Abstract
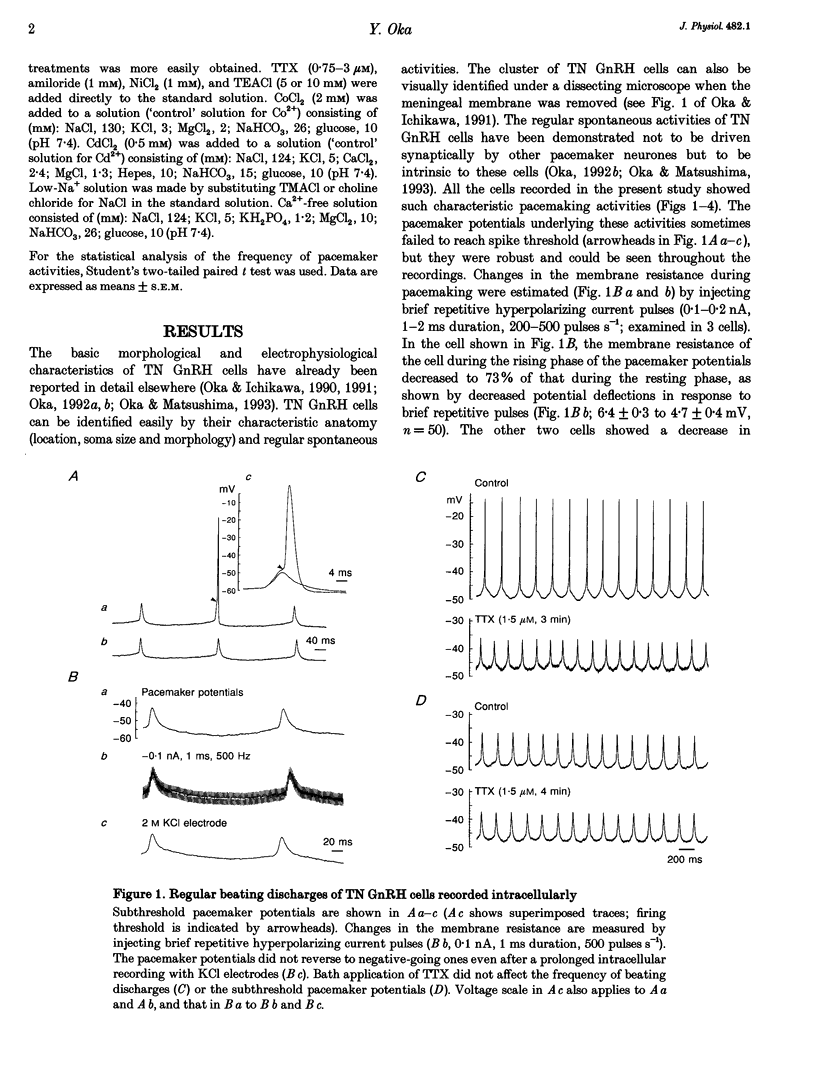
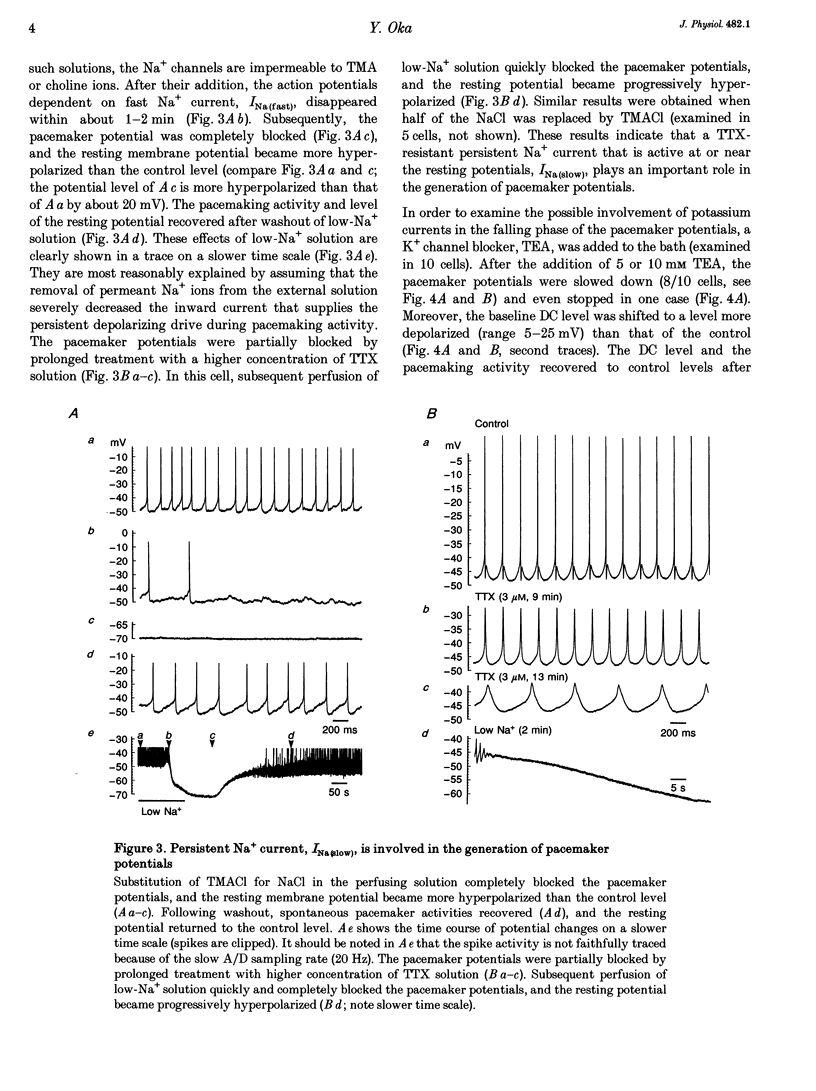
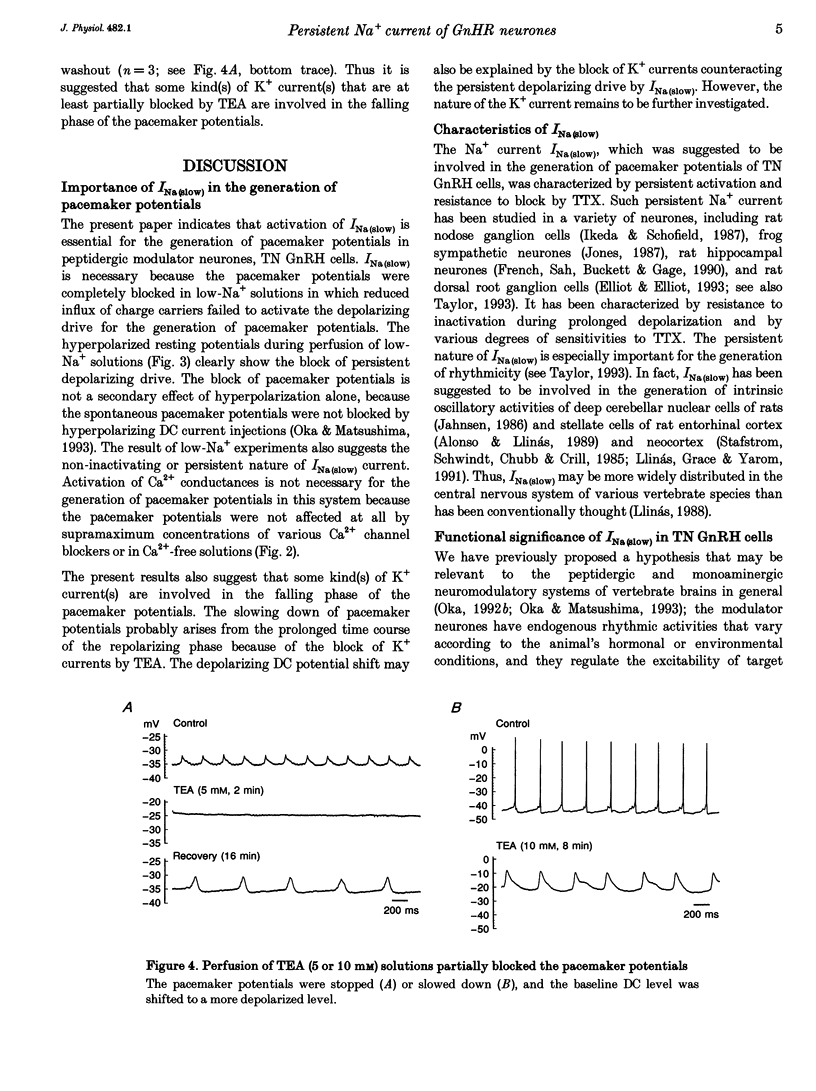
1. Gonadotrophin-releasing hormone (GnRH)-immunoreactive terminal nerve (TN) cells show endogenous regular beating discharges, which may be related to their putative neuromodulator functions. The ionic mechanism underlying the pacemaker potential was studied using intracellular and patch-pipette current clamp recordings from a whole brain in vitro preparation of a small fish brain. 2. The pacemaker potentials were resistant to 1.5-3 microM tetrodotoxin (TTX) and were not affected by Ca2+ channel blockers (amiloride, Ni2+, Co2+, Cd2+) or in Ca(2+)-free solution. In contrast, the pacemaker potentials were readily blocked by substituting tetramethylammonium or choline for Na+ in the perfusing solution, and the resting membrane potential became more hyperpolarized than the control level. 3. The present results suggest that the TTX-resistant persistent Na+ current, INa(slow), supplies the persistent depolarizing drive and plays an important role in the generation of pacemaker potentials in TN GnRH cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso A., Llinás R. R. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature. 1989 Nov 9;342(6246):175–177. doi: 10.1038/342175a0. [DOI] [PubMed] [Google Scholar]
- Alreja M., Aghajanian G. K. Opiates suppress a resting sodium-dependent inward current and activate an outward potassium current in locus coeruleus neurons. J Neurosci. 1993 Aug;13(8):3525–3532. doi: 10.1523/JNEUROSCI.13-08-03525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alreja M., Aghajanian G. K. Pacemaker activity of locus coeruleus neurons: whole-cell recordings in brain slices show dependence on cAMP and protein kinase A. Brain Res. 1991 Aug 16;556(2):339–343. doi: 10.1016/0006-8993(91)90327-r. [DOI] [PubMed] [Google Scholar]
- Elliott A. A., Elliott J. R. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol. 1993 Apr;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French C. R., Sah P., Buckett K. J., Gage P. W. A voltage-dependent persistent sodium current in mammalian hippocampal neurons. J Gen Physiol. 1990 Jun;95(6):1139–1157. doi: 10.1085/jgp.95.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G. Tetrodotoxin-resistant sodium current of rat nodose neurones: monovalent cation selectivity and divalent cation block. J Physiol. 1987 Aug;389:255–270. doi: 10.1113/jphysiol.1987.sp016656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H. Electrophysiological characteristics of neurones in the guinea-pig deep cerebellar nuclei in vitro. J Physiol. 1986 Mar;372:129–147. doi: 10.1113/jphysiol.1986.sp016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W. Sodium currents in dissociated bull-frog sympathetic neurones. J Physiol. 1987 Aug;389:605–627. doi: 10.1113/jphysiol.1987.sp016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R., Grace A. A., Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988 Dec 23;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y. Gonadotropin-releasing hormone (GnRH) cells of the terminal nerve as a model neuromodulator system. Neurosci Lett. 1992 Aug 17;142(2):119–122. doi: 10.1016/0304-3940(92)90353-9. [DOI] [PubMed] [Google Scholar]
- Oka Y., Ichikawa M. Gonadotropin-releasing hormone (GnRH) immunoreactive system in the brain of the dwarf gourami (Colisa lalia) as revealed by light microscopic immunocytochemistry using a monoclonal antibody to common amino acid sequence of GnRH. J Comp Neurol. 1990 Oct 22;300(4):511–522. doi: 10.1002/cne.903000406. [DOI] [PubMed] [Google Scholar]
- Oka Y., Ichikawa M. Ultrastructure of the ganglion cells of the terminal nerve in the dwarf gourami (Colisa lalia). J Comp Neurol. 1991 Feb 8;304(2):161–171. doi: 10.1002/cne.903040202. [DOI] [PubMed] [Google Scholar]
- Oka Y., Matsushima T. Gonadotropin-releasing hormone (GnRH)-immunoreactive terminal nerve cells have intrinsic rhythmicity and project widely in the brain. J Neurosci. 1993 May;13(5):2161–2176. doi: 10.1523/JNEUROSCI.13-05-02161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggenbass M., Dreifuss J. J. Mechanism of action of oxytocin in rat vagal neurones: induction of a sustained sodium-dependent current. J Physiol. 1992 Nov;457:131–142. doi: 10.1113/jphysiol.1992.sp019368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggenbass M., Goumaz M., Sermasi E., Tribollet E., Dreifuss J. J. Vasopressin generates a persistent voltage-dependent sodium current in a mammalian motoneuron. J Neurosci. 1991 Jun;11(6):1609–1616. doi: 10.1523/JNEUROSCI.11-06-01609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Chubb M. C., Crill W. E. Properties of persistent sodium conductance and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985 Jan;53(1):153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- Taylor C. P. Na+ currents that fail to inactivate. Trends Neurosci. 1993 Nov;16(11):455–460. doi: 10.1016/0166-2236(93)90077-y. [DOI] [PubMed] [Google Scholar]



