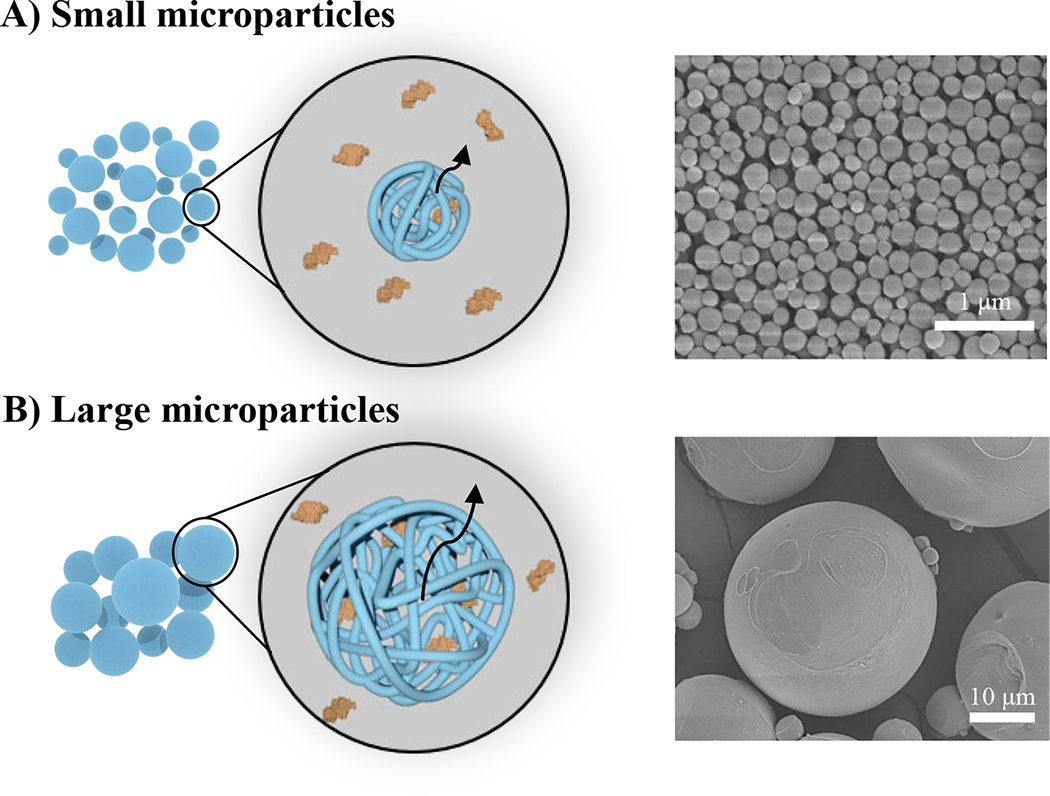
Gelatin microparticles offer several advantages as a growth factor delivery vehicle such as a high surface area-to-volume ratio. Typically, smaller microparticles display faster growth factor release rates due to an increase in surface area and a shorter diffusional path length of embedded growth factors (Figure 4) [44, 46–48]. This was demonstrated in a study that showed microparticles that were 0.20 ± 0.04 μm in average diameter resulted in release of 70% of bone morphogenetic protein 2 (BMP-2) (see Glossary) as compared to 12% from microparticles with an average diameter of 26 ± 6.0 μm after four weeks [44]. Microsphere size and shape can be tuned through various manufacturing techniques with the most common technique being water-in-oil emulsions induced by mechanical agitation such as high-speed stirring of a gelatin solution and an organic phase. These emulsions are then cooled to allow for gelation of the microparticles followed by precipitation [44, 49, 50]. Processing parameters such as mixing speed and solvent selection are used to control microparticle size; however, this technique typically results in a large particle size distribution [46, 51]. As an alternative to high-speed stirring, microfluidic devices have been used to achieve monodispersed particle size distributions. This technique consists of coaxial flow between an aqueous gelatin solution and an oil-based sheath with each phase set to different flow rates to control the droplet size [52, 53]. Particles are collected in a coagulation bath prior to chemical crosslinking. If smaller microparticles are desired, then electrospraying can be used to form microparticles. This technique applies an electric field to a low viscosity gelatin solution as it is being extruded from a syringe. Charge repulsion within the solution droplet at the end of the capillary overcomes the solution surface tension leading to a solution droplets erupting from the droplet towards a ground or oppositely charged collector. As the solvent evaporates from the droplet during flight to the collector, a repulsive threshold is reached within the droplets leading to solution fission into smaller dried particles [46, 54]. Gelatin microparticles are most commonly crosslinked following fabrication by chemical reagents (e.g. glutaraldehyde) [55–57]. It can be difficult to control the crosslinking density and these chemical reagents are typically cytotoxic [24, 58]. Genipin and carbodiimides are alternative reagents used to provide greater control over crosslinking of gelatin microparticles with less cytotoxicity [23, 59, 60].
Figure 4.
Effect of construct surface area-to-volume ratio on growth factor diffusion from gelatin microparticles. A) Smaller microparticles have shorter diffusion path lengths leading to rapid release of growth factors; B) larger microparticles have longer diffusion path lengths and slower release profiles. Representative scanning electron micrographs reproduced with permission from [44].
The tunable nature of gelatin microparticles makes them suitable for a range of tissue engineering applications such as angiogenesis [61], cartilage repair [62, 63], bone regeneration [13], ocular repair [14], and nerve regeneration [60, 64]. Most notably, their size enables incorporation into larger scaffolds as a method to decouple growth factor release kinetics from other scaffold design criteria [46, 65, 66]. This was demonstrated in a study that incorporated gelatin microparticles loaded with VEGF into a porous lithium calcium polyphosphate scaffold for bone repair associated with glucocorticoids-induced osteonecrosis of the femoral head. Microparticles were fabricated through emulsion templating and crosslinked in glutaraldehyde prior to diffusional loading of VEGF [57]. In addition to the use of gelatin microparticles in composite scaffolds, microparticles can also be directly injected as a slurry of particles for more rapid growth factor delivery. Hirose and colleauges reported the delivery of basic fibroblast growth factor (bFGF) (see Glossary) and interferon-beta (IFNβ) from gelatin microparticles as a method to establish a proliferative vitreoretinopathy disease model. The microparticles were fabricated using emulsion templating and crosslinked in glutaraldehyde prior to diffusional loading of bFGF or IFNβ [14]. As a general consideration, direct application of gelatin microparticles may result in a higher initial burst release that results from immediate exposure to aqueous solutions as compared to microparticles embedded within a composite.
Gelatin scaffolds
Several researchers have explored the use of gelatin constructs to act as both a controlled growth factor delivery vehicle and as a scaffolding material. Gelatin scaffolds have been fabricated using a variety of methods including electrospinning, microfluidics, freeze-drying, and porogen leaching. The resulting porous, three-dimensional architectures template new tissue formation by supporting cell attachment, proliferation, and migration [67, 68]. These same pores can alter the diffusion path length that affects growth factor release profiles as previously described in delivery vehicles. Electrospinning has become one of the most widely used techniques to fabricate gelatin scaffolds that serve as growth factor matrices. During electrospinning, an electric potential is applied to a gelatin solution that is constantly flowing from a syringe. Charge repulsion within the solution droplet at the end of the capillary overcomes the solution surface tension leading to a solution jet erupting from the droplet towards a ground or oppositely charged collector. As the solvent evaporates from the solution jet during flight to the collector, nanometer to micron-sized solid polymer fibers are generated [69]. Electrospun scaffolds are commonly crosslinked post-fabrication using glutaraldehyde [70] or EDC/NHS [40, 71]. If gelatin-methacrylate is used, then fibers can be in-situ crosslinked by UV photo-polymerization [26, 72]. Alternatively, reactive electrospinning utilizes a bi-functional crosslinker such as a diisocyanate to initiate in-situ crosslinking of gelatin fibers during the electrospinning process [26]. Similar to the microparticles, the electrospinning parameters such as solvent, distance, and flow rate can be modulated to generate a range of fiber diameters with larger fibers resulting in longer diffusion path lengths for sustained release profiles (Figure 5) [45, 67, 68]. This was demonstrated in a study that showed electrospun fibers with an average diameter of 1.0 ± 0.1 μm resulted in 7.7 ng of platelet derived growth factor (PDGF) as opposed to 4.8 ng from thicker fibers with an average diameter of 3.0 ± 0.2 μm after 20 days [45]. Although electrospinning is the most-widely used fabrication technique for gelatin scaffolds that serve as growth factor matrices, a variety of fabrication techniques have been used to produce fibrous gelatin constructs with a range of surface-area-to-volume ratio and shapes. For example, microfluidic spinning is another common manufacturing process that is similar to microfluidic microparticle fabrication in that an aqueous gelatin solution is flowed through an oil-based sheath or in a silicone microchannel [68, 73]. Differences in flow rates, surface tension, and energy dissipation keeps the two streams separated. This technique allows for precise control over the architecture and uniform size of the resultant fibers. Precipitation of the gelatin fibers can also be achieved through a coagulation bath. Fibers produced by microfluidic spinning range from nanometers to hundreds of microns [68] and are generally crosslinked by glutaraldehyde [73], UV photo-initiation [74, 75] following precipitation.
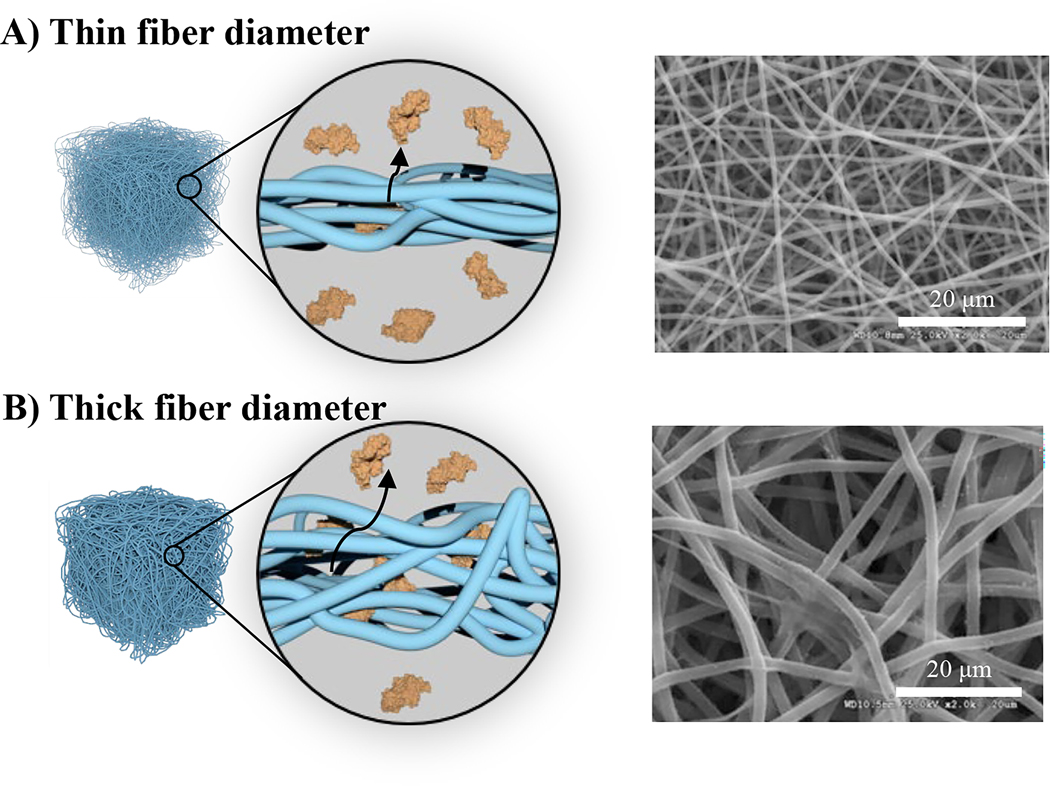
Figure 5.
Effect of construct surface area-to-volume ratio on growth factor diffusion from gelatin fibers. The diffusion path length in electrospun constructs are controlled by fiber diameter with A) thin fibers having shorter diffusion path lengths and rapid release; B) thick fibers have longer diffusion path lengths and slower release profiles. Representative scanning electron micrographs reproduced with permission from [45].
The breadth of architectures available enables the use of gelatin scaffolds in a range of applications such as wound healing [70], bone regeneration [72, 76], and angiogenesis [40, 71]. A primary advantage of fibrous gelatin matrices is that they have the potential to be applied as a stand-alone treatment for tissue engineering grafts [77]. For example, electrospun gelatin fiber meshes loaded with FGF-2 were fabricated for potential use as a tissue engineering construct. Gelatin fibers were crosslinked by both EDC and glutaraldehyde and FGF-2 was bound to gelatin fibers by electrostatic avidin-biotin-complexes. These composite meshes displayed enhanced cell attachment and proliferation, key targets for enhanced tissue regeneration [70]. In another application, electrospun gelatin wraps containing transforming growth factor beta 2 (TGFβ2) were fabricated and crosslinked by genipin. The scaffolds displayed enhanced proliferation and migration with potential application as a medial layer of vascular grafts for modulation of the hemostatic environment [78]. Although fibrous gelatin grafts permit controlled release of growth factors and support cell proliferation and migration, densely packed fibers can limit cell infiltration [79, 80].
Concluding Remarks and Future Perspectives
Multiple growth factor delivery matrices for tissue engineering are currently being assessed in preclinical research. Among these, gelatin has evolved as one of the most widely studied growth factor delivery vehicles due to its native physiochemical properties that enable high loading efficiencies and tunable crosslinking and fabrication processes that provide a broad range of mechanisms to sequester growth factors for temporal release. As opposed to cell transplantation, which involves implanting autologous or exogenous sources of pluripotent cells to replace damaged or lost cell populations in injured tissues, gelatin-based growth factor delivery circumvents risks of tumorigenecity, cost and translational hurdles with ex vivo expansion, and ethical concerns [81]. Upregulation of growth factor expression through gene transfection has also been investigated as an alternative to growth factor delivery. Despite the potential to reprogram tissue, finite control over growth factor expression, risks of tumorigenecity, and insufficient experimental models to demonstrate vector stability in physiological relevant conditions limits clinical translation [82].
Translation of gelatin-based growth factor delivery is projected to advance in the near future as the commercialization of Infuse™, a BMP-2-laden collagen bone graft, has set the foundation for growth factor delivery in clinical settings. Despite its broad clinical use, the poor affinity of collagen to BMP-2 resulted in poor control over the release of BMP-2 [83]. Supraphysiological concentrations released from the device resulted in a number of complications including ectopic bone formation, paralysis, sexual dysfunction, respiratory failure, inflammation of adjacent tissues, excessive bleeding, and even death [84]. These complications further highlight the advantage of gelatin-for sequestration and controlled release of growth factors. Most notable is the ability to generate gelatin with positive or negative charge expands its affinity for growth factors based on IEPs. Moreover, incorporation of the moieties previously discussed in this review further enhance the potential of gelatin matrices to overcome the limitations of commercial collagen matrices and may accelerate clinical translation.
Although we have highlighted the advantages of affinity-based approaches to sequester growth factors and preserve bioactivity, a primary general drawback to consider is that these interactions are not always stable across physiological conditions (see Outstanding Questions). Conventional amine-targeting conjugation modalities institute covalent bonds for more stable interactions. The lack of specificity of these conjugation reactions can results in partial or full masking of the active site or can denature the protein; both of which constitute a loss of bioactivity [29]. Recent investigation of bio-orthogonal crosslinking has highlighted the promise of click chemistry as an alternative to stably sequester growth factors with retained bioactivity. In particular, artificial amino acids that contain functional groups like alkyne or keto permit covalent conjugation to a specific reactive site on growth factors that is distinguished from naturally present amino groups. These reactive sites rapidly react with a complementary functional groups like azide or aldehyde, respectively [85, 86]. The high specificity and fast reaction kinetics limits the possibility for conjugation at other reactive sites (e.g. amino groups). Although this technology has recently been investigated for in-situ crosslinking of gelatin carriers for cell encapsulation, incorporation of these reactive functional groups into gelatin consisted of conventional chemical conjugation that non-specifically binds amino groups [87]. Therefore, extension of bio-orthogonal crosslinking chemistry to gelatin matrices for covalent growth factor immobilization should consider mechanisms that will not impact the bioactive sites on growth factors. Post-translational mutagenesis is one suggested mechanism, but its use for tethering complementary functional groups onto gelatin matrices requires further investigation.
Outstanding Questions.
In addition to its role as a carrier, the gelatin matrix itself and its degradation products can influence cell behavior. What are the possible synergistic effects of integrin-mediated cell behavior of the gelatin matrix when in the presence of growth factors?
How do current issues with batch-to-batch variability in animal-derived gelatin affect its commercialization potential?
Will processes used to generate recombinant gelatin for stabilizers and coatings translate to regenerative medicine applications to address limitations of animal-derived products?
How does chemical modification of gelatin to introduce targeted affinity affect its degradation, clearance, cell interactions, and possible immunogenicity?
In addition to serving as a growth factor carrier, there is a growing body of research that suggests integrin-mediated cell signaling initiated by the extracellular matrix and products can affect tissue regeneration. For example, α2β1 integrin interaction with a collagen-mimetic protein has been shown to enhance bone regeneration [88]. To further capitalize on this natural response, additional research should be conducted on α5β1 and αvβ3 responses to gelatin peptides containing the RGD sequence. In particular, investigation of the integrin-response to gelatin scaffolds versus composites containing gelatin peptides will further divulge the potential for enhanced tissue regeneration using gelatin. A similar consideration is the cellular response to gelatin by-products. Furthermore, evaluation of the potential synergistic interactions between integrin and growth factors will also increase the fundamental knowledge of gelatin matrices for tissue regeneration. This can be achieved by antibody blocking studies, as demonstrated in [88], where blocking the α2 subunit on α2β1 was shown to inhibit VEGF-mediated chemotaxis indicating synergy. Another consideration is the specificity of these integrin-mediated responses with specific growth factors.
Another concern with the clinical use of gelatin matrices is the large molecular weight distribution and other batch-to-batch variability that results due to its derivation from animal sources [89]. Homogeneity can be improved through genetic recombination technology to fabricate recombinant human gelatin. Large-scale preparation of recombinant gelatin holds promise in terms of customizing properties for specific applications. Although this technique has been explored, commercially available forms are primarily used as a stabilizer or coating for cell attachment [90]. Advances in these research areas as well as continued refinement of fabrication processes will continue to expand the utility of gelatin-based growth factor delivery in tissue engineering applications.
Highlights.
Traditional methods of growth factor sequestration and delivery in gelatin matrices relied on controlling hydrogel mesh size via chemical crosslinking with corollary changes to the physical properties of the hydrogel.
Growth factors are generally not directly conjugated to gelatin matrices due to the potential loss of bioactivity from non-specific reaction of amino groups.
Elucidating non-covalent growth factor interactions has led to the development of affinity-based methods to increase sequestration in gelatin matrices.
Modifying gelatin matrices to confer targeted growth factor affinity has the potential to improve tissue regeneration over traditional crosslinking methods.
Glossary
- Affinity sequestration
utilization of non-covalent bonds between growth factors and a material to sequester the growth factor within the delivery vehicle and sustain release
- Aptamer
a peptide or oligonucleotide capable of binding target proteins or small molecules with high specificity through complementary tertiary structures of the aptamer and target
- Bone morphogenic protein 2 (BMP-2)
a growth factor of the transforming growth factor beta (TGF-β) superfamily of proteins that play a role in bone and cartilage remodeling and promoting osteogenic differentiation
- Collagen-binding domain
a region of a protein, such as fibronectin, with high affinity to bind collagen or gelatin
- Epidermal growth factor (EGF)
a growth factor that stimulates mitosis of various cell types enabling cell growth, proliferation, and differentiation
- Fibroblast growth factor 2/basic fibroblast growth factor (FGF-2/bFGF)
a growth factor that promotes angiogenesis as well as mitosis of endothelial cells
- Intermolecular crosslinking
covalent crosslinking that takes place between gelatin molecules in a solution
- Intramolecular crosslinking
covalent crosslinking that takes place within a gelatin molecule
- Non-zero length crosslinker
describes a chemical crosslinker that assists intramolecular or intermolecular bonding within a gelatin solution using intermediate molecules
- Vascular endothelial growth factor (VEGF)
a potent growth factor that promotes angiogenesis and has been used to promote new blood vessel formation
- Zero-length crosslinker
describes a chemical crosslinker that directly facilitates intramolecular or intermolecular bonding within a gelatin solution without using intermediate molecules
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khademhosseini A. and Langer R, A decade of progress in tissue engineering. Nature Protocols, 2016. 11: p. 1775. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, et al. , Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Materials, 2017. 9(10): p. e435–e435. [Google Scholar]
- 3.Hubbell JA, Bioactive biomaterials. Current opinion in biotechnology, 1999. 10(2): p. 123–129. [DOI] [PubMed] [Google Scholar]
- 4.Richardson TP, et al. , Polymeric system for dual growth factor delivery. Nature Biotechnology, 2001. 19: p. 1029. [DOI] [PubMed] [Google Scholar]
- 5.Luginbuehl V, et al. , Localized delivery of growth factors for bone repair. European journal of pharmaceutics and biopharmaceutics, 2004. 58(2): p. 197–208. [DOI] [PubMed] [Google Scholar]
- 6.Caballero Aguilar LM, Silva SM, and Moulton SE, Growth factor delivery: Defining the next generation platforms for tissue engineering. Journal of Controlled Release, 2019. 306: p. 40–58. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S. and Uludağ H, Nanoparticulate systems for growth factor delivery. Pharmaceutical Research, 2009. 26(7): p. 1561. [DOI] [PubMed] [Google Scholar]
- 8.Bajpai AK, et al. , Responsive polymers in controlled drug delivery. Progress in Polymer Science, 2008. 33(11): p. 1088–1118. [Google Scholar]
- 9.Hoque ME, et al. , Gelatin based scaffolds for tissue engineering-a review. Polymers Research Journal, 2015. 9(1): p. 15. [Google Scholar]
- 10.Alemdar N, Fabrication of a novel bone ash-reinforced gelatin/alginate/hyaluronic acid composite film for controlled drug delivery. Carbohydrate Polymers, 2016. 151: p. 1019–1026. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi T, et al. , Drug delivery system of basic fibroblast growth factor using gelatin hydrogel for restoration of acute vocal fold scar. Auris Nasus Larynx, 2017. 44(1): p. 86–92. [DOI] [PubMed] [Google Scholar]
- 12.Hiwatashi N, et al. , Biocompatibility and Efficacy of Collagen/Gelatin Sponge Scaffold With Sustained Release of Basic Fibroblast Growth Factor on Vocal Fold Fibroblasts in 3-Dimensional Culture. Annals of Otology, Rhinology & Laryngology, 2015. 124(2): p. 116–125. [DOI] [PubMed] [Google Scholar]
- 13.Dang PN, et al. , Controlled dual growth factor delivery from microparticles incorporated within human bone marrow-derived mesenchymal stem cell aggregates for enhanced bone tissue engineering via endochondral ossification. Stem cells translational medicine, 2016. 5(2): p. 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirose F, et al. , Experimental proliferative vitreoretinopathy in rabbits by delivery of bioactive proteins with gelatin microspheres. European Journal of Pharmaceutics and Biopharmaceutics, 2018. 129: p. 267–272. [DOI] [PubMed] [Google Scholar]
- 15.Rehmann MS, et al. , Tuning and Predicting Mesh Size and Protein Release from Step Growth Hydrogels. Biomacromolecules, 2017. 18(10): p. 3131–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sionkowska A, et al. , The review of versatile application of collagen. Polymers for Advanced Technologies, 2017. 28(1): p. 4–9. [Google Scholar]
- 17.Lalande M, et al. , Isolated Collagen Mimetic Peptide Assemblies Have Stable Triple-Helix Structures. Chemistry – A European Journal, 2018. 24(52): p. 13728–13733. [DOI] [PubMed] [Google Scholar]
- 18.Aramwit P, et al. , A comparative study of type A and type B gelatin nanoparticles as the controlled release carriers for different model compounds. Materials Express, 2015. 5(3): p. 241–248. [Google Scholar]
- 19.Foox M. and Zilberman M, Drug delivery from gelatin-based systems. Expert Opinion on Drug Delivery, 2015. 12(9): p. 1547–1563. [DOI] [PubMed] [Google Scholar]
- 20.Nikkhah M, Akbari M. , Paul A. , Memic A. , Dolatshahi-Pirouz A. and Khademhosseini A, Gelatin-Based Biomaterials For Tissue Engineering And Stem Cell Bioengineering, in Biomaterials from Nature for Advanced Devices and Therapies. 2016. p. 37–62. [Google Scholar]
- 21.Afewerki S, et al. , Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioengineering & Translational Medicine, 2019. 4(1): p. 96–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikkhah M, et al. , Gelatin-Based Biomaterials For Tissue Engineering And Stem Cell Bioengineering. Biomaterials from Nature for Advanced Devices and Therapies, 2016: p. 37–62. [Google Scholar]
- 23.Turner PA, Thiele JS, and Stegemann JP, Growth factor sequestration and enzyme-mediated release from genipin-crosslinked gelatin microspheres. Journal of biomaterials science. Polymer edition, 2017. 28(16): p. 1826–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen AH, et al. , Gelatin methacrylate microspheres for controlled growth factor release. Acta biomaterialia, 2015. 13: p. 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaipan P, Nguyen A, and Narayan RJ, Gelatin-based hydrogels for biomedical applications. MRS Communications, 2017. 7(3): p. 416–426. [Google Scholar]
- 26.Kishan AP, et al. , Development of a bi-modal, in situ crosslinking method to achieve multi-factor release from electrospun gelatin. Journal of Biomedical Materials Research Part A, 2018. [DOI] [PubMed] [Google Scholar]
- 27.Su J, et al. , Poly(ethylene glycol)-crosslinked gelatin hydrogel substrates with conjugated bioactive peptides influence endothelial cell behavior. Biomaterials, 2019. 201: p. 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prakash Parthiban S, et al. , Covalently immobilized VEGF-mimicking peptide with gelatin methacrylate enhances microvascularization of endothelial cells. Acta Biomaterialia, 2017. 51: p. 330–340. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell AC, et al. , Engineering growth factors for regenerative medicine applications. Acta Biomaterialia, 2016. 30: p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Addi C, et al. , A highly versatile adaptor protein for the tethering of growth factors to gelatin-based biomaterials. Acta Biomaterialia, 2017. 50: p. 198–206. [DOI] [PubMed] [Google Scholar]
- 31.Lee J. h., et al. , The incorporation of bFGF mediated by heparin into PCL/gelatin composite fiber meshes for guided bone regeneration. Drug delivery and translational research, 2015. 5(2): p. 146–159. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, et al. , Injectable gelatin derivative hydrogels with sustained vascular endothelial growth factor release for induced angiogenesis. Acta Biomaterialia, 2015. 13: p. 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claaßen C, et al. , Controlled Release of Vascular Endothelial Growth Factor from Heparin-Functionalized Gelatin Type A and Albumin Hydrogels. Gels (Basel, Switzerland), 2017. 3(4): p. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, et al. , Chimeric aptamer–gelatin hydrogels as an extracellular matrix mimic for loading cells and growth factors. Biomacromolecules, 2016. 17(3): p. 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belair DG, Le NN, and Murphy WL, Design of growth factor sequestering biomaterials. Chemical communications (Cambridge, England), 2014. 50(99): p. 15651–15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacelli S, et al. , Nanodiamond-based injectable hydrogel for sustained growth factor release: Preparation, characterization and in vitro analysis. Acta Biomaterialia, 2017. 58: p. 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basu S, et al. , Adoption of nanodiamonds as biomedical materials for bone repair. Nanomedicine, 2017. 12(24): p. 2709–2713. [DOI] [PubMed] [Google Scholar]
- 38.Gaharwar AK, et al. , 2D Nanoclay for Biomedical Applications: Regenerative Medicine, Therapeutic Delivery, and Additive Manufacturing. Advanced Materials, 2019. 31(23): p. 1900332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waters R, et al. , Stem cell secretome-rich nanoclay hydrogel: a dual action therapy for cardiovascular regeneration. Nanoscale, 2016. 8(14): p. 7371–7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang K, et al. , Enhanced vascularization in hybrid PCL/gelatin fibrous scaffolds with sustained release of VEGF. BioMed research international, 2015. 2015: p. 865076–865076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cidonio G, et al. , Osteogenic and angiogenic tissue formation in high fidelity nanocomposite Laponite-gelatin bioinks. Biofabrication, 2019. 11(3): p. 035027. [DOI] [PubMed] [Google Scholar]
- 42.Bragg JC, et al. , In situ formation of silk-gelatin hybrid hydrogels for affinity-based growth factor sequestration and release. RSC Advances, 2016. 6(115): p. 114353–114360. [Google Scholar]
- 43.Thakur G, Rousseau D, and Rafanan RR, Gelatin based matrices for drug delivery applications. Gelatin: Production, Applications and Health Implications; 2013. [Google Scholar]
- 44.Wang H, et al. , Comparison of micro- vs. nanostructured colloidal gelatin gels for sustained delivery of osteogenic proteins: Bone morphogenetic protein-2 and alkaline phosphatase. Biomaterials, 2012. 33(33): p. 8695–8703. [DOI] [PubMed] [Google Scholar]
- 45.Lee J, et al. , The effect of controlled release of PDGF-BB from heparin-conjugated electrospun PCL/gelatin scaffolds on cellular bioactivity and infiltration. Biomaterials, 2012. 33(28): p. 6709–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliveira MB and Mano JF, Polymer-based microparticles in tissue engineering and regenerative medicine. Biotechnology progress, 2011. 27(4): p. 897–912. [DOI] [PubMed] [Google Scholar]
- 47.Hossain KMZ, Patel U, and Ahmed I, Development of microspheres for biomedical applications: a review. Progress in biomaterials, 2015. 4(1): p. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Echave MC, et al. , Progress of gelatin-based 3D approaches for bone regeneration. Journal of Drug Delivery Science and Technology, 2017. 42: p. 63–74. [Google Scholar]
- 49.Habraken W, et al. , In vitro growth factor release from injectable calcium phosphate cements containing gelatin microspheres. Journal of Biomedical Materials Research Part A, 2009. 91(2): p. 614–622. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, et al. , Gelatin microgel incorporated poly(ethylene glycol)-based boadhesive with enhanced adhesive property and bioactivity. ACS Applied Materials & Interfaces, 2016. 8(19): p. 11980–11989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta V, et al. , Microsphere-based scaffolds in regenerative engineering. Annual review of biomedical engineering, 2017. 19: p. 135–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao X, et al. , Injectable Stem Cell-Laden Photocrosslinkable Microspheres Fabricated Using Microfluidics for Rapid Generation of Osteogenic Tissue Constructs. Advanced Functional Materials, 2016. 26(17): p. 2809–2819. [Google Scholar]
- 53.Park K-S, et al. , Synthesis and characterization of thermosensitive gelatin hydrogel microspheres in a microfluidic system. Macromolecular Research, 2016. 24(6): p. 529–536. [Google Scholar]
- 54.Bock N, et al. , Electrospraying, a reproducible method for production of polymeric microspheres for biomedical applications. Polymers, 2011. 3(1): p. 131–149. [Google Scholar]
- 55.Holland TA, Tabata Y, and Mikos AG, In vitro release of transforming growth factor-β1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. Journal of Controlled Release, 2003. 91(3): p. 299–313. [DOI] [PubMed] [Google Scholar]
- 56.Kimura Y, et al. , Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials, 2003. 24(14): p. 2513–2521. [DOI] [PubMed] [Google Scholar]
- 57.Luo Y, et al. , Porous, lithium-doped calcium polyphosphate composite scaffolds containing vascular endothelial growth factor (VEGF)-loaded gelatin microspheres for treating glucocorticoid-induced osteonecrosis of the femoral head. Biomedical Materials, 2019. 14(3): p. 035013. [DOI] [PubMed] [Google Scholar]
- 58.De Clercq K, et al. , Genipin-crosslinked gelatin microspheres as a strategy to prevent postsurgical peritoneal adhesions: In vitro and in vivo characterization. Biomaterials, 2016. 96: p. 33–46. [DOI] [PubMed] [Google Scholar]
- 59.Annamalai RT, et al. , Harnessing macrophage-mediated degradation of gelatin microspheres for spatiotemporal control of BMP2 release. Biomaterials, 2018. 161: p. 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhuang H, et al. , Gelatin-methacrylamide gel loaded with microspheres to deliver GDNF in bilayer collagen conduit promoting sciatic nerve growth. International journal of nanomedicine, 2016. 11: p. 1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y, et al. , Preparation of gelatin microspheres encapsulated with bFGF for therapeutic angiogenesis in a canine ischemic hind limb. Journal of Biomaterials Science, Polymer Edition, 2011. 22(4–6): p. 665–682. [DOI] [PubMed] [Google Scholar]
- 62.Solorio LD, et al. , Engineered cartilage via self-assembled hMSC sheets with incorporated biodegradable gelatin microspheres releasing transforming growth factor-β1. Journal of Controlled Release, 2012. 158(2): p. 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsaryk R, et al. , Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomaterialia, 2015. 20: p. 10–21. [DOI] [PubMed] [Google Scholar]
- 64.Matsumine H, et al. , Facial nerve regeneration using basic fibroblast growth factor-impregnated gelatin microspheres in a rat model. Journal of tissue engineering and regenerative medicine, 2016. 10(10): p. E559–E567. [DOI] [PubMed] [Google Scholar]
- 65.Vo TN, Kasper FK, and Mikos AG, Strategies for controlled delivery of growth factors and cells for bone regeneration. Advanced drug delivery reviews, 2012. 64(12): p. 1292–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banerjee I, Mishra D, and Maiti TK, PLGA Microspheres Incorporated Gelatin Scaffold: Microspheres Modulate Scaffold Properties. International Journal of Biomaterials, 2009. 2009: p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghassemi Z. and Slaughter G, Cross-linked electrospun gelatin nanofibers for cell-based assays. Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference, 2018. 2018: p. 6088–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng J, et al. , Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. Biomaterials, 2017. 114: p. 121–143. [DOI] [PubMed] [Google Scholar]
- 69.Erencia M, et al. , Electrospinning of gelatin fibers using solutions with low acetic acid concentration: Effect of solvent composition on both diameter of electrospun fibers and cytotoxicity. Journal of Applied Polymer Science, 2015. 132(25). [Google Scholar]
- 70.Lee H, et al. , Fabrication of FGF-2 immobilized electrospun gelatin nanofibers for tissue engineering. International Journal of Biological Macromolecules, 2016. 93: p. 1559–1566. [DOI] [PubMed] [Google Scholar]
- 71.Tsao CJ, et al. , Electrospun patch functionalized with nanoparticles allows for spatiotemporal release of VEGF and PDGF-BB promoting in vivo neovascularization. ACS applied materials & interfaces, 2018. 10(51): p. 44344–44353. [DOI] [PubMed] [Google Scholar]
- 72.Lin W-H, et al. , Fabrication of multi-biofunctional gelatin-based electrospun fibrous scaffolds for enhancement of osteogenesis of mesenchymal stem cells. Colloids and Surfaces B: Biointerfaces, 2016. 138: p. 26–31. [DOI] [PubMed] [Google Scholar]
- 73.Hiramatsu H, et al. Microfluidics-based wet spinning of protein microfibers as solid scaffolds for 3D cell cultivation. in 2016 International Symposium on Micro-NanoMechatronics and Human Science (MHS). 2016. IEEE. [Google Scholar]
- 74.Zuo Y, et al. , Microfluidic-based generation of functional microfibers for biomimetic complex tissue construction. Acta biomaterialia, 2016. 38: p. 153–162. [DOI] [PubMed] [Google Scholar]
- 75.Rickman J, et al. , Rotation-assisted wet-spinning of UV-cured gelatin fibres and nonwovens. Journal of Materials Science, 2019. 54(14): p. 10529–10547. [Google Scholar]
- 76.An G, et al. , Influence of VEGF/BMP-2 on the proliferation and osteogenetic differentiation of rat bone mesenchymal stem cells on PLGA/gelatin composite scaffold. Eur Rev Med Pharmacol Sci, 2017. 21(10): p. 2316–2328. [PubMed] [Google Scholar]
- 77.Norouzi M, et al. , PLGA/gelatin hybrid nanofibrous scaffolds encapsulating EGF for skin regeneration. Journal of biomedical materials research Part A, 2015. 103(7): p. 2225–2235. [DOI] [PubMed] [Google Scholar]
- 78.Ardila DC, et al. , Modulating smooth muscle cell response by the release of TGFβ2 from tubular scaffolds for vascular tissue engineering. Journal of Controlled Release, 2019. 299: p. 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu J. and Hong Y, Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioactive materials, 2016. 1(1): p. 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan Z, et al. , Electrospun vein grafts with high cell infiltration for vascular tissue engineering. Materials Science and Engineering: C, 2017. 81: p. 407–415. [DOI] [PubMed] [Google Scholar]
- 81.Guerado E. and Caso E, Challenges of bone tissue engineering in orthopaedic patients. World journal of orthopedics, 2017. 8(2): p. 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hill AB, et al. , Overcoming Gene-Delivery Hurdles: Physiological Considerations for Nonviral Vectors. Trends in Biotechnology, 2016. 34(2): p. 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han X, et al. , Accelerated Postero-Lateral Spinal Fusion by Collagen Scaffolds Modified with Engineered Collagen-Binding Human Bone Morphogenetic Protein-2 in Rats. PLOS ONE, 2014. 9(5): p. e98480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Epstein NE, Pros, cons, and costs of INFUSE in spinal surgery. Surgical neurology international, 2011. 2: p. 10–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Graaf AJ, et al. , Nonnatural Amino Acids for Site-Specific Protein Conjugation. Bioconjugate Chemistry, 2009. 20(7): p. 1281–1295. [DOI] [PubMed] [Google Scholar]
- 86.Braun AC, et al. , Bioorthogonal strategies for site-directed decoration of biomaterials with therapeutic proteins. Journal of Controlled Release, 2018. 273: p. 68–85. [DOI] [PubMed] [Google Scholar]
- 87.Koshy ST, et al. , Click-Crosslinked Injectable Gelatin Hydrogels. Advanced Healthcare Materials, 2016. 5(5): p. 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shekaran A, et al. , Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials, 2014. 35(21): p. 5453–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Su K. and Wang C, Recent advances in the use of gelatin in biomedical research. Biotechnology Letters, 2015. 37(11): p. 2139–2145. [DOI] [PubMed] [Google Scholar]
- 90.Olsen D, et al. , Recombinant collagen and gelatin for drug delivery. Advanced Drug Delivery Reviews, 2003. 55(12): p. 1547–1567. [DOI] [PubMed] [Google Scholar]
- 91.Ratanavaraporn J, et al. , Influences of physical and chemical crosslinking techniques on electrospun type A and B gelatin fiber mats. International Journal of Biological Macromolecules, 2010. 47(4): p. 431–438. [DOI] [PubMed] [Google Scholar]
- 92.Grover CN, et al. , Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomaterialia, 2012. 8(8): p. 3080–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campiglio CE, et al. , Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials (Basel, Switzerland), 2019. 12(15): p. 2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yue K, et al. , Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials, 2015. 73: p. 254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]