Abstract
Background
Total shoulder arthroplasty (TSA) with a nonspherical humeral head component and inlay glenoid is a bone preserving treatment for glenohumeral arthritis. This study aims to describe minimum two year patient reported outcomes, patient acceptable symptomatic state (PASS) achievement, and complications following TSA with this prosthesis.
Methods
A retrospective review of patients undergoing TSA with nonspherical humeral head and inlay glenoid was performed. Outcomes included Single Assessment Numeric Evaluation (SANE) scores, American Shoulder and Elbow Surgeons (ASES) scores, and complications. SANE and ASES scores were compared to established PASS threshold values to determine PASS achievement.
Results
56 TSA in 53 patients were identified. The mean age was 64.5 years, 64% were male, and mean follow-up was 29.2 ± 4.9 months (24.0–42.8). Two complications (3.6%) were observed: one subscapularis tear requiring revision to reverse TSA and one traumatic minimally displaced greater tuberosity fracture successfully treated nonoperatively. The mean SANE score was 84.3 ± 16.9 (40–100) and 77% of patients surpassed the PASS threshold of 75.5. The mean ASES score was 85.3 ± 15.7 (40–100) and 77% of patients surpassed the PASS threshold of 76.
Discussion
Patients undergoing TSA with a nonspherical humeral head and inlay glenoid demonstrated high PASS achievement rates and few complications at short-term follow-up.
Keywords: Total shoulder arthroplasty, nonspherical humeral head, inlay glenoid, complications, patient reported outcomes
Introduction
Total shoulder arthroplasty (TSA) is an effective surgical treatment for end stage glenohumeral osteoarthritis which has failed conservative management.1,2 Anatomic TSA prosthesis designs are evolving and the current options include stemmed and stemless anatomic TSA, and humeral head resurfacing components with a glenoid replacement.3–5 There is a growing trend towards the use of bone preserving TSA prostheses as alternatives to traditional stemmed prostheses.6–8 Advantages of bone preserving prostheses, including humeral head resurfacing designs and stemless TSA, include shorter anesthesia and operative time, less intraoperative blood loss, a lower risk of periprosthetic fracture, decreased stress shielding, and preserved anatomy for easier revision procedures.3,4,8–13 Potential disadvantages however include a reliance on adequate proximal humeral bone stock. 3
TSA with a nonspherical humeral head and an inlay glenoid is one available bone preserving prosthesis option. This implant combination aims to replicate native humeral and glenoid anatomy, restore natural shoulder movement, and improve component stability.14,15 Initial reports from the few studies available describing this prosthesis suggest excellent short term outcomes however the current evidence is limited.4,16–18 There is a paucity of literature on this implant describing complications, patients reported outcomes (PROs), and patient acceptable symptomatic state (PASS) achievement, which is one of the most widely validated and important tools for demonstrating clinically relevant success following TSA. 19 The purpose of this study is to assess minimum two year PROs reported outcome measures, PASS achievement, and complications following TSA with a nonspherical humeral head and an inlay glenoid. We hypothesized that patients would experience high PASS achievement rates and few postoperative complications after undergoing TSA with this prosthesis.
Materials and methods
Patient selection
Institutional review board (IRB) approval was obtained for this study (IRB #5248). A retrospective chart review was conducted in a consecutive cohort of patients who underwent TSA with a nonspherical humeral head implant and an inlay glenoid replacement (Hemi-CAP OVO/Inlay Glenoid Total Shoulder System; Arthrosurface, Franklin, MA, USA). Procedures were performed by a single surgeon between November 2017 and June 2021. Inclusion criteria were patients with primary glenohumeral osteoarthritis refractory to conservative treatment with an intact rotator cuff. Patients with Walch type A1, A2, B1, B2, and B3 glenoids, as diagnosed on preoperative axillary radiographs, were included. 20 Axillary radiographs have substantial agreement with computed tomography scans and can be used effectively for glenoid staging. 21 Use of this implant in Walch type A1, A2, B1, B2, and B3 glenoids is supported in the literature.4,16–18 Exclusion criteria included rotator cuff tear arthropathy, proximal humeral bone deficiency or deformity prohibitive to humeral head component fixation, and Walch C glenoids. All anatomic TSA candidates who met the inclusion criteria which treated with this prosthesis during the study period.
Surgical technique 22
All TSA utilized a nonspherical humeral head implant with an all-polyethylene inlay glenoid component. Patients receive regional anesthesia with or without general anesthesia, as determined by anesthesiologist recommendations, and are placed in the beach-chair position. A standard deltopectoral approach with a subscapularis tenotomy and biceps tenodesis is performed. Sizing guides are used to determine the true superoinferior (SI) and anteroposterior (AP) dimensions of the humeral head. For the nonspherical humeral head component, the SI dimension is 4 mm larger than the AP dimension, with varying radiuses of curvature. After sizing, a guide pin is placed matching the patient's native version and inclination. The humeral head is reamed to match the spherical undersurface of the component and a tapered post is inserted into the humerus. The glenoid is exposed and the reamer guide pin is placed on the center point on the glenoid. The glenoid is reamed ensuring that the glenoid trial sits flush with the surrounding native glenoid. The glenoid component is implanted using third-generation cement technique. Attention is returned to the humerus and the definitive ovoid humeral head component is impacted over the tapered post, engaging the morse taper. The subscapularis is repaired and standard closure is performed.
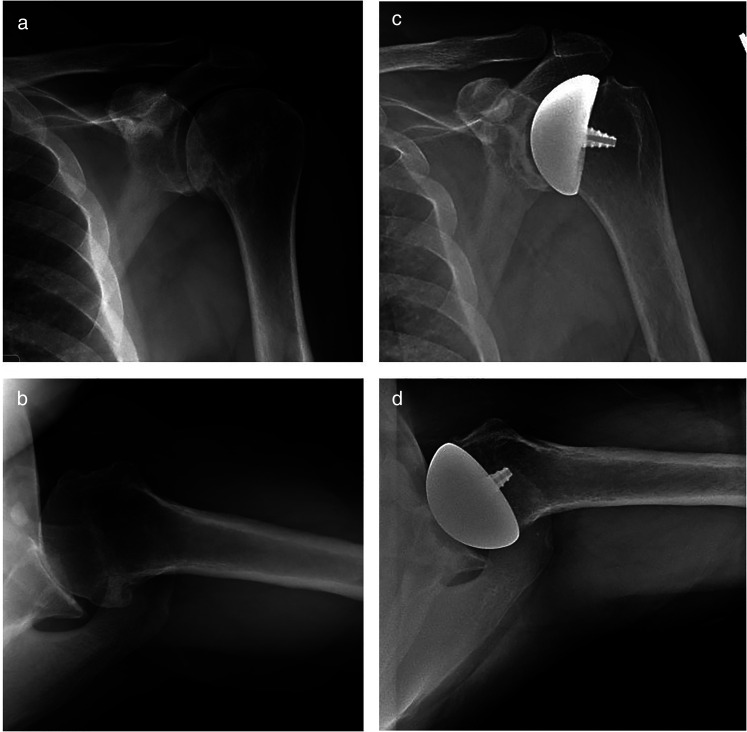
Preoperative and postoperative anterior-posterior and axillary radiographs are presented in Figure 1. Postoperatively, the patients are placed into a sling and are immediately allowed pendulum exercises and range of motion of the elbow, wrist, and hand. At two weeks postoperatively, wall walks are allowed and formal physical therapy with a rotator cuff strengthening program is initiated. External rotation is limited for six weeks to protect the subscapularis repair.
Figure 1.
Preoperative anteroposterior (a) and axillary (b) radiographs demonstrating glenohumeral osteoarthritis. Postoperative anteroposterior (c) and axillary (d) radiographs following humeral head resurfacing arthroplasty with a non-spherical humeral head and an inlay glenoid component.
Data collection
Baseline patient characteristics were collected from electronic medical records (EMR) and included age, sex, body mass index (BMI), Charlson Comorbidity Index (CCI) score.
All patients who underwent surgery were invited to complete electronic PRO and complication reporting which was integrated into the EMR and was available for review. All patients were also contacted via telephone to complete a last follow-up questionnaire which included final PROs and complications. Patients were deemed lost to follow up if they failed to complete electric surveys, did not return to the office greater than two years following their TSA, or remained unreachable by telephone after multiple contact attempts.
Collected complications included dislocations, wound complications, infections, rotator cuff failure, tenodesis failure, humerus or glenoid implant failures, periprosthetic fractures, arthrofibrosis, and all cause reoperations. Collected PROs included Single Assessment Numeric Evaluation (SANE) scores and American Shoulder and Elbow Surgeons (ASES) scores. The SANE and ASES scores are valid and reliable PROs following TSA.19,23–25 The patient acceptable symptom state (PASS) was developed to improve interpretation of PROs. The PASS is defined as an absolute patient reported outcome threshold which is associated with achievement of a clinical outcome that the patient considers acceptable. 19 Previous studies have demonstrated that PASS is a reliable metric to predict postoperative satisfaction following TSA.23–25 PASS thresholds of 75.5 for the SANE score 24 and 76 for the ASES score 23 two years following TSA have been established from prior studies. The postoperative SANE and ASES scores from our cohort were compared to these established threshold SANE and ASES threshold scores to evaluate PASS achievement. Preoperative SANE and ASES scores were not available in our patient cohort; thus, preoperative and postoperative comparisons, minimally clinically important difference (MCID), and substantial clinical benefits could not be provided and are not described.
Statistical analysis
Descriptive statistics of continuous variables were reported with mean, standard deviation, and range. Descriptive statistics of categorical variables were reported with frequencies and percentages. Categorical variables were compared using chi-squared tests and continuous variables were compared using two tailed t-tests and Fisher Exact tests as statistically appropriate. P values < 0.05 were considered to be statistically significant. All analyses were conducted with use of JMP software (Version 12; SAS Institute).
Results
Patient demographics
During the study period, TSA with nonspherical HH and an inlay glenoid component was performed in 74 shoulders in 71 patients. Data on 56 shoulders (76%) in 53 patients with minimum two year follow-up were available for analysis and included in this study. 18 patients (24%) were lost to follow-up. Patient demographics are presented in Table 1. The mean age was 64.5 ± 9.2 years (40–80). 36 (64%) of patients were male, and mean BMI was 31.2 ± 6.3 kg/m2 (20.3–52.0). Mean CCI score was 2.8 ± 1.6 (0–7). Mean follow-up was 29.2 ± 4.9 months (24.0–42.8).
Table 1.
Patient characteristics.
| Patient Characteristics | |
|---|---|
| Number of shoulders: | 56 |
| Age (years): Mean ± SD (Range) | 64.5 ± 9.2 (40–80) |
| Gender | |
| Male: (n, %) | 36/56 (64%) |
| Female: (n, %) | 20/56 (36%) |
| BMI (kg/m2): Mean ± SD (Range) | 31.2 ± 6.3 (20.3–52.0) |
| Charlson Comorbidity Index: Mean ± SD (Range) | 2.8 ± 1.6 (0–7) |
| Length of Follow Up (months): Mean ± SD (Range) | 29.2 ± 4.9 (24.0–42.8) |
BMI: Body Mass Index; SD: Standard Deviation.
Complications
Minimum two year complications are presented in Table 2. Two complication (3.6%) were observed. One patient sustained a late atraumatic subscapularis tear without dislocation 28 months after the index surgery requiring reoperation and conversion to reverse TSA. One patient sustained a minimally displaced greater tuberosity fracture following a fall 27 months after the index surgery which was successfully treated nonoperatively. There were no dislocations, posterosuperior rotator cuff tears, implant failures, infections, tenodesis failures, arthrofibrosis, or other reoperations.
Table 2.
Minimum 2 year complications and patient reported outcomes measures.
| Measures | Value |
|---|---|
| Complication Rates: | |
| Overall Complication Rate: (n, %) | 2/56 (3.6%) |
| Posterosuperior rotator cuff failure, dislocation, infection, implant failure, arthofibrosis | 0/56 (0%) |
| Fracture (minimally displaced greater tuberosity) | 1/56 (1.8%) |
| Subscapular failure, Conversion to RTSA | 1/56 (1.8%) |
| All Cause RTOR | 1/56 (1.8%) |
| Patient Reported Outcomes: | |
| SANE Score: Mean ± SD (Range) | 84.3 ± 16.9 (40–100) |
| SANE PASS Achievement (SANE > 75.5) | 43/56 (76.8%) |
| ASES Score: Mean ± SD (Range) | 85.3 ± 15.7 (40–100) |
| ASES PASS Achievement by (ASES > 76) | 41/53 (77.4%) |
ASES: American Shoulder and Elbow Surgeons; BMI: Body Mass Index; PASS: Patient Acceptable Symptom State; RTOR: Return to Operating Room; RTSA: Reverse Total Shoulder Arthroplasty; SANE: Single Assessment Numeric Evaluation; SD: standard deviation.
Patient reported outcome measures
PROs are presented in Table 2. Last follow-up and time for completion of SANE and ASES scores was a mean of 29.2 ± 4.9 months (24.0–42.8) postoperatively. 53 patients completed both SANE and ASES questionnaires; 3 patients only completed the SANE questionnaires. The mean SANE score was 84.3 ± 16.9 (40–100). 43/56 (76.8%) of patients surpassed the established SANE threshold score of 75.5 to achieve the PASS. 24 The mean ASES score was 85.3 ± 15.7 (40–100). 41/53 (77.4%) of patients surpassed the established ASES threshold score of 76 to achieve the PASS. 23 There were no differences in baseline age, gender, BMI, CCI, or glenoid Walch classification between patients who achieved or failed to achieve a PASS by either ASES or SANE score (Table 3). Both patients who suffered complications failed to achieve the PASS by ASES and SANE.
Table 3.
Comparison of patients who achieved or failed to achieve PASS by SANE and ASES.
| SANE PASS | |||
|---|---|---|---|
| Patient Characteristics | Achieved PASS (n = 43) | Failed to meet PASS (n = 13) | P Value |
| Age (years): Mean ± SD | 64.8 ± 9.0 | 63.5 ± 10.4 | 0.327 |
| Gender: Female (n, %) | 17 (39.5%) | 3 (23.1%) | 0.278 |
| BMI (kg/m2): Mean ± SD | 31.0 ± 6.0 | 31.6 ± 7.7 | 0.378 |
| Charlson Comorbidity Index: Mean ± SD | 2.9 ± 1.7 | 2.2 ± 1.4 | 0.177 |
| ASES PASS | |||
| Patient Characteristics | Achieved PASS (n = 41) | Failed to meet PASS (n = 12) | P Value |
| Age (years): Mean ± SD | 64.9 ± 9.1 | 62.9 ± 10.2 | 0.263 |
| Gender: Female (n, %) | 17 (41.5%) | 2 (16.7%) | 0.115 |
| BMI (kg/m2): Mean ± SD | 31.4 ± 5.9 | 30.9 ± 8.3 | 0.413 |
| Charlson Comorbidity Index: Mean ± SD | 2.8 ± 1.7 | 2.6 ± 1.3 | 0.312 |
ASES: American Shoulder and Elbow Surgeons; BMI: Body Mass index; PASS: Patient Acceptable Symptomatic State; SANE: Single Assessment Numeric Evaluation; SD: Standard deviation.
Discussion
In this study, anatomic TSA with a nonspherical humeral head component and an inlay glenoid demonstrated high PASS achievement rates and few complications at an average of 29.2 months follow-up. The majority of patients were satisfied with their shoulder. Only two complications were observed and there were no dislocations, posterosuperior rotator cuff tears, or implant failures.
Our study strengthens the growing literature evaluating the use of TSA with nonspherical humeral head and inlay glenoid replacement for treatment of glenohumeral osteoarthritis. There are few available studies in the literature evaluating the use of the prosthesis described in this study; however, each have reported excellent patient clinical outcomes, postoperative complications, and radiographic follow-up.4,16–18 Cvetanovich et al., in an analysis of 27 shoulders in a young active patient population, demonstrated improvements in all patient reported outcomes, improved range of motion, high rates of return to activity, and no reoperations or radiographic signs of loosening. 16 Mean follow up was 40.4 months, mean ASES score improved from 39.5 preoperatively to 85.7 postoperatively, and mean SANE score improved from 25.1 preoperatively to 80.4 postoperatively. Egger et al., in an analysis of 31 shoulders with both concentric and nonconcentric glenoids, demonstrated significant clinical benefit in all patients, with no readmissions and only 1 reoperation – open biceps tenodensis following biceps rupture. 4 Mean follow up was 42.6 months, mean Penn Shoulder Score Total improved from 42.2 preoperatively to 88.6 postoperatively, and mean visual analog scale for pain (VAS-Pain) improved from 6.4 preoperatively to 1.0 postoperatively. Yalcin et al., in an analysis of 29 patients with eccentric glenoid wear and posterior subluxation, demonstrated consistent glenohumeral re-centering at mean follow up of 37.9 months. That study did however report an overall complication rate of 10.3%, including two infections, one of which required a revision. 18 Lastly Uribe et al., in an analysis of 39 shoulders with various glenoid morphology types also demonstrated significant functional improvement, excellent pain relief, and patient satisfaction. 17 Mean follow up was 41.0 months, mean ASES score improved from 29.9 preoperatively to 77.1 postoperatively, and mean VAS-Pain improved from 8.1 preoperatively to 1.5 postoperatively. At the final assessment, 94.3% patients met or exceeded the ASES MCID, and 87.1% met or exceeded or exceeded VAS-Pain MCID. Between all studies, no shoulder dislocations or signs of radiographic loosening were reported.
A novel contribution from our study is the evaluation of PASS achievement rates as defined by SANE and ASES scores, which had not been described in the previously listed studies evaluating this implant. In our study, 77% of patients achieved the PASS by SANE score and 77% achieved the PASS by ASES score at minimum two year follow-up. The percentages of patients successfully achieving the PASS for SANE and ASES in this cohort is similar to the previous studies evaluating PASS following TSA with other implants. Cole et al. evaluated 301 patients two years following TSA with short or standard length humeral stems reported that 69% of patients achieved SANE PASS and 87% achieved ASES PASS. 19 Gowd et al. evaluated 207 patients one year following TSA or reverse TSA reported that 78% of patients achieved SANE PASS and 66% of patients achieved ASES PASS. 24 Finally, Polce et al. evaluated 204 patients two years following TSA or reverse TSA reported that 57% of patients achieved SANE PASS and 71% achieved ASES PASS. 25 Overall, the SANE and ASES PASS achievement rates reported in our study are within the ranges previously reported in the TSA literature. While analysis of PASS achievement is encouraging, the lack of preoperative scores limits treatment outcome analysis. Preoperative and postoperative comparisons, including MCID, and substantial clinical benefits could not be provided and may have enabled further analysis.
In our cohort, two complications (3.6%) and one reoperation (1.8%) were observed: one patient sustained a late atraumatic subscapularis tear without dislocation at 28 months postoperatively requiring conversion to reverse TSA and one patient sustaining a minimally displaced greater tuberosity fracture following at 27 months postoperatively which was successfully treated nonoperatively. There were no, posterosuperior rotator cuff tears, implant failures, infections, arthrofibrosis, or other reoperations. Bohsali et al. in a meta-analysis including 19,262 TSA and RSA reported a complication rate of 10.3% at a mean follow-up of 40.3 months following TSA. 26 The most common complications were component loosening (4.0%), glenoid wear (2.3%), instability (1.0%), and rotator cuff tear (0.9%). Parada et al. in a database analysis of 2224 TSA reported complication rate and revision rates at a mean of 34 months to be 10.7% and 5.6%, respectively. 27 Rotator cuff tear / subscapularis failure was the most common complication, occurring in 3.1% of patients at a mean of 23 months postoperatively. In the literature evaluating stemless TSA, a meta-analysis including 962 shoulders reported a complication rate of 8.3% and revision rate of 5.6%, and no statistical difference in complications between stemless and stemmed TSA. 8 The most common complication and indication for revision was rotator cuff failure. The rates of complications and revisions from our study compare similarly to reported values for stemmed and stemless TSA. However, our small cohort size and shorter follow-up period limits this comparison.
The TSA prosthesis used in this study utilizes a combination of a nonspherical humeral head and an inlay glenoid, which may have several advantages over the traditional TSA combination of a spherical humeral and an onlay glenoid. 4 Cadaveric and biomechanical studies show that the native humeral head is nonspherical and that ovoid humeral head components have a 3 times better fit than spherical components.15,28,29 Furthermore, a nonspherical humeral head more closely replicates the natural anatomic center of rotation of the glenohumeral joint, thus more accurately restores native anatomy and contact mechanics. 30 The glenoid component is a historic area of concern and glenoid loosening is citied as the most common reason for revision surgery. 4 Repetitive eccentric loading on the onlay glenoid can lead to the “rocking-horse phenomenon” which leads to glenoid loosening. 14 Unlike the onlay glenoid, the inlay glenoid design is implanted to sit flush with the adjacent, native glenoid articular surface. This decreases implant edge loading and lift off forces responsible for glenoid loosening. Cadaveric studies comparing onlay and inlay glenoid components have demonstrated improved glenoid biomechanical stability and decreased loosening with an inlay glenoid.14,31 Clinically, medium and long term studies of inlay glenoid have confirmed low rates of revision due to glenoid loosening.32,33 Use of this implant may have particular utility in younger, active males who are at higher risk for early glenoid wear and loosening. 16 In our study, there were no implant failures or loosening however, 29.2 months follow-up may be too early to fully assess this outcome. In addition, for our study, all anatomic TSA candidates who met the inclusion criteria were treated with this prosthesis regardless of age and gender. There were no age, gender, or other demographic differences between patients who achieved or failed to achieve PASS suggesting the wide demographic applicability of this implant.
The strengths of our study are that we report PROs and complications on the largest cohort to receive this TSA prosthesis to date. Furthermore, we are the first to our knowledge to report on PASS achievement rates following the use of this implant. However, despite including 56 patients, our cohort size is small in comparison to available studies evaluating stemless and traditional stemmed TSA. Furthermore, our study averaged only 29.2 month follow-up; additional patient recruitment and long-term follow-up are warranted.
In addition, our study is limited by the lack of reoperative SANE and ASES scores. Postoperative SANE and ASES scores were reported, however, the absence of preoperative scores limits patient baseline evaluation and analysis of treatment effects. Postoperative SANE and ASES scores were compared to established PASS thresholds, and new literature may report changing SANE and ASES threshold cutoff scores to achieve PASS. In addition, PASS thresholds may inaccurately estimate patient satisfaction and may not be generalizable to all patient populations. Finally, while two year range of motion measurements was available for most patients, they were not universally available; and thus these are not reported.
Conclusion
Patients undergoing TSA with a nonspherical humeral component and an inlay glenoid experienced few complications and achieved PASS for SANE and ASES at a high rate at minimum two year follow up. Our study provides additional short term evidence supporting the successful use of this implant as a treatment option for glenohumeral arthritis.
Footnotes
Manuscript category: Original Article.
Previous communication to a society or meeting: None.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JPZ reports a relationship with Arthrosurface that includes speaking and lecture fees, unrelated to this submitted work. All other authors, their immediate families, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article. There was no funding source for this study.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Andrew D Posner https://orcid.org/0000-0002-6021-3273
References
- 1.Brolin TJ, Cox RM, Zmistowski BM, et al. Surgeons’ experience and perceived barriers with outpatient shoulder arthroplasty. J Shoulder Elbow Surg 2018; 27: S82–S87. [DOI] [PubMed] [Google Scholar]
- 2.Antonacci CL, Cu BJ, Erickson BJ, et al. Complications and readmissions after reverse and anatomic total shoulder arthroplasty with same-day discharge. J Am Acad Orthop Surg 2021; 29: 116–122. [DOI] [PubMed] [Google Scholar]
- 3.Brabston EW, Fehringer E V, Owen MT, et al. Stemless humeral implants in total shoulder arthroplasty. J Am Acad Orthop Surg 2020; 28: e277–e287. [DOI] [PubMed] [Google Scholar]
- 4.Egger AC, Peterson J, Jones MH, et al. Total shoulder arthroplasty with nonspherical humeral head and inlay glenoid replacement: clinical results comparing concentric and nonconcentric glenoid stages in primary shoulder arthritis. JSES Open Access 2019; 3: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markarian GG, Bab AD, Uribe JW, et al. Current trends in shoulder replacement: the rational for inlay arthroplasty. Acta Shoulder Elb Surg 2016; 1: 14–19. [Google Scholar]
- 6.Wiater JM, Levy JC, Wright SA, et al. Prospective, blinded, randomized controlled trial of stemless versus stemmed humeral components in anatomic total shoulder arthroplasty: results at short-term follow-up. J Bone Joint Surg Am 2020; 102: 1974–1984. [DOI] [PubMed] [Google Scholar]
- 7.Peebles LA, Arner JW, Haber DB, et al. Glenohumeral resurfacing in young, active patients with end-stage osteoarthritis of the shoulder. Arthrosc Tech 2020; 9: e1315–e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu EY, Kord D, Horner NS, et al. Stemless anatomic total shoulder arthroplasty: a systematic review and meta-analysis. J Shoulder Elbow Surg 2020; 29: 1928–1937. [DOI] [PubMed] [Google Scholar]
- 9.Burgess DL, McGrath MS, Bonutti PM, et al. Shoulder resurfacing. J Bone Joint Surg Am 2009; 91: 1228–1238. [DOI] [PubMed] [Google Scholar]
- 10.Harmer L, Throckmorton T, Sperling JW. Total shoulder arthroplasty: are the humeral components getting shorter? Curr Rev Musculoskelet Med 2016; 9: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miniaci A, Scarcella MJ. Shoulder resurfacing for treatment of focal defects and diffuse osteoarthritis. Orthopade 2021; 50: 112–118. [DOI] [PubMed] [Google Scholar]
- 12.Mullett H, Levy O, Raj D, et al. Copeland surface replacement of the shoulder. Results of an hydroxyapatite-coated cementless implant in patients over 80 years of age. J Bone Joint Surg Br 2007; 89: 1466–1469. [DOI] [PubMed] [Google Scholar]
- 13.Saltzman BM, Leroux TS, Verma NN, et al. Glenohumeral osteoarthritis in the young patient. J Am Acad Orthop Surg 2018; 26: e361–e370. [DOI] [PubMed] [Google Scholar]
- 14.Gunther SB, Lynch TL, O’Farrell D, et al. Finite element analysis and physiologic testing of a novel, inset glenoid fixation technique. J Shoulder Elbow Surg 2012; 21: 795–803. [DOI] [PubMed] [Google Scholar]
- 15.Humphrey CS, Gale AL. Spherical versus elliptical prosthetic humeral heads: a comparison of anatomic fit. J Shoulder Elbow Surg 2018; 27: S50–S57. [DOI] [PubMed] [Google Scholar]
- 16.Cvetanovich GL, Naylor AJ, O’Brien MC, et al. Anatomic total shoulder arthroplasty with an inlay glenoid component: clinical outcomes and return to activity. J Shoulder Elbow Surg 2020; 29: 1188–1196. [DOI] [PubMed] [Google Scholar]
- 17.Uribe JW, Zvijac JE, Porter DA, et al. Inlay total shoulder arthroplasty for primary glenohumeral arthritis. JSES Int 2021; 5: 1014–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yalcin S, Scarcella M, Miniaci A. Does non-spherical humeral head with inlay glenoid re-center the glenohumeral joint? Semin Arthroplast JSES 2021; 31: 310–316. [Google Scholar]
- 19.Cole EW, Moulton SG, Werner BC, et al. Why patients fail to achieve a patient acceptable symptom state (PASS) after total shoulder arthroplasty? JSES Int 2022; 6: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walch G, Badet R, Boulahia A, et al. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 1999; 14: 756–760. [DOI] [PubMed] [Google Scholar]
- 21.Aronowitz JG, Harmsen WS, Schleck CD, et al. Radiographs and computed tomography scans show similar observer agreement when classifying glenoid morphology in glenohumeral arthritis. J Shoulder Elbow Surg 2017; 26: 1533–1538. [DOI] [PubMed] [Google Scholar]
- 22.Posner AD, Kuna MC, Carroll JD, et al. Anatomic total shoulder arthroplasty with a nonspherical humeral head and inlay glenoid: 90-day complication profile in the inpatient versus outpatient setting. Clin Shoulder Elb 2023; 26: 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chamberlain AM, Hung M, Chen W, et al. Determining the patient acceptable symptomatic state for the ASES, SST, and VAS pain after total shoulder arthroplasty. J Shoulder Elb Arthroplast 2017; 1: 2471549217720042. [Google Scholar]
- 24.Gowd AK, Charles MD, Liu JN, et al. Single assessment numeric evaluation (SANE) is a reliable metric to measure clinically significant improvements following shoulder arthroplasty. J Shoulder Elbow Surg 2019; 28: 2238–2246. [DOI] [PubMed] [Google Scholar]
- 25.Polce EM, Wolfson TS, Skallerud WK, et al. Establishing thresholds for achievement of clinically significant satisfaction at two years following shoulder arthroplasty: the patient acceptable symptomatic state. Semin Arthroplast JSES 2021; 31: 159–170. [Google Scholar]
- 26.Bohsali KI, Bois AJ, Wirth MA. Complications of shoulder arthroplasty. J Bone Joint Surg Am 2017; 99: 256–269. [DOI] [PubMed] [Google Scholar]
- 27.Parada SA, Flurin P-H, Wright TW, et al. Comparison of complication types and rates associated with anatomic and reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2021; 30: 811–818. [DOI] [PubMed] [Google Scholar]
- 28.Jun BJ, Lee TQ, McGarry MH, et al. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25: 1084–1093. [DOI] [PubMed] [Google Scholar]
- 29.Büchler P, Farron A. Benefits of an anatomical reconstruction of the humeral head during shoulder arthroplasty: a finite element analysis. Clin Biomech 2004; 19: 16–23. [DOI] [PubMed] [Google Scholar]
- 30.Hammond G, Tibone JE, McGarry MH, et al. Biomechanical comparison of anatomic humeral head resurfacing and hemiarthroplasty in functional glenohumeral positions. J Bone Joint Surg Am 2012; 94: 68–76. [DOI] [PubMed] [Google Scholar]
- 31.Gagliano JR, Helms SM, Colbath GP, et al. A comparison of onlay versus inlay glenoid component loosening in total shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26: 1113–1120. [DOI] [PubMed] [Google Scholar]
- 32.Ross M, Glasson J-M, Alexander J, et al. Medium to long-term results of a recessed glenoid for glenoid resurfacing in total shoulder arthroplasty. Shoulder Elbow 2020; 12: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunther SB, Tran SK. Long-term follow-up of total shoulder replacement surgery with inset glenoid implants for arthritis with deficient bone. J Shoulder Elbow Surg 2019; 28: 1728–1736. [DOI] [PubMed] [Google Scholar]