Abstract
Background
Heart failure (HF) is a chronic disease characterized by high mortality and healthcare expenditures. Digital health solutions, including mobile health applications (apps), offer opportunities to enhance patients’ self-care and quality of life. This qualitative study aimed to explore expectations, experiences, and usage behaviour of HF-patients regarding a self-care app (DoctorME app).
Methods
Semi-structured interviews were conducted at 2-3 weeks (initial: n = 38), and 4–6 months (post: n = 45) of app use across four European countries. Most patients were male (initial: 84%; post: 78%), aged 60–69 years (initial and post: 29%), with mild HF symptoms. Interviews were transcribed, pseudonymised, and analysed using qualitative content analysis.
Results
Five key themes were identified: 1) expectations, 2) perceived usability and benefit, 3) usage behaviour and experiences, 4) self-care, and 5) social influences. Patients expected and valued continuous monitoring of vital signs and weight, early detection of deterioration, and quick feedback. The app was considered user-friendly, with most patients using it as recommended (eight times per month). Those reporting improved self-care attributed it to increased awareness and a sense of security. Patients with established self-care routines did not perceive any additional benefit. Patients’ perceptions on the impact of healthcare professionals’ and relatives opinions on app use were divided.
Conclusions
User-friendliness, continuous monitoring, rapid feedback, and e-learning modules are crucial for integrating self-care apps into daily HF care. While technical reliability and individualisation may enhance long-term use, most HF patients considered the app as a complement to, not a replacement for, professional healthcare guidance.
Keywords: Heart failure, mobile applications, decision support systems, telemedicine, self-care, usage behaviour, patient acceptance of health care
Background
Heart failure (HF) represents a multifaceted chronic condition with an increasing prevalence worldwide.1–3 In 2017, 64.3 million people globally suffer from HF. 4 Although the incidence of HF has remained stable or slightly declined, the 5-year mortality rate remains high. 1 In Western countries, annual healthcare expenditure is estimated to be up to €25,000 per patient, with a continuing upward trend. 1 The majority of costs are linked to inpatient care, (re-)hospitalizations, and non-cardiovascular comorbidities.1,5 HF is also the leading cause of hospitalisation in patients aged 65 or older. 6 Patients with HF often have comorbidities (e.g., hypertension, diabetes)7,8 and may experience a poor health-related quality of life (QoL; 9–11). Effective self-care behaviours, such as adhering to prescribed therapies, monitoring vital signs and symptoms and responding appropriately, 12 can improve QoL. 13 Therefore, the importance of patient education and self-care is recognised within recent European Society of Cardiology (ESC) HF guidelines. 14 Digital health solutions such as remote monitoring devices, digital health tools and artificial intelligence (AI) are transforming HF care, for example by facilitating close monitoring of symptoms, vital signs, and other data as well as timely treatment recommendations. 15 A recent meta-analysis showed that eHealth interventions designed to assist HF management can reduce all-cause mortality and HF-related hospitalization, as well as improve patients’ QoL and self-care skills. 16 Moreover, implementing mobile health applications (mHealth apps) to engage patients in HF care has also been found to improve HF knowledge, QoL, 17 and symptoms as measured according to the New York Heart Association (NYHA) classification. 18 In principle, ensuring a high level of user-friendliness and considering patient preferences are essential prerequisites for sustainable app usage.19,20
As part of the PASSION-HF project (Interreg NWE 702), a decision support system (DSS) called DoctorME was developed and trialled in four European countries: Germany (DE), Ireland (IRL), the Netherlands (NL), and the United Kingdom (UK). The smartphone and web-based HF app (DoctorME app) aims to assist patients in the daily care management of their condition. In addition to e-learning modules on various topics, patients can enter their vital signs and weight in the app and perform a so called QuickScan guided by the app if they feel uncomfortable. Based on the values entered by patients, they receive feedback in the form of recommendations for action for better self-care. Additionally, healthcare professionals (HCPs) involved in patient treatment receive clinical guideline-based recommendations via a web interface (DSS) based on regular measurements of the patient's vital signs and clinical parameters, such as serum creatinine, NYHA class, and glucose. As part of PASSION-HF, the aim of this qualitative study was to better understand the expectations, experiences, and usage behaviour of patients with HF regarding the DoctorME app at the beginning and after 4–6 months of use.
Methods
Approach and study design
This qualitative study implemented semi-structured interviews with HF patients. The qualitative research approach was selected for the specific purpose of analysing the individual person with their individual experiences, attitudes and patterns of action within their familiar living environment. 21 Interviews were conducted after 2-3 weeks (initial) and after 4–6 months (post) using the DoctorME app. All material for both measurement points were developed by a multidisciplinary research team from DE, IRL, NL, and UK comprising experts in cardiology, psychology, sociology, engineering, and innovation management. A first draft of the interview guidelines was prepared by AN, a female health scientist with interviewing expertise, and BS, a female medical informatics specialist also skilled in interviewing. Initial drafts of all documents were created in English and German and underwent critical revision by the research team. Subsequently, the Dutch team translated final versions in Dutch. The interview guides were built upon the theoretical framework of the Unified Theory of Acceptance and Use of Technology (UTAUT). 22 However, no questions were taken directly from the UTAUT questionnaire; instead, the constructs of performance expectancy, effort expectancy, social influence and facilitating conditions served as an overarching orientation for the guide. Initial interviews focused on examining patients’ expectations regarding use of the app for assisting their HF self-care, the first impression of the user-friendliness after the first 2-3 weeks, and their planned usage behaviour and implementation in everyday life. After 4–6 months, patients were asked about their actual usage behaviour and experiences with the DoctorME app. Both interview guides can be found in Additional File 1. No pilot tests took place. The interview study is presented in accordance with the criteria for reporting qualitative research (COREQ; 23 see Additional file 2).
Sampling strategy and recruitment
The recruitment process incorporated a purposive maximum variation approach to sample a diverse set of patients with HF that represented different ages, gender, and HF severity as defined by the NYHA classification. 24 Patients who had already been enrolled in the PASSION-HF clinical trial were asked to participate in the interviews on a voluntary basis. For initial interviews, potential participants were contacted by treating HCPs of the respective clinical site (DE, IRL, NL, UK) either during HF-related hospitalisation or routine outpatient visits. For post-interviews, participants were contacted by email and/or telephone. Criteria for inclusion were: (1) ≥ 18 years; (2) diagnosis of HF; (3) cognitive receptivity; (4) fluency in the language of the interview; (5) able to consent to participate; (6) agree to audio-recording of the interview and (7) 4–6 months using the DoctorME app (only for post-interviews). Patients who did not fulfil the inclusion criteria were not eligible to participate in this study. There were no further specific exclusion criteria. To achieve theoretical data saturation, 40–48 interviews (initial and post) were planned (n = 10–12 per clinical site and time point).
Data collection
After ethical approval at each clinical site, initial interviews took place from August 2022 to March 2023 and post-interviews from February 2023 to July 2023. Interviews were carried out by 1 to 3 HF nurses or researchers per clinical site (DE: BS, AN, YM; IRL: MM, CM; NL: JB; UK: LH, AM, DF). As the interviewers had different expertise and experiences in interviewing, BS and AN led virtual training sessions for all interviewers and closely supervised the interview process in their role as interview study leaders. While BS, AN, YM, JB, LH, and DF had no relationship with the participants prior to study commencement, AM, MM and CM were already in contact with the participants as part of their participation in the clinical study of PASSION-HF. Participants were informed that the interviewers conducted this study in their position as participating HF nurses or researchers in PASSION-HF.
Initial interviews lasted an average of 33 min (± 14 min) and post-interviews 23 min (± 9 min). The initial interviews were conducted in conjunction with an usability test of the app, which must be taken into account given the interview duration. However, the usability test is not part of this analysis. Throughout the interviews, each question was audibly presented in an identical sequence, unless it had already been addressed. No field notes were made. Interviews were carried out either at participants’ homes, hospitals or through virtual meetings or telephone calls, based on the preferences of participants. The majority of interviews were conducted through virtual meetings or telephone calls. In a few cases, the patient's informal caregiver (relative) was also present during the interview if the patient so wished. Interviews continued until theoretical saturation was reached.
Data analysis
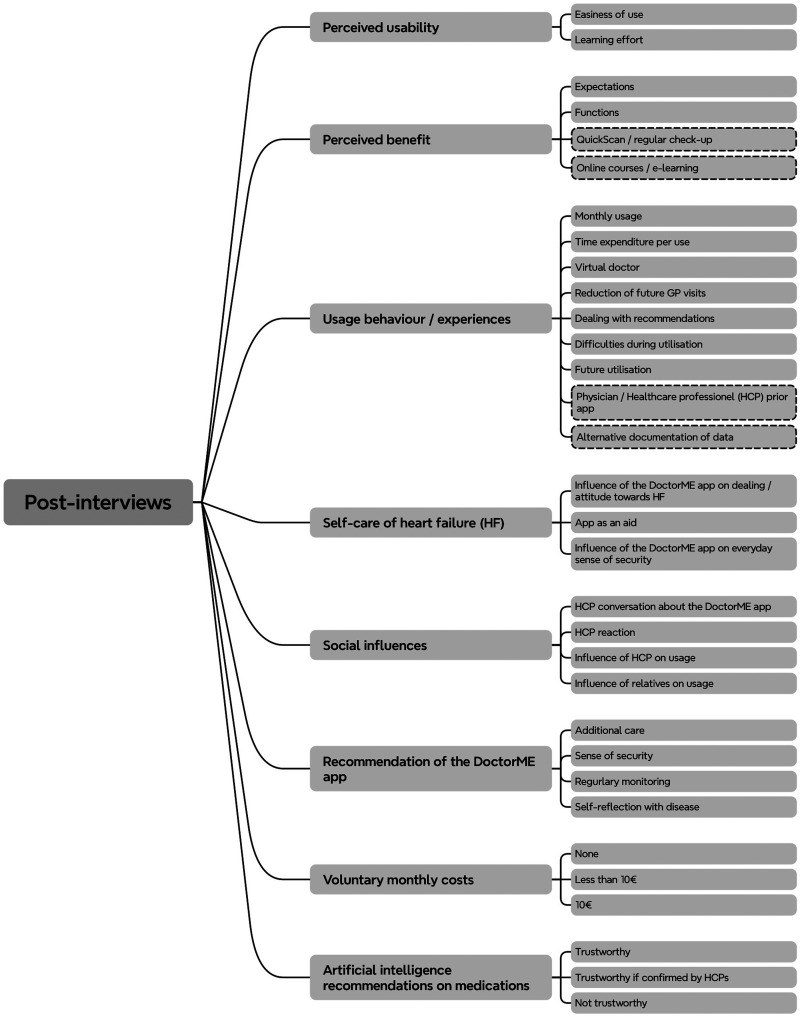
Interviews were audiotaped and transcribed verbatim by an independent transcription office. Dutch transcripts were translated into English afterwards. A qualitative content analysis based on Mayring 21 combining deductive and inductive coding was used to transform interview transcripts into a highly organized and concise summary of findings. Utilizing Schreier's toolbox for qualitative content structuring, 25 this basic procedure has been adjusted and is presented in Table 1. Analysis of the interviews was performed using the MAXQDA 24 software version 24.3.0 and completed in November 2023. All initial interviews were analysed by AN and BS, post-interviews by AN, BS and YM, with the majority being coded by AN. The final code structure was discussed between AN and BS and revised if necessary. In the following description of results, only (sub-)categories containing at least three statements (i.e., three patients) were considered. Participants’ data were pseudonymised. Transcripts and data analysis were not returned to participants. A visualisation of the code structure for post-interviews can be seen in Figure 1. Additional File 3 contains the code structure for the initial interviews. All categories with corresponding example quotes can be found in Additional Files 4 and 5. The following pronouns were used to give an impression for the response frequencies of the participants: some (less than one-third), many (between one-third and two-thirds), most (more than two-thirds) and almost all (approximately 90%).
Table 1.
Coding strategy.
| Main steps | Used approach | |
|---|---|---|
| Creating the category system | ||
| Basic strategy | Deductive-inductive | |
| Deductive categorization | Initial interviews:
|
Post-interviews:
|
| Inductive categorization | Subsumption | |
| Persons involved | 2 (health scientists, medical informatician) | |
| Termination criterion | None | |
| Division of material | ||
| Unit | Coding unit | |
| Coding unit | Individual words and phrases | |
| Systematic | Marking and coding in one step | |
| Persons involved | 1 | |
| Coding | ||
| Test coding | Yes | |
| Person involved in main coding | 2 | |
Figure 1.
Code structure for the post-interviews. Dashed categories = interview did not explicitly ask about these topics.
Results
Sample characteristics
38 patients were interviewed after 2-3 weeks (initial), and 45 patients after 4–6 months of using the DoctorME app (post). Due to the recruitment of additional patients at the end of the utilisation period (n = 10), the withdrawal of patients from PASSION-HF (n = 4), and the unavailability of individual audio recordings (n = 2), the same patients were not necessarily interviewed in both the initial and post-interviews. Initial and post-interviews with the same patients were conducted with a total of 34 patients. Four patients participated in the initial interviews only, while 11 patients participated in the post-interviews only. The majority of interviewed patients were male (initial: 84%; post: 78%) and aged between 60 and 69 years (initial and post: 29%). Most patients (initial: 50%; post: 40%) suffer from HF with mild symptoms (NYHA class I; Table 2). Almost all patients had previously used digital devices such as smartphones and tablets. In most cases, such devices were used for communication, reading messages or e-mails, or surfing/searching the Internet.
Table 2.
Characteristics of patients, overall and per country.
| Germany | Netherlands | United Kingdom | Ireland | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| initial | post | initial | post | initial | post | initial | post | initial | post | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Patients | 11 (29%) | 10 (22%) | 11 (29%) | 11 (24%) | 9 (24%) | 9 (20%) | 7 (18%) | 15 (33%) | 38 (100%) | 45 (100%) |
| Gender | ||||||||||
| Male | 10 (91%) | 9 (90%) | 8 (73%) | 8 (73%) | 8 (89%) | 8 (89%) | 6 (86%) | 10 (67%) | 32 (84%) | 35 (78%) |
| Female | 1 (9%) | 1 (10%) | 3 (27%) | 3 (27%) | 1 (11%) | 1 (11%) | 1 (14%) | 5 (33%) | 6 (16%) | 10 (22%) |
| Age (years) | ||||||||||
| 18–49 | 3 (27%) | 3 (30%) | 1 (9%) | 1 (9%) | 2 (22%) | 2 (22%) | 0 (0%) | 0 (0%) | 6 (16%) | 6 (13%) |
| 50–59 | 2 (18%) | 2 (20%) | 1 (9%) | 2 (18%) | 1 (11%) | 1 (11%) | 1 (14%) | 3 (20%) | 5 (13%) | 8 (18%) |
| 60–69 | 4 (36%) | 4 (40%) | 3 (27%) | 3 (27%) | 3 (33%) | 2 (22%) | 1 (14%) | 4 (27%) | 11 (29%) | 13 (29%) |
| 70–79 | 2 (18%) | 1 (10%) | 5 (45%) | 4 (36%) | 1 (11%) | 2 (22%) | 2 (29%) | 3 (20%) | 10 (26%) | 10 (22%) |
| 80–89 | 0 (0%) | 0 (0%) | 1 (9%) | 1 (9%) | 2 (22%) | 2 (22%) | 3 (43%) | 5 (33%) | 6 (16%) | 8 (18%) |
| Heart failure severity | ||||||||||
| NYHA I | 5 (45%) | 4 (40%) | 6 (55%) | 4 (36%) | 3 (33%) | 2 (22%) | 5 (71%) | 8 (53%) | 19 (50%) | 18 (40%) |
| NYHA I-II | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) | 0 (0%) | 1 (2%) |
| NYHA II | 4 (36%) | 4 (40%) | 2 (18%) | 3 (27%) | 1 (11%) | 2 (22%) | 1 (14%) | 4 (27%) | 8 (21%) | 13 (29%) |
| NYHA II-III | 0 (0%) | 0 (0%) | 0 (0%) | 3 (27%) | 3 (33%) | 0 (0%) | 0 (0%) | 1 (7%) | 3 (8%) | 4 (9%) |
| NYHA III | 0 (0%) | 0 (0%) | 3 (27%) | 1 (9%) | 0 (0%) | 3 (33%) | 0 (0%) | 0 (0%) | 3 (8%) | 4 (9%) |
| NYHA IV | 2 (18%) | 2 (20%) | 0 (0%) | 0 (0%) | 2 (22%) | 2 (22%) | 1 (14%) | 1 (7%) | 5 (13%) | 5 (11%) |
Legend: NYHA = New York Heart Association; initial = 2-3 weeks of app use; post = 4–6 months of app use; The same patients were not always interviewed in both the initial and post-interviews. Initial and post-interviews with the same patients were conducted with 9 patients in Germany, 10 patients in the Netherlands, 8 patients in the United Kingdom and 7 patients in Ireland.
In accordance with the semi-structured interview guidelines, the following key themes were identified from the data: 1) expectations, 2) perceived usability and benefit, 3) usage behaviour and experiences, 4) self-care of HF, and 5) social influence.
Expectations
At the beginning of using the DoctorME app, patients did not necessarily have specific expectations. Furthermore, the expressed expectations differed. Above all, patients expected the DoctorME app to provide continuous monitoring and an associated early warning system in the event of deterioration. Thereby, the combination of telemedicine and a kind of patient record was considered to be particularly important.
“Well, hopefully, it will keep an eye if I put on my blood pressures and stuff. And if something is wrong, somebody will react to it.” (initial, UK, male, 60–69 years)
Further expectations from using the DoctorME app were: (1) app as a source of information regarding HF, (2) promote patients’ motivation/self-discipline to adhere to therapy, and (3) additional HF care. After 4–6 months of using the DoctorME app, the continuous monitoring and the associated early warning system with prompt feedback were also the most frequently mentioned expectations, which have been satisfactorily met.
Perceived usability and benefit
The first impression of the DoctorME app was predominantly positive. However, the opinions on the app design were divided. While some patients found the app easy to use and clearly structured, others found the app not intuitive. After 4–6 months of use, most patients described the DoctorME app as generally easy to use or easy to use due to its simplicity, clarity, and the simple input of data. They also became quickly familiar with it.
“It was very user-friendly. I think even if you weren’t digitally aware, you could manage it.” (post, IRL, female, 50–59 years)
In terms of patient benefit, the most valuable functions were: (1) continuous monitoring, (2) e-learning, (3) feedback from the app, (4) entering vital signs, and (5) a chat function or quick medical response. These findings were largely consistent with the statements made in the initial interviews. At the beginning of using the DoctorME app, the majority of patients saw the greatest benefit in the direct feedback and the associated sense of security, in addition to the continuous monitoring and input of their vital signs, which were perceived to increase self-discipline. The e-learning modules were also perceived as highly beneficial and informative.
“It makes me feel safer. That it indicates when the weight gets too heavy, or the blood pressure gets too high and then it gives advice.” (post, NL, male, 60–69 years)
In both initial and post-interviews, many patients were unable to provide any information on missing functions, as they could not think of any. Some patients expressed a desire for the integration of additional data, including other vital parameters (e.g., blood sugar and oxygen levels), a medication plan, and data of external devices. They also requested the integration of a history of their values. Furthermore, some patients proposed improvements to the e-learnings. The most common requests were for more information on specific HF topics, such as nutrition and exercise recommendations.
Usage behaviour and experiences
Most patients used the DoctorME app eight times per month in accordance with the recommended frequency and only when prompted to do so. In comparison with the initial interviews, the actual frequency of usage after 4–6 months corresponded to the originally planned frequency of usage. In doing so, patients usually integrated the DoctorME app into their normal daily care routines.
“I have now already integrated them into the breakfast ritual.” (initial, DE, male, 60–69 years)
Moreover, for almost half of the patients, the average duration of a session was 5 min or less. The specified duration per use was deemed acceptable for almost all patients. At the start of using the DoctorME app, the majority of patients stated that a session should last a maximum of 10 min on average.
There was a notable divergence of opinion regarding the communication with the virtual doctor, with approximately half expressing a positive view and the other half a negative one. Many patients considered the idea of a virtual doctor (avatar) or virtual communication with the app to be good in principle. However, some also stated that they did not like it, citing several reasons, including the irritating voice, and/or opted to switch the avatar off during the 4–6 months of usage.
“I think the content that was provided through the doctor was fine. The delivery method of visually the doctor just didn’t work for me.” (post, UK, male, 18–49 years)
After entering their vital signs, patients may have received recommendations from the app. In the event that recommendations were made, patients generally adhered to them unless prior arrangements had been made with their HCP.
“I once had the recommendation to get in touch. My blood pressure was low then. […] I’ve also spoken to my cardiologist about it once. That's within normal limits. […] That's why I didn’t contact the team at the hospital.” (post, DE, male, 60–69 years)
Many patients reported no difficulties when using the DoctorME app. However, some patients did highlight instances where the app ‘froze’ or did not always run smoothly, had long loading times or encountered difficulties with the login process. These findings align with the statements of the initial interviews. Technical issues and overly frequent data entry were identified by patients as the primary factors that could negatively impact their use of the DoctorME app. Nevertheless, most patients could imagine continuing to use the DoctorME app in the future.
“There were a couple of times the system was down and wasn’t working properly. I found that very frustrating.” (post, IRL, male, 70–73 years)
Furthermore, patients were asked as to whether the virtual doctor might reduce future GP visits. Their opinions were divided, with almost half of the patients agreeing to this question, while the other patients disagreed and expressed a preference for direct contact with their GP.
“Yes. It's very hard to get a doctor now, as you know, but the virtual definitely makes it a lot easier.” (post, UK, male, 60–69 years)
Both the initial and post-interviews indicated that some patients exhibited a lack of trust in the app, preferring to contact an HCP directly rather than using the app first or following its recommendations. It is noteworthy that the relationship between HCPs and patients was not directly addressed in the interviews.
“From a patient's point of view, there is nothing like a human looking at you. I have to say that. This artificial intelligence is great and there is a place for it, but you can’t replace the human, in my view.” (post, IRL, female, 50–59 years)
Self-care of HF
Almost a third of patients reported that using the DoctorME app for 4–6 months had no influence on their attitude towards HF or their HF management. The primary reason was that many patients had been living with HF for several years and had already established daily care routines. Other patients described a positive influence, predominantly through continuous monitoring of vital signs, and that they had become more aware and sensitized to HF through the DoctorME app.
“It's definitely got me to be way more proactive and more aware […]. It has made me think more about things I should be doing more. I should be exercising more. I would be a healthy eater anyway, but just even watching how much sodium I use. Little things like that. It's definitely been an encouragement.” (post, IRL, female, 60–69 years)
From the patients’ perspective, the majority also described the DoctorME app as a helpful tool especially when they experienced worrying situations in the last 4–6 months, which enabled them to additionally control and maintain their HF management. However, almost as many patients reported that they had not experienced any worrying situations in the last 4–6 months because their condition was stable, or that the DoctorME app had no influence in these situations. There was a divided opinion on the influence of the DoctorME app on their everyday sense of security. If the app has increased the feeling of security, this is often due to the continuous monitoring and prompt feedback.
“Well, as I say, it's like my own early warning system, but I don’t have that rush to any or rush to my GP because if I think that I have any episodes, I think that's the first protocol to check.” (post, UK, male, 50–59 years)
Social influences
Despite nearly half of the patients having not discussed the use of the DoctorME app with their treating HCP, most had informed them. Some patients specifically mentioned using the DoctorME app only to their cardiologist or to their GP. From the patients’ point of view, their HCPs reacted predominantly positively to the use of the DoctorME app.
“Yes, my GP also thought that was a good thing.” (post, DE, male, 60–69 years)
Opinions were divided regarding the impact of HCP's opinions on the use of the DoctorME app. While almost half of the patients indicated that the HCP's opinion had an influence on the use of the DoctorME app, either in general, or in motivating or discouraging them (see following quote), other patients used the DoctorME app regardless of their HCP's opinions, particularly if their HCPs had a negative attitude towards the app.
“I think if she had advised me against it, I would have stopped.” (post, DE, male, 60–69 years)
Furthermore, most patients stated that the opinions of their relatives had no influence on their use of the DoctorME app. This was due to the fact that their own opinion was deemed more important, or that the opinion of their HCP exerted a greater influence on the use of an app than that of their relatives. Nevertheless, some patients were also positively influenced and motivated by their relatives’ positive attitudes towards the DoctorME app.
Recommendation of the DoctorME app and voluntary monthly costs
Almost all patients stated they would recommend the DoctorME app to their relatives or acquaintances, given the additional care, perceived sense of security, or regular monitoring the app offers. However, from the patients’ perspective, it was evident that half of them would not be willing to pay anything for a self-care app like the DoctorME app. Most patients who said they would be willing to pay for the DoctorME app, did not specify the amount. Some patients indicated that they would be willing to pay less than €10 to €15 per month if there was an interaction between the treating HCPs and the app.
Trust of artificial intelligence recommendations on medications
Finally, patients were asked if they would trust AI-based medication recommendations provided by the app. The responses were divided (approx. 1/3 trustworthy; 1/3 trustworthy with conditions; 1/3 not trustworthy). While some patients’ preference was to always speak to their HCP when changing medications, some patients said they would trust the AI-based medication recommendations if the recommendation had been previously confirmed by an HCP.
“As long as there is someone involved in that, like a person at some point, so maybe it flags up to the relevant consultant or the relevant GP that if you look at all these statistics it looks like maybe that needs a change, and it just flags it for someone to review. I don’t think I’d be happy with an algorithm deciding to change my dosage.” (post, UK, male, 18–49 years)
Table 3 summarises the key findings regarding the usability and usage behaviour of the DoctorME app, which can be used for future developments and implementations of digital health solutions in HF care
Table 3.
Summary of usability and usage behaviour of the DoctorME app for heart failure (HF) care.
| Perceived usability |
|
| Length of daily usage |
|
| Frequency of monthly usage |
|
| Perceived benefit of the DoctorME app |
|
| Negative influence of the DoctorME app usage |
|
| Missing functions / suggestions for improvements |
|
| Physician-patient relationship / Trust in the DoctorME app |
|
Legend: This information represents the key findings of the initial and post-interviews.
Discussion
This study aimed to explore patients’ expectations, experiences, and utilization patterns regarding the DoctorME app, both initially and after a period of 4–6 months.
The DoctorME app is designed to continuously record vital signs and other data relevant to HF, enabling timely identification of early deterioration and application of clinical strategies. This approach aligns with other mHealth apps developed for enhancing patients’ self-management in HF care.17,18 Patients perceived the greatest benefit of using the app in the continuous monitoring of their vital signs and weight, as well as the integrated early warning system with quick feedback and e-learning modules. These findings are consistent with those of another qualitative study, 19 wherein patients highlighted communication ability, personalized feedback and education, and automated self-monitoring as the predominant factors facilitating app utilization. Up to this point, the DoctorME app has been unable to perform automated self-monitoring via networked wireless devices. This technical advancement would be beneficial in order to adapt the app to the needs of patients and HCPs. 19
The present results demonstrate that most patients required an average of five minutes for a session, found this duration to be acceptable, and were able to integrate the app into their daily care routines. This is consistent with experiences of users of other self-care apps, such as the Habits Heart App for patient engagement in HF care, who also indicated that they needed approximately five minutes for similar sessions. 17
Moreover, app usage was found to have a partially positive effect on patients’ self-care and sense of security. Greater awareness of the disease appears to be a contributing factor. However, it was mainly patients who had already established their own daily care routines who reported that the app had no additional influence on their self-care. Thereby, patients gave the impression that a mHealth app designed to support HF care would be particularly beneficial right after diagnosis in order to assist patients in understanding how to manage their condition and what to be aware of. This approach could help to alleviate patients’ initial uncertainties following a diagnosis. 12 Once a daily care routine has been established, customising the app becomes a crucial aspect of its utilisation. A qualitative analysis has indicated that patients are more likely to desire added value from individualised app functions than from generic support. 19 The Heart Failure Association of the ESC underscores the importance of self-care for patients diagnosed with HF from the outset of their diagnosis. It provides current recommendations for action on the three concepts of self-care maintenance, self-care monitoring and self-care management. 12 Digital health solutions with integrated reminder functions, early warning systems, and quick feedback can also assist patients in adopting healthy behaviour to maintain physical and emotional stability (self-maintenance), observe changes in signs and symptoms (self-monitoring) and react to symptoms accordingly (self-care management). 12 The implementation of new health-promoting behaviour or the adaptation of habitual behaviour takes time. One meta-analysis also indicated that longer intervention to improve patients’ self-care appears to improve the effects on various outcomes. 26 It would have been beneficial to conduct a further qualitative survey approximately 10–12 months of app use to ascertain the long-term consistency of our results.
The app's added value is not yet apparent in the stand-alone solution, but rather it is evident in the fact that an HCP monitors or controls it. In a previous interview study, determinants of acceptance among patients with HF were examined. 20 Building trust was identified as a key prerequisite for the implementation and long-term usage of a digital solution. The process of building trust requires time and it is enhanced when HCPs are involved in the development of the solution. 20 Nevertheless, the physician-patient relationship is characterised by a particularly high level of trust and loyalty on the part of patients. 27 It is therefore unsurprising to find that a meta-analysis has demonstrated that personal contact during treatment demonstrably improves patient outcomes. 28 Furthermore, older HF patients with a low affinity for technology tend to be sceptical about the implementation of mHealth apps in their daily care routines, particularly if it means seeing their HCP less frequently in person. This was observed in our sample and in the literature. 29 At the same time, older patients also acknowledge the advantages of utilizing new technologies in healthcare. 30
Strengths and limitations
The main strength of this interview study is the collection of patient perspectives from four European countries after 2-3 weeks and after 4–6 months of using a self-care app for HF. Despite the intended purposive maximum variation approach, the present sample does not reflect the average HF population9,31 in terms of average age and HF severity. Participants were slightly younger, and the majority belonged to NYHA class I, thus suffering from mild symptoms. The selection bias can be attributed to the predetermined recruitment time and the fact that only patients who volunteered to participate were interviewed. In addition, it was not possible to consistently ensure that the same patients were interviewed at both measurement points. This can be seen as a further limitation of this study and must be considered when interpreting the results. Nevertheless, of the 38 patients who were interviewed at the beginning of the study and the 45 patients who were interviewed after 4–6 months, a total of 34 patients were identical.
Another strength is the relatively large sample size, which is particularly notable in the context of qualitative research.32,33 However, the data collection methods employed in the participating countries and between the measurement points differed, which may have affected the results. Moreover, the potential interviewer bias remains a factor that cannot be entirely mitigated. Nevertheless, all interviewers adhered to standardized interview guides (regardless of the method of data collection), underwent virtual training, and received supervision from two experienced researchers (BS, AN).
In the process of data analysis, only (sub-)categories with a minimum of three statements (i.e., three patients) were taken into account. Consequently, it is possible that an innovative idea or statement from individual patients may have been be overlooked. However, the aim of the qualitative study was to provide a comprehensive overview of patients’ expectations, experiences, and actual usage behaviour regarding a self-care app for HF.
Conclusions
This qualitative study provides valuable insights into the patients’ preferences and actual usage behaviour of a self-care app for HF. The results indicate that user-friendliness of the app is a key element in the successful implementation of the patient's daily care routines. Patients perceive the continuous monitoring of their HF-relevant data, the prompt feedback on their condition, and e-learnings as particularly beneficial. Furthermore, enhanced technical reliability of the app and its ability to be tailored to the patient's requirements and needs, may also have a positive impact on long-term use. However, it became evident that the majority of patients did not regard the app as a stand-alone method, but rather as one that complements their physician's guidance in the management of their HF. The integration of a self-care app, such as the DoctorME app, as a supportive tool alongside traditional medical care, shows much potential to enhance the overall management and QoL of patients with HF.
Acknowledgements
This article was written on behalf of the PASSION-HF consortium. We are grateful to all participants for their contribution to the interview study.
Abbreviations
- AI
Artificial intelligence
- AN
Anne Neumann
- AM
Anne McNulty
- App
Application
- BS
Bianca Steiner
- BZS
Bettina Zippel-Schultz
- CM
Cathy Mulhall
- DE
Germany
- DF
Donna Fitzsimons
- DSS
Decision support system
- ESC
European Society of Cardiology
- GP
General practitioner
- HCP
Healthcare professional
- HF
Heart failure
- HPBLR
Hans-Peter Brunner-LaRocca
- IRL
Ireland
- JB
Josiane J. J. Boyne
- LH
Loreena Hill
- mHealth apps
Mobile health applications
- MM
Marguerite Murphy
- NL
the Netherlands
- NYHA
New York Heart Association
- PASSION-HF
Patient Self Care Using eHealth in Chronic Heart Failure; Initial First measurement point (after 2-3 weeks of app’ usage) Post Second measurement point (after 4–6 months of app’ usage)
- QoL
Quality of Life
- UK
United Kingdom
- UTAUT
Unified Theory of Acceptance and Use of Technology
- YM
Yannick Maaser
Footnotes
Contributorship: AN, BS, BZS and CS contributed to the conceptualization and design of the study. The following authors were responsible for conducting the study at the respective country: MV, NK and KS in Germany; LH, AM and DF in the UK; JB and HPBLR in the Netherlands; and MM and MB in Ireland. AN, BS, YM, JB, MM, LH, AM and DF collected the data. AN, BS and YM analysed the data. AN and BS were involved in the interpretation of results. BZS, TH and HPBLR supervised the project. AN wrote the first manuscript draft. All authors were engaged in reviewing and editing the manuscript. All authors have read the final version of the manuscript and approved its submission.
Consent to participate: Participants were given written information consent obtained before interviews were conducted. Please refer to the Ethical Considerations section.
Consent for publication: Not applicable
Data availability: The datasets used and/or analysed during the current study are available from the corresponding author (AN) upon reasonable request. Signing a data use/sharing agreement will be necessary, and data security regulations both in the four European countries (DE, IRL, NL, UK) and in the country of the investigator who proposes to use the data must be complied with. The data is not publicly available as some of the information could compromise research participant privacy/consent.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical considerations: The protocol for this study was reviewed and approved by the Ethics Committees of Queen's University Belfast (Health & Social Care Ethics Committee B (HSC REC B), REC reference: 21 /NI/0028), St Vincent's Hospital Group, Research Ethics Committee, Maastricht University Medical Centre + (academisch ziekenhuis Maastricht, Number METC20-098) and University Hospital Aachen (Ethik-Kommission der an der medizinischen Fakultät der rheinisch-westfälischen technischen Hochschule Aachen, EK 418/20). Respondents gave written consent for review and signature before starting interviews. This study was conducted in accordance with the 1964 Helsinki Declaration and its later amendments.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is part of the PASSION-HF project funded by Interreg VB North-West Europe (grant number NWE 702). For more information, visit: http://www.nweurope.eu/projects/project-search/passion-hf-patient-self-care-using-ehealth-in-chronic-heart-failure/.
Interreg VB North-West Europe, (grant number NWE 702).
ORCID iDs: Anne Neumann https://orcid.org/0000-0002-6884-079X
Bettina Zippel-Schultz https://orcid.org/0000-0003-4791-7222
Guarantor: AN, BS and BZS
References
- 1.Savarese G, Becher PM, Lund LH, et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 2023; 118: 3272–3287. [DOI] [PubMed] [Google Scholar]
- 2.Shahim B, Kapelios CJ, Savarese G, et al. Global public health burden of heart failure: an updated review. Card Fail Rev 2023; 9: 20230727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steiner B, Neumann A, Pelz Y, et al. Challenges in heart failure care in four European countries: a comparative study. Eur J Public Health 2023; 33: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018; 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesyuk W, Kriza C, Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord 2018; 18: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braunwald E. The war against heart failure: the lancet lecture. Lancet 2015; 385: 812–824. [DOI] [PubMed] [Google Scholar]
- 7.Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis 2019; 62: 298–302. [DOI] [PubMed] [Google Scholar]
- 8.Khan MS, Samman Tahhan A, Vaduganathan M, et al. Trends in prevalence of comorbidities in heart failure clinical trials. Eur J Heart Fail 2020; 22: 1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson I, Joseph P, Balasubramanian K, et al. Health-related quality of life and mortality in heart failure: the global congestive heart failure study of 23 000 patients from 40 countries. Circulation 2021; 143: 2129–2142. [DOI] [PubMed] [Google Scholar]
- 10.Freedland KE, Rich MW, Carney RM. Improving quality of life in heart failure. Curr Cardiol Rep 2021; 23: 59. [DOI] [PubMed] [Google Scholar]
- 11.Moradi M, Daneshi F, Behzadmehr R, et al. Quality of life of chronic heart failure patients: a systematic review and meta-analysis. Heart Fail Rev 2020; 25: 993–1006. [DOI] [PubMed] [Google Scholar]
- 12.Jaarsma T, Hill L, Bayes-Genis A, et al. Self-care of heart failure patients: practical management recommendations from the heart failure association of the European Society of Cardiology. Eur J Heart Fail 2021; 23: 157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vellone E, Fida R, Ghezzi V, et al. Patterns of self-care in adults with heart failure and their associations with sociodemographic and clinical characteristics, quality of life, and hospitalizations: a cluster analysis. J Cardiovasc Nurs 2017; 32: 180–189. [DOI] [PubMed] [Google Scholar]
- 14.McDonagh TA, Metra M, Adamo M, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 15.Cowie MR, Lam CSP. Remote monitoring and digital health tools in CVD management. Nat Rev Cardiol 2021; 18: 457–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding X, Wen Y, Tian Z, et al. Effect of e-health intervention on disease management in patients with chronic heart failure: a meta-analysis. Front Cardiovasc Med 2022; 9: 1053765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei KS, Ibrahim NE, Kumar AA, et al. Habits heart app for patient engagement in heart failure management: pilot feasibility randomized trial. JMIR Mhealth Uhealth 2021; 9: e19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi E-Y, Park J-S, Min D, et al. Heart failure-smart life: a randomized controlled trial of a mobile app for self-management in patients with heart failure. BMC Cardiovasc Disord 2023; 23: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezerra Giordan L, Ronto R, Chau J, et al. Use of mobile apps in heart failure self-management: qualitative study exploring the patient and primary care clinician perspective. JMIR Cardio 2022; 6: e33992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zippel-Schultz B, Palant A, Eurlings C, et al. Determinants of acceptance of patients with heart failure and their informal caregivers regarding an interactive decision-making system: a qualitative study. BMJ Open 2021; 11: e046160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayring P. Qualitative content analysis: theoretical foundation, basic procedures and software solution. Klagenfurt: SSOAR, 2014, p.143. [Google Scholar]
- 22.Venkatesh V, Thong JYL, Xu X. Consumer acceptance and use of information technology: extending the unified theory of acceptance and use of technology. MIS Q 2012; 36: 157–178. [Google Scholar]
- 23.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007; 19: 349–357. [DOI] [PubMed] [Google Scholar]
- 24.White PD, Myers MM. The classification of cardiac diagnosis. J Am Med Assoc 1921; 77: 1414–1415. [Google Scholar]
- 25.Schreier M. Ways of doing qualitative content analysis: disentangling terms and terminologies. Forum Qualitative Sozialforschung / Forum: Qualitative Social Research 2014; 15: 1–27. [Google Scholar]
- 26.Jonkman NH, Westland H, Groenwold RH, et al. What are effective program characteristics of self-management interventions in patients with heart failure? An individual patient data meta-analysis. J Card Fail 2016; 22: 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chipidza FE, Wallwork RS, Stern TA. Impact of the doctor-patient relationship. Prim Care Companion CNS Disord 2015; 17: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sochalski J, Jaarsma T, Krumholz HM, et al. What works in chronic care management: the case of heart failure. Health Aff (Millwood) 2009; 28: 179–189. [DOI] [PubMed] [Google Scholar]
- 29.Vaportzis E, Clausen MG, Gow AJ. Older adults perceptions of technology and barriers to interacting with tablet computers: a focus group study. Front Psychol 2017; 8: 1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalaitzaki A, Rovithis M, Dimitropoulos A, et al. Promoting self-management and independent living of older individuals with chronic diseases through technology: a study of self-reported needs, priorities, and preferences. Medicina (Kaunas) 2023; 59: 1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the framingham heart study. Circulation 2002; 106: 3068–3072. [DOI] [PubMed] [Google Scholar]
- 32.Mayring P. Qualitative Content Analysis: Demarcation, Varieties, Developments. Forum: Qualitative Social Research, 2019. [Google Scholar]
- 33.Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med 2022; 292: 114523. [DOI] [PubMed] [Google Scholar]