Abstract
Patient: Male, 23-year-old
Final Diagnosis: Mycobacterium marinum infection
Symptoms: Multiple sporotrichoid skin lesions
Clinical Procedure: —
Specialty: Infectious Diseases
Objective:
Unknown etiology
Background:
Mycobacterium marinum is a slow-growing non-tuberculous mycobacterium that is known to cause skin and soft tissue infections, even in healthy patients, and is commonly associated with fish and aquatic environments.
Case Report:
A 23-year-old man working in aquarium management presented with a chronic progression of multiple skin nodules on his right forearm and thumb. The patient was referred from the Dermatology Department to the Outpatient Clinic due to suspected skin tuberculosis, as indicated by a positive T-SPOT.TB test. A second excisional biopsy tested positive for M. marinum via PCR sequencing by the National Institute of Infectious Diseases, confirming the diagnosis. The initial treatment consisted of rifabutin at 300 mg/day and clarithromycin at 800 mg/day. However, due to liver dysfunction, the regimen was changed to moxifloxacin at 400 mg/day and rifabutin. Moxifloxacin was discontinued due to nausea. Finally, the treatment was adjusted to linezolid at 1200 mg/day and clarithromycin. The patient’s skin condition improved, with the nodular lesions showing a trend toward resolution. Culturing is time-consuming, and the sensitivity can be reduced when using N-acetyl-l-cysteine–sodium hydroxide in the pre-treatment process; therefore, caution with its use is necessary. Pathological examination can initially show inflammatory changes, and granulomatous lesions with caseous necrosis are not always present. Antibiotics such as rifampicin, rifabutin, moxifloxacin, and clarithromycin are used, but there is scant evidence for treatment regimens, often resulting in prolonged monotherapy or combination therapy.
Conclusions:
In cases presenting chronic lesions resembling multiple sporotrichoid forms, repeated biopsies are crucial due to the challenges associated with culturing.
Key words: Mycobacterium Infections; Nontuberculous, Diagnosis; Nontuberculous, Drug Therapy; Mycobacterium marinum
Introduction
Mycobacterium marinum is a slow-growing non-tuberculous mycobacterium known to cause skin and soft tissue infections and is commonly associated with fish and aquatic environments. M. marinum was first isolated in 1926 from deceased saltwater fish during a necropsy at an aquarium in Philadelphia [1]. The first case of human infection was reported in 1951 by Norden and Linell [2]. M. marinum grows optimally at 30°C, its growth is inhibited at 37°C [3], and it often causes lesions localized to the skin. It is microbiologically characterized by the development of photochromogenic colonies within 7 to 14 days. Differentiating M. marinum from Mycobacterium ulcerans and other mycolactone-producing non-tuberculous mycobacteriums on a molecular level is, however, challenging [3]. The characteristic of M. marinum in skin infection is a painful skin nodule, called a sporotrichoid form [4]. Due to the difficulty of diagnosis, a case series that gathered 25 instances of skin and soft tissue infections caused by non-tuberculous mycobacterium revealed that approximately 16 were due to M. marinum. The mean interval between the initial clinical presentation and the diagnosis of these infections was reported as 7.1 months, with a range from 1 to 27.3 months [5]. We report the case of a 23-year-old man working in aquarium management who presented with a chronic progression of multiple skin nodules on his right forearm and right thumb, which were diagnosed as M. marinum after repeated skin biopsies.
Case Report
A 23-year-old man who was employed in aquarium management and had no significant medical history was referred from the Dermatology Department to our Outpatient Clinic on suspicion of cutaneous Mycobacterium tuberculosis infection. This suspicion arose due to a positive T-SPOT.TB test, along with a skin nodule and ulcer on the right upper extremity and right thumb. He did not show any signs of lymph node involvement or lymphangitis. Approximately 2 years before his visit, he had sustained an arm injury while working with aquariums. Subsequently, he noticed a rash on his right forearm. He had been under the care of a local physician and our Dermatology Department for the past year, and an initial excisional biopsy had been performed 3 months ago. The procedure was conducted at 37°C. Cultures for acid-fast bacilli and the polymerase chain reaction test for tuberculosis were negative. One month before visiting our clinic, a T-SPOT.TB test was conducted and returned positive. The nodules on the right forearm and right thumb had been enlarging over several months, associated with discharge and slight pain upon palpation. Vital signs show no atypical findings. The physical examination revealed a 3-cm nodule on the radial side of the right forearm with a crusted surface, mild surrounding erythema, no spontaneous pain, and tenderness upon palpation. Additionally, a similar 1-cm nodule was noted near the proximal phalanx of the right thumb (Figure 1). Laboratory test results revealed a white blood cell count of 6000/μL (53.8% neutrophils, 37.0% lymphocytes), hemoglobin of 14.8 g/dL, platelet count of 254×103/μL, creatinine of 0.81 mg/dL, total bilirubin of 0.8 mg/dL, alkaline phosphatase of 75 U/L, lactate dehydrogenase of 158 U/L, aspartate aminotransferase of 22 U/L, alanine aminotransferase of 45 U/L, gamma-glutamyl transferase of 25 U/L, and C-reactive protein less than 0.04 mg/dL. Whole-trunk computed tomography with contrast revealed no findings in the chest, or lymphadenopathy. Magnetic resonance imaging of the right forearm showed increased fat tissue density, but no evidence of subcutaneous abscess or osteomyelitis. The second excisional biopsy of the right upper extremity was performed, and the specimen was stored at around 30°C; M. marinum infection was suspected. Histopathology of the specimen revealed that moderate infiltration of inflammatory cells was observed, but epithelioid granulomas, multinucleated giant cells, and necrosis were not observed (Figure 2). The specimen was sent to a domestic company and the National Institute of Infectious Diseases, where DNA was extracted from the specimen, but the result was negative. The specimen was incubated on 2% Ogawa PS medium “Nissui” (Shimadzu Diagnostics Corporation, Japan) and in Middlebrook 7H9 broth (Becton, Dickinson and Company, USA) at 32°C with 5% CO2. Subsequent cultures detected colonies on the Ogawa medium since day 11 from incubation, and DNA extraction followed by PCR sequencing confirmed M. marinum. Susceptibility testing was performed using BrothMIC SGM (Kyokuto Pharmaceutical Industrial, Japan; Table 1). The result of another sample specimen sent to the domestic company was negative. We treated with rifabutin 300 mg/day plus clarithromycin 800 mg/day.
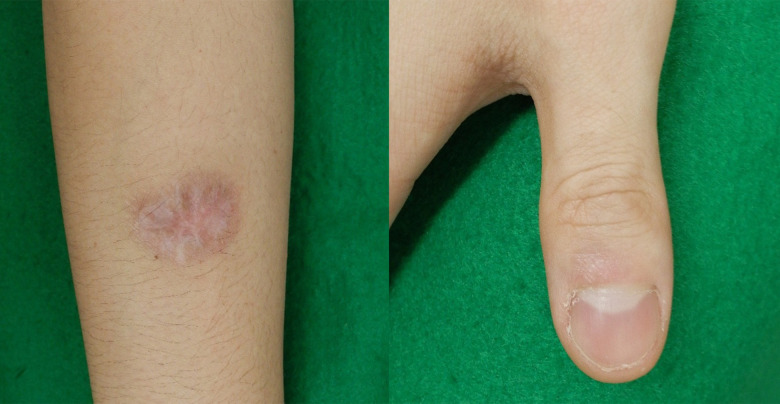
Figure 1.
Skin nodule on the right upper extremity and right digit before treatment. The nodule is firm to the touch. There is no tenderness upon palpation.
Figure 2.

Pathological examination of the skin biopsy. Moderate infiltration of inflammatory cells is observed in the relatively superficial part of the dermis. Epithelioid granulomas, multinucleated giant cells, and necrosis are not observed. No significant organisms are identified with periodic acid-Schiff, Grocott, or Ziehl-Neelsen staining.
Table 1.
Laboratory values.
| Antimicrobial | Minimum inhibitory concentration (μg/mL) |
|---|---|
| Clarithromycin | 0.5 |
| Azithromycin | 16 |
| Moxifloxacin | 0.5 |
| Amikacin | ≤8 |
| Minocycline | 2 |
| Isoniazid | >4 |
| Ethambutol | 2 |
| Sitafloxacin | 1 |
| Kanamycin | ≤2 |
| Doxycycline | 2 |
| Linezolid | ≤2 |
| Ethionamide | 2 |
| Rifampicin | 0.5 |
| Rifabutin | ≤0.25 |
Due to liver dysfunction 1 month after starting the treatment, the regimen was changed to moxifloxacin 400 mg/day plus clarithromycin 800 mg/day. Three months later, the patient developed nausea due to moxifloxacin. The treatment was then switched to linezolid 1200 mg/day and clarithromycin. Following these changes, there were no further complications, and the skin findings demonstrated a trend of improvement. The nodule flattened, and the postinflammatory pigmentation had faded over 7 months (Figure 3). We are now continuing treatment until the skin lesions are diminished.
Figure 3.
Skin nodule after treatment. The nodule has flattened, but scarring remains.
Discussion
We experienced the case of a 23-year-old man working in aquarium management with a diagnosis of M. marinum after 2 excisional biopsies. Due to the optimal growth at 30 °C and poor growth at 37°C of M. marinum, infection with M. marinum in humans is primarily localized to the skin. Skin findings include nodules (67%), of which sporotrichoid lesions (representing 39% of the nodules) occur when the infection advances along the lymphatic vessels to the regional lymph nodes, creating multiple nodules that resemble sporotrichosis, as well as ulcers (16%) and abscesses (14%) [6].
Although M. marinum can infect healthy individuals, since 2002, there have been infrequent reports of infections in patients undergoing treatment with tumor necrosis factor-alpha inhibitors [3]. Deep tissue infections due to M. marinum, such as tenosynovitis (the most frequent), osteomyelitis, arthritis, and bursitis, occur in 20% to 40% of cases [3]. Our case did not involve conditions such as tendinitis or deep abscesses, and the course was favorable with antibiotic therapy alone. However, since there are reports in case series that some cases require surgery [7], imaging studies can be considered for M. marinum infection.
In our case, the T-SPOT.TB test, which is designed primarily for M. tuberculosis detection, yielded a false positive for M. marinum. Interferon-gamma release assays, including T-SPOT.TB and QFT-G, exhibit a higher sensitivity than the tuberculin skin test for the specific detection of latent tuberculosis, as well as active pulmonary and extrapulmonary tuberculosis. The higher sensitivity of these tests is attributed to the measurement of T-cell-mediated interferon-gamma release, which is induced by specific M. tuberculosis antigens, such as the 6-kDa early secretory antigenic target and the 10-kDa culture filtrate protein. However, it is known that false positives can occur with M. kansasii, M. szulgai, M. marinum, and M. gordonae [8]. In cases of skin and soft tissue infections with a history of water exposure, the T-SPOT.TB test for diagnosis of M. marinum infection could be useful for diagnosis.
Pathological findings from biopsies can be obtained more rapidly than culture results and are therefore considered valuable. Certain case series have specifically identified the histopathology of M. marinum, revealing poorly formed granulomas (96%), neutrophils (75%), and necrosis (79%) as common findings [9]. On the other hand, in the initial months, there may only be non-specific inflammatory cell infiltration, and with time, necrotizing granulomas with multinucleated giant cells become evident [10]. Thus, even though granulomas were not present in our case, the absence of granulomas alone does not rule out M. marinum.
M. marinum has a low sensitivity to Ziehl-Neelsen staining and acid-fast bacillus culture [9], and as a slow-grower, it can take 1 to 2 months for colonies to form. Furthermore, in our case, although the details of the procedure were unclear, the results of the culture sent to the domestic company were negative. The guidelines [11] recommend the use of N-acetyl-l-cysteine–sodium hydroxide (NALC-NaOH) for the pre-treatment of samples for the Mycobacterium genus. One of the most common decontaminants is NaOH, which also serves as a mucolytic agent; however, NaOH can also be detrimental to mycobacteria unless used with caution, particularly concerning concentration and exposure time. There have been studies comparing the culture positivity rates of acid-fast bacilli using NALC-NaOH and sulfuric acid, with some studies finding that NALC-NaOH had lower culture sensitivity than sulfuric acid [12]. Additionally, reports indicate that M. marinum is sensitive to alkaline environments, which can result in a low yield of cultures [13]. This underscores the importance of careful pre-treatment of samples.
In general, the antibiotic regimen should avoid monotherapy and instead prefer a combination of 2 or 3 drugs. Regarding antimicrobial susceptibility, in vitro studies suggest that the minimum inhibitory concentrations for antibiotics such as rifampicin, rifabutin, moxifloxacin, minocycline, doxycycline, and clarithromycin tend to be low [14]. Although there is no established evidence for the optimal treatment regimen, recent retrospective studies have frequently cited doxycycline, clarithromycin plus ethambutol, clarithromycin, trimethoprim/sulfamethoxazole, and minocycline as frequently used in the initial therapy [14]. Regarding the duration of therapy, there is no clear evidence, with retrospective studies indicating an average of 25±14 weeks [14]. Conversely, another review suggests treatment durations ranging from 1 to 25 months, with a median of 3.5 months; significantly longer durations were noted in cases in which the infection had spread to deep tissues [6]. As mentioned above, infections that have spread to many deep structures require caution, as surgery or debridement is often performed [7]. In our case, magnetic resonance imaging confirmed the absence of deep abscesses and tendinitis, and the treatment duration was determined in consultation with the Dermatology Department, until the nodular lesions improved. We are currently considering concluding the treatment.
Conclusions
We presented a case of M. marinum infection in a 23-year-old man employed in aquarium management, who presented with multiple sporotrichoid forms. Diagnosing M. marinum is challenging; therefore, we considered it essential to make a comprehensive assessment, including the patient’s history, T-SPOT.TB test, accurate temperature and processing of the specimen, and pathological analysis.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Aronson JD. Spontaneous tuberculosis in salt water fish. The Journal of Infectious Diseases. 1926;39(4):315–20. [Google Scholar]
- 2.Norden A, Linell F. A new type of pathogenic Mycobacterium. Nature. 1951;168(4280):826. doi: 10.1038/168826a0. [DOI] [PubMed] [Google Scholar]
- 3.Aubry A, Mougari F, Reibel F, Cambau E. Mycobacterium marinum . Microbiol Spectr. 2017;5(2) doi: 10.1128/microbiolspec.tnmi7-0038-2016. TNMI7-0038-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su Q, Wang F. Painful nodules on the arms. N Engl J Med. 2021;384(11):e41. doi: 10.1056/NEJMicm2028530. [DOI] [PubMed] [Google Scholar]
- 5.Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: A retrospective study of 25 cases. J Am Acad Dermatol. 2007;57(3):413–20. doi: 10.1016/j.jaad.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 6.Aubry A, Chosidow O, Caumes E, et al. Sixty-three cases of Mycobacterium marinum infection: Clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med. 2002;162(15):1746–52. doi: 10.1001/archinte.162.15.1746. [DOI] [PubMed] [Google Scholar]
- 7.Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: Retrospective case series and literature review. Infection. 2015;43(6):655–62. doi: 10.1007/s15010-015-0776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Ingen J, de Zwaan R, Dekhuijzen R, et al. Region of difference 1 in nontuberculous Mycobacterium species adds a phylogenetic and taxonomical character. J Bacteriol. 2009;191(18):5865–67. doi: 10.1128/JB.00683-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sia TY, Taimur S, Blau DM, et al. Clinical and pathological evaluation of Mycobacterium marinum group skin infections associated with fish markets in New York City. Clin Infect Dis. 2016;62(5):590–95. doi: 10.1093/cid/civ937. [DOI] [PubMed] [Google Scholar]
- 10.Cribier B, Aubry A, Caumes E, et al. [Histopathological study of Mycobacterium marinum infection.] Ann Dermatol Venereol. 2011;138(1):17–22. doi: 10.1016/j.annder.2010.10.025. [in French] [DOI] [PubMed] [Google Scholar]
- 11.Forbes BA, Hall GS, Miller MB, et al. Practical guidance for clinical microbiology laboratories: Mycobacteria. Clin Microbiol Rev. 2018;31(2):e00038-17. doi: 10.1128/CMR.00038-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorn NA, Warberg J. Effect of metabolic inhibitors on the release of vasopressin in vitro. Acta Physiol Scand. 1969;76(1):31A–32A. [PubMed] [Google Scholar]
- 13.Chiyoji A, Ikuo K. [Effect of sample pretreatment solution NALC-NaOH on the survival of antimicrobial bacteria.] Kekkaku. 2011;86(3):368. [in Japanese] [Google Scholar]
- 14.Hendrikx L, van Hees CLM, de Steenwinkel JEM, et al. Treatment and outcome of culture-confirmed Mycobacterium marinum disease. Open Forum Infect Dis. 2022;9(4) doi: 10.1093/ofid/ofac077. ofac077. [DOI] [PMC free article] [PubMed] [Google Scholar]