Abstract
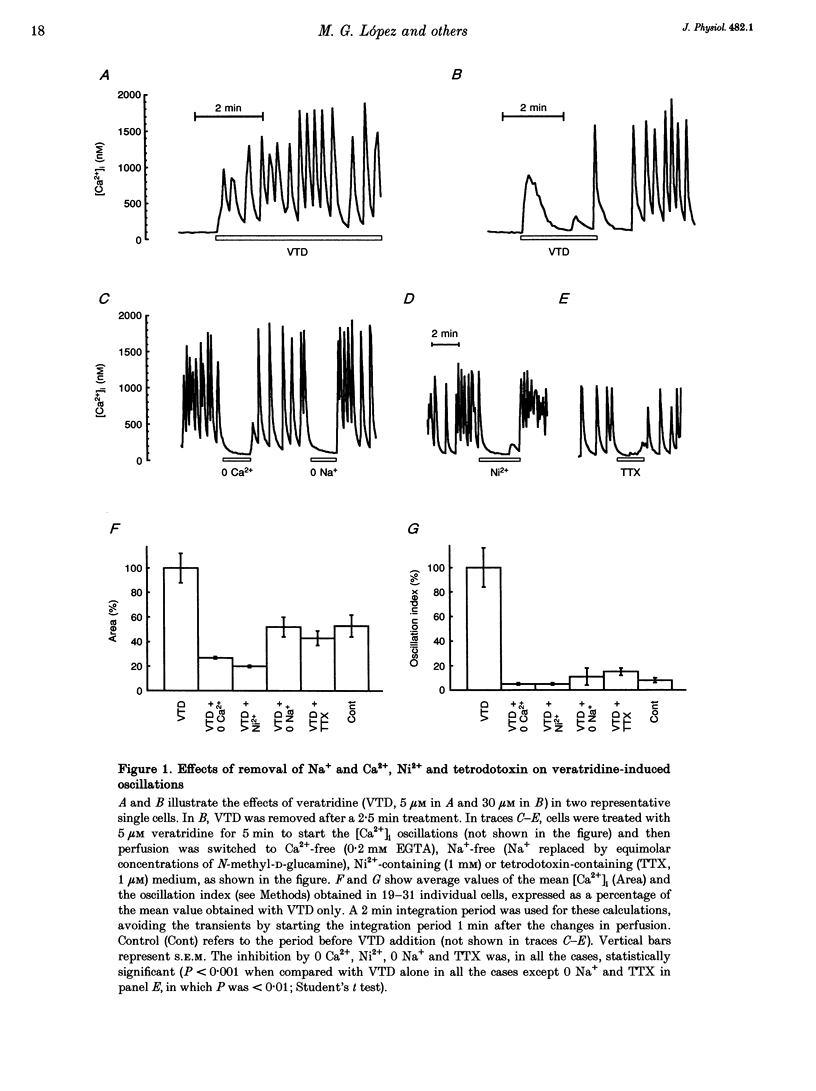
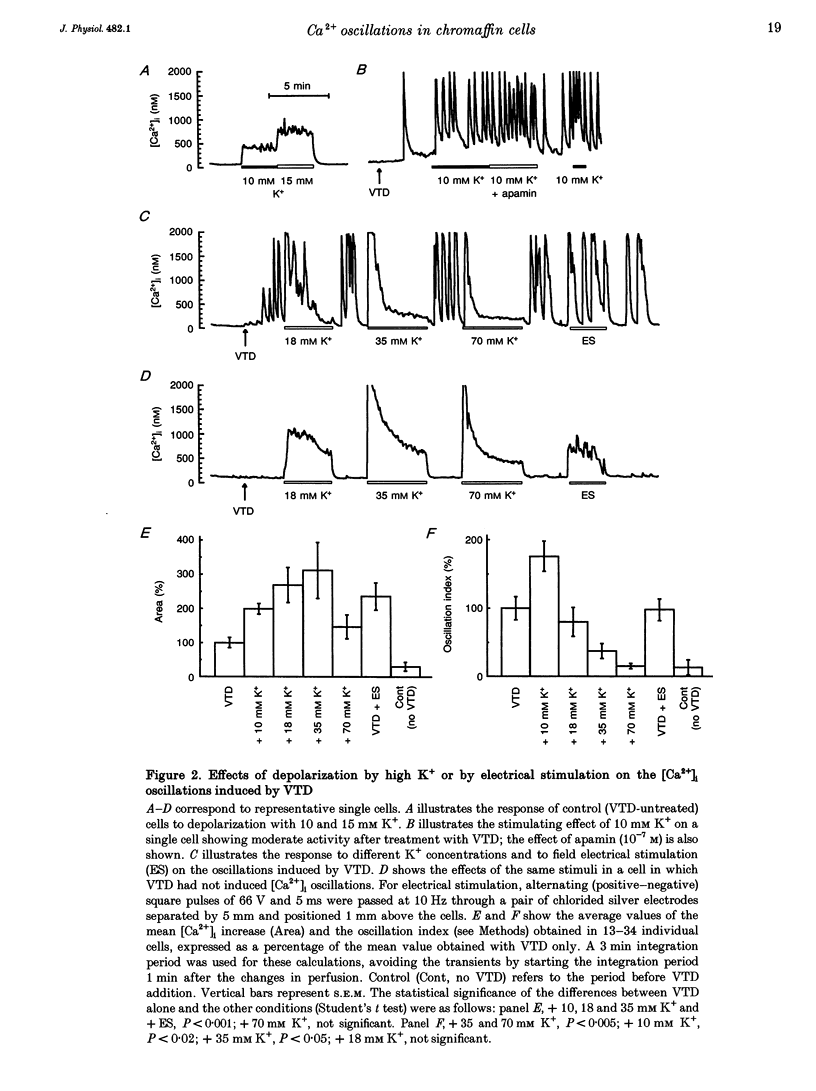
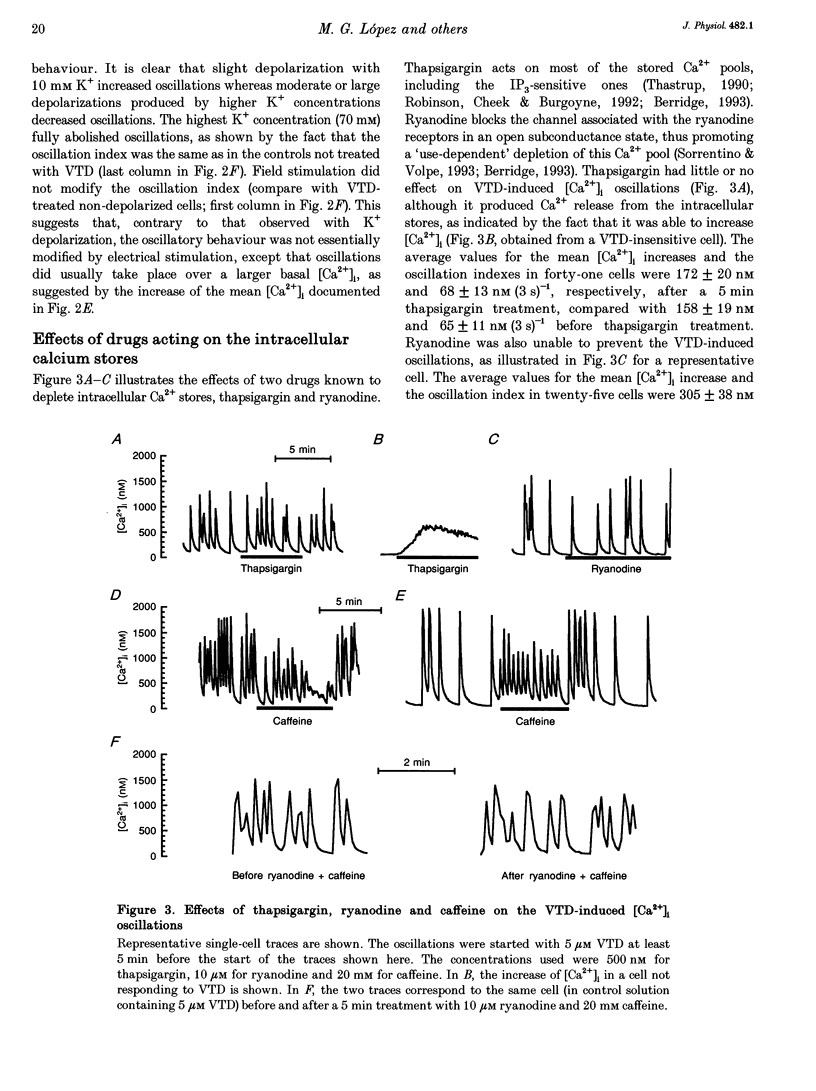
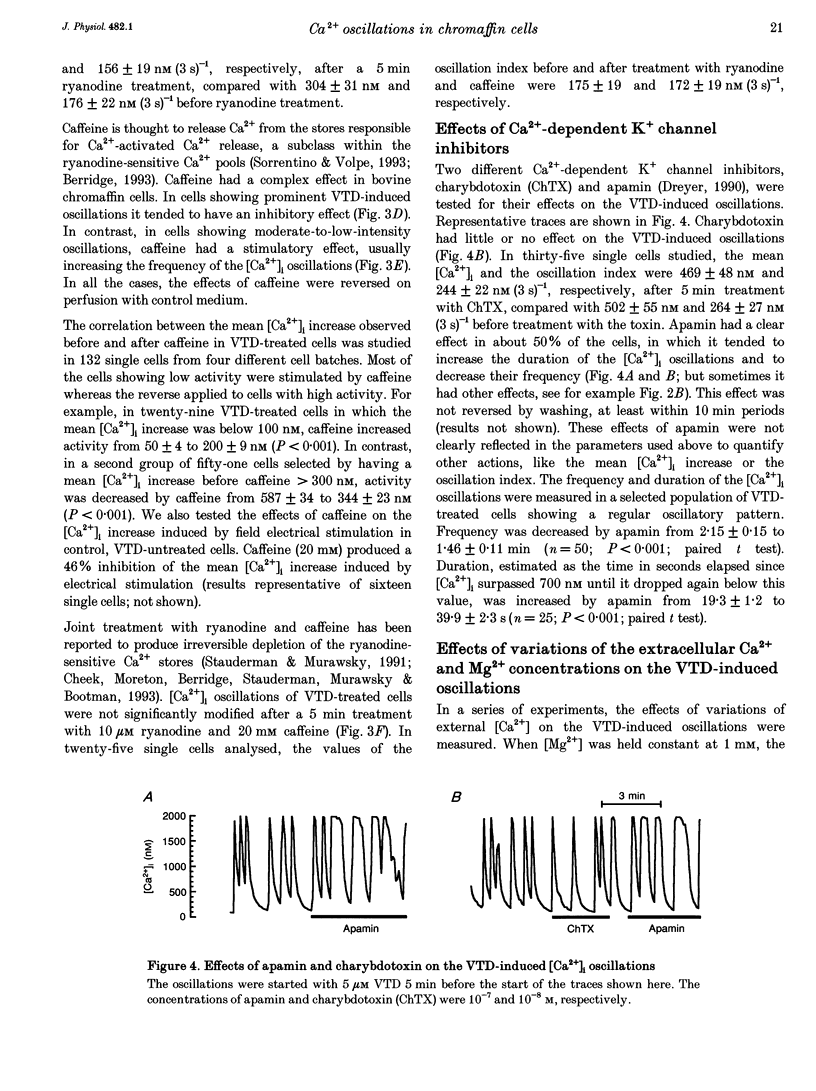
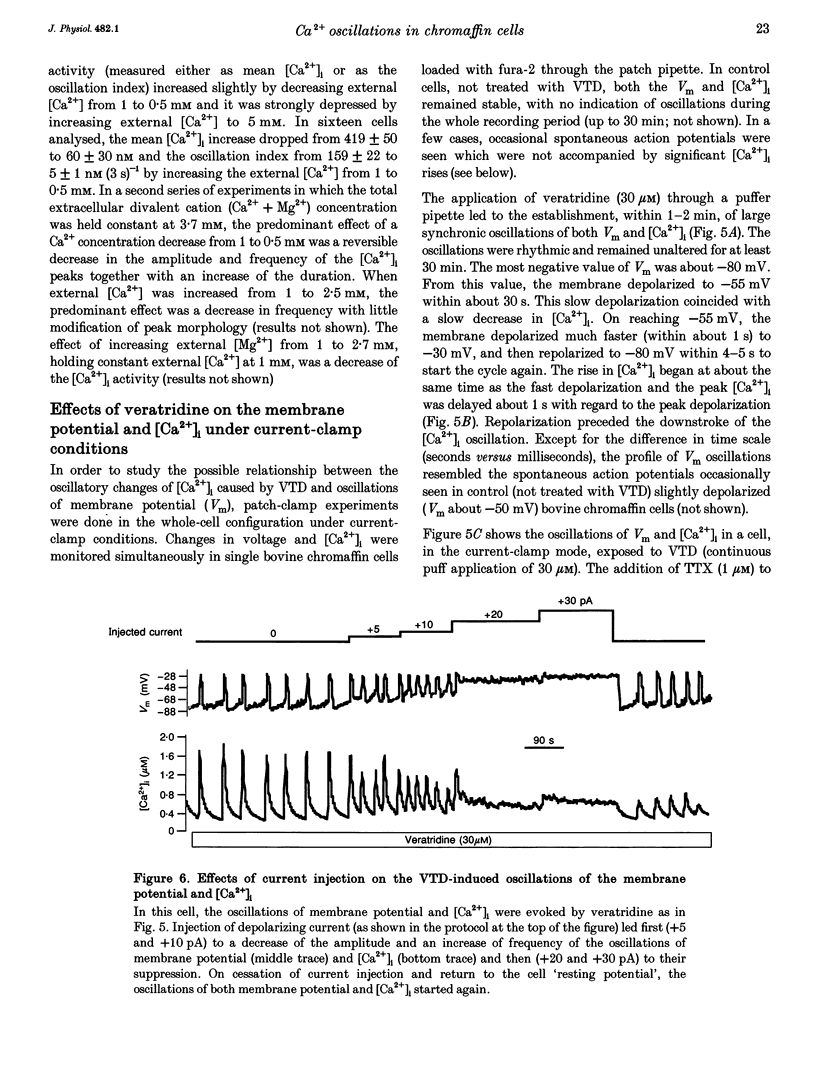
1. Veratridine (VTD) induced large oscillations of the cytosolic Ca2+ concentration ([Ca2+]i) and the membrane potential (Vm) in otherwise silent bovine chromaffin cells loaded with fura-2. 2. Depletion of the intracellular Ca2+ stores by thapsigargin or ryanodine did not affect these oscillations. Caffeine had a complex effect, decreasing them in cells with high activity but increasing them in cells with low activity. 3. The [Ca2+]i oscillations required extracellular Ca2+ and Na+ and were blocked by Ni2+ or tetrodotoxin. They were antagonized by high external concentrations of Mg2+ and/or Ca2+. 4. The oscillations of Vm had three phases: (i) slow depolarization (20 mV in 10-40 s); (ii) further fast depolarization (30 mV in 1 s); and (iii) rapid (5 s) repolarization. [Ca2+]i decreased during (i), increased quickly during (ii) with a 1 s delay with regard to the peak depolarization, and decreased during (iii). 5. Slight depolarizations increased the frequency of the oscillations whereas large depolarizations decreased it. 6. The Ca(2+)-dependent K+ channel blocker apamin increased the duration and decreased the frequency of the oscillations. 7. We propose the following mechanism for the oscillations: (i) the membrane depolarizes slowly by a decrease of potassium conductance (gK), perhaps due to a gradual decrease of [Ca2+]i; (ii) the threshold for activation of Na+ channels (decreased by VTD) is reached, producing further depolarization and recruiting Ca2+ channels, and inactivation of both Ca2+ and VTD-poisoned Na+ channels is slow; and (iii) gK increases, aided by activation of Ca(2+)-dependent K+ channels by the increased [Ca2+]i, and the membrane repolarizes. The contribution of the Na+ channels seems essential for the generation of the oscillations. 8. Bovine chromaffin cells have the machinery required for [Ca2+]i oscillations even though the more physiological stimulus tested here (high K+, field electrical stimulation, nicotinic or muscarinic agonists) produced mainly non-oscillatory responses.
Full text
PDF


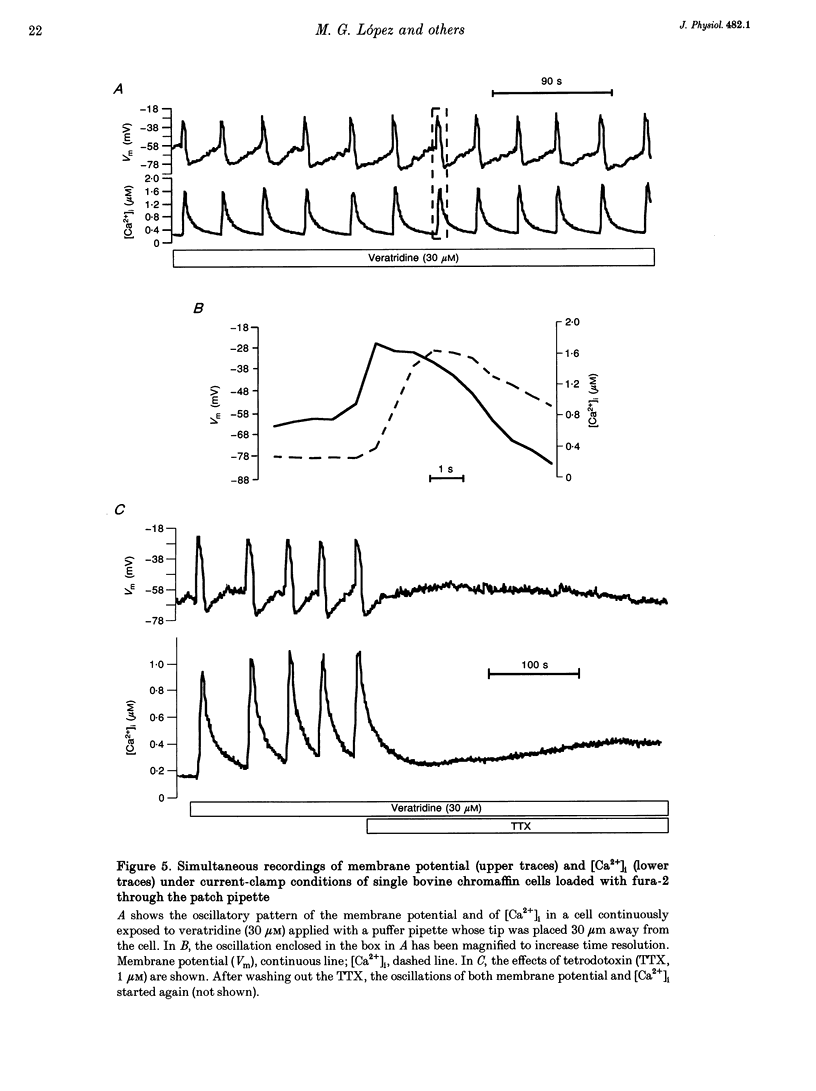




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artalejo A. R., García A. G., Neher E. Small-conductance Ca(2+)-activated K+ channels in bovine chromaffin cells. Pflugers Arch. 1993 Apr;423(1-2):97–103. doi: 10.1007/BF00374966. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bommer M., Herz A. Neurotensin affects metabolism of opioid peptides, catecholamines and inositol phospholipids in bovine chromaffin cells. Life Sci. 1989;44(5):327–335. doi: 10.1016/0024-3205(89)90226-9. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., Moreton R. B., Berridge M. J., Stauderman K. A., Murawsky M. M., Bootman M. D. Quantal Ca2+ release from caffeine-sensitive stores in adrenal chromaffin cells. J Biol Chem. 1993 Dec 25;268(36):27076–27083. [PubMed] [Google Scholar]
- Cheek T. R., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. The caffeine-sensitive Ca2+ store in bovine adrenal chromaffin cells; an examination of its role in triggering secretion and Ca2+ homeostasis. FEBS Lett. 1990 Jun 18;266(1-2):91–95. doi: 10.1016/0014-5793(90)81514-o. [DOI] [PubMed] [Google Scholar]
- D'Andrea P., Zacchetti D., Meldolesi J., Grohovaz F. Mechanism of [Ca2+]i oscillations in rat chromaffin cells. Complex Ca(2+)-dependent regulation of a ryanodine-insensitive oscillator. J Biol Chem. 1993 Jul 15;268(20):15213–15220. [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard D. A., Holz R. W. Cholinergic stimulation of inositol phosphate formation in bovine adrenal chromaffin cells: distinct nicotinic and muscarinic mechanisms. J Neurochem. 1987 Nov;49(5):1634–1643. doi: 10.1111/j.1471-4159.1987.tb01037.x. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteriz R. I., Garcia-Sancho J., Gandia L., Lopez M. G., Garcia A. G. Permeation and inactivation by calcium and manganese of bovine adrenal chromaffin cell calcium channels. Am J Physiol. 1992 Oct;263(4 Pt 1):C818–C824. doi: 10.1152/ajpcell.1992.263.4.C818. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. Phase-dependent contributions from Ca2+ entry and Ca2+ release to caffeine-induced [Ca2+]i oscillations in bullfrog sympathetic neurons. Neuron. 1992 Jun;8(6):1109–1125. doi: 10.1016/0896-6273(92)90132-w. [DOI] [PubMed] [Google Scholar]
- Garrido B., López M. G., Moro M. A., de Pascual R., García A. G. Voltage-dependent inactivation of catecholamine secretion evoked by brief calcium pulses in the cat adrenal medulla. J Physiol. 1990 Sep;428:615–637. doi: 10.1113/jphysiol.1990.sp018231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Changes in the intracellular concentration of free calcium ions in a pace-maker neurone, measured with the metallochromic indicator dye arsenazo III. J Physiol. 1978 Feb;275:357–376. doi: 10.1113/jphysiol.1978.sp012194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Irvine R. F. Inositol phosphates and Ca2+ entry: toward a proliferation or a simplification? FASEB J. 1992 Sep;6(12):3085–3091. doi: 10.1096/fasebj.6.12.1325932. [DOI] [PubMed] [Google Scholar]
- Livett B. G. Adrenal medullary chromaffin cells in vitro. Physiol Rev. 1984 Oct;64(4):1103–1161. doi: 10.1152/physrev.1984.64.4.1103. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Fesce R., Meldolesi J. Spontaneous [Ca2+]i fluctuations in rat chromaffin cells do not require inositol 1,4,5-trisphosphate elevations but are generated by a caffeine- and ryanodine-sensitive intracellular Ca2+ store. J Biol Chem. 1990 Feb 25;265(6):3005–3008. [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty T. J., Taylor C. W. Caffeine-stimulated Ca2+ release from the intracellular stores of hepatocytes is not mediated by ryanodine receptors. Biochem J. 1993 May 1;291(Pt 3):799–801. doi: 10.1042/bj2910799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro M. A., López M. G., Gandía L., Michelena P., García A. G. Separation and culture of living adrenaline- and noradrenaline-containing cells from bovine adrenal medullae. Anal Biochem. 1990 Mar;185(2):243–248. doi: 10.1016/0003-2697(90)90287-j. [DOI] [PubMed] [Google Scholar]
- Neely A., Lingle C. J. Effects of muscarine on single rat adrenal chromaffin cells. J Physiol. 1992;453:133–166. doi: 10.1113/jphysiol.1992.sp019221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan A. J., Burgoyne R. D. A comparison of bradykinin, angiotensin II and muscarinic stimulation of cultured bovine adrenal chromaffin cells. Biosci Rep. 1989 Apr;9(2):243–252. doi: 10.1007/BF01116001. [DOI] [PubMed] [Google Scholar]
- Ota M., Narahashi T., Keeler R. F. Effects of veratrum alkaloids on membrane potential and conductance of squid and crayfish giant axons. J Pharmacol Exp Ther. 1973 Jan;184(1):143–154. [PubMed] [Google Scholar]
- Pauwels P. J., Van Assouw H. P., Peeters L., Leysen J. E. Neurotoxic action of veratridine in rat brain neuronal cultures: mechanism of neuroprotection by Ca++ antagonists nonselective for slow Ca++ channels. J Pharmacol Exp Ther. 1990 Dec;255(3):1117–1122. [PubMed] [Google Scholar]
- Plevin R., Boarder M. R. Stimulation of formation of inositol phosphates in primary cultures of bovine adrenal chromaffin cells by angiotensin II, histamine, bradykinin, and carbachol. J Neurochem. 1988 Aug;51(2):634–641. doi: 10.1111/j.1471-4159.1988.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Robinson I. M., Burgoyne R. D. Characterisation of distinct inositol 1,4,5-trisphosphate-sensitive and caffeine-sensitive calcium stores in digitonin-permeabilised adrenal chromaffin cells. J Neurochem. 1991 May;56(5):1587–1593. doi: 10.1111/j.1471-4159.1991.tb02055.x. [DOI] [PubMed] [Google Scholar]
- Robinson I. M., Cheek T. R., Burgoyne R. D. Ca2+ influx induced by the Ca(2+)-ATPase inhibitors 2,5-di-(t-butyl)-1,4-benzohydroquinone and thapsigargin in bovine adrenal chromaffin cells. Biochem J. 1992 Dec 1;288(Pt 2):457–463. doi: 10.1042/bj2880457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Sorrentino V., Volpe P. Ryanodine receptors: how many, where and why? Trends Pharmacol Sci. 1993 Mar;14(3):98–103. doi: 10.1016/0165-6147(93)90072-r. [DOI] [PubMed] [Google Scholar]
- Stauderman K. A., Murawsky M. M. The inositol 1,4,5-trisphosphate-forming agonist histamine activates a ryanodine-sensitive Ca2+ release mechanism in bovine adrenal chromaffin cells. J Biol Chem. 1991 Oct 15;266(29):19150–19153. [PubMed] [Google Scholar]
- Stauderman K. A., Pruss R. M. Different patterns of agonist-stimulated increases of 3H-inositol phosphate isomers and cytosolic Ca2+ in bovine adrenal chromaffin cells: comparison of the effects of histamine and angiotensin II. J Neurochem. 1990 Mar;54(3):946–953. doi: 10.1111/j.1471-4159.1990.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Stauderman K. A., Pruss R. M. Dissociation of Ca2+ entry and Ca2+ mobilization responses to angiotensin II in bovine adrenal chromaffin cells. J Biol Chem. 1989 Nov 5;264(31):18349–18355. [PubMed] [Google Scholar]
- Stoehr S. J., Smolen J. E., Holz R. W., Agranoff B. W. Inositol trisphosphate mobilizes intracellular calcium in permeabilized adrenal chromaffin cells. J Neurochem. 1986 Feb;46(2):637–640. doi: 10.1111/j.1471-4159.1986.tb13014.x. [DOI] [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Varro A., Hester S., Papp J. G. Caffeine-induced decreases in the inward rectifier potassium and the inward calcium currents in rat ventricular myocytes. Br J Pharmacol. 1993 Aug;109(4):895–897. doi: 10.1111/j.1476-5381.1993.tb13702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C., Fonteriz R., López M. G., García A. G., García-Sancho J. Inhibition of voltage-gated Ca2+ entry into GH3 and chromaffin cells by imidazole antimycotics and other cytochrome P450 blockers. FASEB J. 1992 Jun;6(9):2742–2747. doi: 10.1096/fasebj.6.9.1319362. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- Zahradník I., Palade P. Multiple effects of caffeine on calcium current in rat ventricular myocytes. Pflugers Arch. 1993 Jul;424(2):129–136. doi: 10.1007/BF00374603. [DOI] [PubMed] [Google Scholar]



