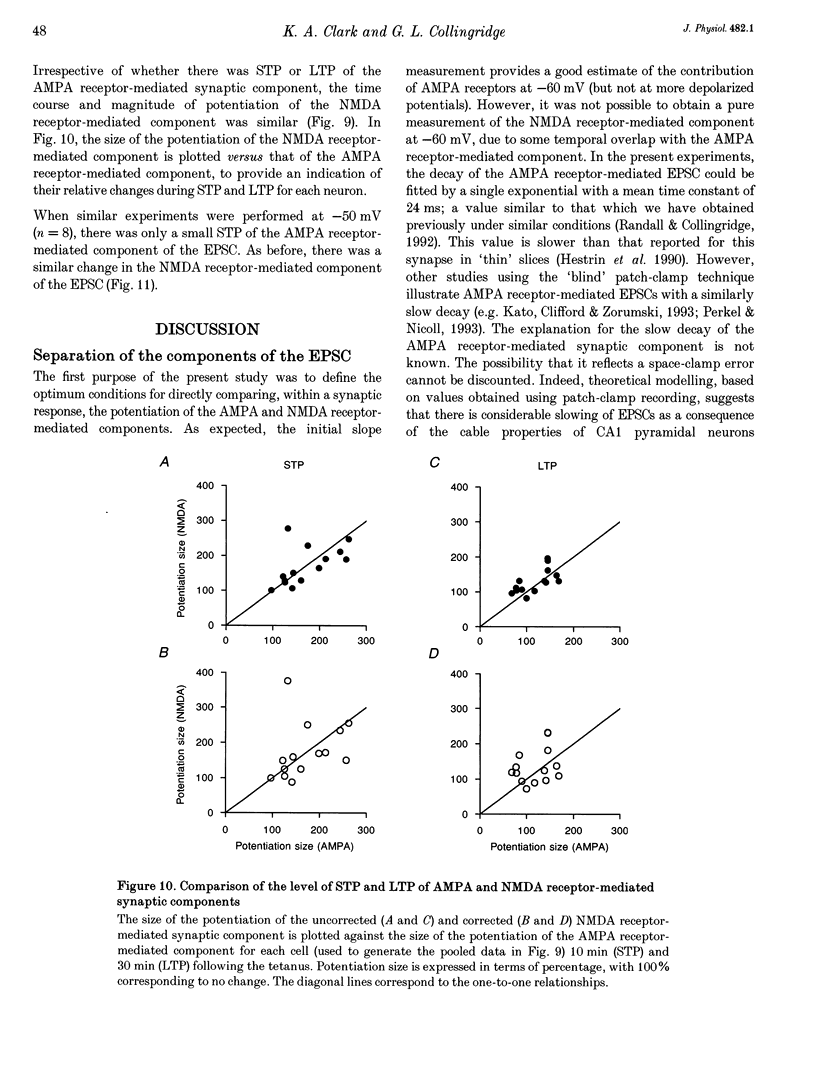
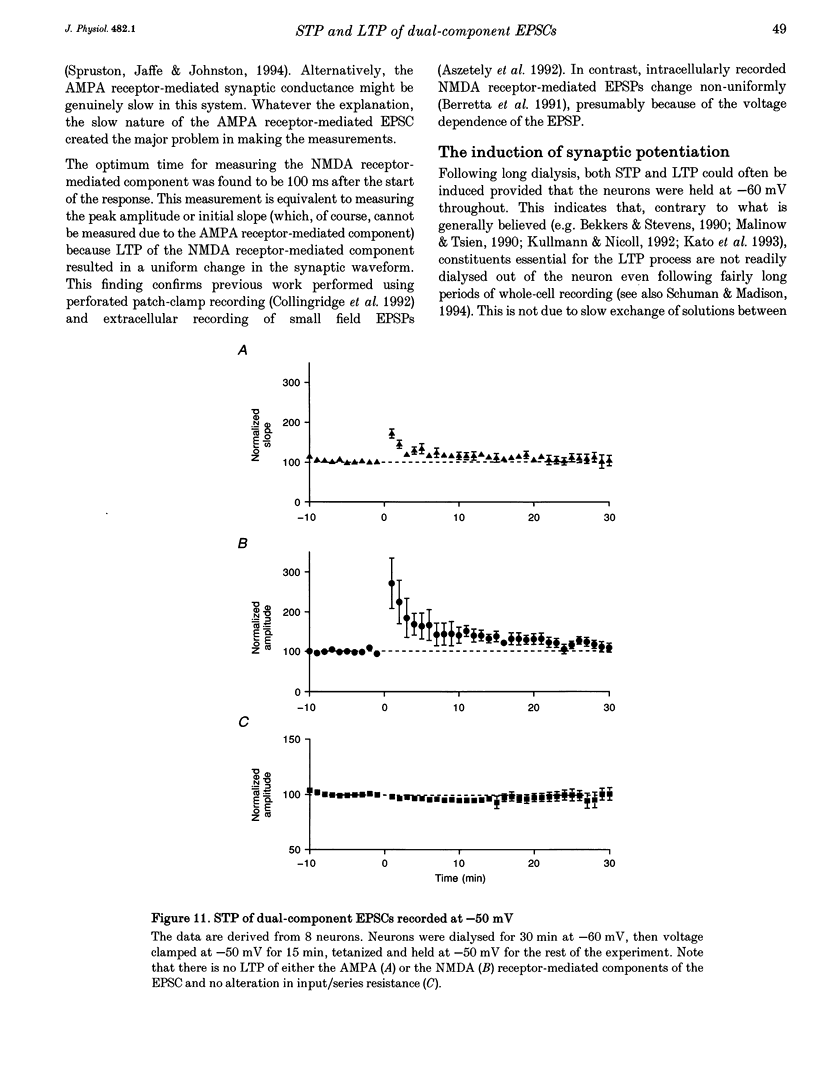
Abstract
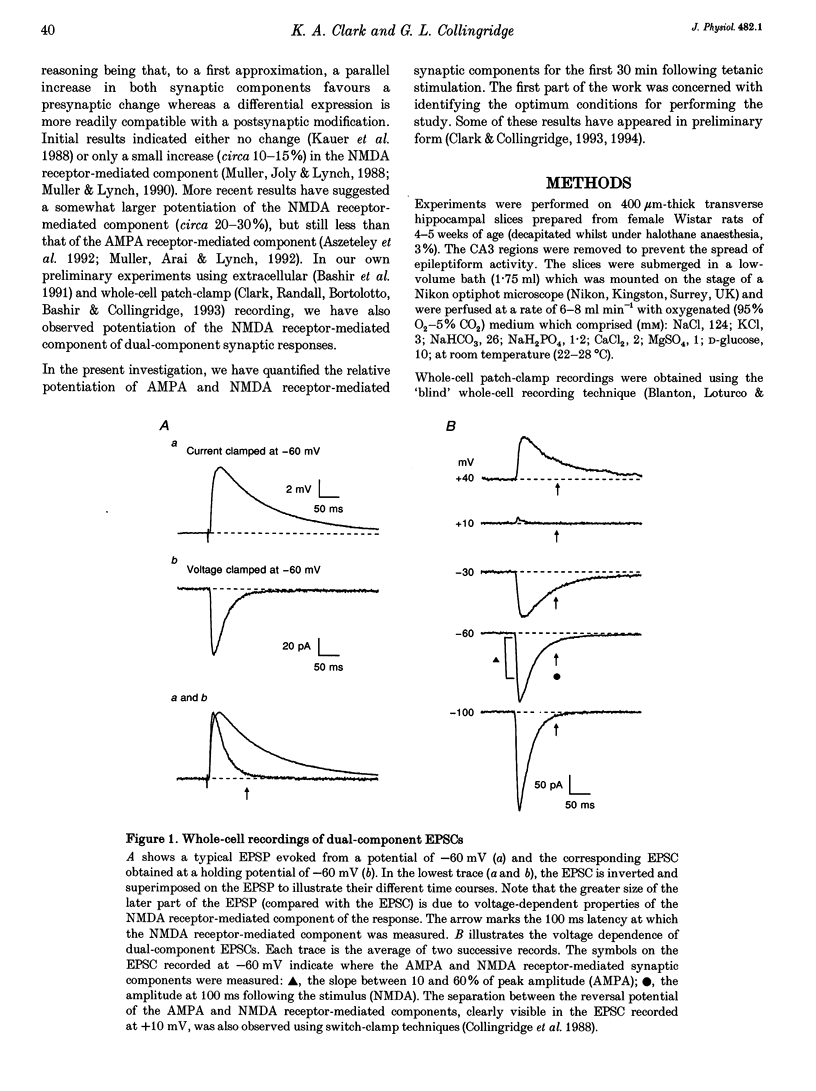
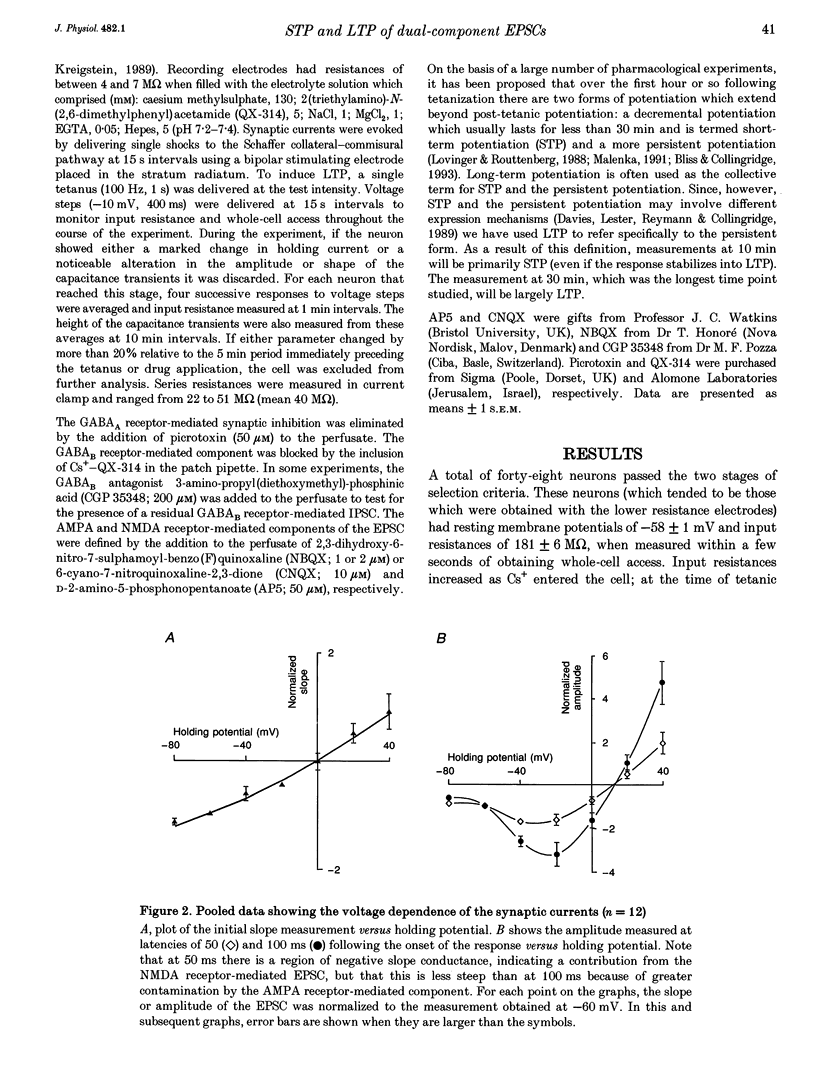
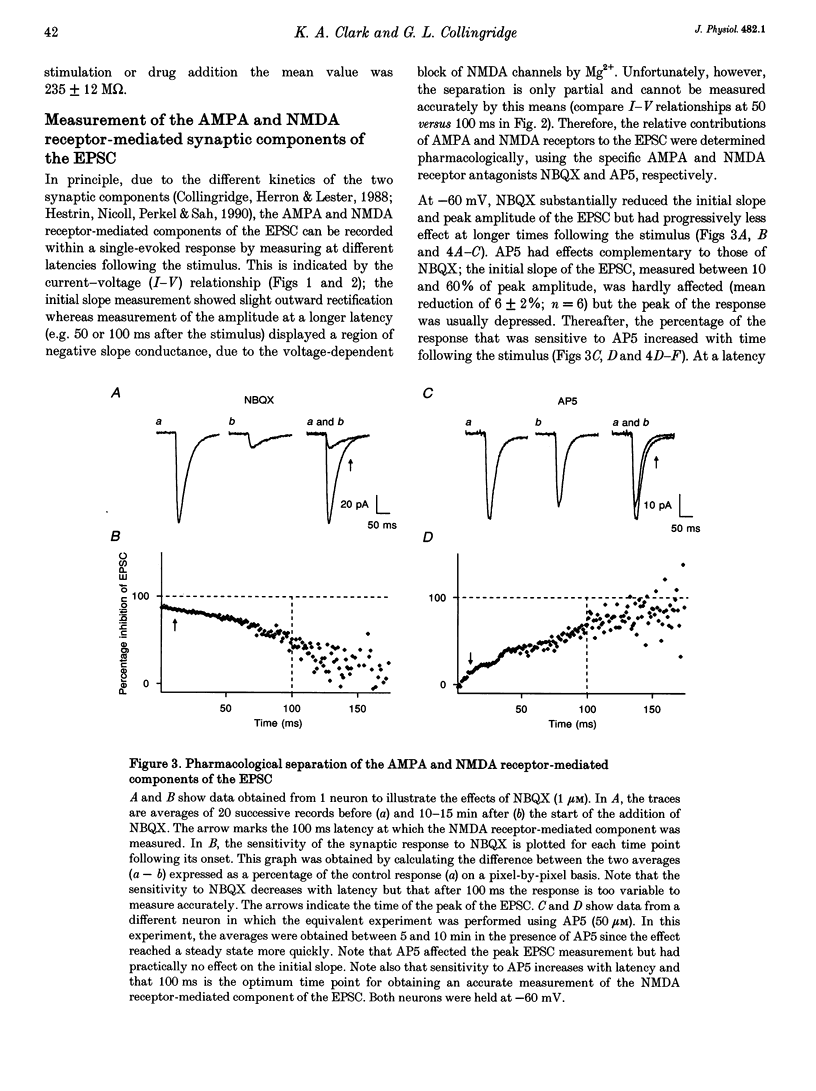
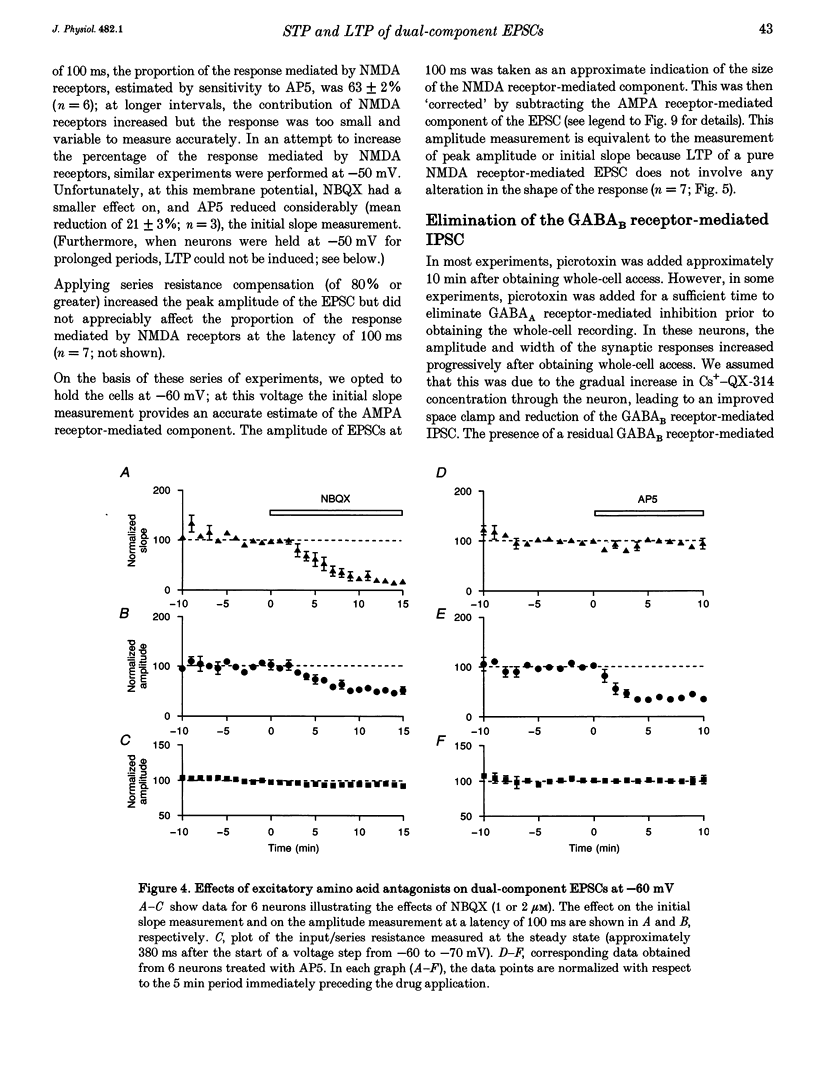
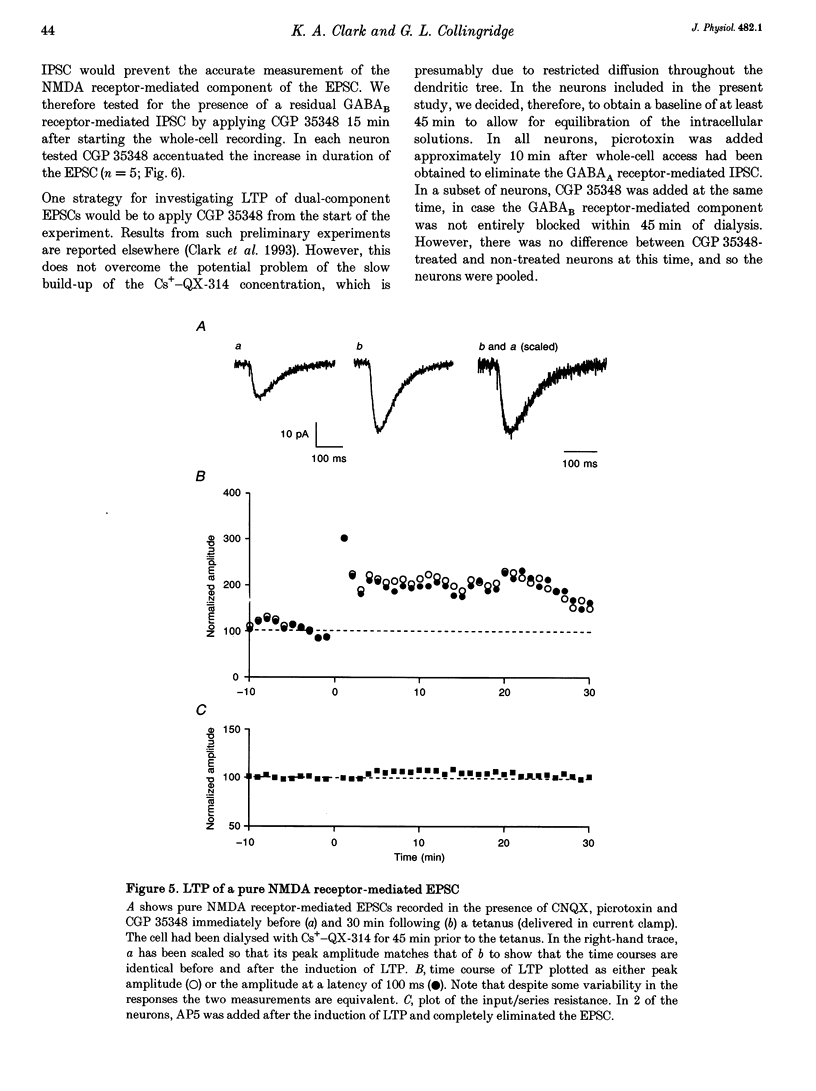
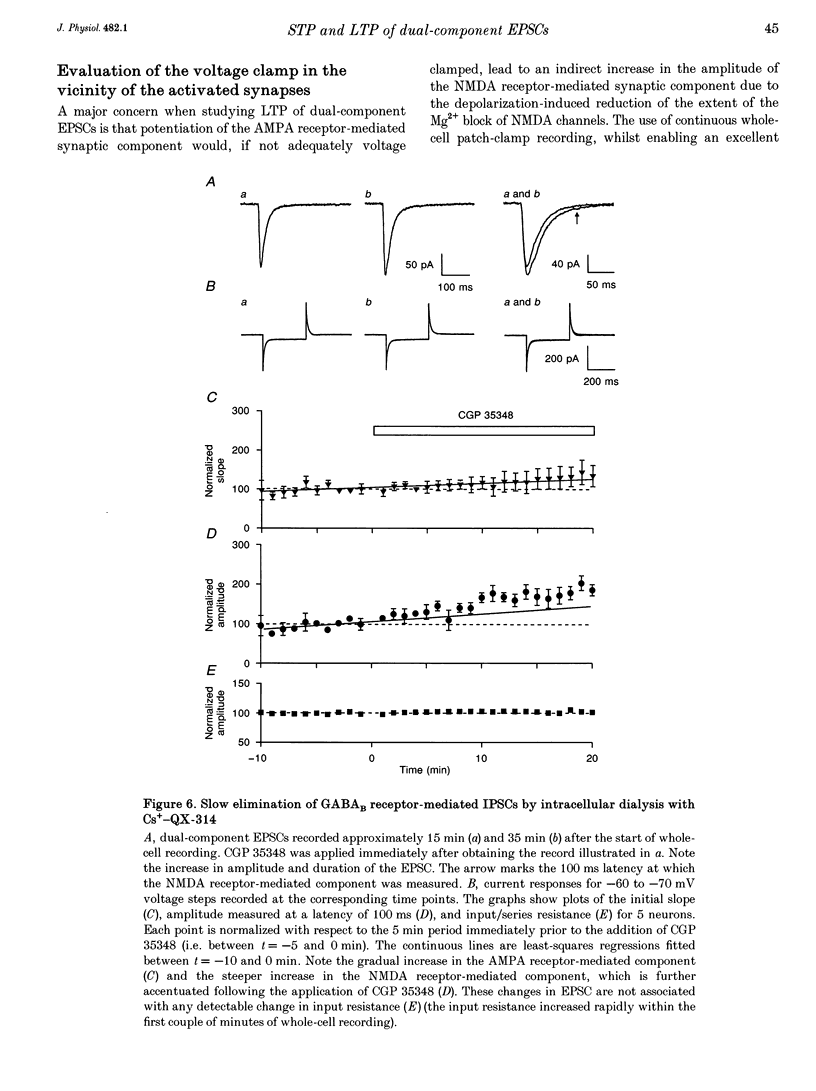
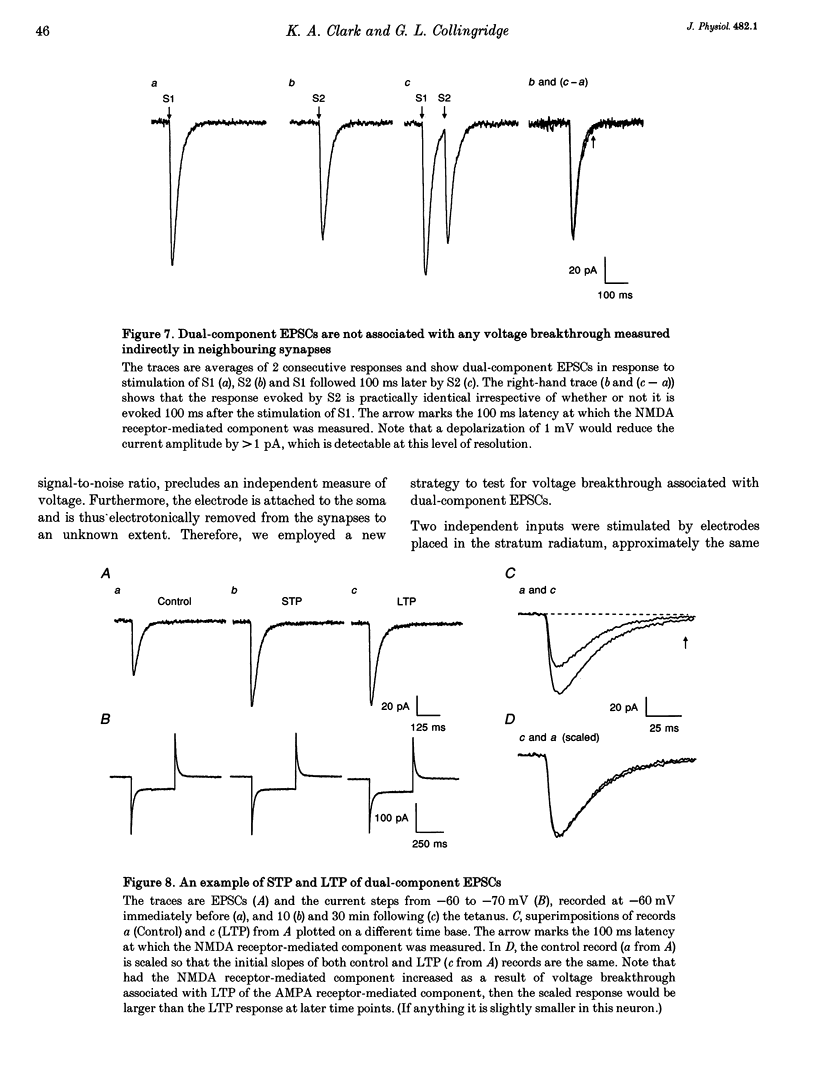
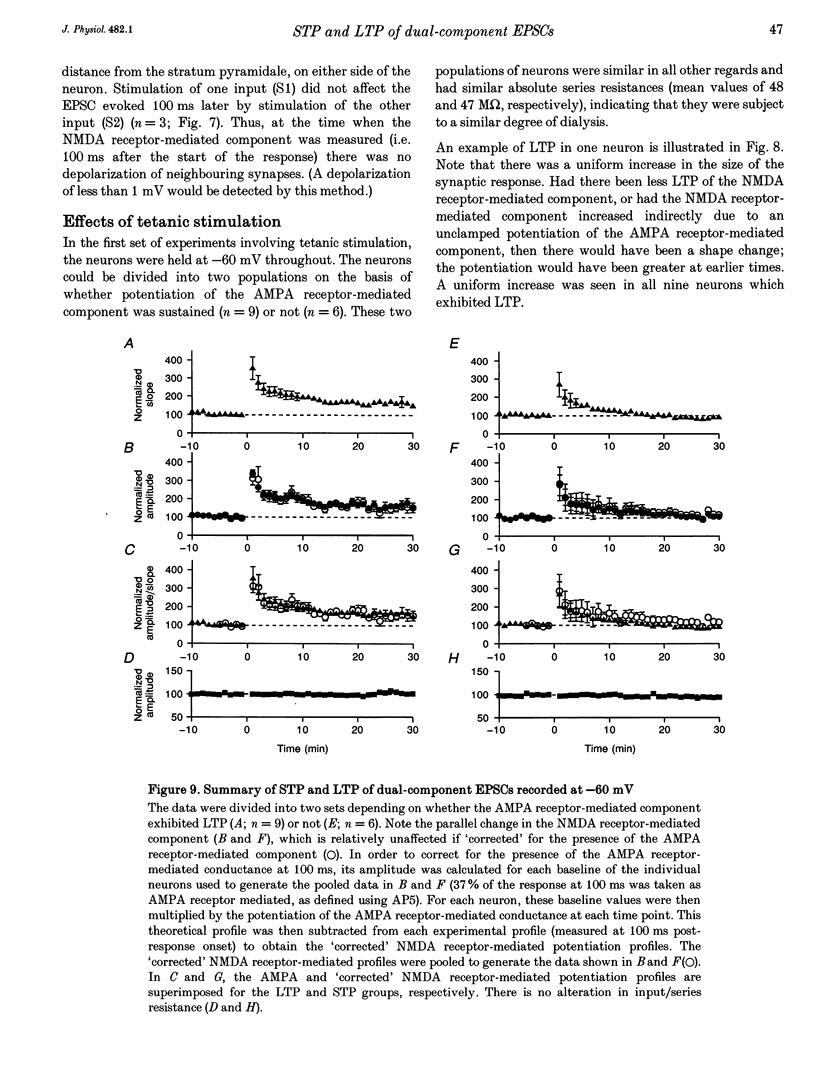
1. Whole-cell patch-clamp recording has been used to study tetanus-induced synaptic potentiation of dual-component excitatory postsynaptic currents (EPSCs) in the CA1 region of rat hippocampal slices, following blockade of GABAA and GABAB receptor-mediated synaptic inhibition. 2. At a holding potential of -60 mV, the initial slope of the EPSC (between 10 and 60% of maximum amplitude) provided an accurate measurement of the AMPA receptor-mediated component, and the amplitude of the EPSC at a latency of 100 ms provided the best estimate of the size of the NMDA receptor-mediated component. 3. Neurons were voltage clamped for at least 45 min prior to delivery of a tetanus (test intensity, 100 Hz, 1 s). Measurements at 10 and 30 min following the tetanus were used as indications of short-term potentiation (STP) and long-term potentiation (LTP), respectively. One set of neurons were voltage clamped at -60 mV throughout. These neurons could be subdivided into two populations on the basis of whether or not there was LTP (n = 9), or only STP (n = 6), of the AMPA receptor-mediated component. A second set of neurons were voltage clamped at -60 mV for 30 min and then at -50 mV for 15 min before, during and for 30 min following tetanization. In these experiments there was STP but not LTP (n = 8). 4. In all neurons (n = 23), the time course of the potentiation of the NMDA receptor-mediated component paralleled that of the AMPA receptor-mediated component. In addition, potentiation of the NMDA and AMPA receptor-mediated components were of a similar magnitude. 5. These data demonstrate that it is possible to induce LTP by high frequency stimulation after 45 min of whole-cell recording. Under these conditions, there is a parallel potentiation of the AMPA and NMDA receptor-mediated components of dual-component EPSCs. This constitutes the first evidence, from studies of dual-component synaptic responses, which is consistent with a presynaptic locus of expression of tetanus-induced STP and LTP in the hippocampus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford S., Frenguelli B. G., Schofield J. G., Collingridge G. L. Characterization of Ca2+ signals induced in hippocampal CA1 neurones by the synaptic activation of NMDA receptors. J Physiol. 1993 Sep;469:693–716. doi: 10.1113/jphysiol.1993.sp019838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asztely Fredrik, Wigström Holger, Gustafsson Bengt. The Relative Contribution of NMDA Receptor Channels in the Expression of Long-term Potentiation in the Hippocampal CA1 Region. Eur J Neurosci. 1992;4(8):681–690. doi: 10.1111/j.1460-9568.1992.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Bashir Z. I., Alford S., Davies S. N., Randall A. D., Collingridge G. L. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991 Jan 10;349(6305):156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990 Aug 23;346(6286):724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- Berretta N., Berton F., Bianchi R., Brunelli M., Capogna M., Francesconi W. Long-term Potentiation of NMDA Receptor-mediated EPSP in Guinea-pig Hippocampal Slices. Eur J Neurosci. 1991;3(9):850–854. doi: 10.1111/j.1460-9568.1991.tb00096.x. [DOI] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Coan E. J., Irving A. J., Collingridge G. L. Low-frequency activation of the NMDA receptor system can prevent the induction of LTP. Neurosci Lett. 1989 Oct 23;105(1-2):205–210. doi: 10.1016/0304-3940(89)90038-4. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. N., Lester R. A., Reymann K. G., Collingridge G. L. Temporally distinct pre- and post-synaptic mechanisms maintain long-term potentiation. Nature. 1989 Apr 6;338(6215):500–503. doi: 10.1038/338500a0. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Y., Colino A., Selig D. K., Malenka R. C. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992 Feb 7;255(5045):730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- Izumi Y., Clifford D. B., Zorumski C. F. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science. 1992 Aug 28;257(5074):1273–1276. doi: 10.1126/science.1519065. [DOI] [PubMed] [Google Scholar]
- Kato K., Clifford D. B., Zorumski C. F. Long-term potentiation during whole-cell recording in rat hippocampal slices. Neuroscience. 1993 Mar;53(1):39–47. doi: 10.1016/0306-4522(93)90282-k. [DOI] [PubMed] [Google Scholar]
- Kauer J. A., Malenka R. C., Nicoll R. A. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988 Dec;1(10):911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M., Nicoll R. A. Long-term potentiation is associated with increases in quantal content and quantal amplitude. Nature. 1992 May 21;357(6375):240–244. doi: 10.1038/357240a0. [DOI] [PubMed] [Google Scholar]
- Larkman A., Hannay T., Stratford K., Jack J. Presynaptic release probability influences the locus of long-term potentiation. Nature. 1992 Nov 5;360(6399):70–73. doi: 10.1038/360070a0. [DOI] [PubMed] [Google Scholar]
- Liao D., Jones A., Malinow R. Direct measurement of quantal changes underlying long-term potentiation in CA1 hippocampus. Neuron. 1992 Dec;9(6):1089–1097. doi: 10.1016/0896-6273(92)90068-o. [DOI] [PubMed] [Google Scholar]
- Lovinger D. M., Routtenberg A. Synapse-specific protein kinase C activation enhances maintenance of long-term potentiation in rat hippocampus. J Physiol. 1988 Jun;400:321–333. doi: 10.1113/jphysiol.1988.sp017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C. Postsynaptic factors control the duration of synaptic enhancement in area CA1 of the hippocampus. Neuron. 1991 Jan;6(1):53–60. doi: 10.1016/0896-6273(91)90121-f. [DOI] [PubMed] [Google Scholar]
- Malinow R., Tsien R. W. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990 Jul 12;346(6280):177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Muller D., Arai A., Lynch G. Factors governing the potentiation of NMDA receptor-mediated responses in hippocampus. Hippocampus. 1992 Jan;2(1):29–38. doi: 10.1002/hipo.450020105. [DOI] [PubMed] [Google Scholar]
- Muller D., Joly M., Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988 Dec 23;242(4886):1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- Muller D., Lynch G. Long-term potentiation differentially affects two components of synaptic responses in hippocampus. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9346–9350. doi: 10.1073/pnas.85.23.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., Lynch G. Synaptic modulation of N-methyl-D-aspartate receptor mediated responses in hippocampus. Synapse. 1990;5(2):94–103. doi: 10.1002/syn.890050203. [DOI] [PubMed] [Google Scholar]
- O'Connor J. J., Rowan M. J., Anwyl R. Long-lasting enhancement of NMDA receptor-mediated synaptic transmission by metabotropic glutamate receptor activation. Nature. 1994 Feb 10;367(6463):557–559. doi: 10.1038/367557a0. [DOI] [PubMed] [Google Scholar]
- Perkel D. J., Nicoll R. A. Evidence for all-or-none regulation of neurotransmitter release: implications for long-term potentiation. J Physiol. 1993 Nov;471:481–500. doi: 10.1113/jphysiol.1993.sp019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall A. D., Collingridge G. L. Amino acid receptor-mediated synaptic currents in the CA1 region of the hippocampus. Ion Channels. 1992;3:63–81. doi: 10.1007/978-1-4615-3328-3_3. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Clements J. D., Westbrook G. L. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993 Oct 29;262(5134):754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. Locally distributed synaptic potentiation in the hippocampus. Science. 1994 Jan 28;263(5146):532–536. doi: 10.1126/science.8290963. [DOI] [PubMed] [Google Scholar]
- Spruston N., Jaffe D. B., Johnston D. Dendritic attenuation of synaptic potentials and currents: the role of passive membrane properties. Trends Neurosci. 1994 Apr;17(4):161–166. doi: 10.1016/0166-2236(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Malinow R. Long-term potentiation: presynaptic enhancement following postsynaptic activation of Ca(++)-dependent protein kinases. Cold Spring Harb Symp Quant Biol. 1990;55:147–159. doi: 10.1101/sqb.1990.055.01.018. [DOI] [PubMed] [Google Scholar]
- Voronin L. L., Kuhnt U., Gusev A. G., Hess G. Quantal analysis of long-term potentiation of "minimal" excitatory postsynaptic potentials in guinea pig hippocampal slices: binomial approach. Exp Brain Res. 1992;89(2):275–287. doi: 10.1007/BF00228244. [DOI] [PubMed] [Google Scholar]
- Xie X., Berger T. W., Barrionuevo G. Isolated NMDA receptor-mediated synaptic responses express both LTP and LTD. J Neurophysiol. 1992 Apr;67(4):1009–1013. doi: 10.1152/jn.1992.67.4.1009. [DOI] [PubMed] [Google Scholar]



