Abstract
Patient: Male, 37-year-old
Final Diagnosis: Coronary aneurysm • coronary artery anomalies
Symptoms: Abdominal pain • hemoptisis • shortness of breath
Clinical Procedure: —
Specialty: Cardiology
Objective:
Congenital defects/diseases
Background:
Giant coronary artery aneurysms (CAA) are extremely rare and can mimic cardiac tumors. Therefore, an unidentified mass in the heart requires a multimodality imaging approach for accurate diagnosis and guidance of further management, which for CAAs often include surgical intervention to prevent complications such as thrombosis or rupture.
Case Report:
A 37-year-old man presented with non-specific symptoms. A CT scan revealed multiple bilateral pulmonary embolisms and an indeterminate mass in the right atrium. Transthoracic echocardiography (TTE) showed a suspected cardiac tumor, and further imaging with transesophageal echocardiography (TEE), magnetic resonance imaging (MRI), and position emission tomography (PET) indicated a local inhomogeneous mass with arterial perfusion. A preoperative cardiac CT found the mass to be a giant thrombosed CAA in the proximal right coronary artery compressing the tricuspid annulus. The patient underwent successful surgical excision of the CAA along with coronary artery bypass grafting. Postoperative management included lifelong administration of acetylsalicylic acid and a 3-month course of anticoagulant therapy. Histopathology excluded systemic vasculitis, indicating a congenital etiology for the CAA.
Conclusions:
This case illustrates the indispensable role of coronary CT angiography in accurately diagnosing and managing complex cardiac conditions. Due to the complex and diverse nature of suspected cardiac tumors, cardiac CT should always be added in the diagnostic workup to describe the coronary anatomy in relation to the tumor and to identify a differential diagnosis such as a giant coronary aneurysm.
Key words: Aneurysm, Cardiac Imaging Techniques
Introduction
A coronary artery aneurysm (CAA) is an uncommon pathology characterized by dilation of the coronary artery that surpasses 50% of the diameter of the reference vessel. When this dilation exceeds 8 mm in diameter or 4 times the reference vessel diameter, it is classified as a giant coronary artery aneurysm, a particularly rare and potentially critical variant of the condition [1,2].
Computed tomography (CT) angiography is increasingly becoming a pivotal tool in assessing complex cardiac diagnostic scenarios [3]. This case report demonstrates the importance of a multimodality imaging approach when evaluating suspected cardiac tumors and discuss the indispensable role of CT angiography in providing definitive diagnostic clarity and guiding the management of a complex condition.
Case Report
History of Presentation
A 37-year-old man presented with dyspnea, fever, night sweats, abdominal pain, and a recent weight loss of 6 kg over 6 months. At the index admission, the patient had abdominal pain, and the echocardiogram (ECG) showed a sinus rhythm with no abnormal findings. Infection biomarkers were elevated, and antibiotics were initiated for suspected pneumonia. Upon readmission within 2 months due to recurrence of abdominal pain, dyspnea, fever, and now hemoptysis, a high-resolution CT scan of the abdomen and chest identified several minor bilateral pulmonary embolisms along with an indeterminate mass in the right atrium. Transthoracic echocardiography (TTE) confirmed an unidentified mass on the right side of the heart, suspected to be a tumor.
Past Medical History
Aside from kidney stones treated years ago, the patient had no prior contact with the hospital.
Differential Diagnosis and Investigations
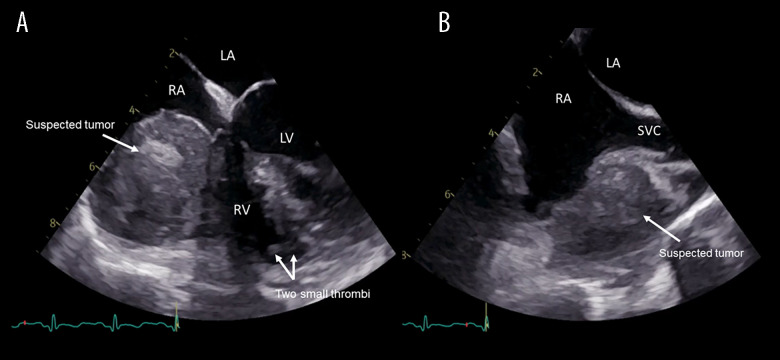
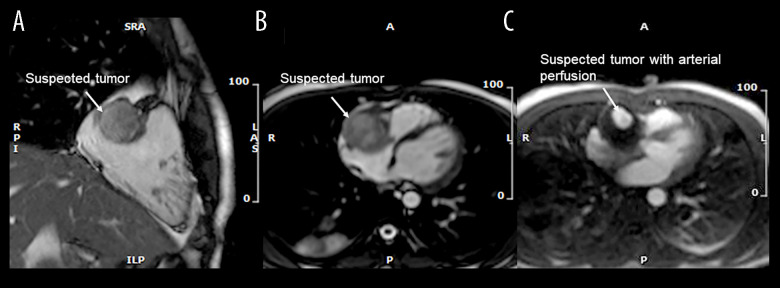
The patient was started on heparin and transferred to Rigshospitalet University Hospital, a tertiary referral center, for further investigations. Upon transfer, the ECG showed abnormal T-waves in the inferior leads III and aVF suggesting a possible inferior infarction. However, TTE found normal structures and functions of valves and chambers, including the lower left ventricular wall, within normal range. Subsequently, transesophageal echocardiography (TEE) found an inhomogeneous 4.3×3.5 cm tumor adherent to the free wall of the right atrium and ventricle (RV), compressing the tricuspid valve annulus, causing turbulent RV inflow (Figure 1). Additionally, a small suspected thrombus was identified apically in the RV cavity. Magnetic resonance imaging (MRI) confirmed the TEE findings and raised suspicion of a primary cardiac tumor, without infiltration to surrounding tissue, but additionally noted that the tumor had a small central cavity with arterial perfusion (Figure 2).
Figure 1.
Transesophageal echocardiography. (A) 4-chamber view. A giant suspected tumor (white arrow) compressing the tricuspid annulus. In the right ventricle (RV) cavity are 2 small thrombi (double arrow) visualized. (B) Bi-caval view. The suspected tumor (white arrow) is located in close proximity to the superior vena cava (SVC). LA – left atrium; RA – right atrium; LV – left ventricle; RV – right ventricle; SVC – superior vena cava.
Figure 2.
Cardiac magnetic resonance. (A) 2-chamber view. A localized non-invasive suspected tumor (white arrow). (B) Transverse view. Suspected tumor (white arrow) compressing the tricuspid annulus. (C) Transverse view with dynamic perfusion. The suspected tumor (white arrow) with arterial perfusion.
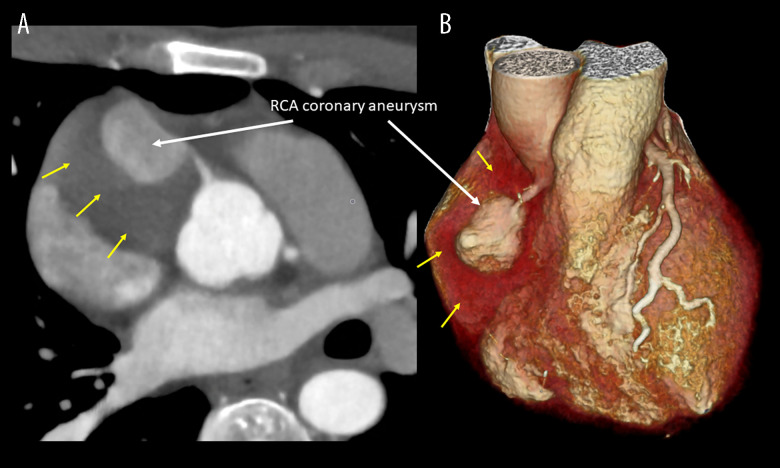
While considering a catheter-based biopsy to obtain a histological diagnosis before attempting surgical removal of the suspected tumor, a fluorodeoxyglucose-positron emission tomography (PET) was performed, which showed the rim of the process had low-moderate metabolic activity but a lack of activity in the center. No extra-cardiac foci were identified, suggesting there was no disseminating cancer, yet not confirming a cancerous or infectious focus. After a final decision to attempt surgical removal of the tumor, an ECG-triggered coronary CT angiography was performed to further investigate the relationship of the tumor with the right coronary artery (RCA). The coronary CT angiography identified the suspected tumor as a thrombosed giant coronary artery aneurysm (CAA) in the proximal RCA (Figure 3). This was a crucial finding that significantly altered the clinical approach. The coronary CT angiography delineated the aneurysm’s size and structure, showing its relationship with the surrounding cardiac structures, confirming that the aneurysm was compressing the right atrium and ventricle without infiltrating the surrounding tissue.
Figure 3.
Cardiac computed tomography. (A) Cross-sectional view illustrating the giant coronary artery aneurysm (CAA) on the right coronary artery (RCA) (white arrow) surrounded by the thrombosed part of the giant CAA yellow arrows). (B) Volume-rendered image illustrating the giant CAA on the RCA (white arrow) surrounded by the thrombosed part (yellow arrows).
An invasive coronary angiogram (ICA) confirmed that the CAA was completely occluded distally, and the distal RCA and the right posterior descending artery were filled by collateral septal branches from the left anterior descending artery.
Management
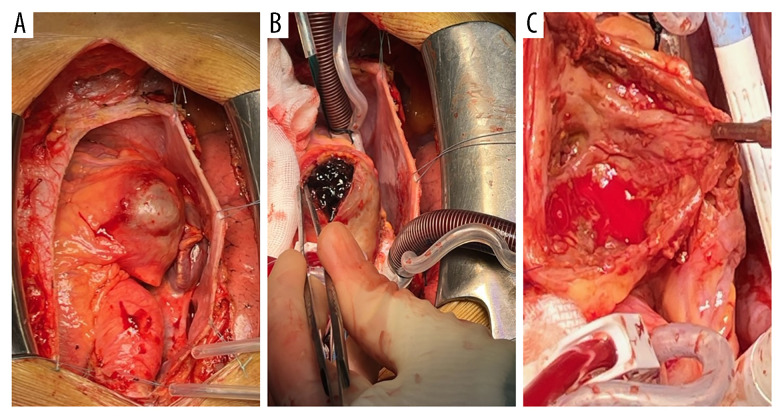
The patient was scheduled for surgical removal of the CAA along with coronary artery bypass grafting. Following median sternotomy, the right internal mammary artery (RIMA) was harvested. After bi-caval cannulation to the ascending aorta, normothermic extracorporeal circulation was begun, the aorta was cross-clamped, and cardioplegia was given in the aortic root. The aneurysm was incised, cleared of old coagulated blood and membranes, and the free wall of the aneurysm was resected (Figure 4). A potential thrombosed fistula to the RV was over-sewn as were the proximal aneurysm inlet and outlet from the RCA. Furthermore, it was noted that a small territory of the infero-lateral RV myocardium had suffered from a myocardial infarction, and 2 small thrombi were removed from the RV cavity in the corresponding region. Finally, the RIMA was anastomosed to the distal RCA.
Figure 4.
Surgical view. (A) Inspection of the aneurysm after sternotomy. Giant coronary artery aneurysm (CAA) (white arrow) on the right coronary artery (RCA). (B) Removal of coagulated blood in the aneurysm sack (white arrow). (C) Inspection of the aneurysm sack upon removal of the coagulated blood (white arrow).
Post-surgery TTE showed a normal left ventricle ejection fraction, and the inflow to the RV was now unimpeded. Because of the bypass-graft, we administered lifelong acetylsalicylic acid and anticoagulant therapy for 3 months as part of the management of the pulmonary embolisms. During the postoperative course, the patient was treated with antibiotics due to a pulmonary Moraxella infection and was discharged 11 days after surgery.
The pathology report for the resected aneurysm wall did not raise any suspicion of systemic vasculitis, and a later MRI of the aorta and branches ruled out additional aneurysms. We concluded that a congenital coronary aneurysm was the most likely diagnosis.
Follow-up
The patient is well and returned to work quickly. At follow-up after 1 year, he had no recurrence of symptoms.
Discussion
In this case report, we investigated an assumed incidentally found cardiac mass suspected to be a cardiac tumor that turned out to be a giant thrombosed RCA aneurysm. The symptoms leading to hospitalization 2 months prior may have been due to thrombosis of the RCA aneurysm, causing ischemia. The infarcted RV myocardium, as noted intraoperatively, together with the presence of 2 minor thrombi in the RV cavity indicates that the pulmonary embolisms came from the RV cavity.
Numerous imaging techniques characterized the suspected tumor. After the initial identification of a cardiac mass on CT, TTE and TEE further described the location and structure of the mass, including the hemodynamic influence on right ventricular inflow. Cardiac MRI confirmed a localized tumor without infiltration to the surrounding tissues and noted a perfused cavity within the suspected tumor, which retrospectively was obviously the non-thrombosed part of the CAA. The PET-CT ruled out metastatic disease, and the coronary CT angiography provided details of the structure, size, and location to alter the suspected diagnosis and subsequent treatment plan and instead obtain the correct diagnosis. This led to successful removal of the giant thrombosed RCA aneurysm. The coronary CT angiography therefore provided invaluable information that ultimately led to the correct diagnosis.
CAAs are rare, occurring in 0.3–4.9% of patients undergoing ICA [1], affecting mostly men, and more commonly the RCA [2]. CAA exceeding 20 mm in diameter is classified as giant and has a prevalence of 0.02%. Our patient presented with a 4.3×3.5 cm inhomogeneous mass, classifying it as a giant aneurysm [1]. The pathogenesis of CAAs involves underlying destruction of the vessel’s media layer and increased wall stress, leading to dilatation of the affected coronary segment. This process is mostly caused by atherosclerosis, although congenital, infectious, or rheumatic causes, such as Kawasaki disease, are more common in younger patients [2].
The pathology report excluded atherosclerosis, in agreement with the coronary CT angiography and the ICA finding, but reported an older inflammatory, degenerative, and reparative process suggesting granulation tissue formation rather than primary vasculitis. Kawasaki disease and systemic vasculitis were ruled out at the Rheumatic Department based on age, absence of skin involvement, and the pathology report. Though the patient did not undergo an assessment for any underlying collagen disease, the pathology report and the blood work suggested no underlying thrombophilia disease during the standard thrombophilia screening. The tentative diagnosis was a congenital coronary aneurysm, supported by the patient’s age, aneurysm size, and pathology findings.
The clinical presentation of patients with CAAs can vary significantly. The patients can be asymptomatic, and the aneurysm can be a coincidental finding. However, patients with a CAA can also have a more severe presentation, including hospitalization for acute coronary syndrome [4]. When a mass in the heart is located, differential diagnoses include tumors, thrombi, or vegetation [5]. We suspect the initial abdominal pain was due to the RCA being affected, and the pulmonary embolisms may have derived from the CAA, as additional blood clots were found within the CAA during surgery. At a check-up visit 1 year after the surgery, the patient was feeling well and had resumed his normal physical activities. Therefore, we assume that the weight loss was associated with the coronary abnormality.
Despite advanced imaging modalities, differentiating between CAAs and intracardiac masses like tumors remains challenging [6]. An unusual giant CAA thrombosis in our case mimicked a tumor with severe, life-threatening clinical implications. Differential diagnosis between CAA and other intracardiac masses is crucial, as the management approach differs significantly.
There have been few studies on CAA treatment, with treatment decisions based on the individual cases and options for surgical removal, percutaneous, and medical approaches. This depends largely on the number of affected vessels and the size, anatomical localization, and form of the aneurysm. Smaller CAA may be treated using covered stent implantation, coil embolization, or stent-assisted coil insertion, while larger ones are surgically removed [2].
Conclusions
This case report demonstrates the important role of CT angiography in evaluating complex cardiac conditions. Given the complex and diverse nature of suspected cardiac tumors, it is essential to incorporate multimodality imaging for optimal characterization of the pathology. As illustrated in this case, the role of cardiac CT is indispensable, not just for preoperative assessment of coronary artery disease but to also describe the relationship of the coronary arteries to the suspected tumor, and on rare occasions to diagnose the presence of a giant coronary artery aneurysm.
Abbreviations:
- CT
computed tomography;
- TTE
transthoracic echocardiography;
- TEE
transesophageal echocardiography;
- MRI
magnetic resonance imaging;
- RV
right ventricle;
- PET
positron emission tomography;
- RCA
coronary artery;
- ICA
invasive coronary angiogram;
- ECG
echocardiogram;
- RIMA
right internal mammary artery
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Sheikh AS, Hailan A, Kinnaird T, et al. Coronary artery aneurysm: Evaluation, prognosis, and proposed treatment strategies. Heart Views. 2019;20(3):101–8. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawsara A, Núñez Gil IJ, Alqahtani F, et al. Management of coronary artery aneurysms. JACC Cardiovasc Interv. 2018;11(13):1211–23. doi: 10.1016/j.jcin.2018.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Tzimas G, Gulsin GS, Takagi H, et al. Coronary CT angiography to guide percutaneous coronary intervention. Radiol Cardiothorac Imaging. 2022;4(1):e210171. doi: 10.1148/ryct.210171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halapas A, Lausberg H, Gehrig T, et al. Giant right coronary artery aneurysm in an adult male patient with non-ST myocardial infarction. Hellenic J Cardiol. 2013;54(1):69–76. [PubMed] [Google Scholar]
- 5.Pino PG, Moreo A, Lestuzzi C. Differential diagnosis of cardiac tumors: General consideration and echocardiographic approach. J Clin Ultrasound. 2022;50(8):1177–93. doi: 10.1002/jcu.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alomar-Melero E, Martin TD, et al. An unusual giant right coronary artery aneurysm resembles an intracardiac mass. Anesth Analg. 2008;107(4):1161–62. doi: 10.1213/ane.0b013e318181f74f. [DOI] [PubMed] [Google Scholar]