Abstract
In patients with a femoropopliteal chronic total occlusion (CTO) after femoro–femoral (FF) bypass surgery, it is often difficult to perform endovascular therapy because of access site problems. We have treated two patients with CTO of the superficial femoral artery (SFA) using an FF crossover bypass graft. The two cases were a man with intermittent claudication and acute limb ischemia, respectively. Enhanced computed tomography showed occlusion of the left SFA and the FF bypass previously performed was patent in both cases. We punctured the right common femoral artery and a guiding sheath was inserted to the left common femoral artery. A guidewire successfully passed through the intraplaque lesion by intravascular ultrasound-guided wiring in both cases. Revascularization was successfully achieved using drug-coated balloons and using drug-eluting stents, respectively. An FF crossover bypass graft may be a good access route for complex femoropopliteal cases, such as CTO lesions.
Keywords: Endovascular therapy, femoro–femoral crossover bypass, chronic total occlusion, acute limb ischemia, chronic limb-threatening ischemia, femoral artery, intravascular ultrasound, drug-eluting balloon
Background
Endovascular therapy (EVT) has become one of the options for revascularization of a femoropopliteal artery lesion as a result of advances in technology and devices.1,2 However, treatment of a femoropopliteal chronic total occlusion (CTO) is often challenging. Contralateral approach is relatively easy to construct the system, although there is a technique of crossover, but it makes procedure difficult due to the distance from the CTO lesion. Pushability of the device and the operability of the guidewire are significantly reduced. Otherwise, ipsilateral antegrade approach is good at pushability of the device and wire maneuverability. However, the ipsilateral antegrade approach is often difficult after bypass surgery. Although there have been reports on techniques involving direct puncture of the bypass graft, there are concerns about complications, such as infection of the graft and issues with hemostasis.3,4 Our experience has been that the contralateral crossover approach via the femoro–femoral (FF) crossover bypass graft can be useful for access and is not inferior to the conventional crossover approach in terms of distance or angle. We have successfully treated two patients with complex femoropopliteal CTOs by FF crossover bypass with progressive wiring. In these cases, although progressive guidewire manipulation was important, a progressive approach via FF crossover bypass seemed to be useful without problems regarding use of devices such as guidewires or balloons. To our knowledge, there have been no reports on treatment of complex femoropopliteal CTOs using an FF crossover bypass graft.
Case presentations
Case 1
The patient was a 78-year-old man with a history of hypertension who was a current smoker and had been aware of intermittent claudication for 6 months. Lower extremity arterial disease was suspected in view of a low ankle brachial index (ABI) value. 5 The patient admitted to our hospital for further evaluation and treatment. The intermittent claudication was Rutherford class 3, and the ABI was not measurable in either lower limb, and the waves of either lower limb were almost flat. Enhanced computed tomography showed the stenosis and occlusion of lower limb vessels. Therefore, we performed angiography and which showed occlusion from the left common iliac artery to the left external iliac artery and stenosis of the right external iliac artery.
We also found that the superficial femoral artery (SFA) was occluded on both sides. Enhanced computed tomography showed a shaggy descending aorta and an abdominal aortic aneurysm with a maximum diameter of 33 mm. After consultation with a vascular surgeon, we considered that revascularization of the left iliac artery carried a high risk of plaque embolization to branch vessels and rupture of the aorta. Therefore, both EVT and bypass surgery were performed. An Epic stent measuring 7.0 × 60 mm (Boston Scientific Corp., Marlborough, MA, USA) was placed in the right external iliac artery, after which FF crossover bypass was performed (Figure 1(a)). The ABI value increased to 0.43 after EVT and to 0.40 after bypass surgery. Although the patient’s symptoms were mildly improved, the intermittent claudication remained, particularly in the left lower limb. EVT in the iliac artery lesions was considered to have a high risk of blood vessel rupture and lateral branch occlusion in this case due to the aneurysm of the terminal aorta and diffuse plaque. In addition, revascularization of the left iliac artery had a risk of occlusion of fem-fem bypass graft. Given that enhanced computed tomography showed the FF crossover bypass graft to be patent (Figure 1(a)), we performed EVT via the FF crossover bypass graft.
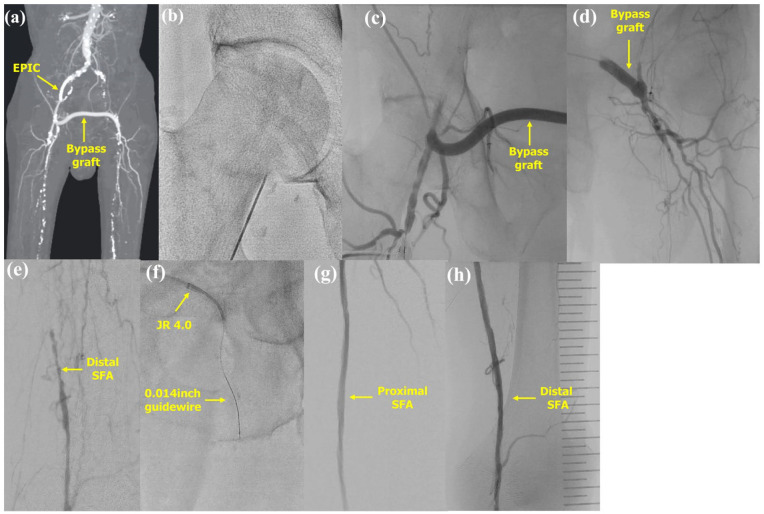
Figure 1.
Endovascular therapy via a femoro–femoral crossover bypass graft in case 1. (a) The femoro–femoral crossover bypass graft was patent. (b) Puncture from the right common femoral artery as distal as possible. (c) The femoro–femoral crossover bypass graft was contrasted. (d) A 4-Fr JR 4.0 guiding catheter was successfully passed through the femoro–femoral crossover bypass graft. (e) The distal left SFA was contrasted by collateral. (f) The guidewire was successfully passed into the chronically occluded lesion. (g) The proximal portion of the SFA after dilation with drug-coated balloon. (h) The distal portion of the SFA after dilation with a drug-coated balloon.
SFA: superficial femoral artery.
Under local anesthesia, we punctured the right common femoral artery (CFA) (Figure 1(b)) with angio-guided. A 6-Fr 10-cm sheath was inserted, followed by a check of flow in the FF crossover bypass graft (Figure 1(c)). A Radifocus guidewire with a 4-Fr JR 4.0 guiding catheter (both from Terumo, Tokyo, Japan) were successfully passed through the FF crossover bypass graft (Figure 1(d)), and a 6-Fr Crossroads guiding sheath (Nipro, Osaka, Japan) was inserted into the left CFA. The occluded lesion extended from the left proximal SFA to the left popliteal artery (Figure 1(e)). During the procedure, a Crosslead penetration 0.014 (ASAHI INTECC Corp., Aichi, Japan) was successfully passed through the intraplaque lesion (Figure 1(f)) with only antegrade guidewire crossing using AnteOwl WR (Terumo) intravascular ultrasound (IVUS)-guided parallel wiring.6,7 The left SFA was dilated using a 6.0 × 200 mm Ranger (drug-coated balloon) and the left popliteal artery using a 5.0 × 200 mm Ranger (Both from Boston Scientific Corp.). After dilation, the left SFA was clearly contrasted (Figure 1(g) and (h)). The procedure was completed using a 6-Fr Exoseal (Kaneka, Tokyo, Japan) as a hemostasis device.
Following the procedure, the ABI value in the left lower limb increased to 0.83, and the patient’s symptoms in the left lower limb were improved. No restenosis of the left SFA was observed in duplex ultrasound even 6 months after treatment.
Case 2
A 68-year-old man with a history of FF crossover bypass surgery and left femoropopliteal bypass for chronic limb-threatening ischemia presented with sudden onset of left limb pain that had started 2 days earlier. His left limb was cold, and the left dorsalis pedis artery was pulseless. Enhanced computed tomography showed total occlusion of the left iliac artery, the left femoropopliteal bypass graft, the left SFA, and the left popliteal artery (Figure 2(a)). Under local anesthesia, we punctured the right CFA with angio-guided and passed through the FF crossover bypass graft with a Gladius MG14 PV ES (ASAHI INTECC Corp.) and a Glidecath (Figure 2(b)). A 6-Fr Crossroads guiding sheath was inserted into the left CFA; then angiogram showed total occlusion from SFA proximal to popliteal artery (Figure 2(c) and (d)). After which we performed AnteOwl WR IVUS-guided parallel wiring and an Astato XS 9-40 (ASAHI INTECC Corp.) successfully passed through the intraplaque lesion. Revascularization was successfully achieved with using two 7.0 × 150 mm Eluvia (drug-eluting stent) and a 7.0 × 80 mm Eluvia (Both from Boston Scientific Corp.) via an antegrade approach alone (Figure 2(e) and (f)). Although final angiography remained infra-popliteal stenosis and occlusions, the treatment for acute limb ischemia was sufficient, so the procedure was completed.
Figure 2.
Endovascular therapy via a femoro–femoral crossover bypass graft in case 2. (a) The left iliac artery and the femoropopliteal bypass graft, the left SFA and the left popliteal artery were occluded. (b) A 0.014-inch guidewire and Glidecath were successfully passed through the femoro–femoral crossover bypass graft. (c) The left SFA was occluded from the proximal portion. (d) The left popliteal artery and tibioperoneal trunk were occluded. (e) The proximal SFA after deploying a drug-eluting stent. (f) The popliteal artery after deploying a drug-eluting stent.
SFA, superficial femoral artery.
The procedure was completed and catheter directed thrombolysis was performed 2 days after procedure using heparin at 10,000 units per day and argatroban hydrate at 20 mg per day. After the catheter directed thrombolysis, the sheath was removed with manual pressure hemostasis. Following the procedure, the ABI value in the left lower limb increased to 0.93, and no restenosis of the left SFA was observed in duplex ultrasound even 1 year after treatment.
Discussions
The frequency of nonanatomical bypass surgery such as FF crossover bypass is decreasing owing to the increasing success rate of aortoiliac intervention and improved safety. However, depending on the nature and anatomy of the aorta, including shaggy aorta as in case 1, FF crossover bypass remains a useful revascularization option. In our two cases, ipsilateral EVT from the left CFA could not be performed because of the short length of the nonoccluded left SFA. Although direct puncture of the bypass graft is a useful technique when the access site for EVT is limited, previous reports have demonstrated the frequency of complications to be approximately 10%–20%. The main complications are bleeding and infection. 3 Other complications of direct graft puncture include pseudoaneurysm and disruption of the anastomotic suture line. 4 As ENSUPRO study demonstrated, endovascular treatment is associated with higher amputation free survival rates than those of hybrid treatment or open surgery. 8 This method may be a reasonable option for revascularization when the bypass graft is occluded.
EVT via FF crossover bypass graft is closer to the cap of the total occlusion lesion than contralateral EVT via the iliac artery. Therefore, the torque response of the guidewire is better than that with the standard contralateral crossover approach. We were able to treat the two cases of complex femoropopliteal CTO described here using only an antegrade approach via FF crossover bypass. In both cases, we were able to successfully pass the intraluminal guidewire with IVUS-guided parallel wiring using an antegrade approach. For crossing a guidewire in CTO lesions, the retrograde approach or using re-entry devices are reasonable options for passing a guidewire for CTO lesions.9–11 In our cases, we chose first IVUS-guided parallel wiring technique which is reliable strategy for passing a guidewire via antegrade approach for CTO cases. IVUS-guided guidewire crossing has been reported to be a useful technique for intentional intraluminal wire passage from an antegrade approach.6,7 There are various treatment strategies of CTO lesions when an EVT is performed; therefore, it is necessary to collect information from prior angiography and computed tomography such as the useful approach site and the morphology of the CTO cap. 12 Considering the above, we should develop a strategy. Among them, antegrade IVUS-guided parallel wiring is an extremely useful method to challenge wire passage via antegrade approach.
However, this technique is dependent on manipulation of the guidewire in the antegrade direction, suggesting that the approach via FF crossover bypass is also sufficient for good manipulation of the guidewire. Moreover, the strength of pushability and deliverability of devices are similar to those of ipsilateral EVT. When a sheath is inserted, it is important to puncture the CFA as distal as possible from the anastomosis of the FF crossover bypass graft. In general, the patency of an FF crossover bypass graft is approximately 70% at 5 years and less than 50% at 10 years.13–15 Therefore, this EVT technique is useful when the access route is limited. Regarding postoperative controls of the endovascular procedures using FF bypass graft, it is essential to measure the ABI of the treated lower limb vessels regularly and to measure the flow velocity in the bypass graft by duplex ultrasound. Enhanced computed tomography for patency of the bypass graft should be performed in consideration of the patient’s renal function.
The main limitation of this technique is that it is unsuitable in cases where the FF crossover bypass anastomosis is distal to the CFA (i.e., proximal to the SFA). In such cases, it is difficult to perform the usual approach from the SFA, so the approach is ipsilateral and proximal to the CFA. However, there is concern about complications at the puncture site. The drawback is that whether FF crossover bypass graft is available or not depends on the angle between the FF crossover bypass anastomosis and the angle at which the FF crossover bypass is connected. A larger study is required to confirm the efficacy and safety of EVT via an FF crossover bypass graft for femoropopliteal lesions.
Conclusions
The EVT technique via an FF crossover bypass graft may be a reasonable option when it is not possible to perform ipsilateral EVT.
Acknowledgments
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Footnotes
Authors’ contributions: Y.T., N.H., and H.M. performed the procedures and the pre-procedure and post-procedure follow-ups. J.K. provided final approval of the submitted manuscript. All authors read and approved the final version of the manuscript.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: All procedures were performed in accordance with the ethical standards of the institutional and/or national research committees and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
ORCID iD: Yasuyuki Tsuchida  https://orcid.org/0009-0004-1905-0945
https://orcid.org/0009-0004-1905-0945
References
- 1. Surowiec SM, Davies MG, Eberly SW, et al. Green, percutaneous angioplasty and stenting of the superficial femoral artery. J Vasc Surg 2005; 41: 269–278. [DOI] [PubMed] [Google Scholar]
- 2. Gerhard-Herman MD, Gornik HL, Barrett C, et al. AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease. Circulation 2017; 135: e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cowling MG, Belli AM, Buckenham TM. Evaluation and complications of direct graft puncture in thrombolysis and other interventional techniques. Cardiovasc Intervent Radiol 1996; 19(2): 82–84. [DOI] [PubMed] [Google Scholar]
- 4. Eisenberg RL, Mani RL, McDonald EJ, et al. The complication rate of catheter angiography by direct puncture through aorto-femoral bypass grafts. AJR Am J Roentgenol 1976; 126(4): 814–816. [DOI] [PubMed] [Google Scholar]
- 5. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2017; 135: e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayakawa N, Kodera S, Takanashi K, et al. Efficacy of navigating through the intraplaque route using AnteOwl WR intravascular ultrasound in femoropopliteal chronic total occlusion. CVIR Endovasc 2021; 4(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayakawa N, Kodera S, Miwa H, et al. Clinical feasibility of endovascular recanalization with intravascular ultrasound-guided wiring for chronic total occlusion of below-the-knee arteries. CVIR Endovasc 2023; 6: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korosoglou G, Torsello G, Saratzis A, et al. Endovascular versus surgical treatment for all comer patients with prosthetic bypass graft occlusion: the multicentre ENSUPRO study. Eur J Vasc Endovasc Surg 2024; 67: 786–796. [DOI] [PubMed] [Google Scholar]
- 9. Tan M, Urasawa K, Koshida R, et al. Anterolateral popliteal puncture technique: a novel retrograde approach for chronic femoropopliteal occlusions. J Endovasc Ther 2017; 24(4): 525–530. [DOI] [PubMed] [Google Scholar]
- 10. Hayakawa N, Kodera S, Sakkya S, et al. Efficacy and safety of angiography-guided retrograde posterior popliteal puncture technique in the supine position. Ann Vasc Surg 2021; 71: 264–272. [DOI] [PubMed] [Google Scholar]
- 11. Kitrou P, Parthipun A, Diamantopoulos A, et al. Targeted true lumen re-entry with the outback catheter: accuracy, success, and complications in 100 peripheral chronic total occlusions and systematic review of the literature. J Endovasc Ther 2015; 22(4): 538–545. [DOI] [PubMed] [Google Scholar]
- 12. Korosoglou G, Schmidt A, Lichtenberg M, et al. Crossing algorithm for infrainguinal chronic total occlusions: an interdisciplinary expert opinion statement. JACC Cardiovasc Interv 2023;16(3): 317–331. [DOI] [PubMed] [Google Scholar]
- 13. Park K-M, Park Y-J, Kim Y-W, et al. Long term outcomes of femorofemoral crossover bypass grafts. Vasc Specialist Int 2017; 33(2): 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mingoli A, Sapienza P, Feldhaus RJ, et al. Femorofemoral bypass grafts: factors influencing long-term patency rate and outcome. Surgery 2001; 129(4): 451–458. [DOI] [PubMed] [Google Scholar]
- 15. Devolfe C, Adeleine P, Henrie M, et al. Ilio-femoral and femoro-femoral crossover grafting. Analysis of an 11-year experience. J Cardiovasc Surg 1983; 24(6): 634–640. [PubMed] [Google Scholar]